Unraveling LncRNA GAS5 in Atherosclerosis: Mechanistic Insights and Clinical Translation
Simple Summary
Abstract
1. Introduction
1.1. The Significance of Atherosclerosis
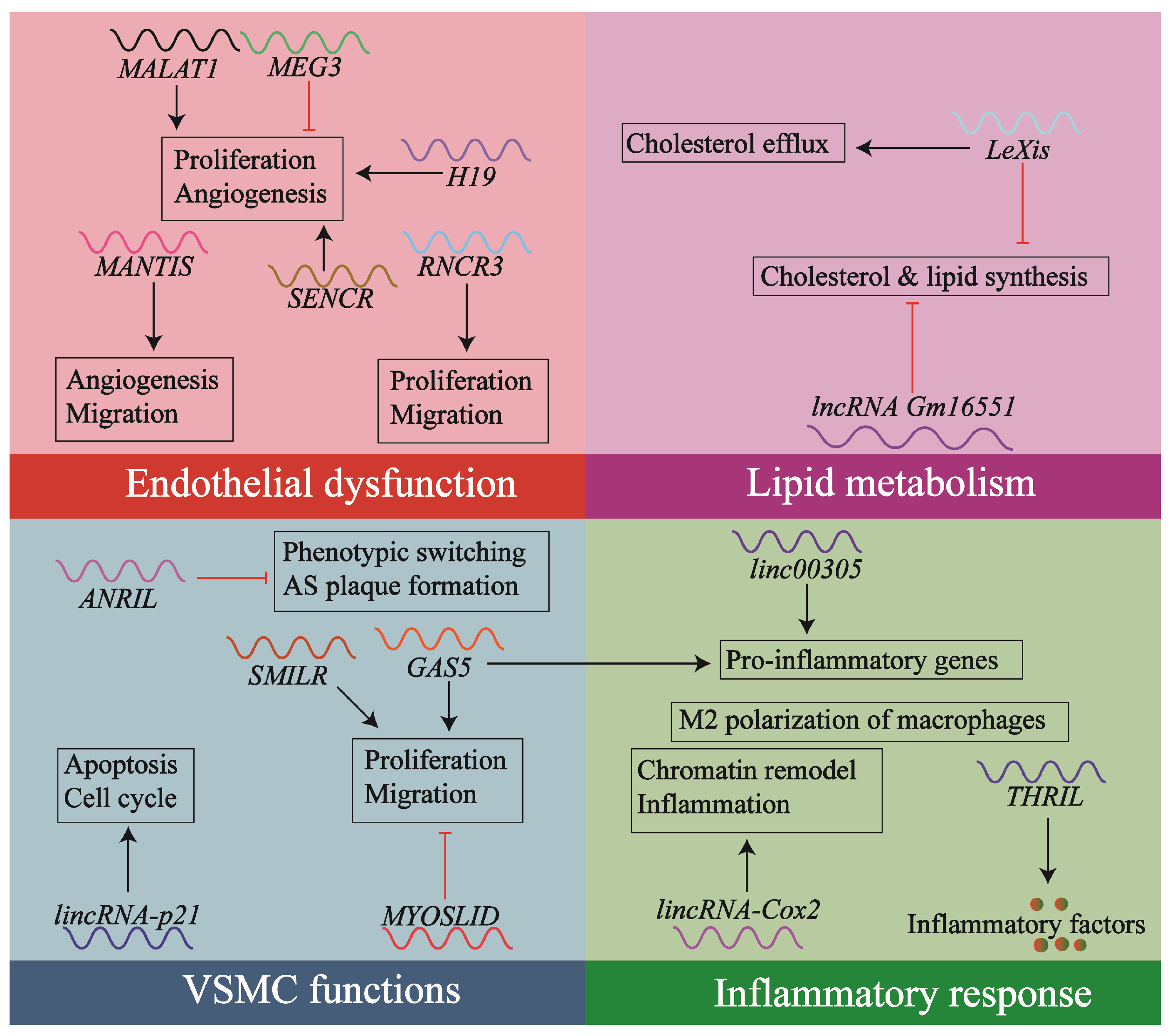
1.2. Contribution of lncRNAs to Atherosclerosis
1.3. The lncRNA GAS5: Involvement in Atherosclerosis
2. The Diverse Functions of lncRNA GAS5
2.1. Genomic and Molecular Characteristics of lncRNA GAS5
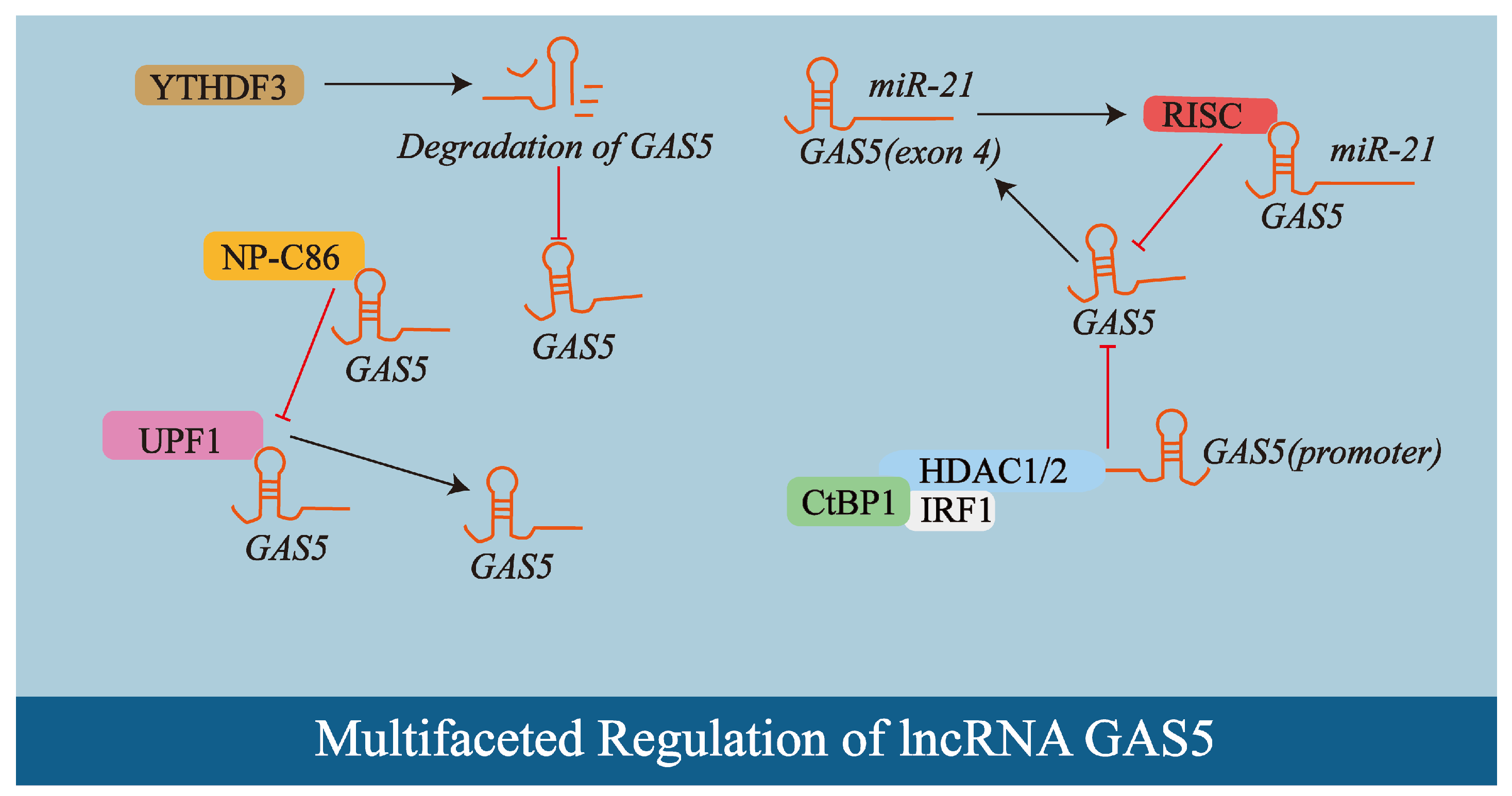
2.2. Multifaceted Regulation of lncRNA GAS5
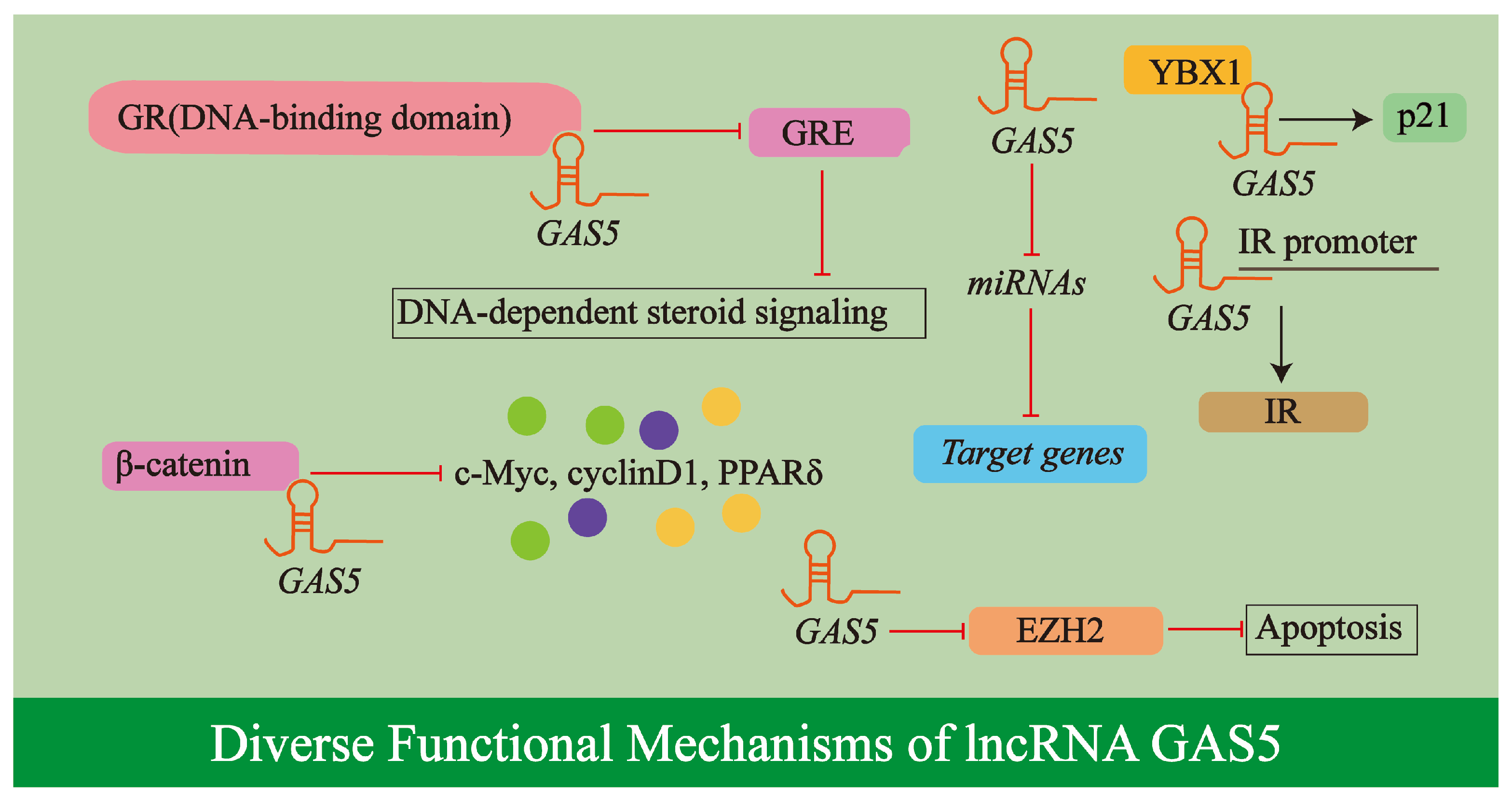
2.3. Diverse Functional Mechanisms of lncRNA GAS5
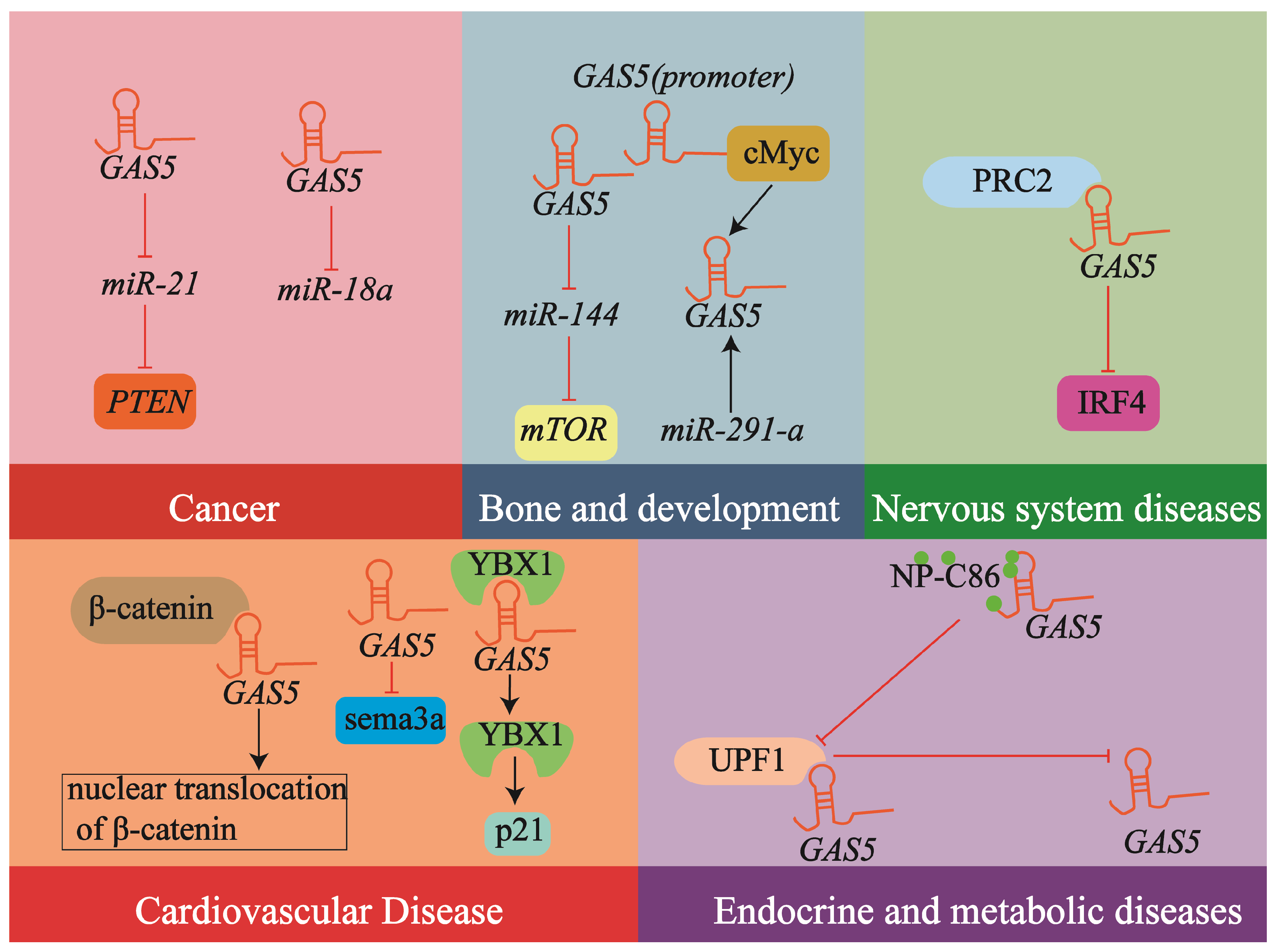
2.4. lncRNA GAS5 in Human Diseases: A Broad Spectrum
2.5. The Potential of lncRNA GAS5 in Atherosclerosis
3. Expression and Impact of lncRNA GAS5 in Atherosclerosis
3.1. Expression in Atherosclerotic Plaques
3.2. Circulating lncRNA GAS5 Levels and Their Clinical Relevance
3.3. Association with Clinical Parameters
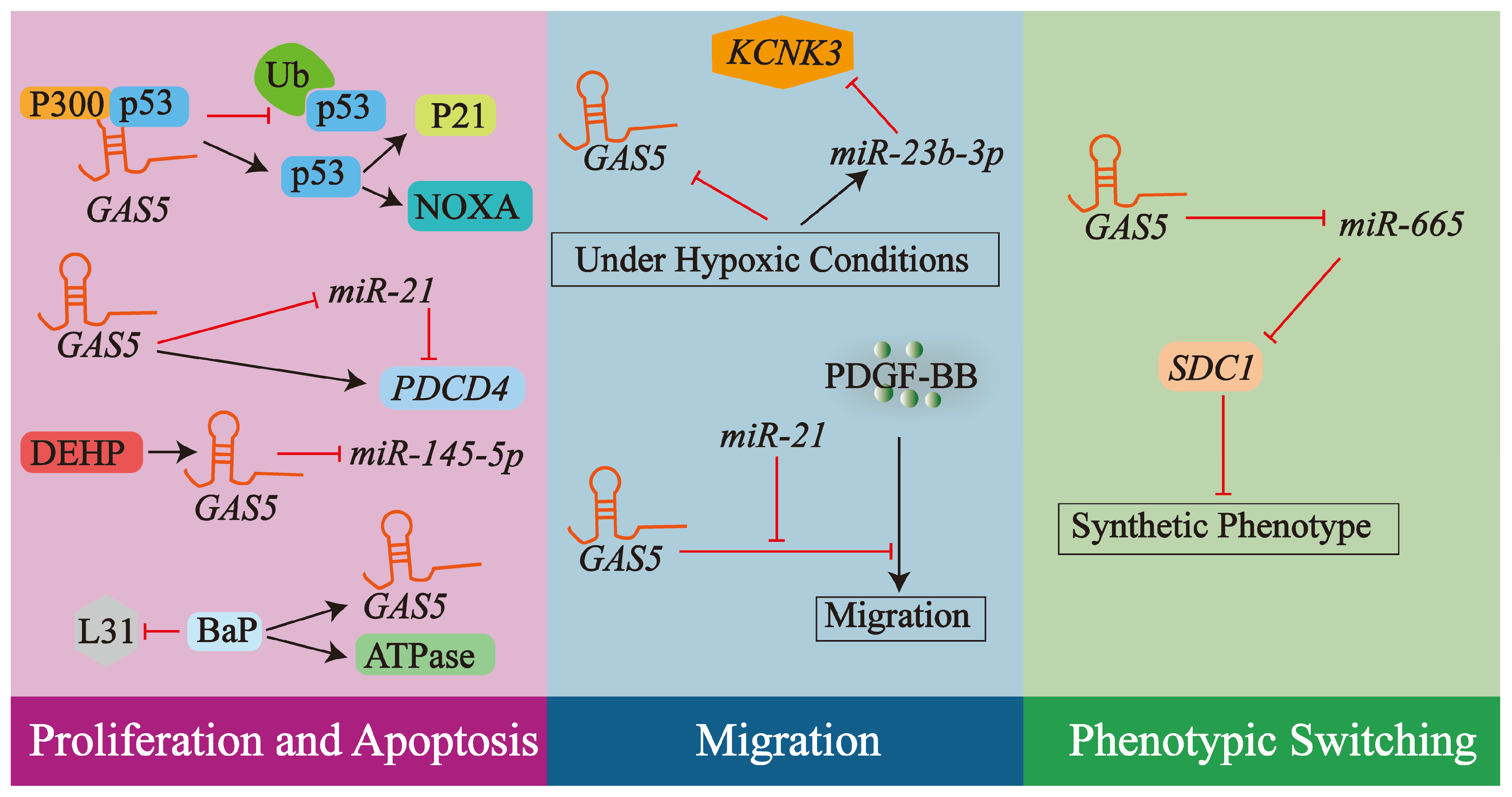
4. Cell-Specific Mechanisms of GAS5 in Atherosclerosis
4.1. lncRNA GAS5 and VSMCs
4.1.1. Regulation of VSMCs Proliferation and Apoptosis
4.1.2. Regulation of VSMCs Migration
4.1.3. Phenotypic Switching of VSMCs
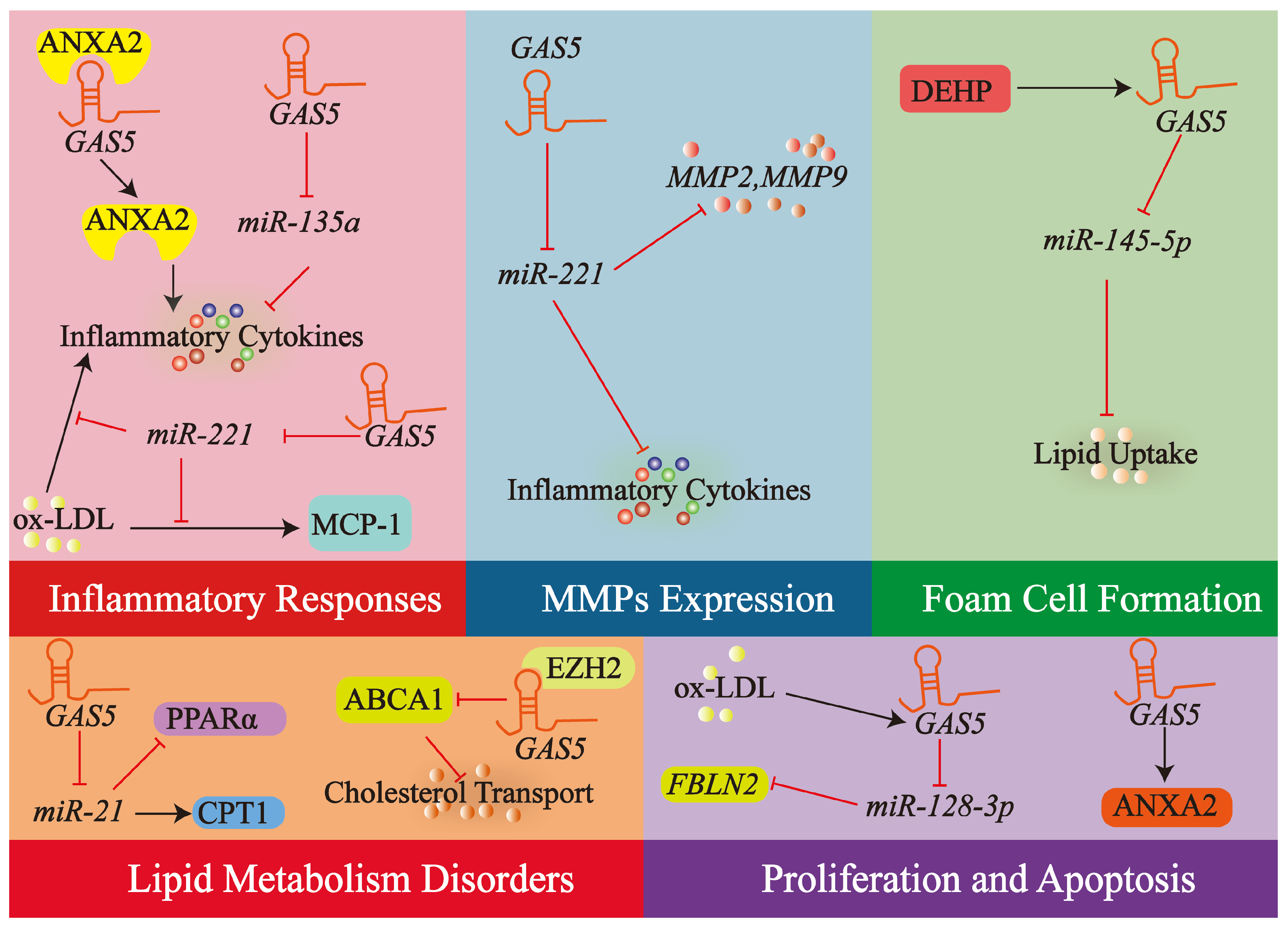
4.2. lncRNA GAS5 in Macrophages
4.2.1. Modulation of Macrophage Inflammatory Responses
4.2.2. Regulation of Macrophage MMPs
4.2.3. Lipid Metabolism Disorders
4.2.4. Macrophage Apoptosis and Proliferation
4.2.5. Regulation of Foam Cell Formation
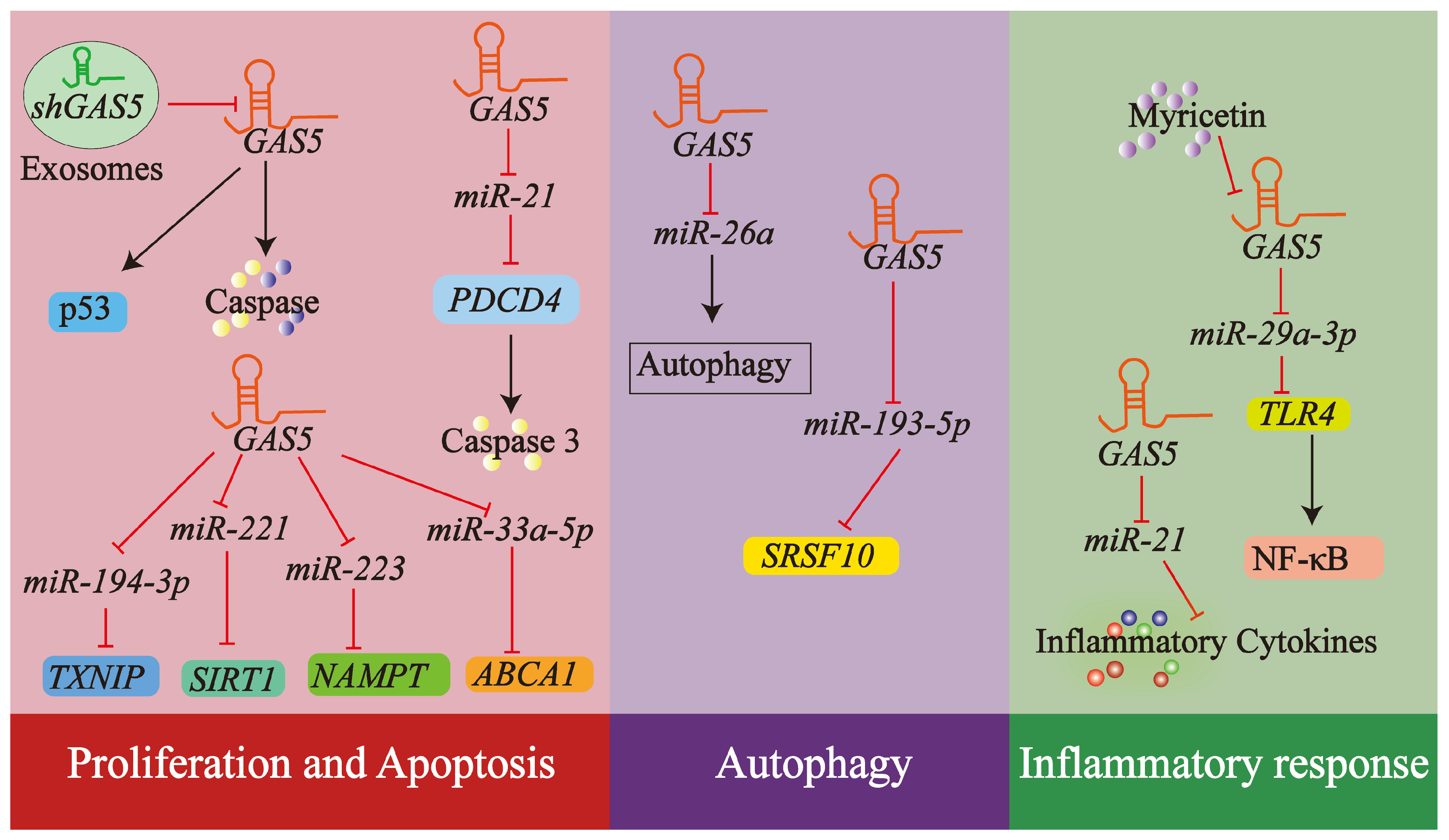
4.3. lncRNA GAS5 and Endothelial Cells
4.3.1. Endothelial Cell Proliferation and Apoptosis
4.3.2. Autophagy in Endothelial Cells
4.3.3. Inflammatory Response in Endothelial Cells
4.4. Summary of the Mechanisms of lncRNA GAS5 in Different Cell Types
5. Current Therapeutic Applications of lncRNA GAS5
5.1. Biomarker for Atherosclerosis
5.2. Use of shRNAs or siRNAs
5.3. Small Molecule Inhibitors or Activators
5.4. Gene Editing Approaches
6. Challenges and Future Directions
6.1. Context-Dependent Expression and Regulation of lncRNA GAS5
6.2. Current Challenges
6.3. Future Directions
- (1)
- Elucidate the precise molecular interaction network of GAS5 in various relevant vascular cells (ECs, VSMCs, macrophages, and other immune cells), identifying additional miRNA targets and protein partners, as well as the downstream signaling pathways they regulate; differentiate the expression patterns and functional differences among various GAS5 splicing isoforms, clarifying their specific roles throughout the various developmental phases of atherosclerosis and within diverse cellular environments.
- (2)
- Conduct large-scale, multicenter, prospective clinical cohort studies to rigorously evaluate the diagnostic and prognostic value of circulating GAS5 (including total levels and exosomal levels) across different subtypes and stages of cardiovascular disease and explore its potential for use in combination with other biomarkers.
- (3)
- Employ various methods, such as those described in Section 5 for regulating GAS5 in other diseases, to achieve precise control of GAS5 expression or function in specific cells; additionally, investigate the feasibility of targeting key downstream effectors of GAS5.
- (4)
- Utilize research models closer to human pathophysiological states, such as humanized mouse models and vascular organoids, along with single-cell multi-omics analysis of human atherosclerotic plaques, to better bridge basic research and clinical practice.
- (5)
- Investigate the interaction (crosstalk) and feedback loops between GAS5 and additional non-coding RNAs, like other lncRNAs and circular RNAs (circRNAs), along with the epigenetic regulatory network, to construct a more comprehensive map of the atherosclerosis regulatory network.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Yang, J.; Lin, X.; Wang, L.; Sun, T.; Zhao, Q.; Ma, Q.; Zhou, Y. LncRNA MALAT1 Enhances ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-κB Pathway in Macrophages. Curr. Vasc. Pharmacol. 2020, 18, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Lin, G.; Wu, T.; Gao, X.; He, Z.; Nong, W. Research Progress of Long Non-Coding RNA GAS5 in Malignant Tumors. Front. Oncol. 2022, 12, 846497. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Aillaud, M.; Schulte, L.N. Emerging Roles of Long Noncoding RNAs in the Cytoplasmic Milieu. Noncoding RNA 2020, 6, 44. [Google Scholar] [CrossRef]
- Karakas, D.; Ozpolat, B. The Role of LncRNAs in Translation. Noncoding RNA 2021, 7, 16. [Google Scholar] [CrossRef]
- Lu, X.; Tan, Q.; Ma, J.; Zhang, J.; Yu, P. Emerging Role of LncRNA Regulation for NLRP3 Inflammasome in Diabetes Complications. Front. Cell Dev. Biol. 2021, 9, 792401. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Li, A.; Shen, K.; Wang, S.; Wang, S.; Wu, P.; Luo, W.; Pan, Q. LncRNA Expression Profiles in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Emerging Biomarkers and Therapeutic Targets. Front. Immunol. 2021, 12, 792884. [Google Scholar] [CrossRef] [PubMed]
- Busscher, D.; Boon, R.A.; Juni, R.P. The multifaceted actions of the lncRNA H19 in cardiovascular biology and diseases. Clin. Sci. 2022, 136, 1157–1178. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Cancer Res. 2022, 41, 100. [Google Scholar] [CrossRef]
- Hu, B.; Chen, W.; Zhong, Y.; Tuo, Q. The role of lncRNA-mediated pyroptosis in cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1217985. [Google Scholar] [CrossRef]
- Lei, H.T.; Wang, J.H.; Yang, H.J.; Wu, H.J.; Nian, F.H.; Jin, F.M.; Yang, J.; Tian, X.M.; Wang, H.D. LncRNA-mediated cell autophagy: An emerging field in bone destruction in rheumatoid arthritis. Biomed. Pharmacother. 2023, 168, 115716. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, B.; Yang, Y.X.; Jia, Q.J.; Zhang, A.; Qi, Z.W.; Zhang, J.P. Long Noncoding RNAs in Pathological Cardiac Remodeling: A Review of the Update Literature. Biomed. Res. Int. 2019, 2019, 7159592. [Google Scholar] [CrossRef]
- Liang, W.; Fan, T.; Liu, L.; Zhang, L. Knockdown of growth-arrest specific transcript 5 restores oxidized low-density lipoprotein-induced impaired autophagy flux via upregulating miR-26a in human endothelial cells. Eur. J. Pharmacol. 2019, 843, 154–161. [Google Scholar] [CrossRef]
- Li, D.; Ma, Y.; Deng, W.; Feng, J. Construction and Analysis of lncRNA-Associated ceRNA Network in Atherosclerotic Plaque Formation. Biomed. Res. Int. 2022, 2022, 4895611. [Google Scholar] [CrossRef]
- Shirazi-Tehrani, E.; Chamasemani, A.; Firouzabadi, N.; Mousaei, M. ncRNAs and polyphenols: New therapeutic strategies for hypertension. RNA Biol. 2022, 19, 575–587. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Mao, Y.; Nan, G. Long Noncoding RNA-H19 Contributes to Atherosclerosis and Induces Ischemic Stroke via the Upregulation of Acid Phosphatase 5. Front. Neurol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Hu, D.J.; Li, Z.Y.; Zhu, Y.T.; Li, C.C. Overexpression of long noncoding RNA ANRIL inhibits phenotypic switching of vascular smooth muscle cells to prevent atherosclerotic plaque development in vivo. Aging 2020, 13, 4299–4316. [Google Scholar] [CrossRef]
- Simion, V.; Haemmig, S.; Feinberg, M.W. LncRNAs in vascular biology and disease. Vasc. Pharmacol. 2019, 114, 145–156. [Google Scholar] [CrossRef]
- Filippova, E.A.; Fridman, M.V.; Burdennyy, A.M.; Loginov, V.I.; Pronina, I.V.; Lukina, S.S.; Dmitriev, A.A.; Braga, E.A. Long Noncoding RNA GAS5 in Breast Cancer: Epigenetic Mechanisms and Biological Functions. Int. J. Mol. Sci. 2021, 22, 6810. [Google Scholar] [CrossRef] [PubMed]
- Elamir, A.M.; Senara, S.; Abdelghaffar, N.K.; Gaber, S.N.; El Sayed, H.S. Diagnostic role of lncRNA GAS5 and its genetic polymorphisms rs2067079, rs6790 and rs17359906 in rheumatoid arthritis. Biomed. Rep. 2021, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, J.; Xu, P.; Gui, R. Long Non-coding RNA GAS5 Maintains Insulin Secretion by Regulating Multiple miRNAs in INS-1 832/13 Cells. Front. Mol. Biosci. 2020, 7, 559267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, T. Relation of circulating lncRNA GAS5 and miR-21 with biochemical indexes, stenosis severity, and inflammatory cytokines in coronary heart disease patients. J. Clin. Lab. Anal. 2022, 36, e24202. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, A.; Liu, M. Plasma Long Non-Coding RNA (lncRNA) GAS5 is a New Biomarker for Coronary Artery Disease. Med. Sci. Monit. 2017, 23, 6042–6048. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Huang, Y.; Liu, D.; Chen, S.; Qin, S. Long Noncoding RNA GAS5: A New Factor Involved in Bone Diseases. Front. Cell Dev. Biol. 2022, 9, 807419. [Google Scholar] [CrossRef]
- Lu, K.P.; Alejandro, N.F.; Taylor, K.M.; Joyce, M.M.; Spencer, T.E.; Ramos, K.S. Differential expression of ribosomal L31, Zis, gas-5 and mitochondrial mRNAs following oxidant induction of proliferative vascular smooth muscle cell phenotypes. Atherosclerosis 2002, 160, 273–280. [Google Scholar] [CrossRef]
- Liu, C.; Qin, Q.; Xu, J.; Li, X.; Cong, H. Phthalate promotes atherosclerosis through interacting with long-non coding RNA and induces macrophage foam cell formation and vascular smooth muscle damage. Chemosphere 2022, 308, 136383. [Google Scholar] [CrossRef]
- Willemsen, L.; de Winther, M.P. Macrophage subsets in atherosclerosis as defined by single-cell technologies. J. Pathol. 2020, 250, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.D.; Yao, H.H.; Wang, L.M.; Yu, M.; Shi, S.; Yuan, Z.X.; Liu, J. Knockdown of GAS5 Inhibits Atherosclerosis Progression via Reducing EZH2-Mediated ABCA1 Transcription in ApoE(−/−) Mice. Mol. Ther. Nucleic Acids 2020, 19, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Wang, J.; Zhang, T.; Ye, T.; Wang, S.; Xing, D.; Chen, W. Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin. Chim. Acta 2021, 516, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Yan, G.; Zheng, J.; Liu, Z. The lncRNA GAS5 Inhibits the Osteogenic Differentiation and Calcification of Human Vascular Smooth Muscle Cells. Calcif. Tissue Int. 2020, 107, 86–95. [Google Scholar] [CrossRef]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef]
- Renganathan, A.; Kresoja-Rakic, J.; Echeverry, N.; Ziltener, G.; Vrugt, B.; Opitz, I.; Stahel, R.A.; Felley-Bosco, E. GAS5 long non-coding RNA in malignant pleural mesothelioma. Mol. Cancer 2014, 13, 119. [Google Scholar] [CrossRef]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The lncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.H.; Zhang, Y.Y.; Zhang, M.Y.; Zhang, L.Y.; Ye, K.H.; Teng, L.; Han, M.M.; Yue, Y.M.; Yang, J.; et al. SNORD80-guided 2′-O-methylation stabilizes the lncRNA GAS5 to regulate cellular stress responses. Proc. Natl. Acad. Sci. USA 2025, 122, e2418996122. [Google Scholar] [CrossRef]
- Dong, S.; Qu, X.; Li, W.; Zhong, X.; Li, P.; Yang, S.; Chen, X.; Shao, M.; Zhang, L. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J. Hematol. Oncol. 2015, 8, 43. [Google Scholar] [CrossRef]
- Shi, Y.; Parag, S.; Patel, R.; Lui, A.; Murr, M.; Cai, J.; Patel, N.A. Stabilization of lncRNA GAS5 by a Small Molecule and Its Implications in Diabetic Adipocytes. Cell Chem. Biol. 2019, 26, 319–330.e6. [Google Scholar] [CrossRef]
- Fang, C.; Wu, W.; Ni, Z.; Liu, Y.; Luo, J.; Zhou, Y.; Gong, C.; Hu, D.; Yao, C.; Chen, X.; et al. Ailanthone inhibits non-small cell lung cancer growth and metastasis through targeting UPF1/GAS5/ULK1 signaling pathway. Phytomedicine 2024, 128, 155333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, K.; Lou, Z.; Ding, K.; Zhang, F.; Zhu, J.; Chang, Z. The CtBP1-HDAC1/2-IRF1 transcriptional complex represses the expression of the long noncoding RNA GAS5 in human osteosarcoma cells. Int. J. Biol. Sci. 2019, 15, 1460–1471. [Google Scholar] [CrossRef]
- Ni, W.; Yao, S.; Zhou, Y.; Liu, Y.; Huang, P.; Zhou, A.; Liu, J.; Che, L.; Li, J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol. Cancer 2019, 18, 143. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Li, M.; Zhong, L.; Zheng, H.; Sun, Y.; Lai, Y.; Chen, X.; Wei, G.; Si, X.; et al. Long noncoding RNA GAS5 induces abdominal aortic aneurysm formation by promoting smooth muscle apoptosis. Theranostics 2019, 9, 5558–5576. [Google Scholar] [CrossRef]
- Wang, W.; Jia, Y.J.; Yang, Y.L.; Xue, M.; Zheng, Z.J.; Wang, L.; Xue, Y.M. LncRNA GAS5 exacerbates renal tubular epithelial fibrosis by acting as a competing endogenous RNA of miR-96-5p. Biomed. Pharmacother. 2020, 121, 109411. [Google Scholar] [CrossRef]
- Chen, T.; Meng, Y.; Zhou, Z.; Li, H.; Wan, L.; Kang, A.; Guo, W.; Ren, K.; Song, X.; Chen, Y.; et al. GAS5 protects against nonalcoholic fatty liver disease via miR-28a-5p/MARCH7/NLRP3 axis-mediated pyroptosis. Cell Death Differ. 2023, 30, 1829–1848. [Google Scholar] [CrossRef]
- Wang, Y.N.; Shan, K.; Yao, M.D.; Yao, J.; Wang, J.J.; Li, X.; Liu, B.; Zhang, Y.Y.; Ji, Y.; Jiang, Q.; et al. Long Noncoding RNA-GAS5: A Novel Regulator of Hypertension-Induced Vascular Remodeling. Hypertension 2016, 68, 736–748. [Google Scholar] [CrossRef]
- Wang, M.; Guo, C.; Wang, L.; Luo, G.; Huang, C.; Li, Y.; Liu, D.; Zeng, F.; Jiang, G.; Xiao, X. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018, 9, 238. [Google Scholar] [CrossRef]
- Cao, L.; Chen, J.; Ou, B.; Liu, C.; Zou, Y.; Chen, Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed. Pharmacother. 2017, 93, 570–579. [Google Scholar] [CrossRef]
- Yang, J.; Hao, T.; Sun, J.; Wei, P.; Zhang, H. Long noncoding RNA GAS5 modulates α-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed. Pharmacother. 2019, 112, 108656. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Liu, X.; Sui, X.; Pei, Y.; Liang, Z.; Zhou, N. Long non-coding RNA GAS5 reduces cardiomyocyte apoptosis induced by MI through sema3a. Int. J. Biol. Macromol. 2018, 120, 371–377. [Google Scholar] [CrossRef]
- Sun, D.; Yu, Z.; Fang, X.; Liu, M.; Pu, Y.; Shao, Q.; Wang, D.; Zhao, X.; Huang, A.; Xiang, Z.; et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017, 18, 1801–1816. [Google Scholar] [CrossRef]
- Ji, Q.; Qiao, X.; Liu, Y.; Wang, D. Expression of long-chain noncoding RNA GAS5 in osteoarthritis and its effect on apoptosis and autophagy of osteoarthritis chondrocytes. Histol. Histopathol. 2021, 36, 475–484. [Google Scholar] [CrossRef]
- Tu, J.; Tian, G.; Cheung, H.H.; Wei, W.; Lee, T.L. Gas5 is an essential lncRNA regulator for self-renewal and pluripotency of mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res. Ther. 2018, 9, 71. [Google Scholar] [CrossRef]
- Shen, S.; Zheng, X.; Zhu, Z.; Zhao, S.; Zhou, Q.; Song, Z.; Wang, G.; Wang, Z. Silencing of GAS5 represses the malignant progression of atherosclerosis through upregulation of miR-135a. Biomed. Pharmacother. 2019, 118, 109302. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef]
- Kraczkowska, W.; Jagodziński, P.P. The Long Non-Coding RNA Landscape of Atherosclerotic Plaques. Mol. Diagn. Ther. 2019, 23, 735–749. [Google Scholar] [CrossRef]
- Petkovic, A.; Erceg, S.; Munjas, J.; Ninic, A.; Vladimirov, S.; Davidovic, A.; Vukmirovic, L.; Milanov, M.; Cvijanovic, D.; Mitic, T.; et al. LncRNAs as Regulators of Atherosclerotic Plaque Stability. Cells 2023, 12, 1832. [Google Scholar] [CrossRef]
- Li, Y.; Geng, Y.; Zhou, B.; Wu, X.; Zhang, O.; Guan, X.; Xue, Y.; Li, S.; Zhuang, X.; Zhou, J.; et al. Long Non-coding RNA GAS5 Worsens Coronary Atherosclerosis Through MicroRNA-194-3p/TXNIP Axis. Mol. Neurobiol. 2021, 58, 3198–3207. [Google Scholar] [CrossRef]
- Fang, P.; Wu, Y.; Zhang, Z.; Cui, C.; Dong, X.; Hu, K.; Jia, J.; Duan, X.; Zhang, Y.; Huo, H. The clinical value of long noncoding RNA GAS5 in acute ischemic stroke: Correlation with disease risk, inflammation, severity, and risk of recurrence. J. Clin. Lab. Anal. 2022, 36, e24171. [Google Scholar] [CrossRef] [PubMed]
- Esawy, M.M.; Abd-elhameed, A.; Gomaa, A.F.; Baioumy, S.A.; ElKot, M.A.; Hegab, M.A.; Alazzouni, A.S.; Thagfan, F.A.; Abdel-Gaber, R.; Dkhil, M.A.; et al. LncRNA-GAS5 and β-Catenin as Independent Predictors of Asymptomatic Organ Damage in Nondiabetic Hypertensive Patients. ACS Omega 2023, 8, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. The Emerging Role of Long Non-coding RNAs and Circular RNAs in Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 632393. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hu, Y.; Yu, S.; Zhu, W.; Liu, L.; Luo, M.; Luo, S.; Shen, J.; Huang, L.; Liu, J.; et al. The lncRNA GAS5 upregulates ANXA2 to mediate the macrophage inflammatory response during atherosclerosis development. Heliyon 2024, 10, e24103. [Google Scholar] [CrossRef]
- Wang, C.H.; Shi, H.H.; Chen, L.H.; Li, X.L.; Cao, G.L.; Hu, X.F. Identification of Key lncRNAs Associated with Atherosclerosis Progression Based on Public Datasets. Front. Genet. 2019, 10, 123. [Google Scholar] [CrossRef]
- Shen, Z.; She, Q. Association Between the Deletion Allele of Ins/Del Polymorphism (Rs145204276) in the Promoter Region of GAS5 with the Risk of Atherosclerosis. Cell. Physiol. Biochem. 2018, 49, 1431–1443. [Google Scholar] [CrossRef]
- Tang, R.; Mei, X.; Wang, Y.C.; Cui, X.B.; Zhang, G.; Li, W.; Chen, S.Y. LncRNA GAS5 regulates vascular smooth muscle cell cycle arrest and apoptosis via p53 pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2516–2525. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.; Zhang, Z. lncRNA GAS5 acts as a ceRNA for miR-21 in suppressing PDGF-bb-induced proliferation and migration in vascular smooth muscle cells. J. Cell. Biochem. 2019, 120, 15233–15240. [Google Scholar] [CrossRef]
- Sun, P.; Tang, L.N.; Li, G.Z.; Xu, Z.L.; Xu, Q.H.; Wang, M.; Li, L. Effects of MiR-21 on the proliferation and migration of vascular smooth muscle cells in rats with atherosclerosis via the Akt/ERK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2216–2222. [Google Scholar] [CrossRef]
- Hao, X.; Li, H.; Zhang, P.; Yu, X.; Jiang, J.; Chen, S. Down-regulation of lncRNA Gas5 promotes hypoxia-induced pulmonary arterial smooth muscle cell proliferation by regulating KCNK3 expression. Eur. J. Pharmacol. 2020, 889, 173618. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Q.; Zhang, Y.; Hong, L.; Lei, D.; Zhang, L. Olmesartan alleviates bleomycin-mediated vascular smooth muscle cell senescence via the miR-665/SDC1 axis. Am. J. Transl. Res. 2020, 12, 5205–5220. [Google Scholar]
- Chen, T.; Liang, Q.; Xu, J.; Zhang, Y.; Zhang, Y.; Mo, L.; Zhang, L. MiR-665 Regulates Vascular Smooth Muscle Cell Senescence by Interacting with LncRNA GAS5/SDC1. Front. Cell Dev. Biol. 2021, 9, 700006. [Google Scholar] [CrossRef]
- Chaterji, S.; Lam, C.H.; Ho, D.S.; Proske, D.C.; Baker, A.B. Syndecan-1 regulates vascular smooth muscle cell phenotype. PLoS ONE 2014, 9, e89824. [Google Scholar] [CrossRef]
- Ye, J.; Wang, C.; Wang, D.; Yuan, H. LncRBA GSA5, up-regulated by ox-LDL, aggravates inflammatory response and MMP expression in THP-1 macrophages by acting like a sponge for miR-221. Exp. Cell Res. 2018, 369, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, X.; Yang, M.; Shangguan, J.; Yin, Y. GAS5 knockdown suppresses inflammation and oxidative stress induced by oxidized low-density lipoprotein in macrophages by sponging miR-135a. Mol. Cell. Biochem. 2021, 476, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Williams, H.; George, S.J. Evidence for the Involvement of Matrix-Degrading Metalloproteinases (MMPs) in Atherosclerosis. Prog. Mol. Biol. Transl. Sci. 2017, 147, 197–237. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, H. Long non-coding RNA GAS5 knockdown facilitates proliferation and impedes apoptosis by regulating miR-128-3p/FBLN2 axis in ox-LDL-induced THP-1 cells. Clin. Hemorheol. Microcirc. 2021, 77, 153–164. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Zhang, S.; Song, Q.; He, X.; Wang, J. WWP2 ameliorates oxidative stress and inflammation in atherosclerotic mice through regulation of PDCD4/HO-1 pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1057–1067. [Google Scholar] [CrossRef]
- Fei, X.; Cen, X.; Zhao, R.; Wang, J.; Cui, H. PRMT5 knockdown enhances cell viability and suppresses cell apoptosis, oxidative stress, inflammation and endothelial dysfunction in ox-LDL-induced vascular endothelial cells via interacting with PDCD4. Int. Immunopharmacol. 2023, 122, 110529. [Google Scholar] [CrossRef]
- Ge, C.; Song, J.; Chen, L.; Wang, L.; Chen, Y.; Liu, X.; Zhang, Y.; Zhang, L.; Zhang, M. Atheroprotective pulsatile flow induces ubiquitin-proteasome-mediated degradation of programmed cell death 4 in endothelial cells. PLoS ONE 2014, 9, e91564. [Google Scholar] [CrossRef]
- Wang, H.; He, F.; Liang, B.; Jing, Y.; Zhang, P.; Liu, W.; Zhu, B.; Dou, D. LincRNA-p21 alleviates atherosclerosis progression through regulating the miR-221/SIRT1/Pcsk9 axis. J. Cell. Mol. Med. 2021, 25, 9141–9153. [Google Scholar] [CrossRef]
- Barangi, S.; Hayes, A.W.; Karimi, G. The role of lncRNAs/miRNAs/Sirt1 axis in myocardial and cerebral injury. Cell Cycle 2023, 22, 1062–1073. [Google Scholar] [CrossRef]
- Yao, J.; Shi, Z.; Ma, X.; Xu, D.; Ming, G. lncRNA GAS5/miR-223/NAMPT axis modulates the cell proliferation and senescence of endothelial progenitor cells through PI3K/AKT signaling. J. Cell. Biochem. 2019, 120, 14518–14530. [Google Scholar] [CrossRef]
- Diao, L.; Bai, L.; Jiang, X.; Li, J.; Zhang, Q. Long-chain noncoding RNA GAS5 mediates oxidative stress in cardiac microvascular endothelial cells injury. J. Cell. Physiol. 2019, 234, 17649–17662. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Zhao, H.; Liu, W.; Xu, W.; Jiang, L.; Xu, R.; Zheng, Y.; Tang, X.; Li, X.; et al. lncR-GAS5 upregulates the splicing factor SRSF10 to impair endothelial autophagy, leading to atherogenesis. Front. Med. 2023, 17, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, X.; Chen, Q.; Chen, T.; Jiang, N.; Guo, Z. Myricetin ameliorates ox-LDL-induced HUVECs apoptosis and inflammation via lncRNA GAS5 upregulating the expression of miR-29a-3p. Sci. Rep. 2021, 11, 19637. [Google Scholar] [CrossRef] [PubMed]
- Haemmig, S.; Simion, V.; Yang, D.; Deng, Y.; Feinberg, M.W. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr. Opin. Cardiol. 2017, 32, 776–783. [Google Scholar] [CrossRef]
- Yu, T.; Xu, B.; Bao, M.; Gao, Y.; Zhang, Q.; Zhang, X.; Liu, R. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: A transcriptomics study. Front. Endocrinol. 2022, 13, 981100. [Google Scholar] [CrossRef]
- Dong, Z.; Li, S.; Wang, X.; Si, L.; Ma, R.; Bao, L.; Bo, A. lncRNA GAS5 restrains CCl4-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G539–G550. [Google Scholar] [CrossRef]
- Patel, R.S.; Lui, A.; Hudson, C.; Moss, L.; Sparks, R.P.; Hill, S.E.; Shi, Y.; Cai, J.; Blair, L.J.; Bickford, P.C.; et al. Small molecule targeting long noncoding RNA GAS5 administered intranasally improves neuronal insulin signaling and decreases neuroinflammation in an aged mouse model. Sci. Rep. 2023, 13, 317. [Google Scholar] [CrossRef]
- Esguerra, J.L.S.; Ofori, J.K.; Nagao, M.; Shuto, Y.; Karagiannopoulos, A.; Fadista, J.; Sugihara, H.; Groop, L.; Eliasson, L. Glucocorticoid induces human beta cell dysfunction by involving riborepressor GAS5 LincRNA. Mol. Metab. 2020, 32, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Goustin, A.S.; Thepsuwan, P.; Kosir, M.A.; Lipovich, L. The Growth-Arrest-Specific (GAS)-5 Long Non-Coding RNA: A Fascinating lncRNA Widely Expressed in Cancers. Noncoding RNA 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wang, T.; Zeng, L.; Zhu, S.; Cao, W.; Wu, W.; Wu, H.; Zou, T. Diagnostic potential of circulating LncRNAs in human cardiovascular disease: A meta-analysis. Biosci. Rep. 2018, 38, BSR20181610. [Google Scholar] [CrossRef] [PubMed]
| Disease Type | Interacting Molecules/Signaling Pathway | Related Functions | Ref |
|---|---|---|---|
| Cancer | miR-21 | Tumor-Inhibiting | [42] |
| miR-21/PTEN | Tumor-Inhibiting | [50] | |
| miR-18a | Tumor-Inhibiting | [51] | |
| Cardiovascular disease | β-catenin signaling | Vascular Remodeling | [48] |
| sema3a | Cardiomyocyte Apoptosis | [52] | |
| YBX1, miR-21 | VSMCs Apoptosis | [45] | |
| Endocrine and metabolic diseases | UPF1 | Glucose Uptake | [40] |
| Nervous system diseases | PRC2/IRF4 | Microglial Polarization | [53] |
| Bone and development | miR-144/mTOR | Autophagy of Chondrocytes | [54] |
| miR-291-a, cMyc | Self-Renewal and Pluripotency of Stem Cells | [55] |
| Cell Type | Species | Key Interacting Molecules/Signaling Pathway | Biological Process Affected | Ref |
|---|---|---|---|---|
| VSMCs | Rat | p53-p300, p21, NOXA | Cell cycle arrest (G1), Apoptosis, Proliferation | [67] |
| Human | miR-21/PDCD4 | Proliferation, Migration | [68] | |
| Mouse | miR-145-5p | Proliferation, Apoptosis | [30] | |
| Rat | ATPase, L31 | Proliferation, Phenotypic switching | [29] | |
| Human/Rat | miR-21, Akt/ERK pathway | Migration | [68,69] | |
| Rat | miR-23b-3p/KCNK3 | Proliferation, Migration, Phenotypic switching | [70] | |
| Human/Mouse | miR-665/SDC1 | Anti-senescence, Phenotypic switching | [71,72,73] | |
| Macrophages | Human | miR-221/MCP-1 | Inflammatory response | [74] |
| Human | miR-135a | Inflammatory response, Oxidative stress | [56,75] | |
| Mouse | ANXA2 | Inflammatory response | [64] | |
| Human | miR-221, MMP-2, MMP-9 | MMP biosynthesis, ECM degradation | [74] | |
| Human | miR-135a | Lipid metabolism | [56] | |
| Human | EZH2, ABCA1, ABCG1 | Lipid accumulation, Cholesterol efflux | [32] | |
| Human | miR-128-3p/FBLN2 | Proliferation, Apoptosis | [77] | |
| Mouse | ANXA2 | Apoptosis | [64] | |
| Mouse | miR-145-5p | Foam cell formation, Lipid uptake, Plasma lipids | [30] | |
| ECs | Human | p53, Caspases | Apoptosis | [57] |
| Human | miR-21/PDCD4, Caspase | Apoptosis, Proliferation | [66] | |
| Rat | miR-194-3p/TXNIP | Proliferation, Apoptosis | [60] | |
| Human | miR-223/NAMPT/PI3K/AKT | Proliferation, Senescence | [83] | |
| Human | miR-221/Sirt1 | Proliferation, Migration | [81] | |
| Rat | miR-33a-5p/ABCA1 | Proliferation, Apoptosis | [84] | |
| Human | miR-26a | Autophagic flux, Apoptosis | [17] | |
| Human | miR-193-5p/SRSF10 | Autophagy | [85] | |
| Human | miR-23a-3p/TLR4 | Endothelial growth, Inflammation, EndMT | [86] |
| Methods | Effect | Disease Domain | Ref |
|---|---|---|---|
| siRNA | GAS5 downregulation and liver fibrosis indicators were upregulated | Hepatic fibrosis | [89] |
| shRNA | GAS5 downregulation, protects endothelial cell function, and reduces atherosclerosis | Coronary atherosclerosis | [60] |
| np-c86 | GAS5 upregulation and promotes insulin receptor activity and glucose absorption | Diabetes | [40] |
| np-c86 | GAS5 upregulation, improves neuronal insulin signaling, and reduces neuroinflammation | Neurodegenerative diseases | [90] |
| Myricetin | GAS5 downregulation, inflammatory response and EndMT reduction | Atherosclerosis | [86] |
| ASO/HREM | GAS5 was downregulated/upregulated, and β cell activity was enhanced when it was upregulated | Diabetes | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Luo, Q.; Li, X.; Liu, X.; Yang, Z.; Tuo, Q.; Chen, W. Unraveling LncRNA GAS5 in Atherosclerosis: Mechanistic Insights and Clinical Translation. Biology 2025, 14, 697. https://doi.org/10.3390/biology14060697
Wei Y, Luo Q, Li X, Liu X, Yang Z, Tuo Q, Chen W. Unraveling LncRNA GAS5 in Atherosclerosis: Mechanistic Insights and Clinical Translation. Biology. 2025; 14(6):697. https://doi.org/10.3390/biology14060697
Chicago/Turabian StyleWei, Yu, Quanye Luo, Xiang Li, Xi Liu, Zheyu Yang, Qinhui Tuo, and Wen Chen. 2025. "Unraveling LncRNA GAS5 in Atherosclerosis: Mechanistic Insights and Clinical Translation" Biology 14, no. 6: 697. https://doi.org/10.3390/biology14060697
APA StyleWei, Y., Luo, Q., Li, X., Liu, X., Yang, Z., Tuo, Q., & Chen, W. (2025). Unraveling LncRNA GAS5 in Atherosclerosis: Mechanistic Insights and Clinical Translation. Biology, 14(6), 697. https://doi.org/10.3390/biology14060697







