The Effect of Radixin on the Function and Expression of Organic Anion Transporting Polypeptide 1B1
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Transfection of Plasmid Constructs into Cells
2.3. Generation of Knockdown Cell Lines
2.4. Uptake Function Measurement, Cell Surface Biotinylation, and Western Blotting
2.5. Co-Immunoprecipitation
2.6. Site-Directed Mutagenesis
2.7. Statistical Analysis
3. Results
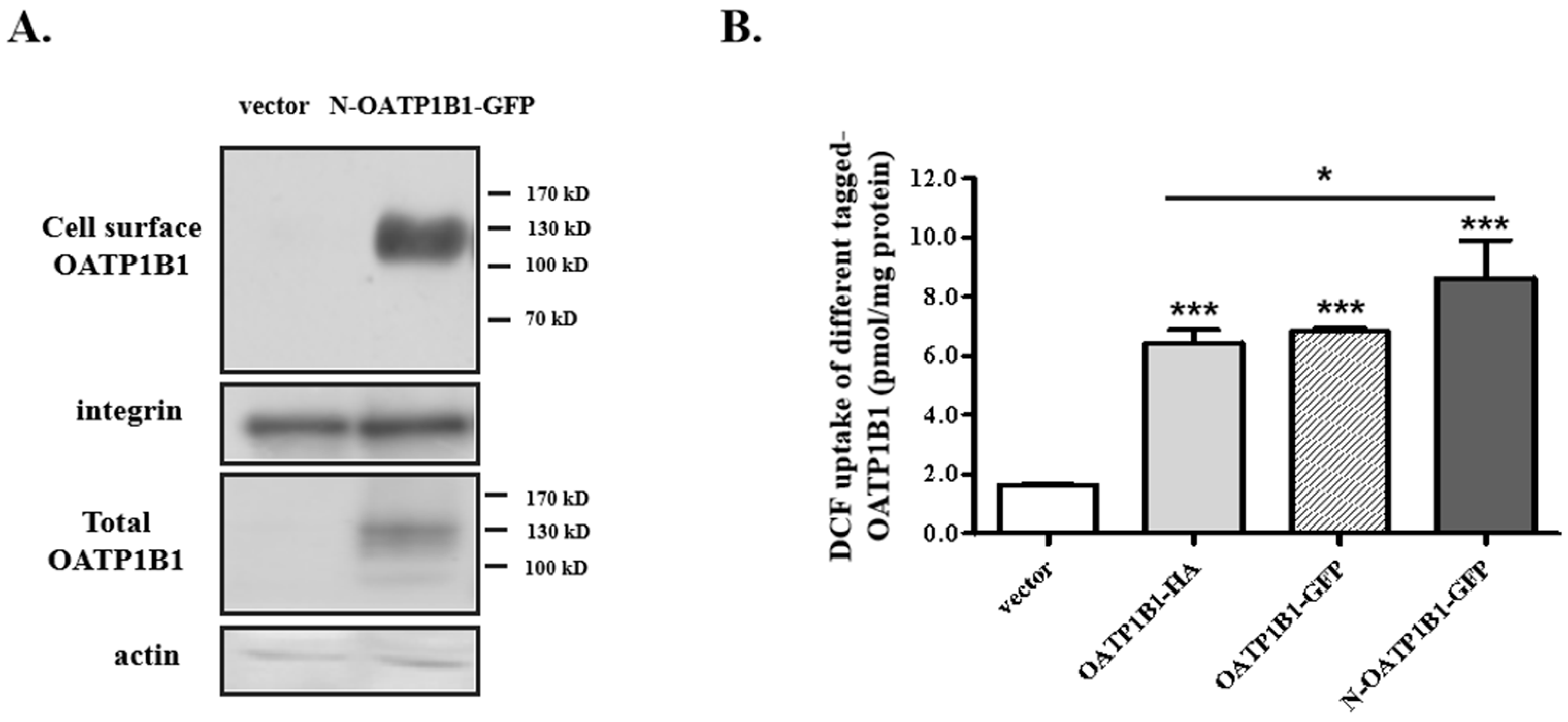
3.1. Characterization of N-OATP1B1-GFP Expression and Uptake Function
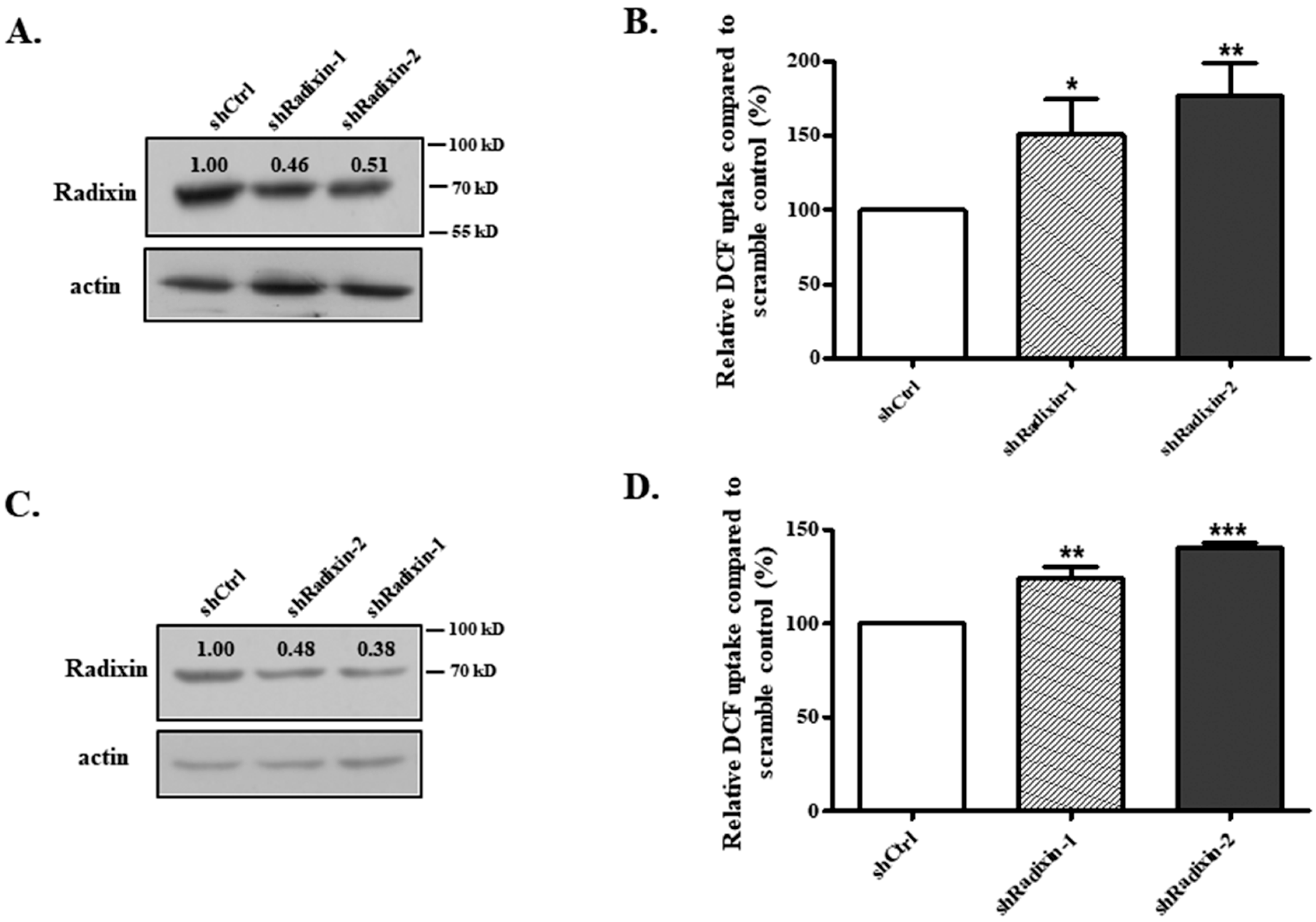
3.2. Knockdown of Radixin Increased OATP1B1 Uptake Function
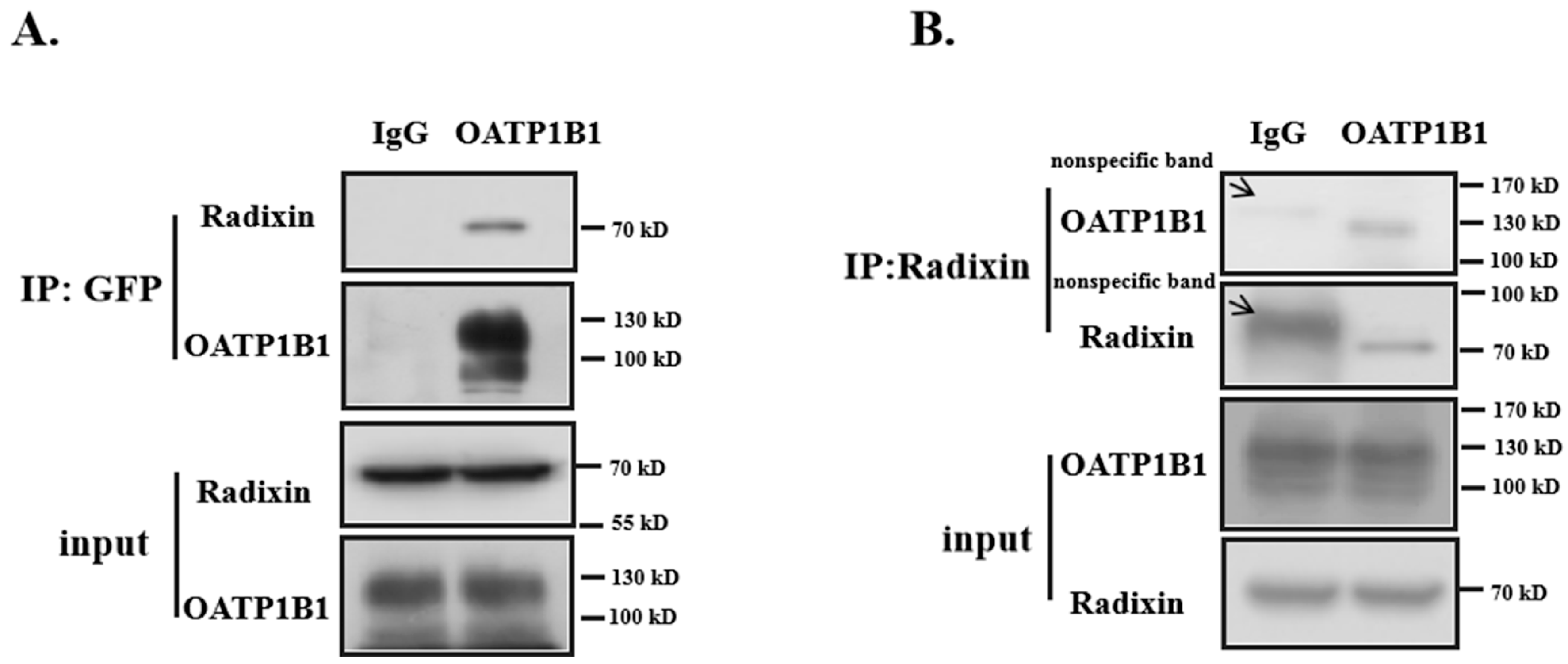
3.3. Radixin Is Associated with OATP1B1
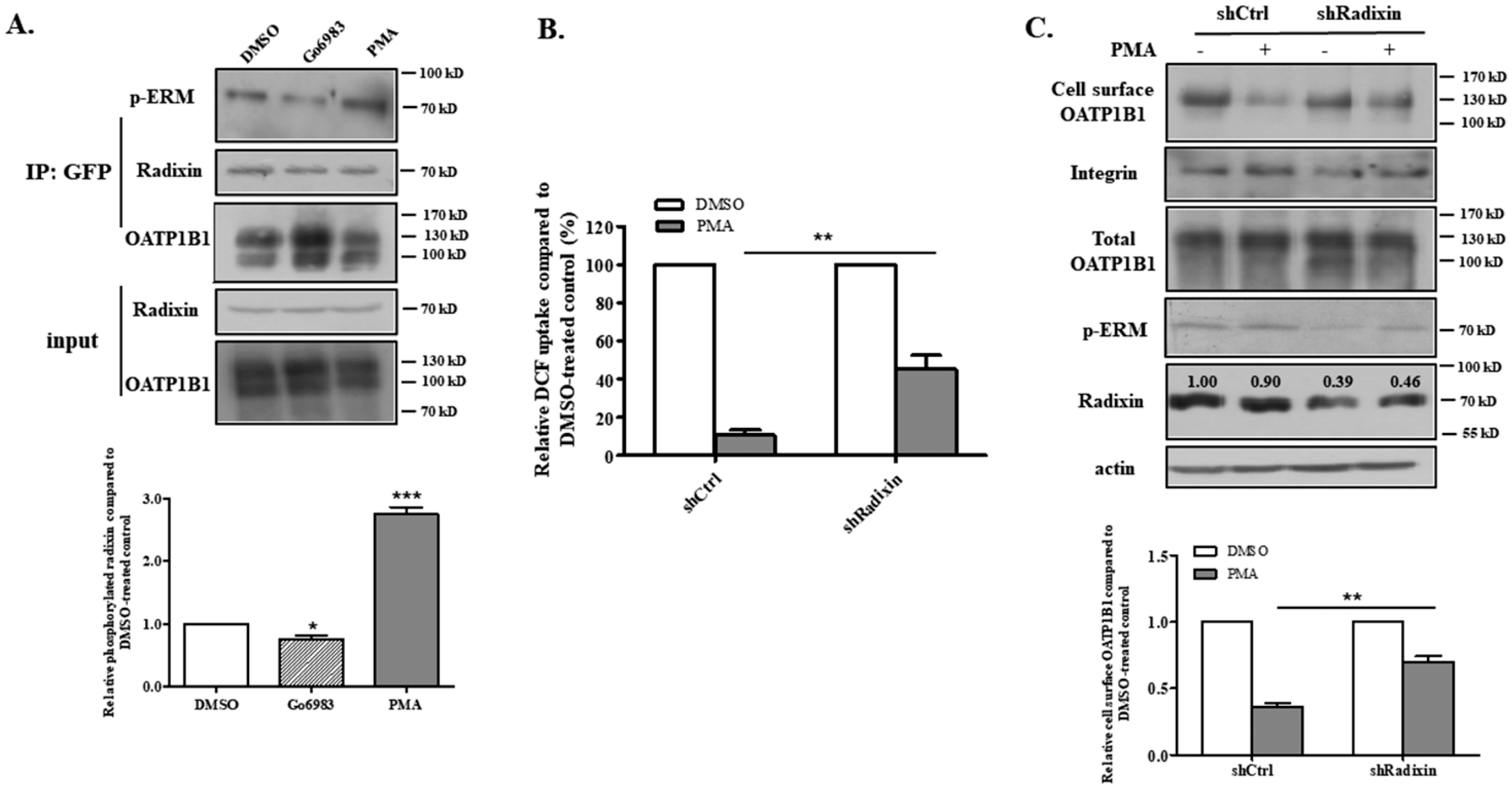
3.4. PKC-Induced OATP1B1 Internalization Is Likely Mediated by Radixin
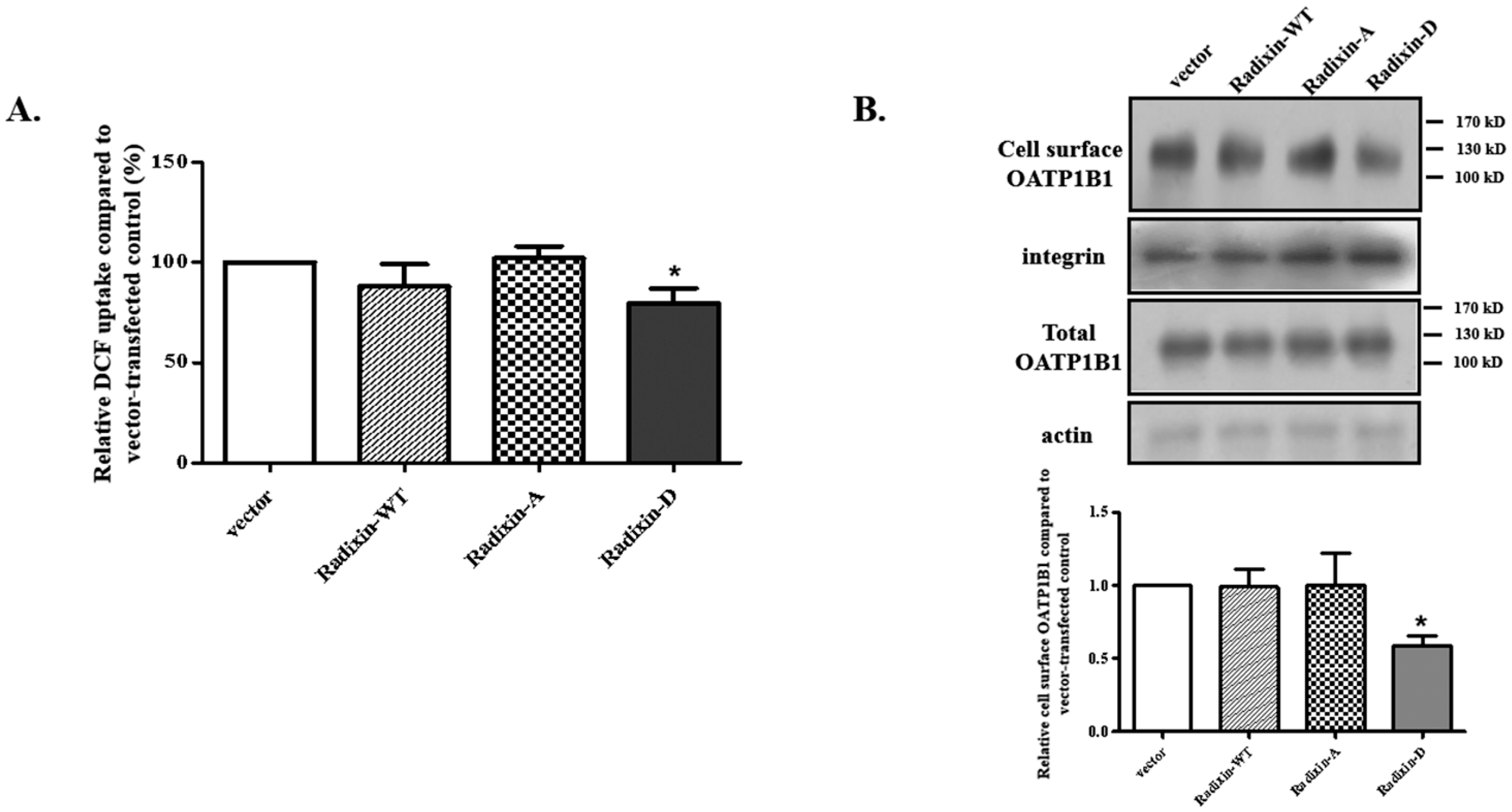
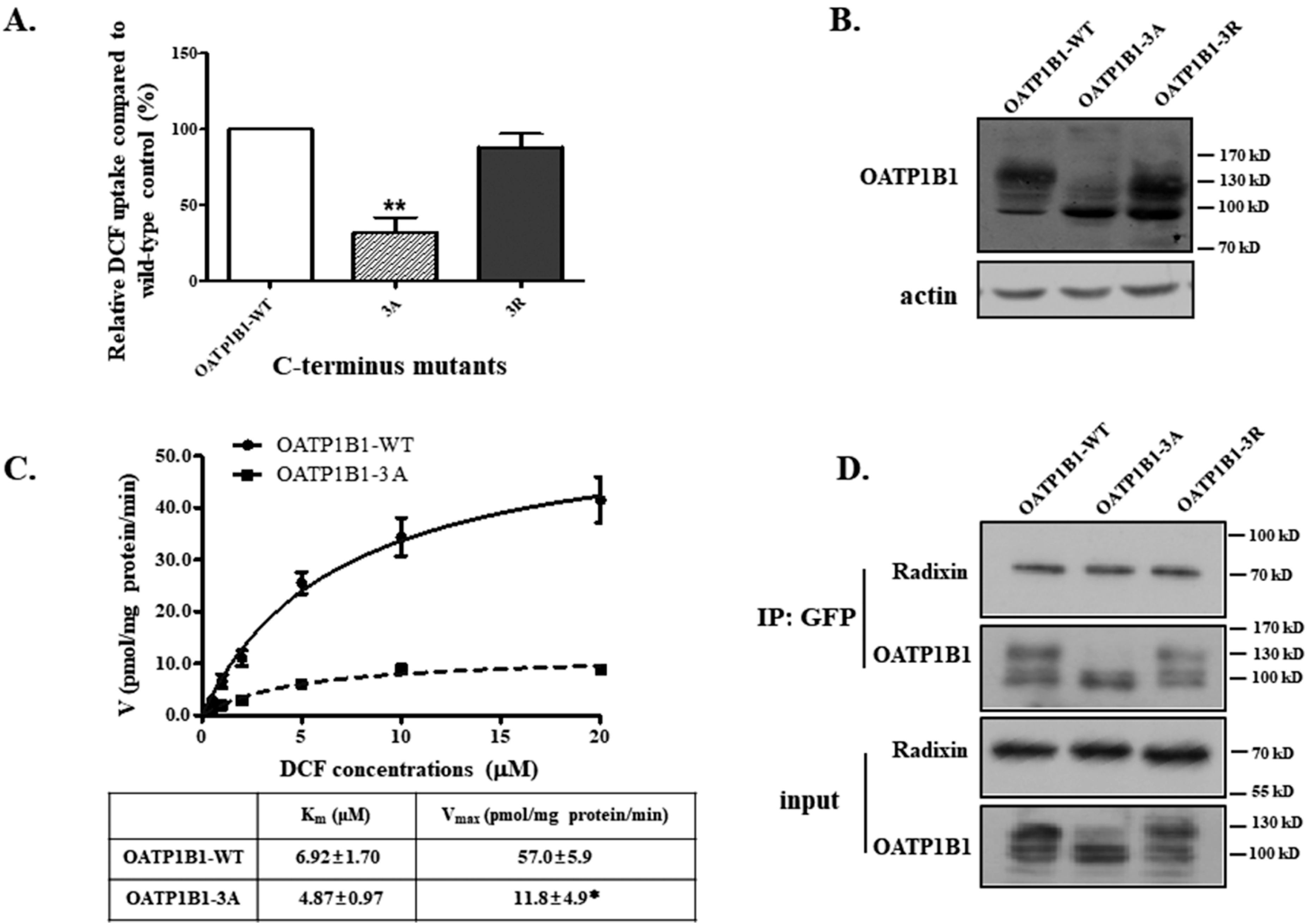
3.5. The Role of C-Terminal Positively Charged Amino Acid Cluster for OATP1B1 Interaction with Radixin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McFeely, S.J.; Ritchie, T.K.; Yu, J.; Nordmark, A.; Levy, R.H.; Ragueneau-Majlessi, I. Identification and evaluation of clinical substrates of organic anion transporting polypeptides 1B1 and 1B3. Clin. Transl. Sci. 2019, 12, 379–387. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, X.; Liu, H.; Yuan, Y.; Xiao, Y.; Nan, J.; Zhang, W.; Song, W.; Wang, J.; Wei, F.; et al. Cryo-EM structures of human organic anion transporting polypeptide OATP1B1. Cell Res. 2023, 33, 940–951. [Google Scholar] [CrossRef]
- Ciută, A.D.; Nosol, K.; Kowal, J.; Mukherjee, S.; Ramírez, A.S.; Stieger, B.; Kossiakoff, A.A.; Locher, K.P. Structure of human drug transporters OATP1B1 and OATP1B3. Nat. Commun. 2023, 14, 5774. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M. Protein kinases and cross-talk between post-translational modifications in the regulation of drug transporters. Mol. Pharmacol. 2023, 103, 9–20. [Google Scholar] [CrossRef]
- Alguel, Y.; Cameron, A.D.; Diallinas, G.; Byrne, B. Transporter oligomerization: Form and function. Biochem. Soc. Trans. 2016, 44, 1737–1744. [Google Scholar] [CrossRef]
- Ni, C.; Yu, X.; Fang, Z.; Huang, J.; Hong, M. Oligomerization study of human organic anion transporting polypeptide 1B1. Mol. Pharm. 2017, 14, 359–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruggiero, M.; Hagenbuch, B. OATP1B3 expression and function is modulated by coexpression with OCT1, OATP1B1, and NTCP. Drug Metab. Dispos. 2020, 48, 622–630. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.J.; Xiao, Y.; Murray, J.W.; Novikoff, P.M.; Angeletti, R.H.; Orr, G.A.; Lan, D.; Silver, D.L.; Wolkoff, A.W. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J. Biol. Chem. 2005, 280, 30143–30149. [Google Scholar] [CrossRef]
- Zheng, J.; Chan, T.; Cheung, F.S.; Zhu, L.; Murray, M.; Zhou, F. PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability. PLoS ONE 2014, 9, e94712. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yoshida, K.; Watanabe, C.; Sai, Y.; Tsuji, A. Screening of the interaction between xenobiotic transporters and PDZ proteins. Pharm. Res. 2004, 21, 1886–1894. [Google Scholar] [CrossRef]
- Wang, P.; Murray, J.W.; Wolkoff, A.W. Interaction of human OATP1B1 with PDZK1 is required for its trafficking to the hepatocyte plasma membrane. Drug Metab. Dispos. 2023, 51, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Ponuwei, G.A. A glimpse of the ERM proteins. J. Biomed. Sci. 2016, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Hata, M.; Fukumoto, K.; Yamane, Y.; Matsui, T.; Tamura, A.; Yonemura, S.; Yamagishi, H.; Keppler, D.; Tsukita, S. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat. Genet. 2002, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sekine, S.; Ito, K.; Horie, T. Correlation between apical localization of Abcc2/Mrp2 and phosphorylation status of ezrin in rat intestine. Drug Metab. Dispos. 2009, 37, 1521–1527. [Google Scholar] [CrossRef]
- Luciani, F.; Molinari, A.; Lozupone, F.; Calcabrini, A.; Lugini, L.; Stringaro, A.; Puddu, P.; Arancia, G.; Cianfriglia, M.; Fais, S. P-glycoprotein-actin association through ERM family proteins: A role in drug resistance? Biol. Pharm. Bull. 2017, 40, 388–395. [Google Scholar]
- Hong, M.; Hong, W.; Ni, C.; Huang, J.; Zhou, C. Protein kinase C affects the internalization and recycling of organic anion transporting polypeptide 1B1. Biochim. Biophys. Acta 2015, 1848, 2022–2030. [Google Scholar] [CrossRef]
- Chen, J.; Xue, Y.; Shuai, X.; Ni, C.; Fang, Z.; Ye, L.; Hong, M. Effect of major components of Tripterygium wilfordii Hook. f on the uptake function of organic anion transporting polypeptide 1B1. Toxicol. Appl. Pharmacol. 2022, 435, 115848. [Google Scholar] [CrossRef]
- Chai, J.; Cai, S.Y.; Liu, X.; Lian, W.; Chen, S.; Zhang, L.; Feng, X.; Cheng, Y.; He, X.; He, Y.; et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis. J. Hepatol. 2015, 63, 1440–1448. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Ikura, M. Radixin: Cytoskeletal adapter and signaling protein. Int. J. Biochem. Cell Biol. 2004, 36, 2131–2136. [Google Scholar] [CrossRef]
- Ng, T.; Parson, M.; Hughes, W.E.; Monypenny, J.; Zicha, D.; Gautreau, A.; Arpin, M.; Gshmeissner, S.; Verveer, P.J.; Bastiaens, P.I.; et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001, 20, 2723–2741. [Google Scholar] [CrossRef]
- Ren, L.; Hong, S.H.; Cassavaugh, J.; Osborne, T.; Chou, A.J.; Kim, S.Y.; Gorlick, R.; Hewitt, S.M.; Khanna, C. The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene 2009, 28, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Adyshev, D.M.; Dudek, S.M.; Moldobaeva, N.; Kim, K.; Ma, S.; Kasa, A.; Garcia, J.G.N.; Verin, A.D. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L240–L255. [Google Scholar] [CrossRef] [PubMed]
- Louvet-Vallée, S. ERM proteins: From cellular architecture to cell signaling. Biol. Cell 2000, 92, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.R.; Chen, M.; Pasquariello, K.Z.; Gibson, A.A.; Petti, J.J.; Shen, S.; Qu, J.; Ong, S.S.; Chen, T.; Jin, Y.; et al. Regulation of OATP1B1 function by tyrosine kinase-mediated phosphorylation. Clin. Cancer Res. 2021, 27, 4301–4310. [Google Scholar] [CrossRef]
- Sekine, K.; Ito, J.; Saeki, T.; Horie, T. Interaction of Mrp2 with radixin causes reversible canalicular Mrp2 localization induced by intracellular redox status. Biochim. Biophys. Acta 2011, 1812, 1427–1434. [Google Scholar] [CrossRef]
- Dellbrügge, F.; Jesse, L.D.; Medyukhina, A.; Liu, N.; Neugebauer, S.; Freißmuth, M.; Höppener, S.; Figge, M.T.; Morrison, H.; Riecken, L.B.; et al. Contribution of radixin and ezrin to the maintenance of hepatocytes’ excretory function in health and disease. Heliyon 2023, 9, e21009. [Google Scholar] [CrossRef]
- Fields, A.P.; Gustafson, W.C. Protein kinase C in disease: Cancer. Methods Mol. Biol. 2003, 233, 519–537. [Google Scholar]
- Thakkar, N.; Slizgi, J.R.; Brouwer, K.L.R. Effect of liver disease on hepatic transporter expression and function. J. Pharm. Sci. 2017, 106, 2282–2294. [Google Scholar] [CrossRef]
- Jeleń, F.; Oleksy, A.; Smietana, K.; Otlewski, J. PDZ domains-common players in the cell signaling. Acta Biochim. Pol. 2003, 50, 985–1017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, C.; Tang, L.; Wang, X.; Li, Z.; Hong, M. The Effect of Radixin on the Function and Expression of Organic Anion Transporting Polypeptide 1B1. Biology 2025, 14, 744. https://doi.org/10.3390/biology14070744
Ni C, Tang L, Wang X, Li Z, Hong M. The Effect of Radixin on the Function and Expression of Organic Anion Transporting Polypeptide 1B1. Biology. 2025; 14(7):744. https://doi.org/10.3390/biology14070744
Chicago/Turabian StyleNi, Chunxu, Longxia Tang, Xuyang Wang, Zichong Li, and Mei Hong. 2025. "The Effect of Radixin on the Function and Expression of Organic Anion Transporting Polypeptide 1B1" Biology 14, no. 7: 744. https://doi.org/10.3390/biology14070744
APA StyleNi, C., Tang, L., Wang, X., Li, Z., & Hong, M. (2025). The Effect of Radixin on the Function and Expression of Organic Anion Transporting Polypeptide 1B1. Biology, 14(7), 744. https://doi.org/10.3390/biology14070744






