Beta Diversity of Tenebrionid Beetles (Coleoptera, Tenebrionidae) in Ningxia Grasslands and Their Driving Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
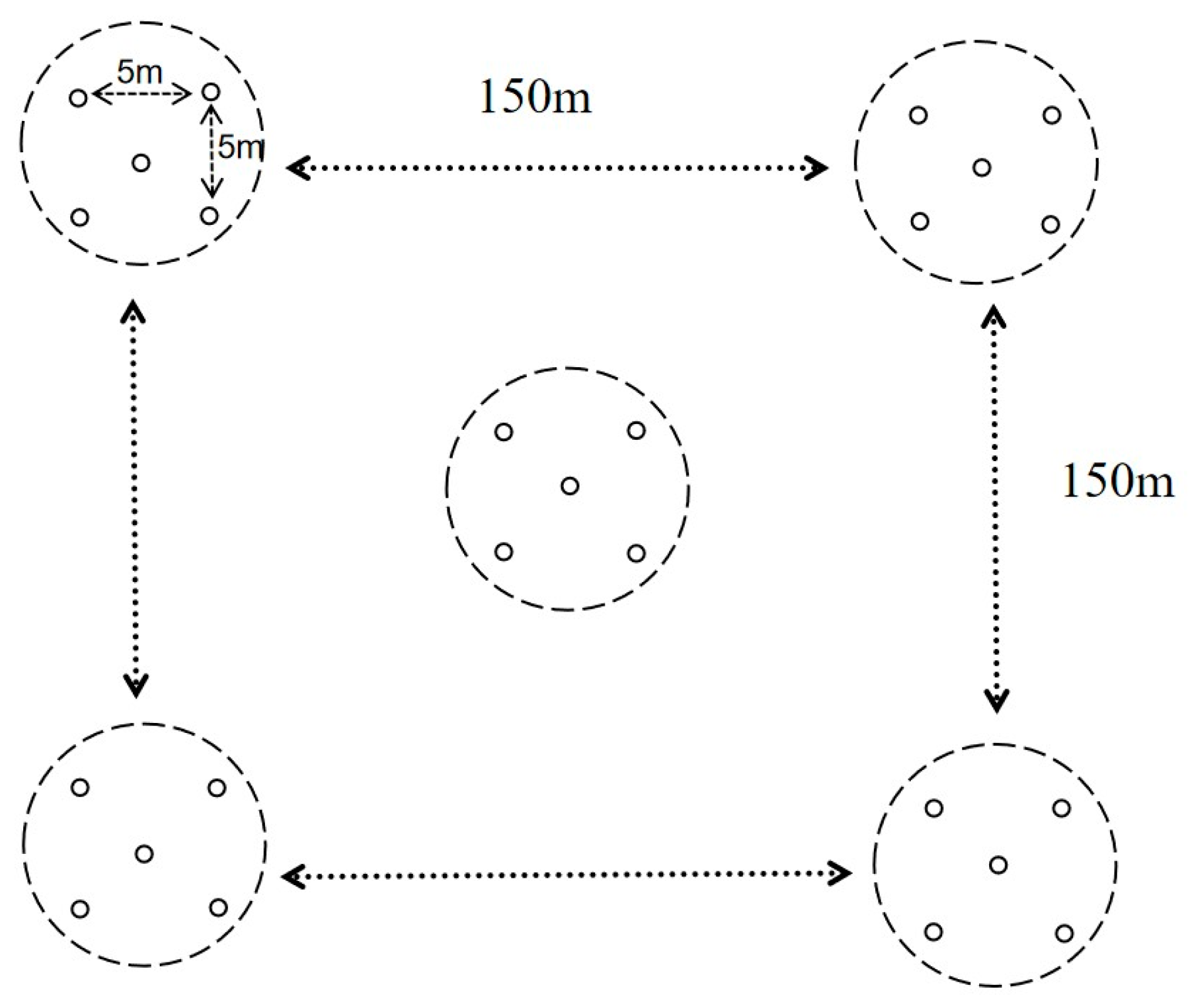
2.1. Monitoring Area
2.2. Tenebrionid Beetle Collection
2.3. Environmental Data Collection
2.3.1. Soil Environmental Data Collection
2.3.2. Climate and Elevation Survey Methods
2.3.3. Vegetation Data Collection
2.4. Data Statistical Analysis
3. Results and Analysis
3.1. Beta Diversity of Tenebrionid Beetle Communities Across Ningxia Grassland Types
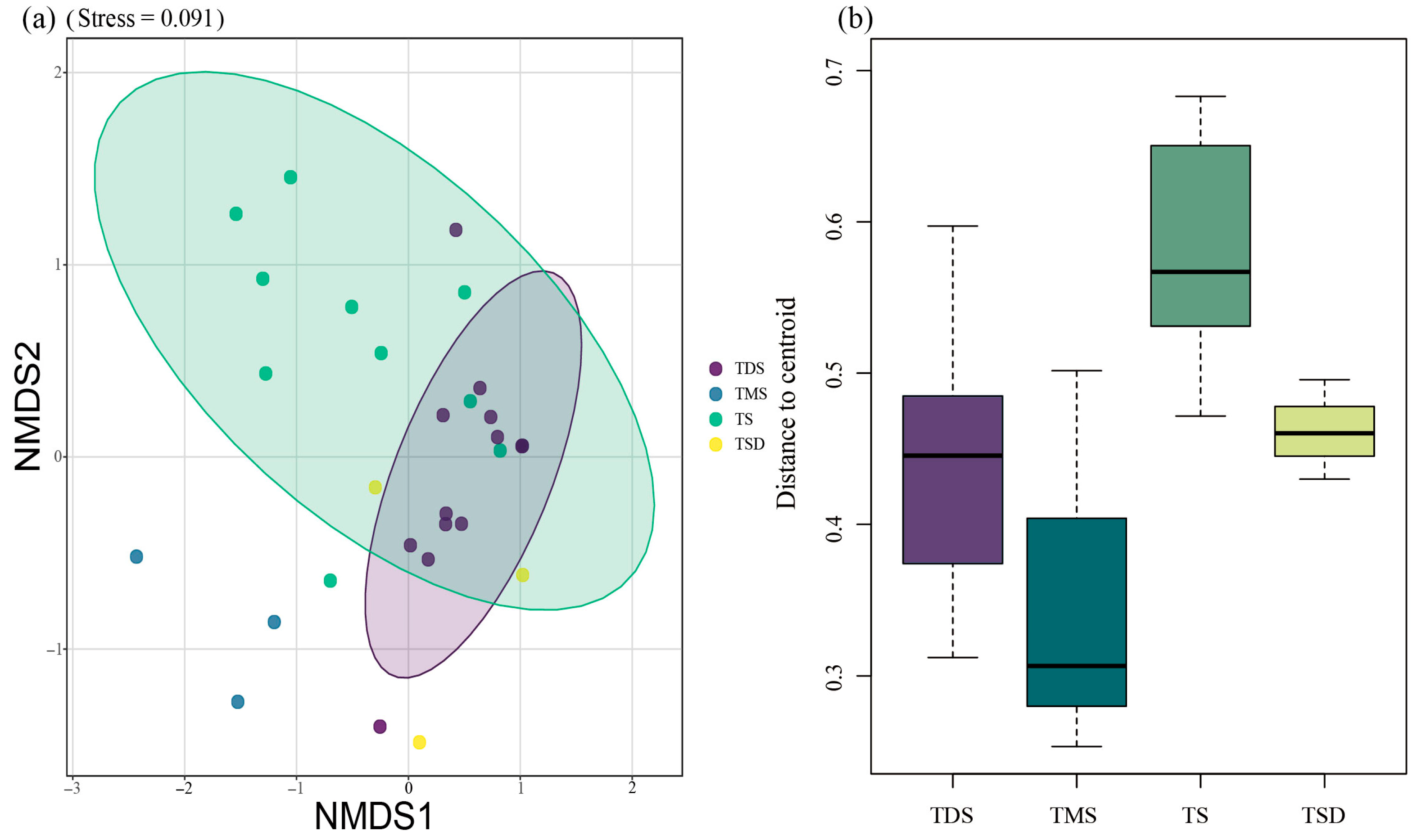
3.1.1. Compositional Differences Among Four Grassland Types
3.1.2. Partitioning of Tenebrionidae Beta Diversity Across Four Ningxia Grassland Types
3.2. Environmental Factors Influencing Tenebrionid Community Diversity in Ningxia Grasslands
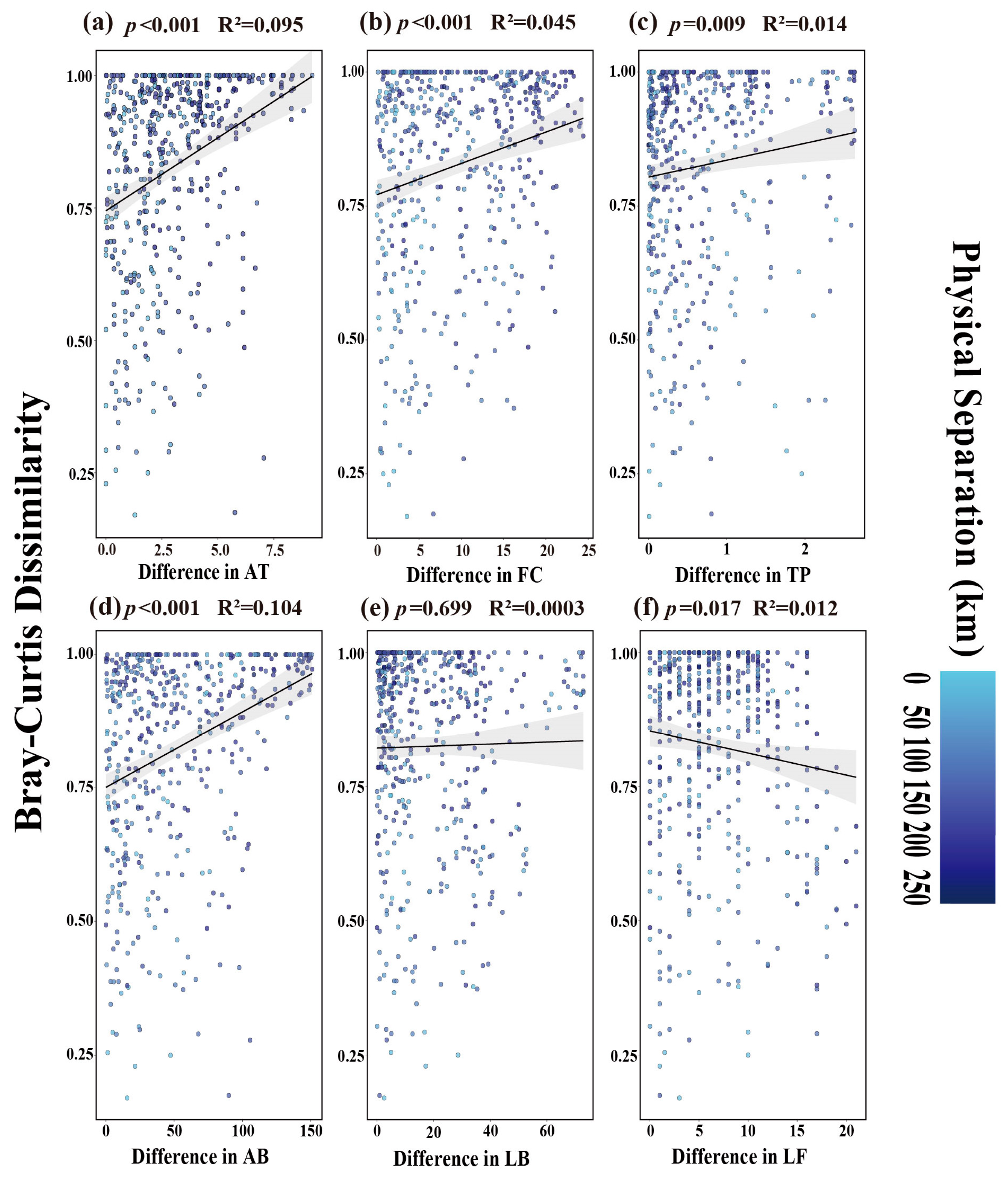
3.2.1. Environmental Factors Affecting Dominant Tenebrionid Species
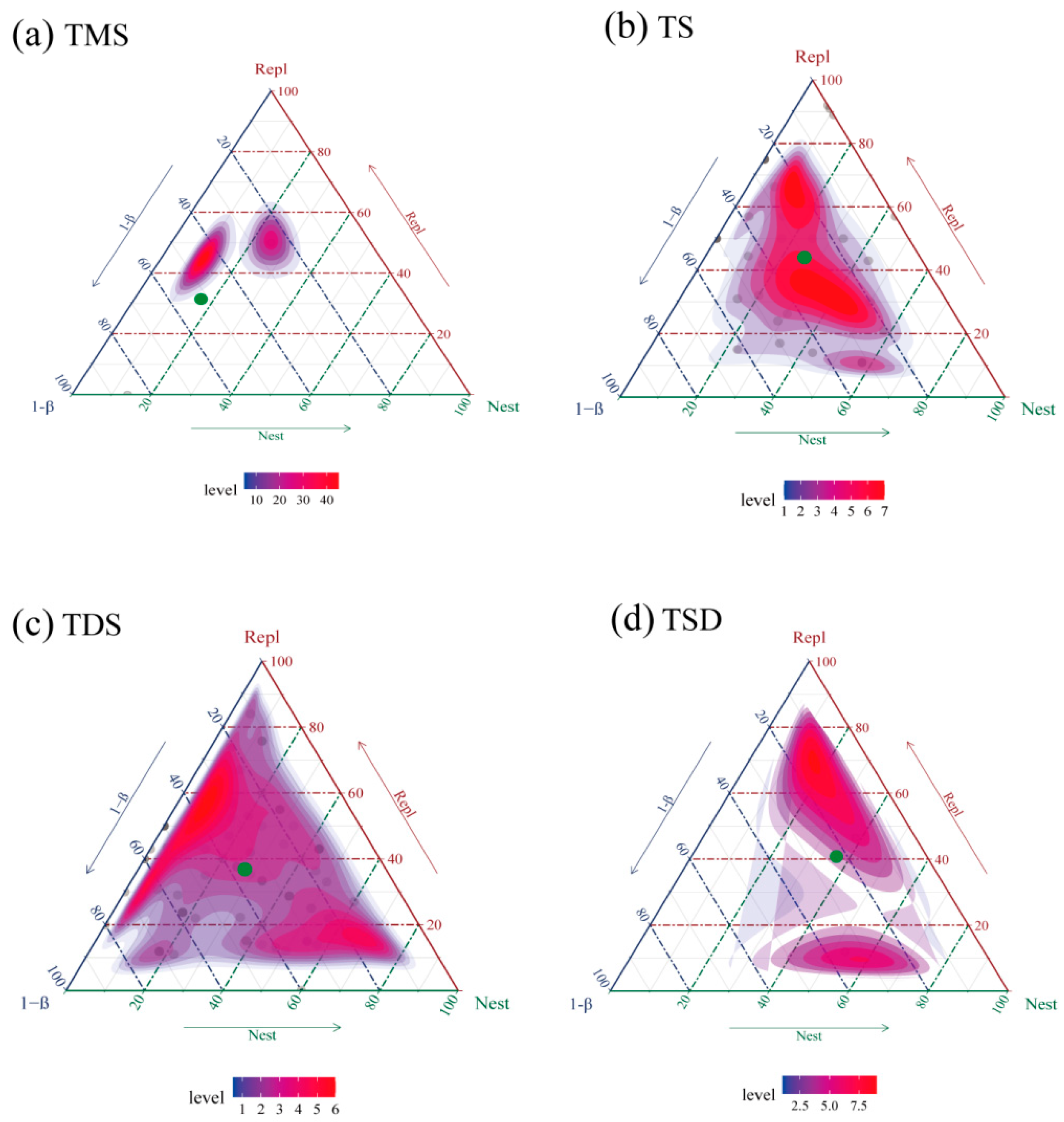
3.2.2. Driving Factors of Tenebrionid Beetles’ Beta Diversity Distribution in Ningxia Grassland
4. Discussion
4.1. Beta Diversity of Tenebrionid Beetle Communities Across Ningxia Grassland Types
4.1.1. Community Composition Differences Among Four Grassland Types
4.1.2. Partitioning of Beta Diversity in Tenebrionid Communities Across Four Grassland Types
4.2. Driving Factors of Beta Diversity in Ningxia Grassland Tenebrionid Communities
4.2.1. Environmental Factors Influencing Dominant Tenebrionid Species
4.2.2. Drivers of Beta Diversity Distribution Patterns in Ningxia Grassland Tenebrionid Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDS | Temperate desert steppe |
| TMS | Temperate meadow steppe |
| TS | Temperate steppe |
| TSD | Temperate steppe desert |
| EL | Elevation (m) |
| PP | Precipitation (mm) |
| AT | Mean daily temperature (°C) |
| HT | Maximum daily temperature (°C) |
| LT | Minimum daily temperature (°C) |
| BD | Soil bulk density |
| WHC | Saturated water holding capacity |
| CHC | Capillary water holding capacity |
| FC | Field water holding capacity |
| NCP | Non-capillary porosity |
| SCP | Capillary porosity |
| STP | Total porosity |
| OM | Organic matter |
| PH | Soil ph |
| AK | Available potassium |
| TP | Total phosphorus |
| AN | Alkali hydrolyzable nitrogen |
| AP | Available phosphorus |
| WC | Soil water content |
| GC | Gramineae cover |
| GF | Gramineae frequency |
| GB | Gramineae biomass |
| GH | Gramineae height |
| GN | Gramineae species number |
| LC | Leguminosae cover |
| LF | Leguminosae frequency |
| LB | Leguminosae biomass |
| LH | Leguminosae height |
| LN | Leguminosae species number |
| AC | Asteraceae cover |
| AF | Asteraceae frequency |
| AB | Asteraceae biomass |
| AH | Asteraceae height |
| AN1 | Asteraceae species number |
| UC | Shrub cover |
| UF | Shrub frequency |
| UB | Shrub biomass |
| UH | Shrub height |
| UN | Shrub species number |
| OC | Other plant cover |
| OF | Other plant frequency |
| OB | Other plant biomass |
| OH | Other plant height |
| ON | Other plant species number |
| sp | Scytosoma pygmaeum |
| cc | Cyphogenia chinensis |
| ap | Anatolica potanini |
| bf | Blaps femoralis |
| bfm | B. femoralis medusula |
| ae | A. ebenina |
| mk | Microdera kraatzi |
| so | Scytosoma opaca |
References
- Wang, W.; Huang, W.; Yang, J.; Ma, H.; Li, X. Distribution patterns and climate explanation of plant species richness in Ningxia grasslands. J. Arid. Land Resour. Environ. 2019, 33, 158–163. [Google Scholar]
- Yang, Y.; Qiu, K.; Li, J.; Xie, Y.; Liu, W.; Huang, Y.; Wang, S.; Bao, P. Relationship between altitudinal distribution characteristics of typical plant community diversity and soil factors on the eastern slope of the Helan Mountains. Acta Ecol. Sin. 2023, 43, 4995–5004. [Google Scholar]
- Liu, H.; Hao, H.; Sun, L.; Zhou, T. Spatial–temporal evolution characteristics of landscape ecological risk in the agro-pastoral region in Western China: A case study of Ningxia Hui Autonomous Region. Land 2022, 11, 1829. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, X.; Zhang, H. Fauna composition and distributiona characters of darklin beetles (Coleoptera: Tenebrionidae) in Ningxia Hui autonomous rigion. Acta Agric. Boreali-Occident. Sin. 1997, 6, 76–81. [Google Scholar]
- Cao, Z.; Zhang, H.; Wang, Y.; Shi, C.; Ban, L.; Zhang, R.; Wei, S. Dominant and indicator species of grasshoppers in Ningxia steppe and their responses to vegetation communities. Acta Agrestia Sin. 2024, 32, 2005–2017. [Google Scholar]
- Cao, Z.; Zhang, H.; Luo, Y.; Xiong, C.; Cui, Y.; Sun, W.; Wang, Y.; Shi, C.; Ban, L.; Zhang, R.; et al. Effects of habitat loss on tenebrionidae in gravel–sand mulching areas of desert steppe in ningxia, China. Agronomy 2024, 14, 2518. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, H.; Lou, C.; Ren, G. Energy availability factors drive the geographical pattern of Tenebrionidae (coleoptera) in the arid and semiarid areas of China. Diversity 2022, 15, 18. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, W.; He, Z.; Wang, Y.; Feng, Y.; Niu, Y.; Xin, W.; Pan, C.; Liu, J. Spatiotemporal variations of tenebrionid beetles (Coleoptera: Tenebrionidae) in the Gobi desert, Northwest China. J. Arid. Land 2025, 17, 112–129. [Google Scholar] [CrossRef]
- Macagno, H.B.; Cruz, I.G.; Rodríguez-Artigas, S.M.; Corronca, J.A.; Flores, G.E. Environmental factors determining the diversity of darkling beetles (Coleoptera: Tenebrionidae) in arid, high-altitude environments in Northwestern Argentina. An. Acad. Bras. Ciências 2023, 95, e20201185. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Bayannasan, E. Diversity and Community Pattern of Darkling Beetles (Coleoptera: Tenebrionidae) Along an Ecological Gradient in Arid Mongolia. Erforsch. Biol. Ress. Mongolei (Halle/Saale) 2012, 12, 251–266. [Google Scholar]
- McGeoch, M.A.; Van Rensburg, B.J.; Botes, A. The verification and application of bioindicators: A case study of dung beetles in a savanna ecosystem. J. Appl. Ecol. 2002, 39, 661–672. [Google Scholar] [CrossRef]
- Nahmani, J.; Lavelle, P.; Rossi, J.P. Does changing the taxonomical resolution alter the value of soil macroinvertebrates as bioindicators of metal pollution? Soil Biol. Biochem. 2006, 38, 385–396. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- El-Barougy, R.F.; Bersier, L.F.; Gray, S.M.; El-Keblawy, A.; Galal, T.; Ullah, F.; Elgamal, I.A.; Dakhil, M.A. Shaping beta diversity in arid landscape through native plant species contributions: Synergy of climate, soil, and species traits. Front. Plant Sci. 2025, 16, 1521596. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Z.; Hao, Q.; Guo, X. Interpretation of environmental factors affecting beta diversity and its components of secondary forest in Lao Mountain. Chin. J. Ecol. 2020, 39, 3211–3220. [Google Scholar]
- Li, Z.; Zhang, Z.; Yang, A.; Pan, S.; Zheng, J.; Mou, L.; Chen, W.; Zhang, T.; Zhou, L.; You, W.; et al. Opposing island biogeographic effects of turnover and nestedness on beta-diversity of soil faunal communities between woodland and deforested grassland. Appl. Soil Ecol. 2025, 207, 105966. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Yang, S.; Chao, X.; Liu, H.; Ba, S. Protozoan community diversity and its impact factor in the middle reaches of the Yarlung Zangbo River in the wet season. Biodiversity 2023, 31, 100–115. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, G.; Guo, K.; Wang, K.; Wijewardene, L.; Wu, N. Scales matter: Regional environment factors affect α diversity but local factors affect β diversity of macroinvertebrates in Thousand Islands Lake catchment area. Ecol. Indic. 2024, 158, 111561. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, S.; Gao, L.; Zhang, Z. Colored Pictorial Handbook of Grassland Insects in Ningxia, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2014; pp. 128–208. [Google Scholar]
- Ren, G.; Bai, X.; Bai, L. Fauna of the Beetles from Ningxia, China, 1st ed.; Publishing House of Electronics Industry: Beijing, China, 2019; pp. 44–538. [Google Scholar]
- Wang, X.; Yang, G. Insects in Helan Mountain of Ningxia Hui Autonomous Region, 1st ed.; Ningxia people’s Publishing House: Yinchuan, China, 2010; pp. 151–255. [Google Scholar]
- Graystock, P.; Ng, W.H.; Parks, K.; Tripodi, A.D.; Muñiz, P.A.; Fersch, A.A.; Myers, C.R.; McFrederick, Q.S.; McArt, S.H. Dominant bee species and floral abundance drive parasite temporal dynamics in plant-pollinator communities. Nat. Ecol. Evol. 2020, 4, 1358–1367. [Google Scholar] [CrossRef]
- Callisto, M.; Linares, M.S.; Kiffer Jr, W.P.; Hughes, R.M.; Moretti, M.S.; Macedo, D.R.; Solar, R. Beta diversity of aquatic macroinvertebrate assemblages associated with leaf patches in neotropical montane streams. Ecol. Evol. 2021, 11, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Lu, M. Complex relationships between beta diversity and dispersal in meta-community models. Ecography 2021, 44, 1769–1780. [Google Scholar] [CrossRef]
- Koutsikos, N.; Vavalidis, T.; Perdikaris, C.; Zogaris, S.; Vardakas, L. Anthropogenic influences reshape lentic fish diversity: Patterns of homogenization and differentiation across a Mediterranean biodiversity hotspot. Sci. Total Environ. 2025, 973, 179154. [Google Scholar] [CrossRef]
- Eibes, P.M.; Oldeland, J.; Irl, S.D.; Twerski, A.; Kühne, N.; Schmiedel, U. Partitioned beta diversity patterns of plants across sharp and distinct boundaries of quartz habitat islands. J. Veg. Sci. 2021, 32, e13036. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Z.; Wang, Y.; Shi, X.; Cheng, R.; Ban, L.; Zhang, R.; Wei, S. Beta diversity of grasshoppers and predatory beetles across steppes is closely associated with altitude and average annual precipitation in Ningxia, northwest China. Glob. Ecol. Conserv. 2024, 51, e02941. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2025. Available online: https://www.R-project.org (accessed on 28 February 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mc, G.D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Packages Version2.6-10. 2025. Available online: https://CRAN.R-project.org/package=vegan/ (accessed on 19 January 2025).
- Dopman, E.B.; Sword, G.A.; Hillis, D.M. The importance of the ontogenetic niche in resource-associated divergence: Evidence from a generalist grasshopper. Evolution 2002, 56, 731–740. [Google Scholar]
- Fattorini, S.; Baselga, A. Species richness and turnover patterns in European tenebrionid beetles. Insect Conserv. Divers. 2012, 5, 331–345. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di, B.L.; Strona, G.; Pace, L.; Biondi, M. Elevational patterns of generic diversity in the tenebrionid beetles (Coleoptera Tenebrionidae) of Latium (Central Italy). Diversity 2020, 12, 47. [Google Scholar] [CrossRef]
- Fattorini, S. Adaptations of tenebrionid beetles to Mediterranean sand dune environments and the impact of climate change (Coleoptera: Tenebrionidae). Fragm. Entomol. 2023, 55, 1–20. [Google Scholar]
- ISari, L.T.; Ribeiro-Costa, C.S.; Medeiros, A.C. Insects associated with seeds of Lonchocarpus muehlbergianus hassl.(Fabaceae) in Três Barras, Paraná, Brazil. Neotrop. Entomol. 2002, 31, 483–486. [Google Scholar]
- Di Iorio, O.R. Survival and emergence of adult seed beetles (Coleoptera: Chrysomelidae: Bruchinae) from legume seeds egested by vertebrates. Boletín Soc. Entomol. Aragonesa 2015, 57, 286–292. [Google Scholar]
- Tamgno, B.R.; Tinkeu, S.N. Application of the flour of four leguminous crops for the control of Tenebrionidae beetle (Tribolium castaneum-Herbst). Afr. J. Food Agric. Nutr. Dev. 2014, 14, 8474–8487. [Google Scholar] [CrossRef]
- Padin, S.B.; Fuse, C.B.; Urrutia, M.I.; Dal Bello, G. Toxicity and repellency of nine medicinal plants against Tribolium castaneum in stored wheat. Bull. Insectology 2013, 66, 45–49. [Google Scholar]
- Stefanazzi, N.; Gutiérrez, M.M.; Stadler, T.; Bonini, A.; Ferrero, A.A. Actividad biologica del aceite esencial de Tagetes terniflora Kunth (Asteraceae) enTribolium castaneum Herbst (Insecta, Coleoptera, Tenebrionidae). Boletín Sanid. Veg. Plagas 2006, 32, 439–447. [Google Scholar]
- ExTRACTS, B. Tenebrio molitor (Coleoptera: Tenebrionidae) as a guinea pig for the analysis of the toxicity of natural products. Vie Milieu-Life Environ. 2013, 63, 193–204. [Google Scholar]
- Zhao, M.; Wang, Y.; Yang, W.; Zhu, Y.; Zhang, S.; Liang, Y.; Yang, G. Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan. Insects 2025, 16, 388. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, C.; Cao, Z.; Zhang, H.; Wang, Y.; Sun, W.; Shi, C.; Cui, Y.; Zhang, R.; Wei, S. Beta Diversity of Tenebrionid Beetles (Coleoptera, Tenebrionidae) in Ningxia Grasslands and Their Driving Factors. Biology 2025, 14, 680. https://doi.org/10.3390/biology14060680
Xiong C, Cao Z, Zhang H, Wang Y, Sun W, Shi C, Cui Y, Zhang R, Wei S. Beta Diversity of Tenebrionid Beetles (Coleoptera, Tenebrionidae) in Ningxia Grasslands and Their Driving Factors. Biology. 2025; 14(6):680. https://doi.org/10.3390/biology14060680
Chicago/Turabian StyleXiong, Changyu, Ziyu Cao, Haixiang Zhang, Ying Wang, Wei Sun, Chun Shi, Yifan Cui, Rong Zhang, and Shuhua Wei. 2025. "Beta Diversity of Tenebrionid Beetles (Coleoptera, Tenebrionidae) in Ningxia Grasslands and Their Driving Factors" Biology 14, no. 6: 680. https://doi.org/10.3390/biology14060680
APA StyleXiong, C., Cao, Z., Zhang, H., Wang, Y., Sun, W., Shi, C., Cui, Y., Zhang, R., & Wei, S. (2025). Beta Diversity of Tenebrionid Beetles (Coleoptera, Tenebrionidae) in Ningxia Grasslands and Their Driving Factors. Biology, 14(6), 680. https://doi.org/10.3390/biology14060680




