Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction
Simple Summary
Abstract
1. Introduction
2. Reactive Oxygen Species (ROS)
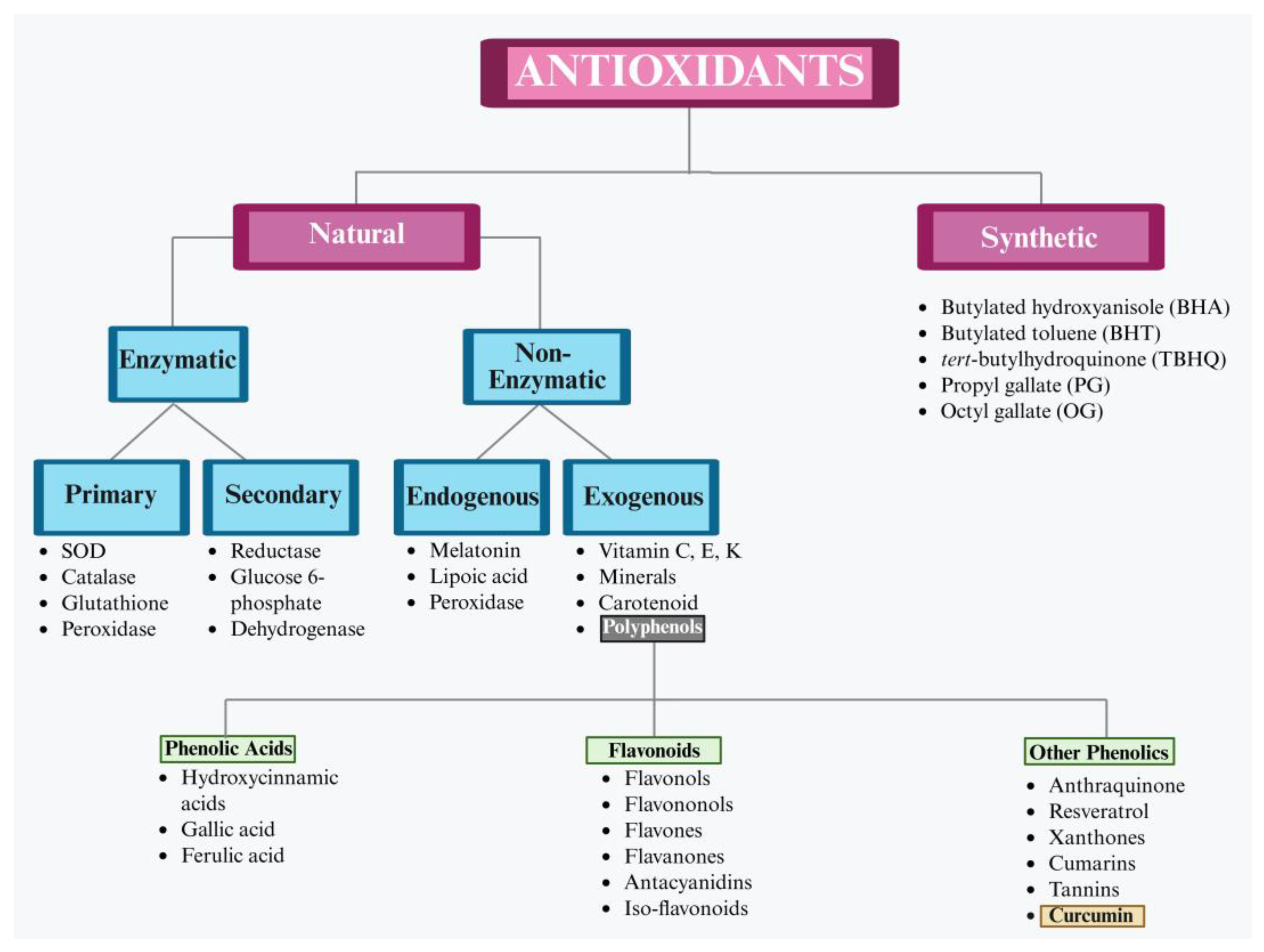
3. Need for Antioxidants
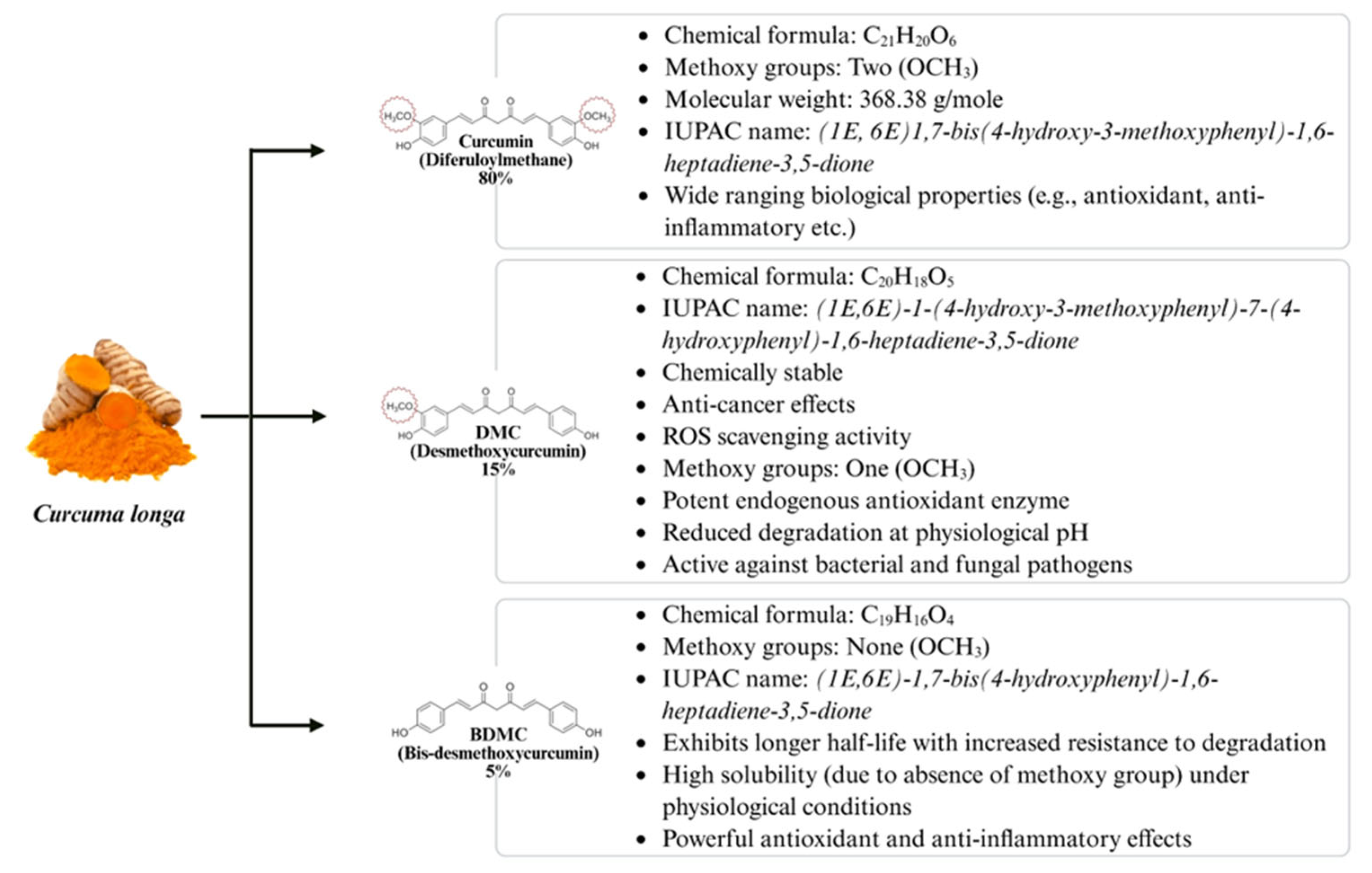
4. Curcumin
5. Curcumin’s Antioxidant Mechanism: Scavenging Free Radicals and ROS Modulation at the Cellular Level
6. Bioavailability of Curcumin
7. The Fate of Curcumin in Cell Culture Systems: Stability, Uptake and Activity
8. ROS in Ovarian Physiology and Reproductive Regulation
9. Protective Role of Antioxidants in Counteracting ROS
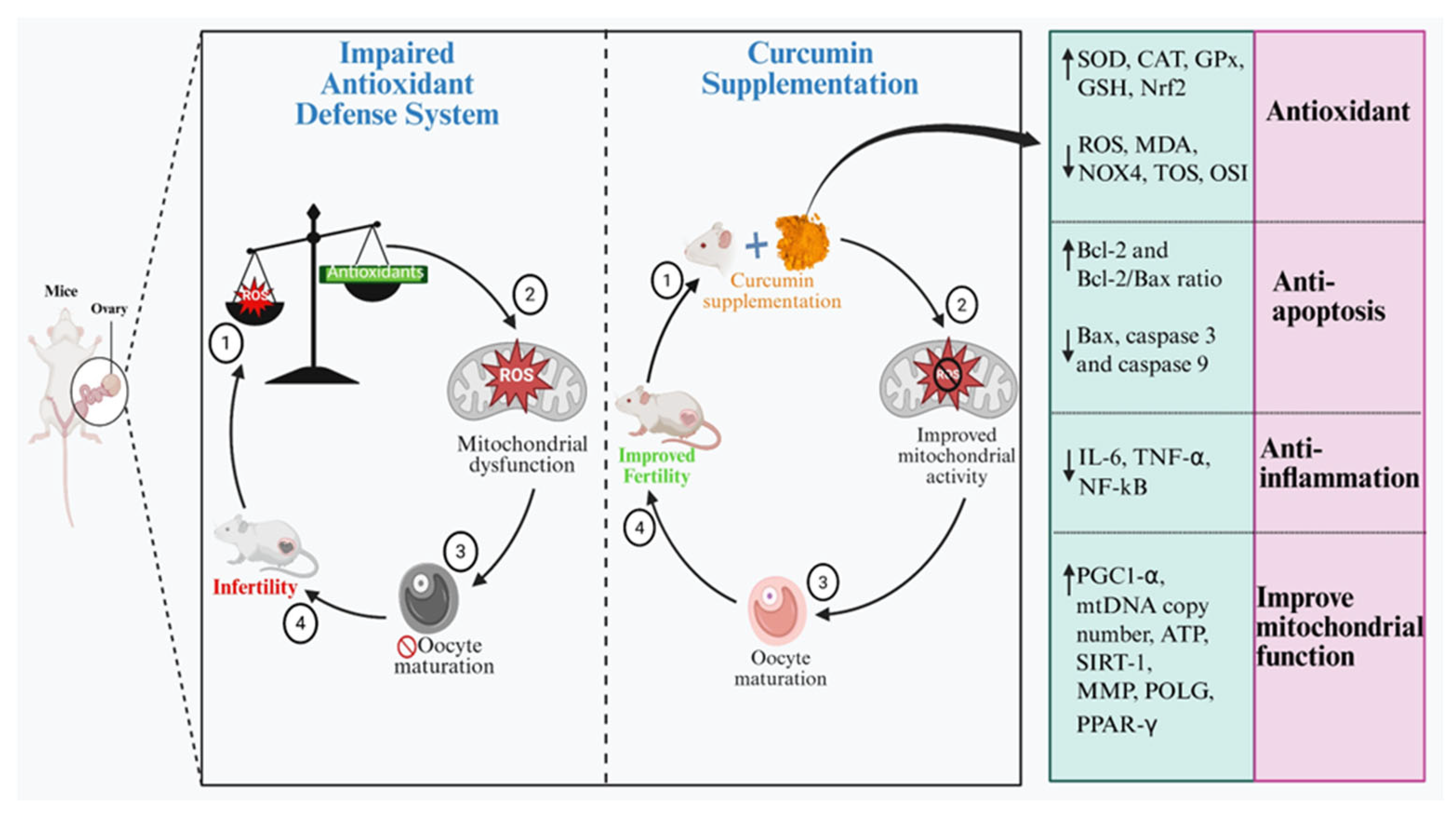
10. Modulatory Effects of Curcumin in Improving the Ovarian Function
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telfer, E.E.; Andersen, C.Y. In Vitro Growth and Maturation of Primordial Follicles and Immature Oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Ara, C.; Butt, A.N.; Ali, S.; Batool, F.; Shakir, H.A.; Arshad, A. Abnormal Steroidogenesis, Oxidative Stress, and Reprotoxicity Following Prepubertal Exposure to Butylparaben in Mice and Protective Effect of Curcuma longa. Environ. Sci. Pollut. Res. 2021, 28, 6111–6121. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Ma, X.; Yang, L.; Ma, J.; Zhao, X.; Zhang, Q. Bibliometric and Visual Analysis on Oxidative Stress in Gynecological and Reproductive Diseases: A Systematic Review. Medicine 2024, 103, 37815. [Google Scholar] [CrossRef] [PubMed]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between Anti-Oxidants and Reactive Oxygen Species: A Requisite for Oocyte Development and Maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Lettieri, G.; D’agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, E4198. [Google Scholar] [CrossRef] [PubMed]
- del Collado, M.; Da Silveira, J.C.; Oliveira, M.L.F.; Alves, B.M.S.M.; Simas, R.C.; Godoy, A.T.; Coelho, M.B.; Marques, L.A.; Carriero, M.M.; Nogueira, M.F.G.; et al. In Vitro Maturation Impacts Cumulus–Oocyte Complex Metabolism and Stress in Cattle. Reproduction 2017, 154, 881–893. [Google Scholar] [CrossRef]
- Sá, N.A.R.; Araújo, V.R.; Correia, H.H.V.; Ferreira, A.C.A.; Guerreiro, D.D.; Sampaio, A.M.; Escobar, E.; Santos, F.W.; Moura, A.A.; Lôbo, C.H.; et al. Anethole Improves the in Vitro Development of Isolated Caprine Secondary Follicles. Theriogenology 2017, 89, 226–234. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.T. Impact of Oxidative Stress on Oocyte Competence for in Vitro Embryo Production Programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Itziou, A.; Balis, V.; Lakioti, E.; Karayannis, V.; Tsanaktsidis, C. Environmental Pollution and Oxidative Stress: Health Effects During Pregnancy: A Review. Appl. Sci. 2024, 14, 9884. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and Therapeutic Activities, and Anticancer Properties of Curcumin (Review). Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Tanvir, E.M.; Prince, M.B.; Paul, S.; Saha, M.; Ali, M.Y.; Gan, S.H.; Khalil, M.I.; Karim, N. Protective Mechanism of Turmeric (Curcuma longa) on Carbofuran-Induced Hematological and Hepatic Toxicities in a Rat Model. Pharm. Biol. 2017, 55, 1937–1945. [Google Scholar] [CrossRef]
- Hashish, E.A.; Elgaml, S.A. Hepatoprotective and Nephroprotective Effect of Curcumin Against Copper Toxicity in Rats. Indian J. Clin. Biochem. 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-Oxidative Effects of Curcumin on Immobilization-Induced Oxidative Stress in Rat Brain, Liver and Kidney. Biomed. Pharm. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- Gülçin, I.; Beydemir, Ş.; Topal, F.; Gagua, N.; Bakuridze, A.; Bayram, R.; Gepdiremen, A. Apoptotic, Antioxidant and Antiradical Effects of Majdine and Isomajdine from Vinca Herbacea Waldst. and Kit. J. Enzyme. Inhib. Med. Chem. 2012, 27, 587–594. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gülçin, İ. Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: Amulti-Biomarker Approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, H. The Stilbene Derivatives, Nucleosides, and Nucleosides Modified by Stilbene Derivatives. Bioorg. Chem. 2019, 90, 103073. [Google Scholar] [CrossRef]
- Barandeh, B.; Amini Mahabadi, J.; Azadbakht, M.; Gheibi Hayat, S.M.; Amini, A. The Protective Effects of Curcumin on Cytotoxic and Teratogenic Activity of Retinoic Acid in Mouse Embryonic Liver. J. Cell. Biochem. 2019, 120, 19371–19376. [Google Scholar] [CrossRef]
- Ran, Z.; Zhang, Y.; Wen, X.; Ma, J. Curcumin Inhibits High Glucose-Induced Inflammatory Injury in Human Retinal Pigment Epithelial Cells through the ROS-PI3K/AKT/MTOR Signaling Pathway. Mol. Med. Rep. 2019, 19, 1024–1031. [Google Scholar] [CrossRef]
- Hsuuw, Y.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin Prevents Methylglyoxal-Induced Oxidative Stress and Apoptosis in Mouse Embryonic Stem Cells and Blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin Stimulates Angiogenesis through VEGF and Expression of HLA-G in First-Trimester Human Placental Trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef]
- Ghaneifar, Z.; Yousefi, Z.; Tajik, F.; Nikfar, B.; Ghalibafan, F.; Abdollahi, E.; Momtazi-Borojeni, A.A. The Potential Therapeutic Effects of Curcumin on Pregnancy Complications: Novel Insights into Reproductive Medicine. IUBMB Life 2020, 72, 2572–2583. [Google Scholar] [CrossRef]
- Park, Y.S.; You, S.Y.; Cho, S.; Jeon, H.J.; Lee, S.; Cho, D.H.; Kim, J.S.; Oh, J.S. Eccentric Localization of Catalase to Protect Chromosomes from Oxidative Damages during Meiotic Maturation in Mouse Oocytes. Histochem. Cell Biol. 2016, 146, 281–288. [Google Scholar] [CrossRef]
- Tiwari, V.; Chopra, K. Attenuation of Oxidative Stress, Neuroinflammation, and Apoptosis by Curcumin Prevents Cognitive Deficits in Rats Postnatally Exposed to Ethanol. Psychopharmacology 2012, 224, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Gantait, A.; Barman, T.; Mukherjee, P.K. Validated Method for Estimation of Curcumin in Turmeric Powder. Indian J. Tradit. Knowl. 2011, 10, 247–250. [Google Scholar]
- Lerin, L.A.; Catani, M.; Oliveira, D.; Massi, A.; Bortolini, O.; Cavazzini, A.; Giovannini, P.P. Continuous Ion-Exchange Resin Catalysed Esterification of Eugenol for the Optimized Production of Eugenyl Acetate Using a Packed Bed Microreactor. RSC Indian Adv. 2015, 5, 76898–76903. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-Free Biology of Oxidative Stress. Am. J. Physiol. Cell Physiol. 2008, 295, 849–868. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 1, 6501046. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar]
- St John, J.C.; Okada, T.; Andreas, E.; Penn, A. The Role of MtDNA in Oocyte Quality and Embryo Development. Mol. Reprod. Dev. 2023, 90, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Rivera, R.M. The Effects of Superovulation and Reproductive Aging on the Epigenome of the Oocyte and Embryo. Mol. Reprod. Dev. 2018, 85, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Yang, Z.; Liu, Z. Antioxidative Effects of Hesperetin against Lead Acetate-Induced Oxidative Stress in Rats. Indian. J. Pharmacol. 2013, 45, 395–398. [Google Scholar]
- Duarte, T.L.; Lunec, J. Review: When Is an Antioxidant Not an Antioxidant? A Review of Novel Actions and Reactions of Vitamin C. Free. Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar]
- Hassanpour, S.H.; Doroudi, A. Review of the Antioxidant Potential of Flavonoids as a Subgroup of Polyphenols and Partial Substitute for Synthetic Antioxidants. Avicenna. J. Phytomed. 2023, 13, 354–376. [Google Scholar]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of Oxidative Stress and Antioxidant Supplementation in Pregnancy Disorders. Am. J. Clin. Nutr. 2011, 94, 1980–1985. [Google Scholar] [CrossRef]
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed in Vivo and in Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374. [Google Scholar] [CrossRef]
- Luberda, Z. The Role of Glutathione in Mammalian Gametes. Reprod. Biol. 2005, 5, 5–7. [Google Scholar]
- Onaolapo, M.C.; Nzekwe, S.C.; Lateef, O.; Olabisi, V.O.; Amos, H.; Ajayi, A.; Folorunsho, A. Importance of Oxidative Stress Mechanism in Reproductive Functions and Infertility; IntechOpen: London, UK, 2022. [Google Scholar]
- Silva, E.; Greene, A.F.; Strauss, K.; Herrick, J.R.; Schoolcraft, W.B.; Krisher, R.L. Antioxidant Supplementation during in Vitro Culture Improves Mitochondrial Function and Development of Embryos from Aged Female Mice. Reprod. Fertil. Dev. 2015, 27, 975–983. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Optimization of Reflux Conditions for Total Flavonoid and Total Phenolic Extraction and Enhanced Antioxidant Capacity in Pandan (Pandanus amaryllifolius Roxb.) Using Response Surface Methodology. Sci. World J. 2014, 1, 523120. [Google Scholar]
- Rahmani, A.; Alsahli, M.; Aly, S.; Khan, M.; Aldebasi, Y. Role of Curcumin in Disease Prevention and Treatment. Adv. Biomed. Res. 2018, 7, 38. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Samini, M.; Farkhondeh, T. Protective Effects of Carnosol against Oxidative Stress Induced Brain Damage by Chronic Stress in Rats. BMC Complement. Altern. Med. 2017, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; González-Burgos, E.; Gómez-Serranillos, M.P. Curcumin: Current Evidence of Its Therapeutic Potential as a Lead Candidate for Anti-Inflammatory Drugs-an Overview. In Discovery and Development of Anti-Inflammatory Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–59. ISBN 9780128169926. [Google Scholar]
- Lehfeldt, C.; Shirley, A.M.; Meyer, K.; Ruegger, M.O.; Cusumano, J.C.; Viitanen, P.V.; Strack, D.; Chapple, C. Cloning of the SNG1 Gene of Arabidopsis Reveals a Role for a Serine Carboxypeptidase-like Protein as an Acyltransferase in Secondary Metabolism. Plant Cell 2000, 12, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Lal, J. Turmeric, Curcumin and Our Life: A Review. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 11–17. [Google Scholar]
- Kumar, A.; Chetia, H.; Sharma, S.; Kabiraj, D.; Talukdar, N.C.; Bora, U. Curcumin Resource Database. Database 2015, 2015, 70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. ISBN 9780128147740. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of Pulsed Ultrasonic and Microwave-Assisted Extraction for Curcuminoids by Response Surface Methodology and Kinetic Study. Food Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef]
- Edey, L.F.; O’Dea, K.P.; Herbert, B.R.; Hua, R.; Waddington, S.N.; MacIntyre, D.A.; Bennett, P.R.; Takata, M.; Johnson, M.R. The Local and Systemic Immune Response to Intrauterine LPS in the Prepartum Mouse. Biol. Reprod. 2016, 95, 125. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Huang, Z.; Wells, D. The Human Oocyte and Cumulus Cells Relationship: New Insights from the Cumulus Cell Transcriptome. Mol. Hum. Reprod. 2010, 16, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C.; Halliwell, B. Mini-Review: Oxidative Stress, Redox Stress or Redox Success? Biochem. Biophys. Res. Commun. 2018, 502, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Mittal, M.; Saraf, P.; Kumari, P. Pesticides Induced Oxidative Stress and Female Infertility: A Review. Toxin Rev. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Bhide, M.K.; Kadam, R.M.; Mohan, H. Reactions of Superoxide Radicals with Curcumin: Probable Mechanisms by Optical Spectroscopy and EPR. Free Radic. Res. 2004, 38, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Chemical and Structural Features Influencing the biological activity of Curcumin. Curr. Pharm. Des. 2013, 11, 2093–2100. [Google Scholar]
- Bengmark, S. Curcumin, an Atoxic Antioxidant and Natural NFκB, Cyclooxygenase-2, Lipooxygenase, and Inducible Nitric Oxide Synthase Inhibitor: A Shield against Acute and Chronic Diseases. JPEN J. Parenter. Enteral Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef]
- Filardi, T.; Varì, R.; Ferretti, E.; Zicari, A.; Morano, S.; Santangelo, C. Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications? Nutrients 2020, 12, 3179. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The Effect of Oxidative and Reductive Stress on Semen Parameters and Functions of Physiologically Normal Human Spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A.; Brown, E.M.; Benedict, S.; Kambal, A. Alterations in Mitochondrial Respiratory Functions, Redox Metabolism and Apoptosis by Oxidant 4-Hydroxynonenal and Antioxidants Curcumin and Melatonin in PC12 Cells. Toxicol. Appl. Pharmacol. 2008, 226, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent Reductive Stress Triggers Mitochondrial Oxidation and Cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Y.; Hu, Y.; Lu, M.; Wang, J.; Dong, J.; Chen, D.; Chen, L.; Fu, F.; Qiu, F. Curcumin and Its Major Metabolites Inhibit the Inflammatory Response Induced by Lipopolysaccharide: Translocation of Nuclear Factor-ΚB as Potential Target. Mol. Med. Rep. 2015, 11, 3087–3093. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H. Curcumin and its derivatives: A review of their biological activities. Syst. Rev. Pharm. 2020, 11, 472–481. [Google Scholar]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In Vitro Permeability Enhancement of Curcumin across Caco-2 Cells Monolayers Using Electrospun Xanthan-Chitosan Nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent Developments in Formulation Design for Improving Oral Bioavailability of Curcumin: A Review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- Fatemi Abhari, S.M.; Khanbabaei, R.; Hayati Roodbari, N.; Parivar, K.; Yaghmaei, P. Curcumin-Loaded Super-Paramagnetic Iron Oxide Nanoparticle Affects on Apoptotic Factors Expression and Histological Changes in a Prepubertal Mouse Model of Polycystic Ovary Syndrome-Induced by Dehydroepiandrosterone—A Molecular and Stereological Study. Life Sci. 2020, 249, 117515. [Google Scholar] [CrossRef]
- Raja, M.A.; Maldonado, M.; Chen, J.; Zhong, Y.; Gu, J. Development and Evaluation of Curcumin Encapsulated Self-Assembled Nanoparticles as Potential Remedial Treatment for Pcos in a Female Rat Model. Int. J. Nanomed. 2021, 16, 6231–6247. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Al-Okaily, B.N. Alterations of VEGF Expression and Ovarian Follicles in Doxorubicin Treated Rats: Role of Curcumin Selenium Nanoparticles. Adv. Anim. Vet. Sci. 2024, 12, 2185–2194. [Google Scholar] [CrossRef]
- Ivm, O. In Vitro Maturation: A Committee Opinion. Fertil. Steril. 2021, 115, 298–304. [Google Scholar]
- Marin, D.; Yang, M.; Wang, T. In Vitro Growth of Human Ovarian Follicles for Fertility Preservation. Reprod. Dev. Med. 2018, 2, 230–236. [Google Scholar] [CrossRef]
- Xu, Y.; Duncan, F.E.; Xu, M.; Woodruff, T.K. Use of an Organotypic Mammalian in Vitro Follicle Growth Assay to Facilitate Female Reproductive Toxicity Screening. Reprod. Fertil. Dev. 2016, 28, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Alex, A.; Abdelhafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-Dimensional in Vitro Follicle Growth: Overview of Culture Models, Biomaterials, Design Parameters and Future Directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; He, L.; Ning, P.; Lin, J.; Li, H.; Lin, Z.; Kang, K.; Zhang, Y. (+)-Catechin Inhibition of Transmissible Gastroenteritis Coronavirus in Swine Testicular Cells Is Involved Its Antioxidation. Res. Vet. Sci. 2015, 103, 28–33. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin Analogues and Derivatives with Anti-Proliferative and Anti-Inflammatory Activity: Structural Characteristics and Molecular Targets. Expert Opin. Drug Discov. 2019, 14, 821–842. [Google Scholar] [CrossRef]
- Ali, A.; Jaber, N. Pharmacological Aspects of Curcumin: Review Article. Int. J. Pharmacogn. 2018, 5, 26–313. [Google Scholar]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Görlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European Contribution to the Study of ROS: A Summary of the Findings and Prospects for the Future from the COST Action BM1203 (EU-ROS). Redox. Biol. 2017, 13, 94–162. [Google Scholar]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Carolina Alves, R.; Perosa Fernandes, R.; Fonseca-Santos, B.; Damiani Victorelli, F.; Chorilli, M. A Critical Review of the Properties and Analytical Methods for the Determination of Curcumin in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2019, 49, 138–149. [Google Scholar] [CrossRef]
- Niranjan, A.; Singh, S.; Dhiman, M.; Tewari, S.K. Biochemical Composition of Curcuma longa L. Accessions. Anal. Lett. 2013, 46, 1069–1083. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A Review on Selected Pharmacological Activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the Aqueous Solubility of Curcumin at Acidic Condition through the Complexation with Whey Protein Nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- de Camargo, L.E.A.; Brustolin Ludwig, D.; Tominaga, T.T.; Carletto, B.; Favero, G.M.; Mainardes, R.M.; Khalil, N.M. Bovine Serum Albumin Nanoparticles Improve the Antitumour Activity of Curcumin in a Murine Melanoma Model. J. Microencapsul. 2018, 35, 467–474. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Baldini, G.M.; Lot, D.; Malvasi, A.; Laganà, A.S.; Vimercati, A.; Dellino, M.; Cicinelli, E.; Baldini, D.; Trojano, G. Abnormalities of Oocyte Maturation: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 12197. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Nottola, S.A.; Tunjung, W.A.S.; Kadowaki, A.; Bianchi, S.; Cecconi, S.; Sato, E.; Macchiarelli, G. EGF-FSH Supplementation Reduces Apoptosis of Pig Granulosa Cells in Co-Culture with Cumulus-Oocyte Complexes. Biochem. Biophys. Res. Commun. 2016, 481, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin Exerts a Protective Effect against Premature Ovarian Failure in Mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef]
- Farshad, Z.; Shahedi, A.; Fesahat, F.; Hassanpour, A.; Anvari, M. Effect of Formaldehyde and Curcumin on Histomorphological Indices, Gene Expression Associated with Ovarian Follicular Development, and Total Antioxidant to Oxidant Levels in Wistar Rats. Int. J. Biomater. 2023, 1, 4662440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Zhang, C.J.; Diao, H.L.; Zhang, Y. Protective Effects of Curcumin against Sodium Arsenite-Induced Ovarian Oxidative Injury in a Mouse Model. Chin. Med. J. 2017, 130, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of Stress on Oocyte Quality and Reproductive Outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef]
- Ávila, J.; González-Fernández, R.; Rotoli, D.; Hernández, J.; Palumbo, A. Oxidative Stress in Granulosa-Lutein Cells from in Vitro Fertilization Patients. Reprod. Sci. 2016, 23, 1656–1661. [Google Scholar] [CrossRef]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. Beneficial Effects of Curcumin and Capsaicin on Cyclophosphamide-Induced Premature Ovarian Failure in a Rat Model. J. Ovarian Res. 2018, 11, 33. [Google Scholar] [CrossRef]
- Mor, E.; Cabilly, Y.; Goldshmit, Y.; Zalts, H.; Modai, S.; Edry, L.; Elroy-Stein, O.; Shomron, N. Species-Specific MicroRNA Roles Elucidated Following Astrocyte Activation. Nucleic Acids Res. 2011, 39, 3710–3723. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Jin, X.; Willmore, W.G. Redox Regulation of Mitochondrial Function with Emphasis on Cysteine Oxidation Reactions. Redox Biol. 2014, 2, 123–139. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef] [PubMed]
- Khopde, S.M.; Priyadarsini, K.I.; Venkatesan, P.; Rao, M.N.A. Free Radical Scavenging Ability and Antioxidant Efficiency of Curcumin and Its Substituted Analogue. Biophys. Chem. 1999, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Kadasi, A.; Stochmalova, A.; Balazi, A.; Földesiová, M.; Makovicky, P.; Makovicky, P.; Chrenek, P.; Harrath, A.H. Effect of Turmeric on the Viability, Ovarian Folliculogenesis, Fecundity, Ovarian Hormones and Response to Luteinizing Hormone of Rabbits. Animal 2018, 12, 1242–1249. [Google Scholar] [CrossRef]
- Li, B.; Weng, Q.; Liu, Z.; Shen, M.; Zhang, J.; Wu, W.; Liu, H. Selection of Antioxidants against Ovarian Oxidative Stress in Mouse Model. J. Biochem. Mol. Toxicol. 2017, 31, 21997. [Google Scholar] [CrossRef] [PubMed]
- Alaee, S.; Khodabandeh, Z.; Dara, M.; Hosseini, E.; Sharma, M. Curcumin Mitigates Acrylamide-Induced Ovarian Antioxidant Disruption and Apoptosis in Female Balb/c Mice: A Comprehensive Study on Gene and Protein Expressions. Food Sci. Nutr. 2024, 12, 4160–4172. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Jiang, J.; Liu, L.; Ma, X.; Wang, T.; Zhao, L.; Li, W.; Niu, D. Curcumin Protects Porcine Granulosa Cells and Mouse Ovary against Reproductive Toxicity of Aflatoxin B1 via PI3K/AKT Signaling Pathway. Environ. Pollut. 2024, 363, 125210. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yang, S.; Yang, S.; Zeng, J.; Yan, Z.; Zhang, L.; Ma, X.; Dong, W.; Zhang, Y.; Zhao, X.; et al. The Mechanism of Curcumin to Protect Mouse Ovaries from Oxidative Damage by Regulating AMPK/MTOR Mediated Autophagy. Phytomedicine 2024, 128, 125210. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte Quality and Aging. JBRA Assist. Reprod. 2022, 26, 105–122. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Sayed, S.; Shukry, M.; El-Sayed, Y.S.; Alshehri, M.A. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef]
- Beeraka, N.M.; Bovilla, V.R.; Doreswamy, S.H.; Puttalingaiah, S.; Srinivasan, A.; Madhunapantula, S.R.V. The Taming of Nuclear Factor Erythroid-2-Related Factor-2 (Nrf2) Deglycation by Fructosamine-3-Kinase (FN3K)-Inhibitors-a Novel Strategy to Combat Cancers. Cancers 2021, 13, 281. [Google Scholar] [CrossRef]
- Lv, Y.; Cao, R.C.; Liu, H.-B.; Su, X.W.; Lu, G.; Ma, J.L.; Chan, W.Y. Single-Oocyte Gene Expression Suggests That Curcumin Can Protect the Ovarian Reserve by Regulating the Pten-Akt-Foxo3a Pathway. Int. J. Mol. Sci. 2021, 22, 6570. [Google Scholar] [CrossRef]
- Baki, K.B.; Sapmaz, T.; Sevgin, K.; Topkaraoglu, S.; Erdem, E.; Tekayev, M.; Guler, E.M.; Beyaztas, H.; Bozali, K.; Aktas, S.; et al. Curcumin and Gallic Acid Have a Synergistic Protective Effect against Ovarian Surface Epithelium and Follicle Reserve Damage Caused by Autologous Intraperitoneal Ovary Transplantation in Rats. Pathol. Res. Pract. 2024, 258, 155320. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, D.; Feng, H.; Wang, X.; Su, J.; Yao, Y.; Ng, E.H.Y.; Yeung, W.S.B.; Li, R.H.W.; et al. Identification of Curcumin as a Novel Potential Drug for Promoting the Development of Small Ovarian Follicles for Infertility Treatment. PNAS Nexus 2022, 1, 108. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Greenshields, A.; Hill, R.; Hilchie, A.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin-Induced Apoptosis in Ovarian Carcinoma Cells Is P53-Independent and Involves P38 Mitogen-Activated Protein Kinase Activation and Downregulation of Bcl-2 and Survivin Expression and Akt Signaling. Mol. Carcinog. 2010, 49, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. The Influence of Turmeric and Curcumin on Female Reproductive Processes. Planta Med. 2022, 88, 1020–1025. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Lateef, R.; Akhtar, M.J.; Rajanahalli, P. Dietary Antioxidant Curcumin Mitigates CuO Nanoparticle-Induced Cytotoxicity through the Oxidative Stress Pathway in Human Placental Cells. Molecules 2022, 27, 7378. [Google Scholar] [CrossRef]

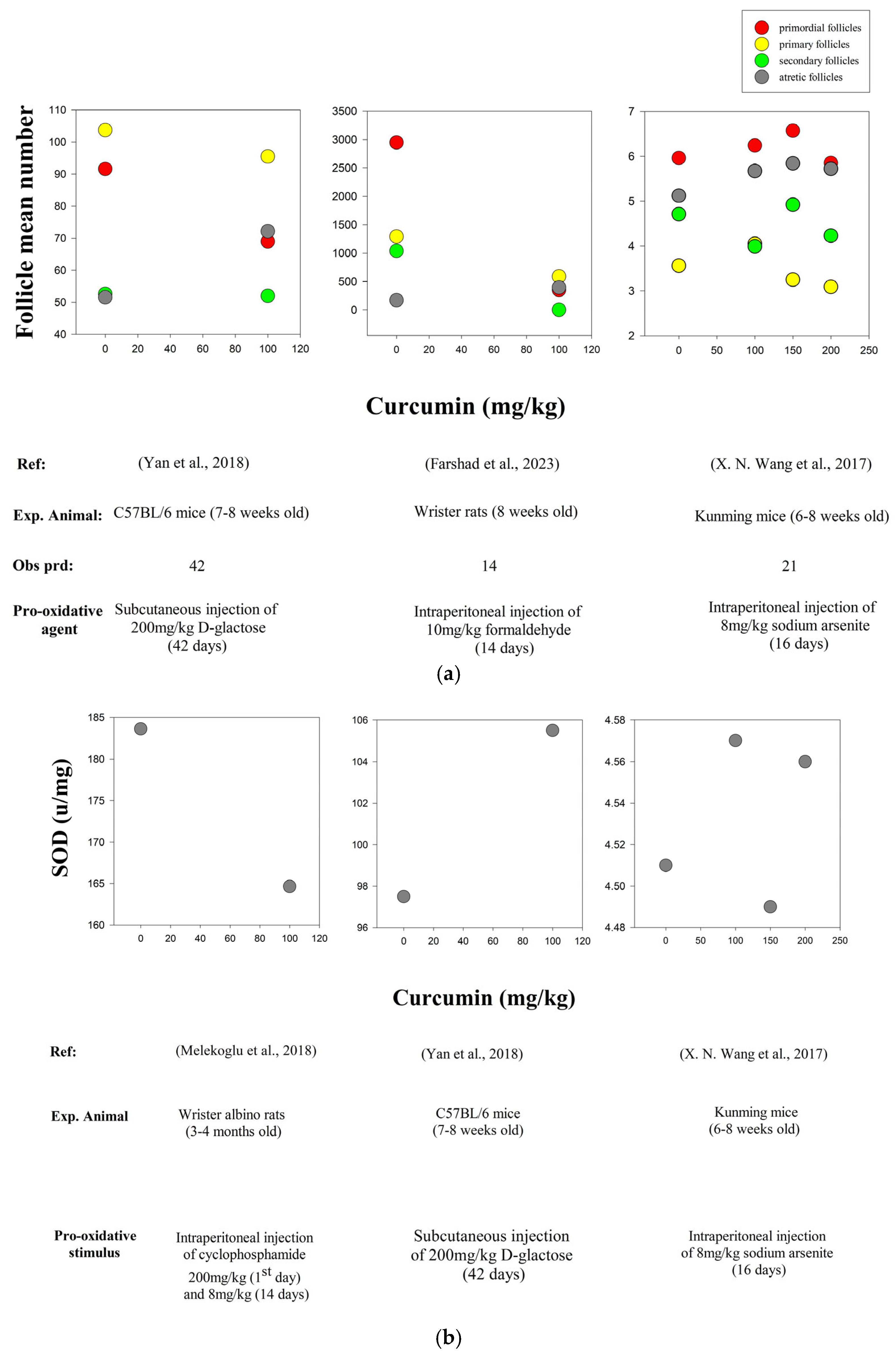
| In vivo/ In vitro | Experimental Animal | Study Period | Pro- Oxidative Stimulus | Curcumin (CUR) Dose | Observations | Increase (↑) and Decrease (↓) | Reference |
|---|---|---|---|---|---|---|---|
| In vivo | Wister Rats (8 weeks old) | 14 d | Intraperitoneal injection of 10 mg/kg formaldehyde (FA) for 14 days | FA + CUR (10 mg/kg/d + 100 mg/kg/d) | Follicular destruction and atresia | ↓ | [106] |
| Ovarian follicle count | ↑ | ||||||
| GPx protein levels | ↑ | ||||||
| FIGLA levels | ↑ | ||||||
| Apoptosis rate | ↓ | ||||||
| TAC/TOS ratio | ↑ | ||||||
| In vivo | Balb/c Mice (6–8 weeks old) | 35 d | Intragastrical injection of 50 mg/kg of Acrylamide (ACR) for 35 days | ACR + CUR (50 mg/kg/d + 100 mg/kg/d) and ACR + CUR (50 mg/kg/d + 200 mg/kg/d) | SOD, CAT and GPx protein levels | ↑ | [119] |
| Bax and Caspase-3 | ↓ | ||||||
| Bcl-2 levels | ↑ | ||||||
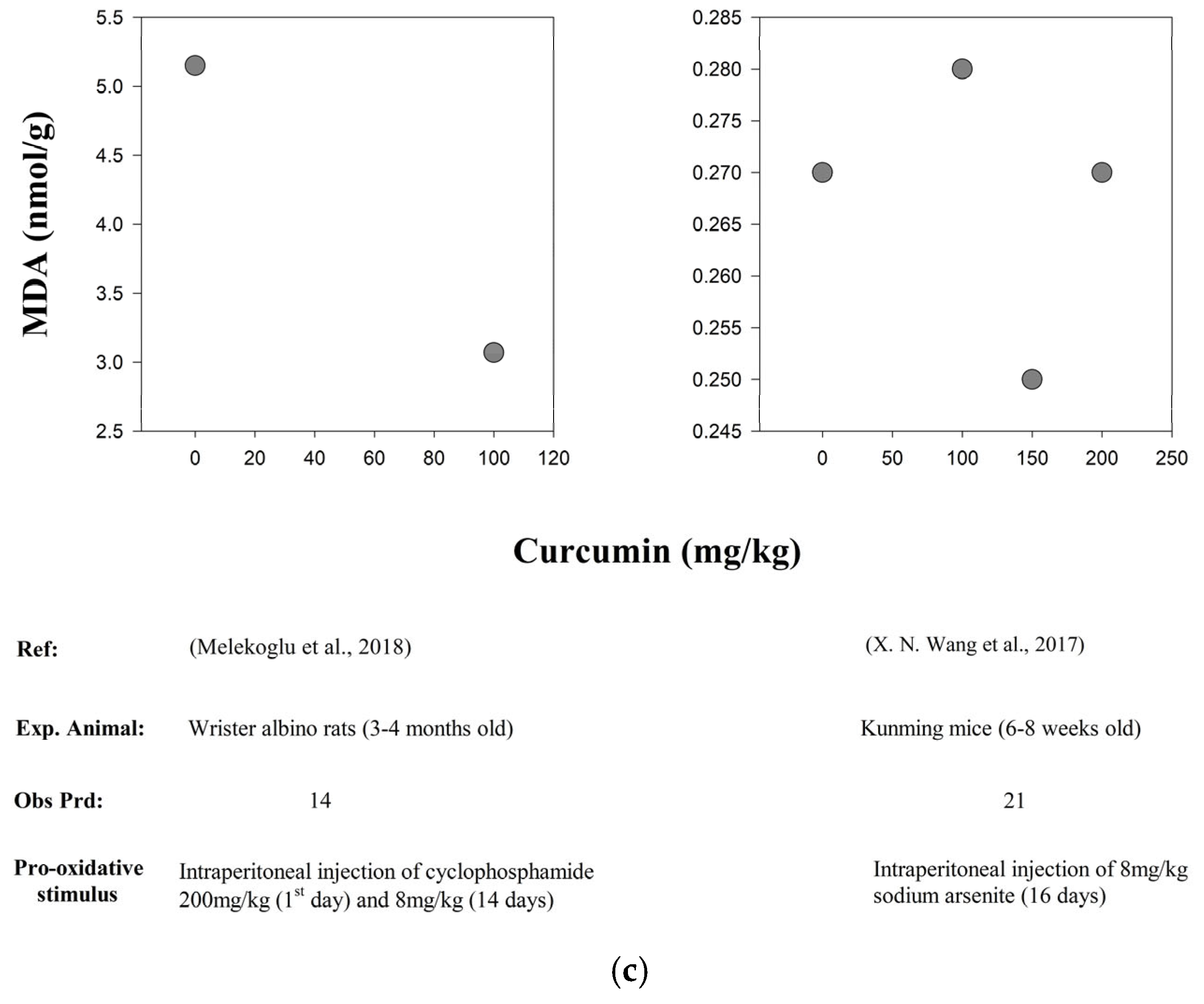
| In vivo | Kunming Mice (6–8 weeks old) | 16 d | Intraperitoneal injection of 8 mg/kg of Sodium arsenite (As) for 16 days | Curcumin dose-dependent groups were as follows: 0 mg/kg/d, 100 mg/kg/d, 150 mg/kg/d and 200 mg/kg/d | SOD and GPx | ↑ | [107] |
| MDA levels | ↓ | ||||||
| P66Shc expression levels | ↓ | ||||||
| Proliferation of granulosa cells | ↑ | ||||||
| In vivo | Wister Albino Rats (3–4 months old) | 14 d | Intraperitoneal injection of 200 mg/kg of Cyclophosphamide (POF) on day 1 and then 8 mg/kg for 14 days | POF + CUR (8 mg/kg/d + 100 mg/kg/d) | ROS levels (SOD, CAT, GPx levels ↑) | ↓ | [111] |
| GSH levels and estradiol levels | ↑ | ||||||
| FSH and LH levels | ↓ | ||||||
| KF-kB levels | ↑ | ||||||
| MAPK levels | ↓ | ||||||
| Bax and Caspase-3 | ↓ | ||||||
| In vivo | C5BL/6 Mice (7–8 weeks old) | 42 d | Subcutaneous Injection of 200 mg/kg D-galactose for 24 days | D-gal + CUR (200 mg/kg/d + 100 mg/kg/d) | SOD activity | ↑ | [105] |
| MDA activity | ↓ | ||||||
| Amh expression level | ↑ | ||||||
| Caspase-3 and -9 protein expression levels | ↓ | ||||||
| E2 levels | ↑ | ||||||
| FSH and LH levels | ↓ | ||||||
| Curcumin inhibited ovarian injury via the Nrf2/HO-1 and P13K/Akt pathways | |||||||
| In vivo | ICR Mice (8 weeks) | Oral administration of 0.75 mg/kg of aflatoxin B1 (AFB1) for 28 days | AFB1 + CUR (0.75 mg/kg/d + 100 mg/kg/d) | Curcumin restored normal follicular structure | [120] | ||
| Atretic follicles | ↓ | ||||||
| ROS and MDA levels | ↓ | ||||||
| PI3K/Akt expression levels | ↑ | ||||||
| Expression of antioxidant markers (including SOD, GPx, NF-kB, Nrf2, HO-1 and HSP70) | ↑ | ||||||
| Bax and Caspase-3 | ↓ | ||||||
| In vitro | Porcine Granulosa Cells | 24 h | Aflatoxin B1 (AFB1) at a concentration of 8 µM for 12 h. | Groups: Control, AFB1 (8 µM), CUR (10 µM) and AFB1+ CUR (8 µM + 10 µM) | Curcumin preserved the AFB1-induced inhibition of cell viability, mitochondrial dysfunction, oxidative stress, cell cycle arrest and apoptosis | ||
| ROS and MDA levels | ↓ | ||||||
| SOD and GPx levels | ↑ | ||||||
| PI3K/Akt expression levels | ↑ | ||||||
| Mitochondrial fusion genes (Mfn1 and Mfn2) | ↑ | ||||||
| Mitochondrial fission genes (Drp1 and Fis1) | ↓ | ||||||
| Bcl-2 and XIAP | ↑ | ||||||
| Expression of apoptosis markers (Bax, BAD, Casepase-3, PTEN and AIF) | ↓ | ||||||
| In vivo | ICR Mice (6–8 weeks old) | 14 d | Intraperitoneal injection of 20 mg/kg/d of 3-nitropropionic acid (3-NPA for 14 days | Curcumin dose-dependent groups were as follows: 50 mg/kg/d, 100 mg/kg/d and 200 mg/kg/d | No. of primary and secondary follicles | ↑ | [121] |
| Atretic follicles | ↓ | ||||||
| ROS and MDA levels | ↓ | ||||||
| Bcl-2 and SOD expression levels in 100 mg/kg/d and 200 mg/kg curcumin-treated groups | ↑ | ||||||
| Bax and Cl-cas3 expression levels | ↓ | ||||||
| LC3B2 and BECNI levels | ↓ | ||||||
| PAMK/AMAK levels | ↓ | ||||||
| pmTOR/mTOR expression levels | ↑ | ||||||
| In vitro | ICR Mice (6–8 weeks old) | 24 h | H2O2 at a concentration of 50 µM, 100 µM, 200 µM and 400 µM for 3 h respectively in groups | Three curcumin dose-dependent groups were as follows: 5 µM, 10 µM and 20 µM | Cell viability | ↓ | |
| Bcl-2 expression levels | ↑ | ||||||
| Bax and Cl-cas3 (dose dependently) | ↓ | ||||||
| LC3B2 and BECN1 expression levels | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virk, T.L.; Liu, Q.; Yuan, Y.; Xu, X.; Chen, F. Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction. Biology 2025, 14, 750. https://doi.org/10.3390/biology14070750
Virk TL, Liu Q, Yuan Y, Xu X, Chen F. Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction. Biology. 2025; 14(7):750. https://doi.org/10.3390/biology14070750
Chicago/Turabian StyleVirk, Tuba Latif, Qi Liu, Yuguo Yuan, Xianyu Xu, and Fenglei Chen. 2025. "Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction" Biology 14, no. 7: 750. https://doi.org/10.3390/biology14070750
APA StyleVirk, T. L., Liu, Q., Yuan, Y., Xu, X., & Chen, F. (2025). Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction. Biology, 14(7), 750. https://doi.org/10.3390/biology14070750






