Feeding and Growth in the Ephyra Stage of Aurelia coerulea: An In Situ Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Polyps and Ephyrae
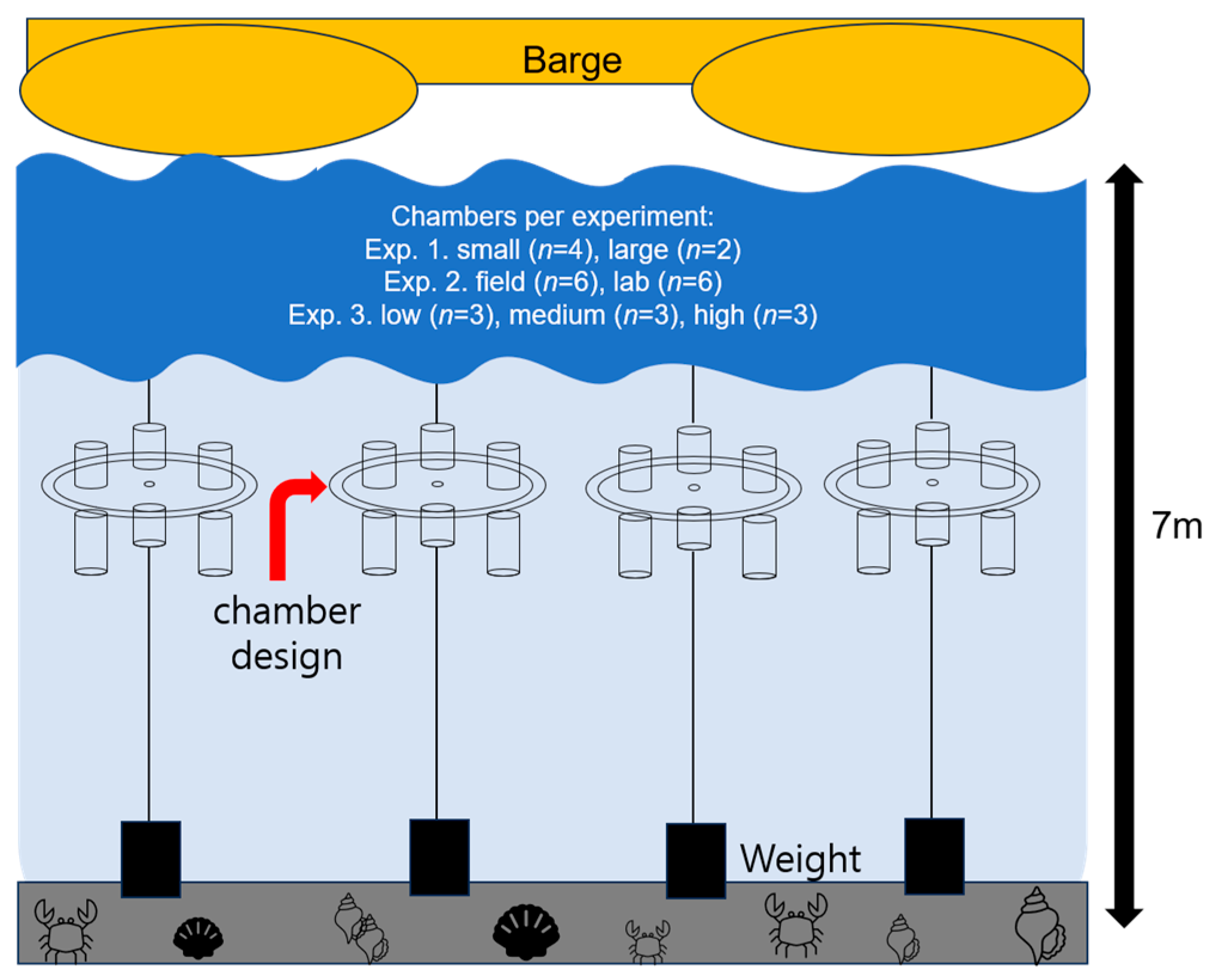
2.2. Experimental Setup
2.3. Prey Organisms
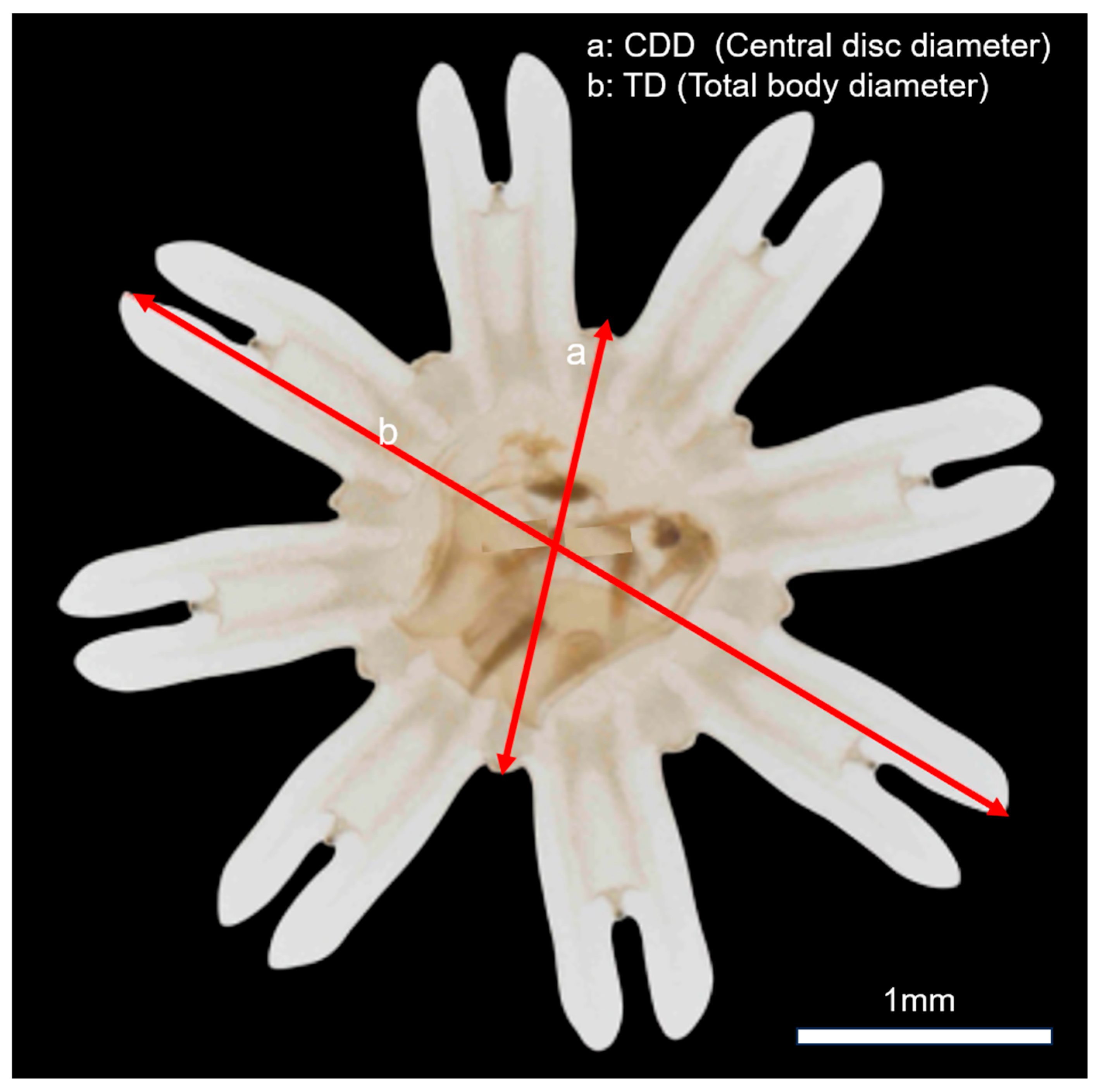
2.4. Grazing and Growth Parameters
2.5. Statistical Analysis
3. Results
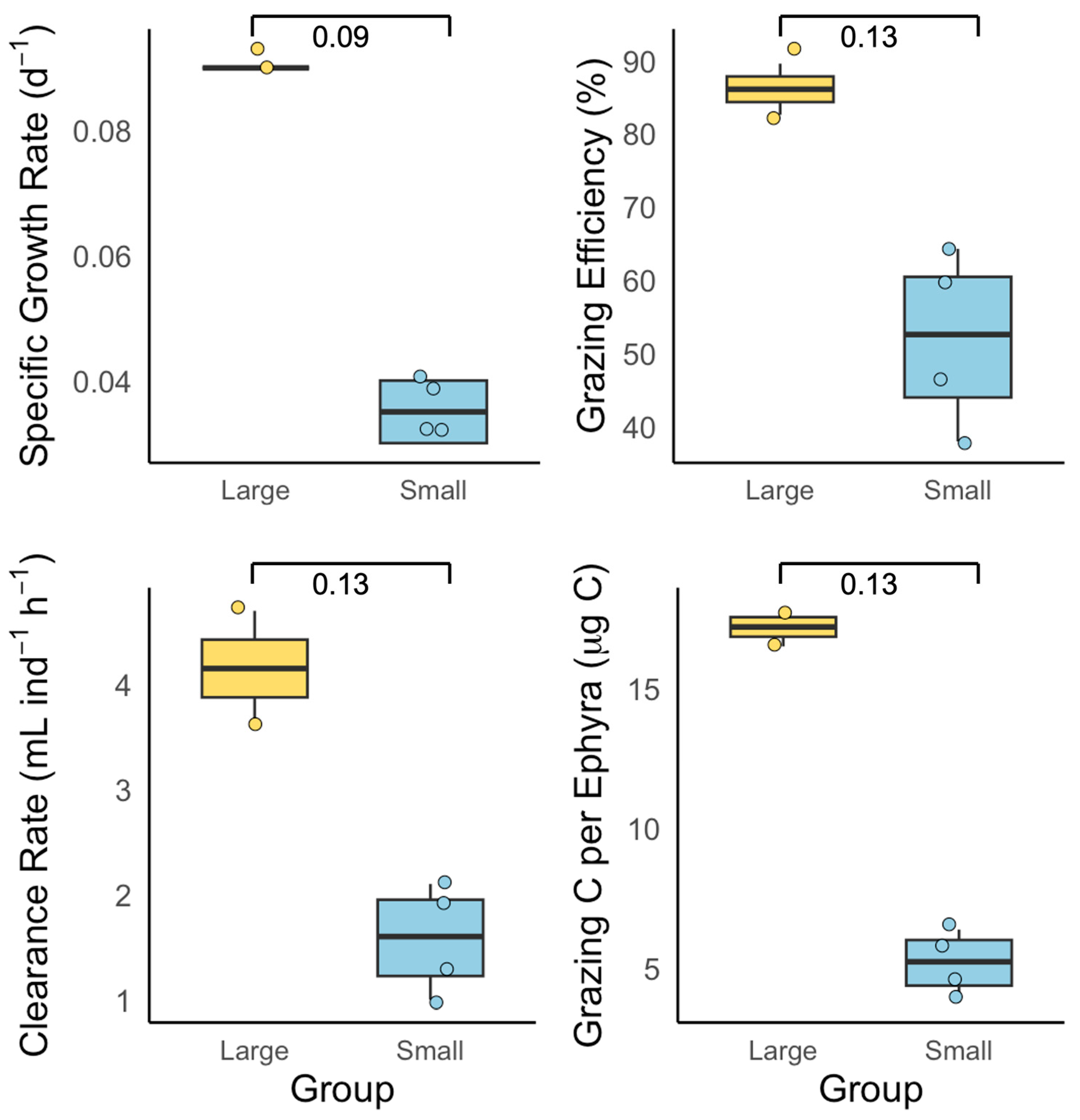
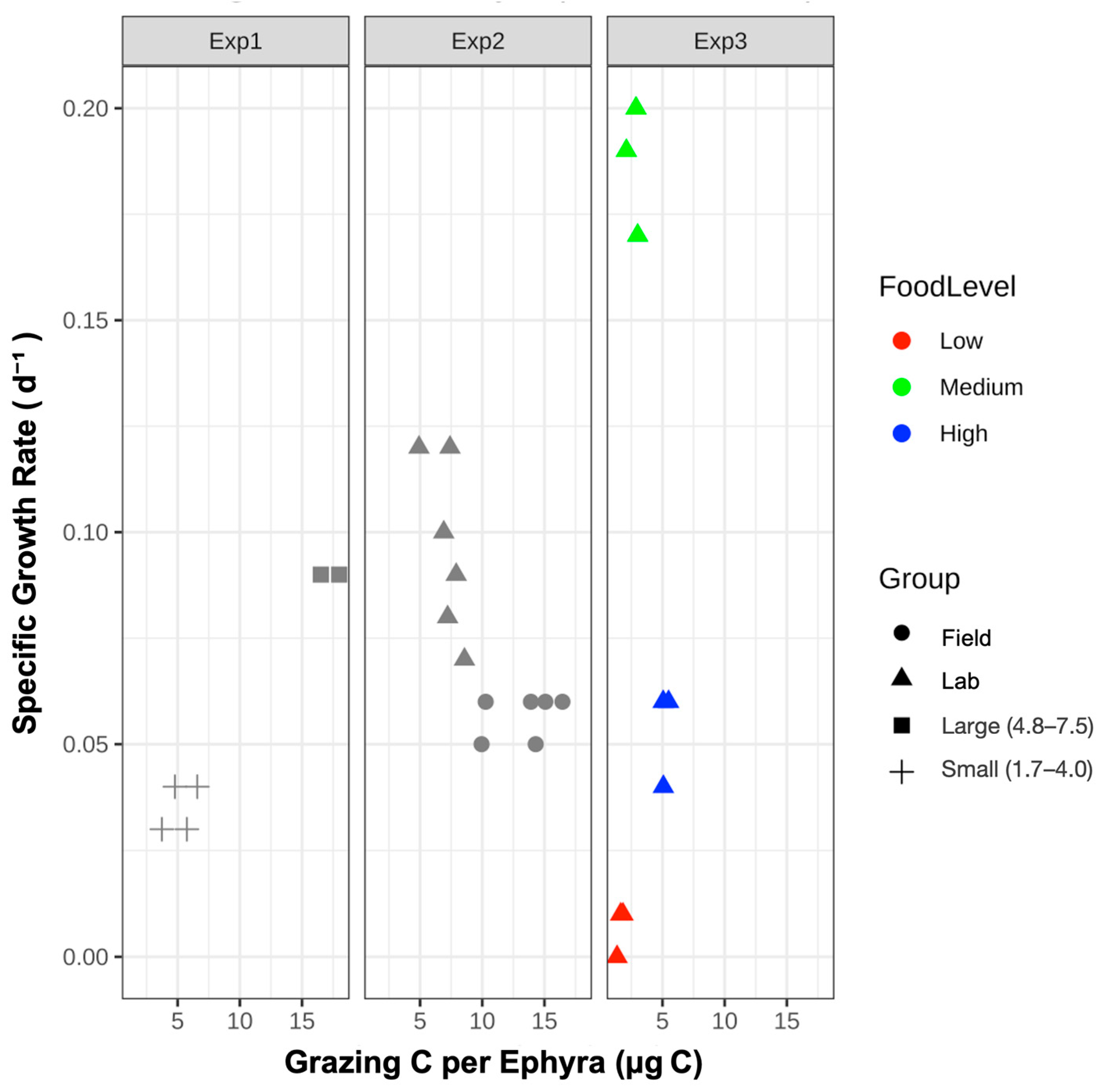
3.1. Exp. 1: Size-Specific Feeding and Growth
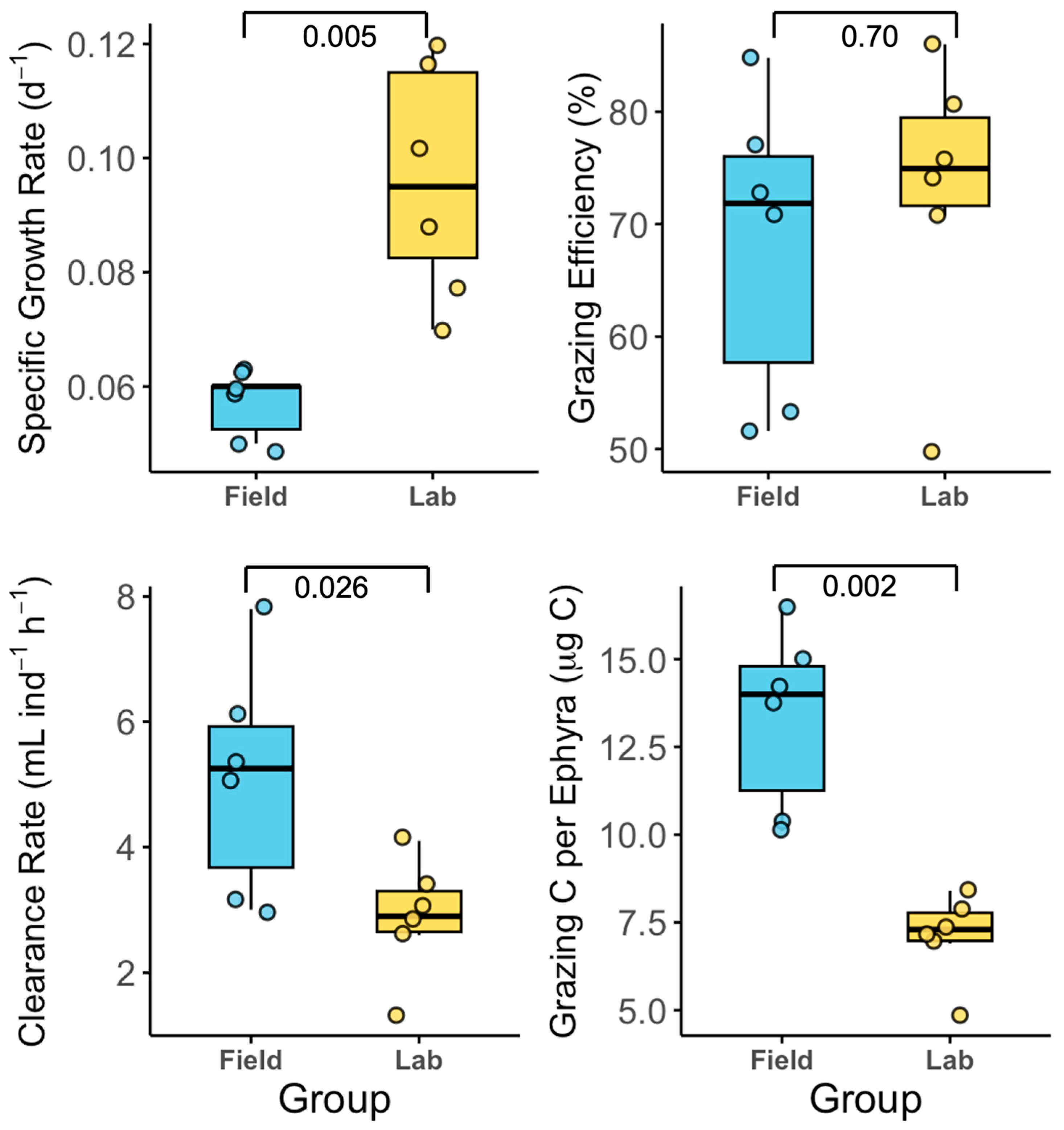
3.2. Exp. 2: Field-Collected Versus Laboratory-Reared Ephyrae
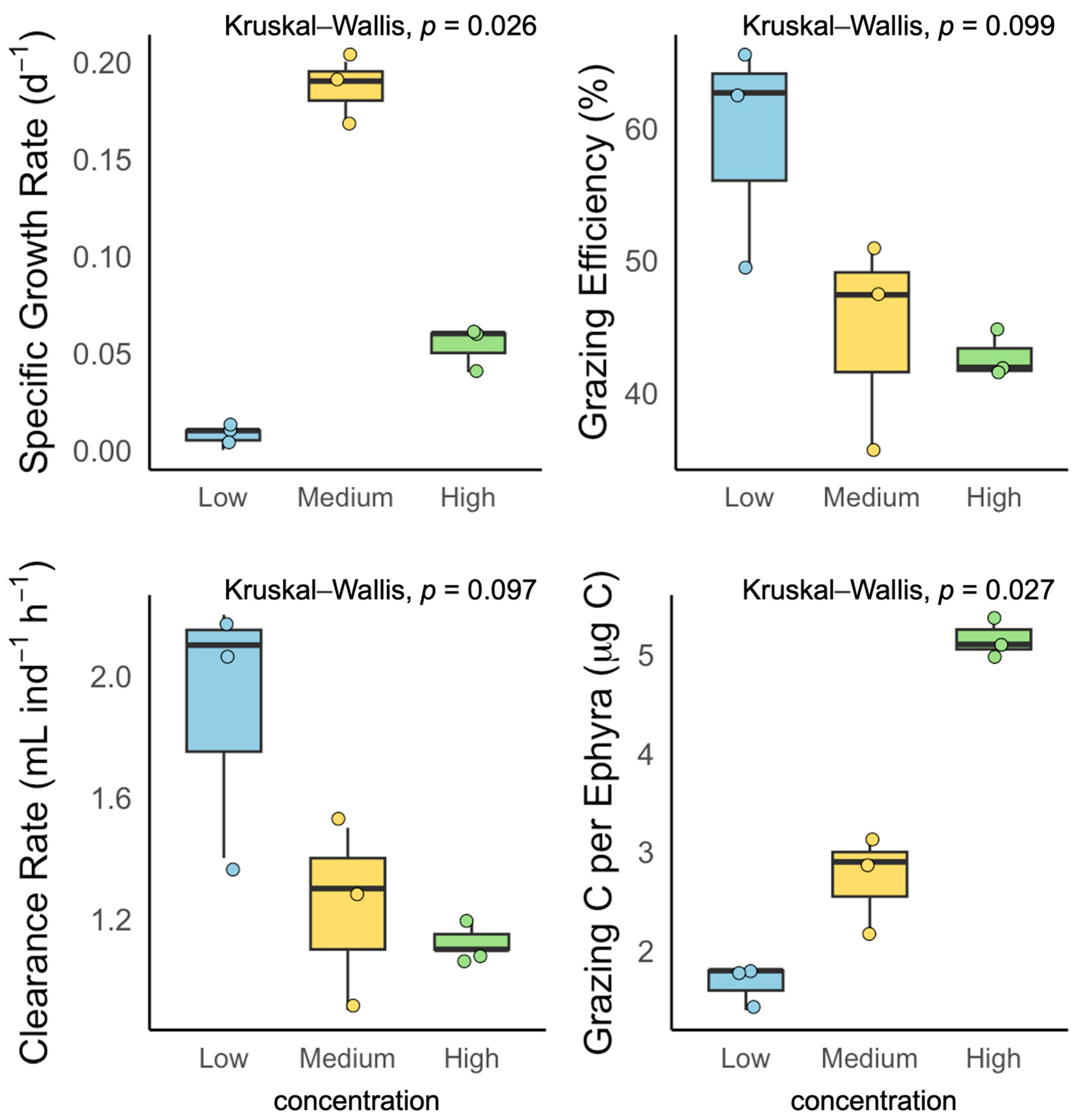
3.3. Exp. 3: Effects of Food Concentration on Laboratory Ephyrae
4. Discussion
4.1. Comparison of Growth and Feeding Efficiency: Size-Dependent
4.2. Comparison of Growth and Feeding Efficiency: Field-Collected and Laboratory-Reared Ephyrae
4.3. Comparison of Growth and Feeding Efficiency: Prey Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Willcox, S.; Moltschaniwskyj, N.A.; Crawford, C. Asexual reproduction in scyphistomae of Aurelia sp.: Effects of temperature and salinity in an experimental study. J. Exp. Mar. Biol. Ecol. 2007, 353, 107–114. [Google Scholar] [CrossRef]
- Kim, K.Y.; Youn, S.H.; Choi, S.Y.; Park, W. Massive Outbreak of Aurelia coerulea in Geoje Bay, Korea. Water 2024, 16, 2846. [Google Scholar] [CrossRef]
- Möller, H. Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany (FRG). Mar. Biol. 1980, 60, 123–128. [Google Scholar] [CrossRef]
- Purcell, J.E. Pelagic cnidarians and ctenophores as predators: Selective predation, feeding rates and effects on prey populations. Ann. Inst. Oceanogr. Paris 1997, 73, 125–137. [Google Scholar]
- Hansson, L.J.; Moeslund, O.; Kiørboe, T.; Riisgård, H.U. Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Mar. Ecol. Prog. Ser. 2005, 304, 117–131. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, F.; Sun, S.; Lü, S. Experimental clearance rate and intraguild predation of jellyfish Cyanea nozakii. J. Oceanol. Limnol. 2024, 42, 128–140. [Google Scholar] [CrossRef]
- Uye, S.; Fujii, N.; Takeoka, H. Unusual aggregations of the scyphomedusa Aurelia aurita in coastal waters along western Shikoku. Plankton Biol. Ecol. 2003, 50, 17–21. [Google Scholar]
- Uye, S.; Ueta, Y. Recent increase of jellyfish populations and their nuisance to fisheries in the Inland Sea of Japan. Bull. Jpn. Soc. Fish. Oceanogr. 2004, 68, 9–19, (In Japanese with English Abstract). [Google Scholar]
- Ishii, H.; Ohba, T.; Kobayashi, T. Effects of low dissolved oxygen on planula settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankton Benthos Res. 2008, 3, 107–113. [Google Scholar] [CrossRef]
- Höhn, D.P.; Lucas, C.H.; Thatje, S. Respiratory response to temperature of three populations of Aurelia aurita polyps in northern Europe. PLoS ONE 2017, 12, e0173092. [Google Scholar] [CrossRef] [PubMed]
- Båmstedt, U.; Wild, B.; Martinussen, M. Significance of food type for growth of ephyrae Aurelia aurita (Scyphozoa). Mar. Biol. 2001, 139, 641–650. [Google Scholar] [CrossRef]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Han, C.-H.; Uye, S.-I. Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita s.l. Plankton Benthos Res. 2010, 5, 98–105. [Google Scholar] [CrossRef]
- Ishii, H.; Kojima, S.; Tanaka, Y. Survivorship and production of Aurelia aurita ephyrae in the innermost part of Tokyo Bay, Japan. Plankton Biol. Ecol. 2004, 51, 26–35. [Google Scholar]
- Schroth, W.; Jarms, G.; Streit, B.; Schierwater, B. Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evol. Biol. 2002, 2, 1–10. [Google Scholar] [CrossRef][Green Version]
- Sullivan, B.K.; Suchman, C.L.; Costello, J.H. Mechanics of prey selection by ephyrae of the scyphomedusa Aurelia aurita. Mar. Biol. 1997, 130, 213–222. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, F.; Sun, S.; Wang, W.; Wan, A.; Li, C. Experimental clearance rates of Aurelia coerulea ephyrae and medusae, and the predation impact on zooplankton in Jiaozhou Bay. J. Oceanol. Limnol. 2020, 38, 1256–1269. [Google Scholar] [CrossRef]
- Kamiyama, T. Planktonic ciliates as food for the scyphozoan Aurelia coerulea: Feeding and growth responses of ephyra and metephyra stages. J. Oceanogr. 2018, 74, 53–63. [Google Scholar] [CrossRef]
- Russell, F.S. Nausithoeïdae. In The Medusae of the British Isles II. Pelagic Scyphozoa with a Supplement to the First Volume on Hydromedusae; Russell, F.S., Ed.; Cambridge University Press: London, UK, 1970; pp. 29–37. [Google Scholar]
- Olesen, N.J.; Frandsen, K.; Riisgård, H.U. Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Mar. Ecol. Prog. Ser. 1994, 105, 9–18. [Google Scholar] [CrossRef]
- Møller, L.F.; Riisgård, H.U. Population dynamics, growth and predation impact of the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa and Aequorea vitrina, in Limfjorden (Denmark). Mar. Ecol. Prog. Ser. 2007, 346, 153–165. [Google Scholar] [CrossRef]
- Fu, Z.; Shibata, M.; Makabe, R.; Ikeda, H.; Uye, S. Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Prog. Ser. 2014, 510, 255–263. [Google Scholar] [CrossRef]
- Båmstedt, U.; Lane, J.; Martinussen, M.B. Bioenergetics of ephyra larvae of the scyphozoan jellyfish Aurelia aurita in relation to temperature and salinity. Mar. Biol. 1999, 135, 89–98. [Google Scholar] [CrossRef]
- Wang, N.; Li, C. The effect of temperature and food supply on the growth and ontogeny of Aurelia sp. 1 ephyrae. Hydrobiologia 2015, 754, 157–167. [Google Scholar] [CrossRef]
- Purcell, J.E. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia 2009, 616, 23–50. [Google Scholar] [CrossRef]
- Shin, H.H.; Han, I.; Oh, W.; Chae, J.; Yoon, E.; Lee, K. Estimation of moon jellyfish Aurelia coerulea using hydroacoustic methods off the coast of Tongyeong, Korea. Korean J. Fish. Aquat. Sci. 2019, 52, 725–734. [Google Scholar] [CrossRef]
- NIFS (National Institute of Fisheries Science). Korean Jellyfish Information System. 2025. Available online: https://www.nifs.go.kr/jelly/main.jely (accessed on 1 May 2025).
- NFRDI. Annual Report of the Sanitation Survey on the Aquaculture Ground of Shellfish in Hansan-Geoje Bay; NFRDI: Busan, Korea, 2011; pp. 1–8. [Google Scholar]
- Lee, D.I.; Choi, Y.H.; Hong, S.J.; Kim, H.C.; Lee, W.C. Spatio-Temporal Variation Characteristics of Primary Productivity and Environmental Factors of Shellfish Mariculture in Jaran Bay, Korea. Korean Soc. Mar. Environ. Energy 2022, 28, 721–731. [Google Scholar] [CrossRef]
- Uye, S.I. Length-weight relationships of important zooplankton from the Inland Sea of Japan. J. Oceanogr. Soc. Jpn. 1982, 38, 149–158. [Google Scholar] [CrossRef]
- Uye, S.I.; Nagano, N.; Tamaki, H. Geographical and seasonal variations in abundance, biomass and estimated production rates of microzooplankton in the Inland Sea of Japan. J. Oceanogr. 1996, 52, 689–703. [Google Scholar] [CrossRef]
- Frost, B.W. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Riisgård, H.U.; Madsen, C.V. Clearance rates of ephyrae and small medusae of the common jellyfish Aurelia aurita offered different types of prey. J. Sea Res. 2011, 65, 51–57. [Google Scholar] [CrossRef]
- Djeghri, N. Variability and Plasticity of the Nutrition of Zooxanthellate Jellyfishes: Insights from Experimental and Field Studies. Ph.D. Thesis, Université de Bretagne occidentale-Brest, Brest, France, 2019. [Google Scholar]
- Olesen, N.J.; Purcell, J.E.; Stoecker, D.K. Feeding and growth by ephyrae of scyphomedusae Chrysaora quinquecirrha. Mar. Ecol. Prog. Ser. 1996, 137, 149–159. [Google Scholar] [CrossRef]
- Leoni, V.; Molinero, J.C.; Crochemore, S.; Meffre, M.; Bonnet, D. Ontogenetic dietary shifts of the medusa Rhizostoma pulmo (Cnidaria: Scyphozoa). Hydrobiologia 2022, 849, 2933–2948. [Google Scholar] [CrossRef]
- Milisenda, G.; Rossi, S.; Vizzini, S.; Fuentes, V.L.; Purcell, J.E.; Tilves, U.; Piraino, S. Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci. Rep. 2018, 8, 12140. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Michaels, A.E.; Davis, L.H. Grazing by the Jellyfish, Aurelia aurita, on Microzooplankton. J. Plankton Res. 1987, 9, 901–915. [Google Scholar] [CrossRef]
- Gordoa, A.; Acuña, J.L.; Farrés, R.; Bacher, K. Burst Feeding of Pelagia noctiluca Ephyrae on Atlantic Bluefin Tuna (Thunnus thynnus) Eggs. PLoS ONE 2013, 8, e74721. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Kim, K.Y.; Youn, S.H. Feeding and Growth in the Ephyra Stage of Aurelia coerulea: An In Situ Study. Biology 2025, 14, 687. https://doi.org/10.3390/biology14060687
Choi SY, Kim KY, Youn SH. Feeding and Growth in the Ephyra Stage of Aurelia coerulea: An In Situ Study. Biology. 2025; 14(6):687. https://doi.org/10.3390/biology14060687
Chicago/Turabian StyleChoi, Seo Yeol, Kyoung Yeon Kim, and Seok Hyun Youn. 2025. "Feeding and Growth in the Ephyra Stage of Aurelia coerulea: An In Situ Study" Biology 14, no. 6: 687. https://doi.org/10.3390/biology14060687
APA StyleChoi, S. Y., Kim, K. Y., & Youn, S. H. (2025). Feeding and Growth in the Ephyra Stage of Aurelia coerulea: An In Situ Study. Biology, 14(6), 687. https://doi.org/10.3390/biology14060687






