Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Measurements and Statistical Analysis
3. Results
3.1. Growth Parameter
3.2. Flowering Parameters
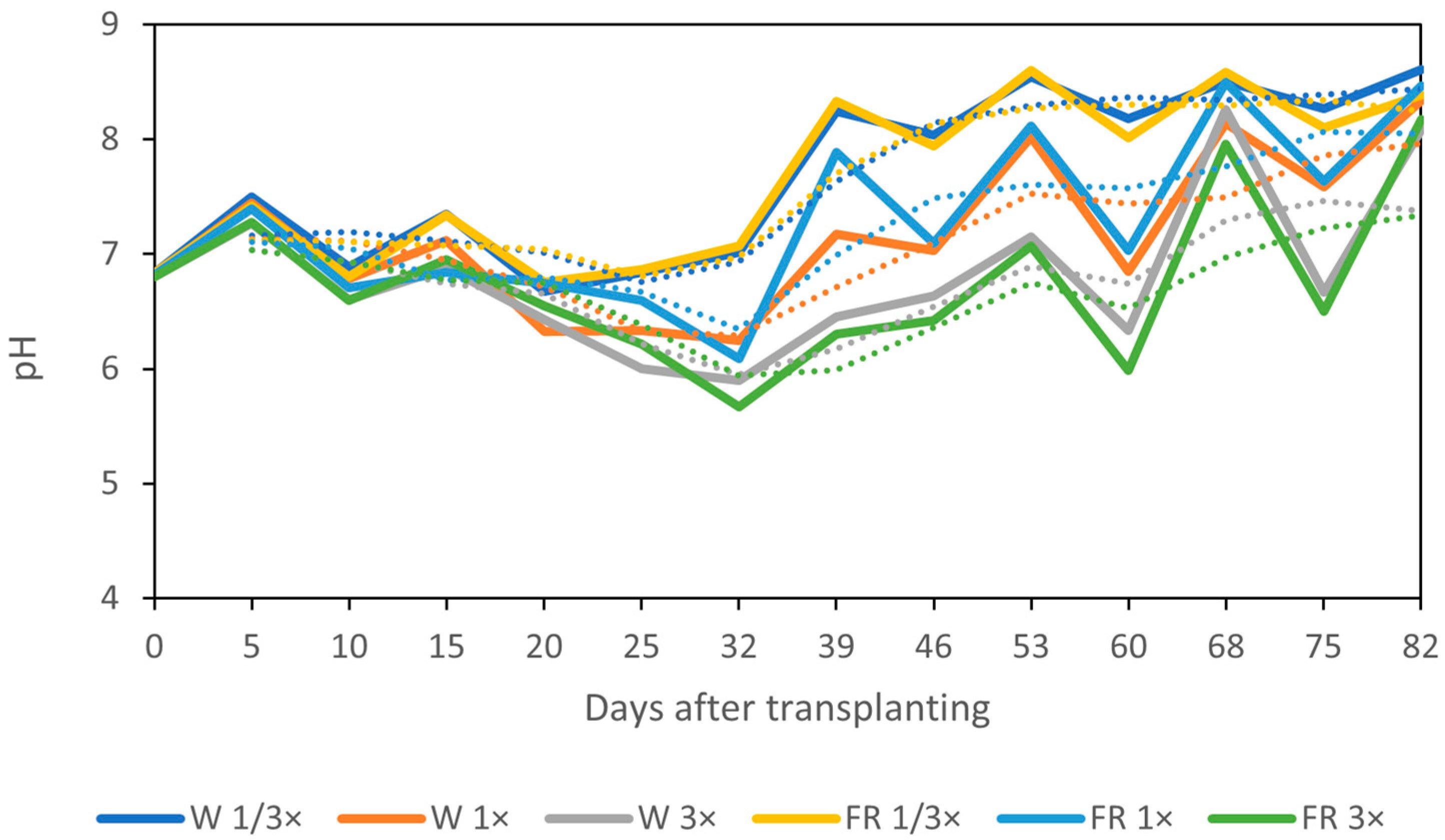
3.3. Change in Nutrient Solution
4. Discussion
4.1. Shade Responses and Biomass Accumulation Under FR Condition
4.2. Flowering Time Regulated by FR Light
4.3. Plant Growth, Flowering, and NUE Were Promoted by Elevated NH4+
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiorentino, M.; Gravina, C.; Piccolella, S.; Pecoraro, M.T.; Formato, M.; Stinca, A.; Pacifico, S.; Esposito, A. Calendula arvensis (Vaill.) L.: A Systematic Plant Analysis of the Polar Extracts from Its Organs by UHPLC-HRMS. Foods 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, D.P.; Gheena, S.; Ramani, P.; Rajeshkumar, S.; Ramalingam, K.; Shanmugam, R. In Vitro Evaluation of Antioxidant and Anti-inflammatory Potentials of Herbal Formulation Containing Marigold Flower (Calendula officinalis L.) Tea. Cureus 2023, 15, e43308. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179. [Google Scholar] [CrossRef]
- Munyanont, M.; Lu, N.; Rachma, D.F.; Ruangsangaram, T.; Takagaki, M. Lighting Patterns Regulate Flowering and Improve the Energy Use Efficiency of Calendula Cultivated in Plant Factories with Artificial Lighting. Agriculture 2024, 14, 2208. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Plant factory as a resource-efficient closed plant production system. In Plant Factory An Indoor Vertical Farming System for Efficient Quality Food Production; Elsevier: London, UK, 2015; pp. 69–90. [Google Scholar]
- Shamshiri, R.; Kalantari, F.; Ting, K.C.; Thorp, K.R.; Hameed, I.A.; Weltzien, C.; Ahmad, D.; Shad, Z.M. Advances in greenhouse automation and controlled environment agriculture: A transition to plant factories and urban agriculture. Int. J. Agric. Biol. Eng. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Boros, I.F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED lighting environments on lettuce (Lactuca sativa L.) in PFAL systems—A review. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Tsukagoshi, S.; Takagaki, M. Growth and nutrient utilization in basil plant as affected by applied nutrient quantity in nutrient solution and light spectrum. Biology 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of Photosynthetic Photon Flux Density and Root-Zone Temperature for Enhancing Secondary Metabolite Accumulation and Production of Coriander in Plant Factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lu, N.; Kikuchi, M.; Takagaki, M.; Tamashiro, T. Productivity potential and economic feasibility of small-sized tomato production in plant factories with artificial lighting: A comparative study with high-tech greenhouse production. J. Clean. Prod. 2024, 470, 143171. [Google Scholar] [CrossRef]
- Rachma, D.F.; Munyanont, M.; Maeda, K.; Lu, N.; Takagaki, M. Estimation of Harvest Time Based on Cumulative Temperatures to Produce High-Quality Cherry Tomatoes in a Plant Factory. Agronomy 2024, 14, 3074. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Lu, N.; Ichikawa, Y.; Watanabe, A.; Kikuchi, M.; Takagaki, M. An evaluation of pollination methods for strawberries cultivated in plant factories: Robot vs hand. Technol. Hortic. 2023, 3, 19. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2009, 61, 11–24. [Google Scholar] [CrossRef]
- Yamada, A.; Tanigawa, T.; Suyama, T.; Matsuno, T.; Kunitake, T. Red: Far-red light ratio and far-red light integral promote or retard growth and flowering in Eustoma grandiflorum (Raf.) Shinn. Sci. Hortic. 2008, 120, 101–106. [Google Scholar] [CrossRef]
- Owen, W.G.; Meng, Q.; Lopez, R.G. Promotion of Flowering from Far-red Radiation Depends on the Photosynthetic Daily Light Integral. HortScience 2018, 53, 465–471. [Google Scholar] [CrossRef]
- Takemura, Y.; Kishimoto, M.; Tamura, F. Selection of cut flower species affected promotion of flowering and stem elongation by far-red lighting or heating treatments on end of day under limited sunshine from autumn to winter. Hort. Sci. 2020, 47, 169–179. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2001, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Cinar, O.; Koksal, N. The effects of nitrogen on rose-scented pelargonium as a potted ornamental plant; growth and development, quality and biochemical characteristics. J. Plant Nutr. 2024, 48, 1409–1424. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Du, M.; Shou, G.; Wang, Z.; Xu, G. Nitrogen as a regulator for flowering time in plant. Plant Soil 2022, 480, 1–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Kong, F.; Chen, L. Nutrient-mediated modulation of flowering time. Front. Plant Sci. 2023, 14, 1101611. [Google Scholar] [CrossRef]
- Lin, Y.; Tsay, Y. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017, 68, 2603–2609. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2011, 42, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Tarkowská, D.; Clarke, J.L.; Blystad, D.; Gislerød, H.R.; Torre, S.; Olsen, J.E. Impact of end-of-day red and far-red light on plant morphology and hormone physiology of poinsettia. Sci. Hortic. 2014, 174, 77–86. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2020, 345, 128727. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Mah, J.J.; Llewellyn, D.; Zheng, Y. Morphology and flowering responses of four bedding plant species to a range of red to far red ratios. HortScience 2018, 53, 472–478. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Ilias, I.F.; Rajapakse, N. The Effects of End-of-the-day Red and Far-red Light on Growth and Flowering of Petunia x hybrida `Countdown Burgundy’ Grown under Photoselective Films. HortScience 2005, 40, 131–133. [Google Scholar] [CrossRef]
- Shibuya, T.; Izumi, M.; Endo, R. Effects of end-of-day far-red light and relative humidity on flowering and stem elongation of petunia (Petunia x hybrida) seedlings. Sci. Hortic. 2023, 324, 112600. [Google Scholar] [CrossRef]
- Cerny, T.A.; Faust, J.E.; Layne, D.R.; Rajapakse, N.C. Influence of Photoselective Films and Growing Season on Stem Growth and Flowering of Six Plant Species. J. Am. Soc. Hortic. Sci. 2003, 128, 486–491. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-day Plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- King, R.W.; Hisamatsu, T.; Goldschmidt, E.E.; Blundell, C. The nature of floral signals in Arabidopsis. I. Photosynthesis and a far-red photoresponse independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT). J. Exp. Bot. 2008, 59, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimising fertigation of hydroponically grown sowthistle (Sonchus oleraceus L.): The impact of the nitrogen source and supply concentration. Agric. Water Manag. 2023, 289, 108528. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3− ratio triggers plant growth and nutrient uptake of flowering Chinese cabbage by optimizing the pH value of nutrient solution. Front. Plant Sci. 2021, 12, 656144. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Zhou, M.; Zhu, Q.Y.; Du, W.X.; Jin, C.W. Improved plant nitrate status involves in flowering induction by extended photoperiod. Front. Plant Sci. 2021, 12, 629857. [Google Scholar] [CrossRef] [PubMed]





| Light Treatments | |||||
| W | EOD-FR | ||||
| Intensity (µmol m−2 s−1) | W | 300 ± 10 | 300 ± 10 | ||
| FR | - | 80 ± 5 | |||
| Photoperiod (h) | W | 12 (6:00–18:00) | 12 (6:00–18:00) | ||
| FR | - | 6 (18:00–0:00) | |||
| NH4+ Treatments | |||||
| 1/3× | 1× | 3× | |||
| NH4+ concentration (me/L) | 0.43 | 1.3 | 3.9 | ||
| NH4+:NO3− ratio | 3:97 | 7.5:92.5 | 20:80 | ||
| EC (dS m−1) | 1.8 | 1.8 | 1.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyanont, M.; Lu, N.; Ruangsangaram, T.; Takagaki, M. Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory. Biology 2025, 14, 716. https://doi.org/10.3390/biology14060716
Munyanont M, Lu N, Ruangsangaram T, Takagaki M. Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory. Biology. 2025; 14(6):716. https://doi.org/10.3390/biology14060716
Chicago/Turabian StyleMunyanont, Maitree, Na Lu, Thanit Ruangsangaram, and Michiko Takagaki. 2025. "Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory" Biology 14, no. 6: 716. https://doi.org/10.3390/biology14060716
APA StyleMunyanont, M., Lu, N., Ruangsangaram, T., & Takagaki, M. (2025). Effect of Far-Red Light and Nutrient Solution Formulas on Calendula Production in a Plant Factory. Biology, 14(6), 716. https://doi.org/10.3390/biology14060716





