Simple Summary
The Igf2r expression pattern in offspring is influenced by the parents and is important for organism growth. In this study, we examined the Igf2r gene in horses, donkeys, and their hybrids. We found that the parent-specific expression of Igf2r was lost in equine brain and mule liver tissues, whereas it was retained in most other tissues. Surprisingly, this was not related to canonical differentially methylated regions (DMRs). However, DNA methylation patterns in CpG island 2 (CpGI2) in the promoter of Igf2r could be transmitted from parents to offspring, thereby determining its expression activity, even in hybrids. This helps us to improve hybrid breeding strategies and address livestock growth issues.
Abstract
Genomic imprinting is critical for mammalian development, but its regulation varies across species. The insulin-like growth factor 2 receptor (IGF2R), which is a maternally expressed imprinted gene critical for cell proliferation and differentiation, as well as embryonic and placental development, is classically regulated by differentially methylated regions (DMRs) and lncRNA-Airn in mice. However, studies on this in equus are scarce, especially in terms of mechanistic studies. In the present study, heart, liver, spleen, lung, kidney, brain, and muscle samples were obtained from horses, donkeys, and hybrids, and gene expression and imprinting state were tested to investigate the imprinting regulation of Igf2r in these animals. Bisulfite sequencing combined with an allele-specific expression analysis revealed a tissue-specific loss of imprinting in the mule liver and hybrid brain tissues. Strikingly, we found that the maternal-specific expression of equine Igf2r did not rely on the canonical DMRs or lncRNA-Airn. Surprisingly, DNA methylation of a specific region called CpG island 2 (CpGI2) in the Igf2r promoter showed cis-acting inheritance, meaning that the DNA methylation patterns of the parental alleles are retained in hybrid tissues. Notably, the DNA methylation of CpGI2 correlated negatively with Igf2r expression in the spleen (R2 = 0.8797, p = 6.46 × 10−6), lung (R2 = 0.8569, p = 1.57 × 10−5), and kidney (R2 = 0.8650, p = 3.85 × 10−6). Our findings suggest that imprinting may work differently in other species. This study provides a framework for understanding imprinting diversity in hybrids and shows that equine hybrids can be used to study how epigenetic inheritance works.
1. Introduction
In mammals, genomic imprinting is an epigenetic phenomenon, leading to the silencing of one of the parental alleles, thereby resulting in the monoallelic expression of numerous genes [1,2]. Imprinted genes have been demonstrated to play critical roles in the regulation of growth, metabolism, and brain development. Dysregulation of these genes has been linked to the development of developmental disorders and diseases [3,4]. DNA methylation imprints are erased in primordial germ cells and re-established differentially during gametogenesis. These regions are known as germline differentially methylated regions (gDMRs) and can resist genome-wide demethylation during preimplantation development. They are also referred to as imprinted control regions (ICRs). Subsequently, ICRs determine the expression of a non-coding gene and multiple coding gene single alleles in a cluster [2]. Therefore, to resolve the regulatory mechanism of imprinted gene expression, studying its DNA methylation pattern is indispensable. Notably, DNA methylation patterns can be inherited, independent of canonical ICRs [5]. Such cis-acting methylation directly modulates gene expression by inhibiting transcription factor binding or recruiting methyl-binding proteins, thereby suppressing promoter activity [6,7].
One classic maternal transmission imprint gene, the insulin-like growth factor 2 receptor (IGF2R) gene, mainly encodes a receptor responsible for insulin-like growth factor 2 (IGF2) degradation, and disruption of its maternal transcription results in over-growth and perinatal lethality [8]. Therefore, the Igf2-Igf2r axis plays a direct role in the regulation of cell proliferation, fetal growth, and placental development [9,10]. In addition, Igf2r has been implicated as a tumor suppressor gene in several human tumors, including breast cancer, hepatocellular carcinoma, and choriocarcinoma [11]. In mice, Igf2r imprinting is governed by two differentially methylated regions (DMR1 in the promoter and DMR2 in intron 2) and the long non-coding RNA-Airn, which recruits chromatin modifiers to silence the paternal allele [12,13,14]. Interestingly, Igf2r is biallelically expressed in humans, although the allelic-differentially methylated region in intron 2 and the non-coding RNA-Airn are still present [15,16].
Although Igf2r imprinting diverged during human and mouse evolution, equine Igf2r exhibits maternal-specific expression, and its regulation remains unexplored [17,18]. Furthermore, the offspring (mules and hinnies) of interspecies hybrids between horses (Equus caballus) and donkeys (Equus asinus) can offer sufficient single-nucleotide variations (SNVs) [10,19], and DNA methylation levels remain unchanged in hybrids [20] but lack identifiable Airn homologs in horses [21]. This system allows us to dissect imprinting mechanisms independent of conserved lncRNAs.
In our study, we found that Igf2r imprints were lost in the liver of mules, except in the brain tissue of mules and hinnies. Furthermore, there was no DMR, except for three CpG islands (CpGIs) in the equine Igf2r promoter, and intronic 2 DMRs were present in tissues, including in the liver in mules and in the brain in mules and hinnies. Given the role of cis-inherited DNA methylation in gene regulation [22], we hypothesize that equine Igf2r imprinting is independent of Airn and its expression is driven by cis-acting methylation in CpGI2 in the promoter region. To examine our hypothesis, we performed pyrosequencing, bisulfite sequencing, and absolute quantification in horses, donkeys, and their hybrids. We aimed to determine (1) whether intronic 2 DMRs regulate the imprinted expression of Igf2r; (2) whether promoter DNA methylation is inherited in cis in equids; and (3) whether the Igf2r expression level is dependent on its DNA methylation of CpGI2 in the promoter region. Our findings provide a framework for understanding epigenetic diversity across species.
2. Materials and Methods
2.1. Sample Collection and Preparation
All experiments and methods involving animals in this study were approved and authorized by the Animal Care and Use Committee of Jining Medical University, and all procedures were conducted in accordance with the guidelines and regulations of Jining Medical University. Tissues (muscle, liver, brain, testis, sperm, heart, spleen, lung, and kidney) were collected from well-documented adult horses (Equus caballus, n = 3), donkeys (Equus asinus, n = 3), mules (horse ♀ × donkey ♂, n = 3), and hinnies (donkey ♀ × horse ♂, n = 3) (mean age = 4 ± 0.5 years), according to ethical guidelines. All samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C until processing.
2.2. Genomic DNA and RNA Extraction
The grinding of the tissue samples (muscle, liver, brain, testis, heart, spleen, lung, and kidney) with liquid nitrogen increased the quality and quantity of DNA and mRNA extracted from these samples. Genomic DNA was isolated from ground samples and sperm using a TaKaRa MiniBEST Universal Genomic DNA Extraction Kit (TaKaRa Bio, Dalian, China, #9765), according to the manufacturer’s protocol. DNA purity and concentration were quantified using a Take 3 plate reader (BioTek Epoch, Santa Clara, CA, USA; absorbance ratios: 260/280 > 1.8, 260/230 > 2.0). Total RNA was extracted from the heart, liver, spleen, lung, kidney, muscle, and brain tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA, #15596026CN), followed by DNase I treatment to eliminate genomic DNA contamination. Then, 1 µg of RNA was reverse-transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA, #4368814).
2.3. Single-Nucleotide Variant (SNV) Identification
2.3.1. SNV Analysis
Reference mRNA and DNA sequences of Igf2r (for DNA: NCBI Accession NC_091714 for horse and NW_014638170 for donkey; for mRNA: NCBI Accession XM_005608119.4 for horse and XM_044760750.2 for donkey) and reference mRNA of H19 (NCBI Accession: NR_027326.2 for horse; NCBI Accession: XM_014830185.1 for donkey) were aligned using BioEdit 7.0.9 to identify interspecies SNVs. Figure 1A,B show the SNVs from a comparison of Igf2r and H19 mRNA sequences between horses and donkeys.
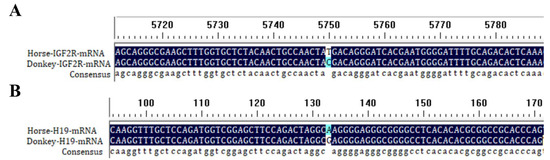
Figure 1.
DNAMAN alignment of Igf2r (A) and H19 (B) at horse and donkey mRNA SNV sites.
2.3.2. Experimental SNV Validation
For DNA-derived SNVs, PCR amplification was performed on genomic DNA using Green Taq Mix (Vazyme, Nanjing, China, #P131), with primers flanking the SNV regions of Igf2r. The cycling conditions were as follows: 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s; and a final extension at 72 °C for 10 min. For cDNA-derived SNVs, PCR was conducted using TB Green Premix Ex Taq (TaKaRa, Dalian, China, #RR820Q) under identical conditions. Amplicons were Sanger-sequenced (Sangon Biotech, Shanghai, China), and chromatograms were analyzed in BioEdit ver.7.0.9.0 to confirm SNVs.
2.4. Allele-Specific Expression (ASE) Quantification
The allele-specific expression of Igf2r and H19 was quantified using pyrosequencing on a PyroMark Q48 Autoprep system (QIAGEN, Hilden, Germany) [17]. Primers (for Igf2r: forward: 5′-GACGCTTTGGTGCTCTAGAACTGC-3′, reverse: 5′-CCTGCCGATACCAGCATCTTCATC-3′, sequencing primer: 5′-TGCTCTACAACTGCCAA-3′, for H19: forward: 5′-ACAGCGAGAAGGACAATGGAATG-3′, reverse: 5′-GAGTATGCAAGAAAACTGCCGAGTG-3′, sequencing primer: 5′-CGGAGCTTCCAGACTAGG-3′ were designed with PyroMark Assay Design Software 2.0 (QIAGEN, Hilden, Germany). PCR amplification was performed in 20 µL reactions with a PyroMark PCR Kit (QIAGEN, Hilden, Germany, #978703) and the following program: 95 °C for 15 min, 45 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Pyrosequencing data were analyzed with PyroMark Q48 Advanced CpG Reagents (QIAGEN, Hilden, Germany, #974002) using the Allele Quantification (AQ) mode. Maternal-to-paternal expression ratios were normalized to SNV-defined allelic inputs.
2.5. DNA Methylation Analysis
2.5.1. CpG Island Prediction and Bisulfite Conversion
CpG islands in the Igf2r promoter and intron 2 were predicted using MethPrimer (http://www.urogene.org/methprimer, accessed on 5 March 2024). Genomic DNA (500 ng) was bisulfite-converted using an EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA, #D5006); the amount of input DNA was 200–500 ng. Conversion efficiency (non-methylated C residues are converted to U, and methylated cytosines are protected) > 99% was confirmed using the control DNA included in the kit.
2.5.2. Bisulfite PCR and Cloning
Bisulfite-treated DNA (2 µL) was amplified with region-specific primers (Table 1) using ZymoTaq DNA Polymerase (Zymo Research, Irvine, CA, USA, #E2002). PCR products were cloned into the pMD™19-T vector (TaKaRa, Dalian, China, #6013), and 10–15 clones per sample were sequenced (Sangon Biotech, Shanghai, China). DNA methylation levels were quantified using QUMA (http://quma.cdb.riken.jp/, accessed on 15 March 2025). Parental allele origins were determined using an SNV analysis.

Table 1.
Primers used to detect methylation levels.
2.6. Absolute Quantification of Igf2r in Tissues
For the absolute quantification of reactions, a CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA) with a TB Green Premix Ex Taq II Kit (TaKaRa, Dalian, China, #RR820) was used. The Igf2r primer sequence was as follows: forward PCR primer: 5’-GACGCTTTGGTGCTCTAGAACTGC-3’; reverse PCR primer: 5’-CCTGCCGATACCAGCATCTTCATC-3’. At first, Igf2r gene fragments were cloned using the pMD™19-T vector. Further, plasmids were extracted with a TlANprep Mini Plasmid Kit (TIANGEN, Beijing, China, #DP103), and concentrations were determined using a biospectrometer (Eppendorf, Hamburg, Germany). The mass concentration of the plasmid containing the Igf2r target fragment was converted to a molecular copy number concentration using the following formula: Copies/mL = (6.02 × 1023 copies/mol) × (concentration (g)/mL)/(molecular weight (g)/mol). The quantified plasmids were subjected to 10-fold serial dilution to obtain standards in the range of 109 to 101, and a standard curve was plotted. An absolute real-time quantitative analysis was performed on various tissue samples, together with standard samples. The threshold cycle (Ct) value of the test sample was compared with that of the standard sample to determine the expression levels in different tissues.
2.7. Statistical Analysis
Data are expressed as mean ± SD. DNA methylation levels and ASE ratios were compared using a one-way ANOVA in GraphPad Prism 8.0.2. Regression analyses between DNA methylation and expression were performed using linear regression. Significance thresholds were set to p < 0.05.
3. Results
3.1. Igf2r Is Maternally Allele-Specifically Expressed in Adult Tissues of Mule and Hinny, Except for in the Brain of Mule and Hinny and the Liver of Mule
Research has shown that Igf2r is only expressed as a biallelic gene in brain tissue, while in other tissues, it is expressed specifically as a maternal allele [23]. In our research, quantitative allele-specific pyrosequencing revealed that Igf2r showed strong maternal allele-specific expression in the heart, spleen, lung, kidney, and muscle tissues of both mules and hinnies (Figure 2A,B). However, imprints were lost in the liver tissue of mules, except in the brain tissue of mules and hinnies (Figure 2B). In contrast, the imprinted gene H19 maintained stable maternal expression across all tissues (Figure 2C,D), confirming tissue-specific rather than global imprinting erosion in hybrids.
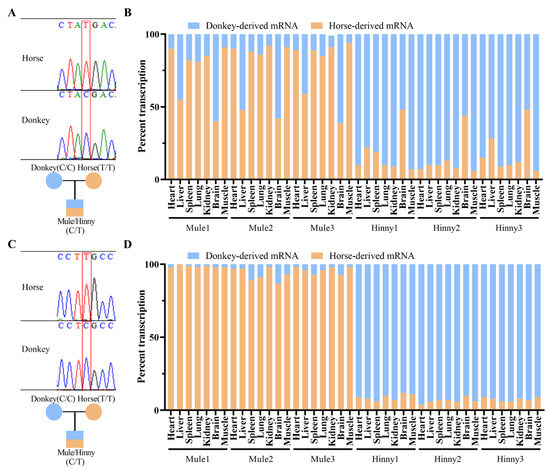
Figure 2.
Allele-specific expression analysis of equine Igf2r and H19 in hybrids. (A) SNV validation of Igf2r. (B) Tissue-specific ASE ratios of Igf2r. (C) SNV validation of H19. (D) Tissue-specific ASE ratios of H19.
3.2. There Is No DMR in the Equine Promoter Region of Igf2r
Bisulfite and Sanger sequencing were performed on DNA from the horse and donkey tissues. The results showed that CpG islands (CpGIs) 1 and 3 in the Igf2r promoter region were all hypermethylated (66.7–100% methylation) without differential DNA methylation in the parent of origin (Figure 3A–C). Species-specific hypermethylation differences were observed in the CpGI2 region, but hypermethylation was maintained in the horse tissues, and tissue-specific hypermethylation differences were observed in the donkey tissues, demonstrating that DMRs were not present in the horse promoter region (Figure 3D). Further assays in the donkey liver and kidney tissues showed that the donkey promoter region was also free of DMRs (Figure 3E).
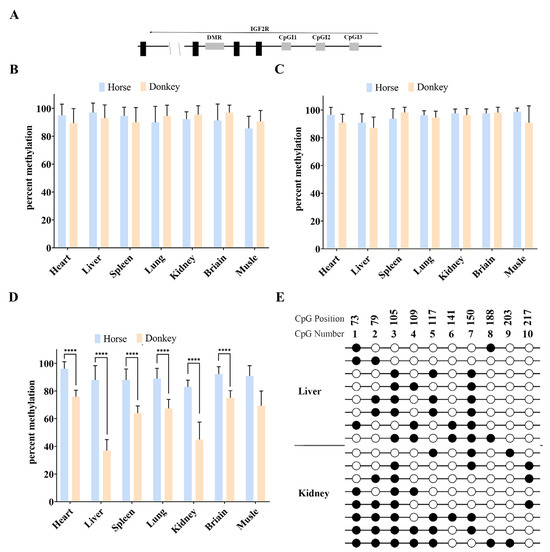
Figure 3.
DNA methylation analysis of Igf2r promoter regions. (A) Schematic of equine Igf2r locus and SNV sites (black triangle), with 12 CpGs in CpGI1, 10 CpGs in CpGI2, and 16 CpGs in CpGI3 (white box). SNVs between horse and donkey are as follows: CpGI1: A (horse)/G (donkey); CpGI2: G (horse)/C (donkey); and CpGI3: A (horse)/G (donkey). (B) Percent methylation of CpGI1 in the Igf2r promoter. (C) Percent methylation of CpGI3 in the Igf2r promoter. (D) Percent methylation of CpGI2 in the Igf2r promoter. Effective readings ≥ 10 (****: p < 5.48 × 10−5). (E) Bisulfite sequencing of CpGI2 in the Igf2r promoter with donkey tissues (liver and kidney). Effective readings = 8.
Studies on the heart tissues of horses, donkeys, mules, and hinnies revealed a maternal allele-specific DMR in the intron 2 region of Igf2r in equines (Figure 4A,B), and this DMR remained unaltered by a 14 bp deletion in the horse (Figure 4B,C). Subsequent studies on testicular tissues and germ cells (sperm hypermethylation: 0.42 ± 0.1%; oocytes: 95 ± 0.1%) revealed that the DMR in the Igf2r intron 2 region was a gametic DMR (Figure 4D) and persisted in the liver and brain tissue (Figure 4E,F). A collective analysis of these results indicated the presence of a maternal allele-specific DMR in the intron 2 region of Igf2r in equids, which does not regulate the imprinted expression of Igf2r.
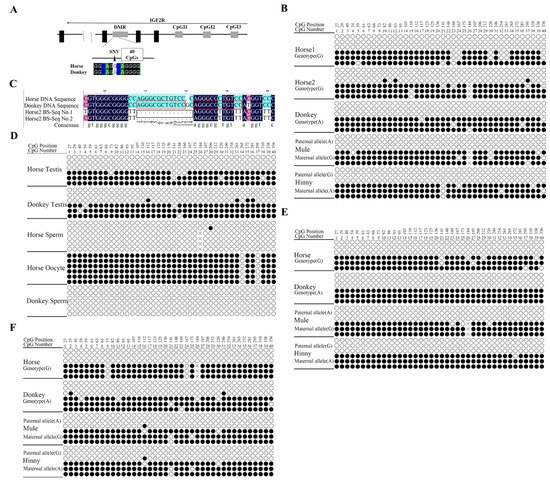
Figure 4.
DNA methylation analysis of Igf2r promoter and intronic regions. (A) Schematic of equine Igf2r locus, including transcriptional direction (arrow), transcription unit (black box), DMR, and CpG islands (gray box). Bisulfite mutagenesis and sequencing are indicated below, including 1 SNV site (black triangle) and 40 CpGs (white box). SNVs between horse and donkey are as follows: G (horse)/A (donkey). (B) Maternal allele-specific methylation in intron 2 of Igf2r in equine hearts; × represents the deletion of a 14 bp repetitive sequence fragment (AGGGCGCTGTCCGC). Each row of circles represents an individual strand sequenced. Black circles represent methylated cytosines, white circles represent unmethylated cytosine. (C) Comparative results of bisulfite sequencing (BS-Seq) of the horse2 intron 2 DMR region: 14 bp deletions are shown in black boxes, and 12 bp repeat sequences are shown in red boxes. (D) Conservation of the intronic DMR in germ cells (sperm and oocytes). (E) Maternal allele-specific methylation in intron 2 of Igf2r in the liver. (F) Maternal allele-specific methylation in intron 2 of Igf2r in the brain. Effective readings ≥ 6.
3.3. Cis-Acting Sequences Govern DNA Methylation Inheritance in Equines
DNA methylation can be divided into two types: imprinted DNA methylation and nonimprinted DNA methylation. The imprinted DNA methylation of the DMR of the Igf2r intron 2 region is mainly determined by cis-acting sequences. However, we found the same pattern in the Igf2r promoter region in equine. Bisulfite and Sanger sequencing showed that the DNA methylation differences between horses and donkeys were not significant in CpGI1 and CpGI3 (Figure 3B,C), while they were significant (p < 5.48 × 10−5) in CpGI2 (Figure 3D). We then investigated the DNA methylation patterns of CpGI1 and CpGI2. The sequencing results showed that CpGI1 DNA methylation exhibited a high methylation state between horses, donkeys, mules, and hinnies (Figure 5A). However, CpGI2 showed species-specific DNA methylation, tending towards the result of horse > hybrids > donkey (Figure 5D). Further research with SNV information showed that high DNA methylation was maintained uniformly across species in CpGI1 (Figure 3A and Figure 5B), with a DNA methylation rate of 71–100% (Figure 5C). In contrast, hybrid tissues retained parental DNA methylation patterns at CpGI2 (Figure 3A and Figure 5E), with horse-derived alleles maintaining hypermethylation (88.66 ± 10.88% in mules and 91.16 ± 8.89% in hinnies) (Figure 5F,G) and donkey-derived alleles showing hypomethylation (56.63 ± 25.48% in mules and 59.2 ± 19.5% in hinnies) (Figure 5F,H). These results underscore that CpGI2 DNA methylation is dictated by species-specific cis-regulatory elements.
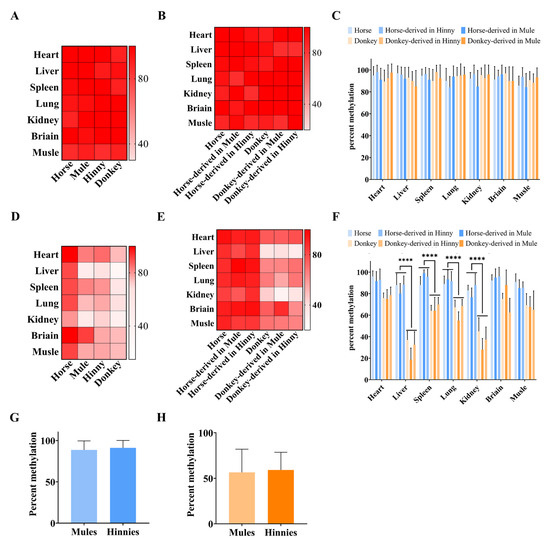
Figure 5.
DNA methylation cis-acting inheritance of Igf2r in promoter CpGI2 in equines. (A) DNA methylation rates of seven equine tissues (heart, liver, spleen, lung, kidney, brain, and muscle) in CpGI1. (B) DNA methylation rates in CpGI1, and DNA methylation in hybrid tissue traced back to parents using SNV information. (C) Bar chart: DNA methylation rates in CpGI1. (D) DNA methylation rates in CpGI2, tending towards the result of horse > hybrids > donkey. (E) DNA methylation rates in CpGI2. (F) Bar chart: DNA methylation rates in CpGI2, (****: p < 6.46 × 10−7). (G) DNA methylation rates of horse-derived alleles of Igf2r in CpGI2 in hybrid tissues. (H) DNA methylation rates of donkey-derived alleles of Igf2r in CpGI2 in hybrid tissues. n = 3.
3.4. Promoter CpGI2 DNA Methylation Drives Igf2r Expression Level in a Tissue-Dependent Manner
DNA methylation has been identified as the principal regulator of gene function, playing a pivotal role in developmental processes and over the course of an individual’s lifetime [24,25,26]. A real-time PCR analysis revealed that the expression levels of Igf2r in mules were analogous to those observed in horses, while hinnies exhibited a profile consistent with that of donkeys (Figure 6A). Furthermore, the results showed that the DNA methylation of CpGI2 of Igf2r in the promoter region in mules or hinnies was consistent with the origin being from horses or donkeys (Figure 6B). Linear regression analyses revealed a strong negative correlation between CpGI2 DNA methylation and Igf2r expression in the spleen (R2 = 0.8797, p = 6.46 × 10−6), lung (R2 = 0.8569, p = 1.57 × 10−5), and kidney (R2 = 0.8650, p = 1.10 × 10−5) (Figure 6C). In the heart and muscle tissues, DNA methylation and expression exhibited weaker correlations in combined linear regression analyses across all species. Nevertheless, when subjected to separate analyses, negative correlations between DNA methylation and expression were observed in horses and donkeys, as well as in mules and hinnies (Figure 6D). All results suggest that the DNA methylation in the promoter region and expression of Igf2r in horses and donkeys can be passed down from generation to generation to mules and hinnies, which also indicates that the DNA methylation in the promoter region of Igf2r can regulate its expression.
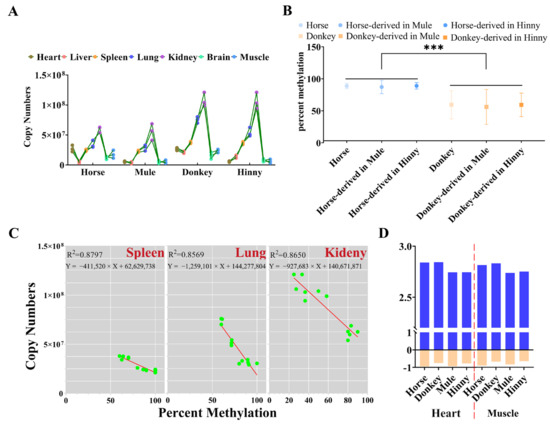
Figure 6.
Tissue-dependent correlation between CpGI2 DNA methylation and Igf2r expression. (A) Igf2r expression levels in mules and hinnies mirror their respective maternal species. (B) CpGI2 DNA methylation states align with parental origin (***: p = 1.82 × 10−4). (C) Linear regression between DNA methylation and expression of Igf2r in spleen, lung, and kidney. (D) Relationship between DNA methylation and Igf2r gene expression. Data transformation methods: copy number values were subjected to double natural log transformation (LN(LN)), while DNA methylation levels were normalized to a range of 0–1, followed by × −1 adjustment.
4. Discussion
The maternal-specific expression of Igf2r and its epigenetic regulation in rodents are well described, whereas in our study, we identified a unique mode of regulation in equine hybrids. Here, we conducted a comparative study of the imprinting and DNA methylation status of Igf2r in the equine genome, focusing on allele-specific expression, promoter CpG island methylation, and the intron 2 DMR. By integrating bisulfite sequencing and allele-specific expression assays, we demonstrated that maternal Igf2r imprinting is present in the majority of somatic tissues of horses and donkeys despite the lack of promoter DMRs and Airn homologues, which is in stark contrast to typical mouse models, suggesting that equine mammals diverged during evolution.
While the maternal allele-specific DMR in intron 2 (DMR2) is conserved in equines, its functional divergence from murine models raises questions about its potential role in gene dosage regulation. Our study identified a maternally inherited DMR in intron 2 of equine Igf2r (DMR2), which is conserved across mice, humans, and equines. While this DMR persists in somatic and germline tissues, its methylation status shows no correlation with Igf2r expression levels. In mice, the intronic DMR2 interacts with lncRNA-Airn to silence the paternal allele by recruiting chromatin modifiers [27]. Intriguingly, we demonstrated that CpGI2 methylation, not canonical DMR2, drives Igf2r dosage in equines. This suggests that equines have evolved a regulatory axis independent of conserved lncRNA-dependent silencing, potentially driven by metabolic or placental adaptations [28,29,30].
The absence of Igf2r imprinting in mule liver and brain tissues raises critical questions about tissue-specific epigenetic stability. In mice, brain-specific imprint relaxation has been attributed to neuron-specific histone modifications and Airn deficiency [23]. Similarly, we observed that equine brain tissue exhibits biallelic Igf2r expression, likely due to chromatin histone modifications during neurogenesis. However, the sole absence of imprints in the mule liver—but not in the hinny liver—suggests interference due to hybridization [31]. In the present study, we found that the most substantial disparities in the parental DNA methylation of the Igf2r promoter CpGI2 locus are present in mule liver tissue. It is evident that DNA methylation plays a pivotal role in various biological processes, including gene expression, histone modifications, transcription factor binding, and chromatin accessibility [32,33,34]. Consequently, it can be hypothesized that this could be a contributing factor to the observed absence of Igf2r imprinting in mule liver tissue. Future research should analyze specific histone modifications, chromatin accessibility, and transcription factors in this tissue to test this hypothesis.
While mouse Igf2r has a differentially methylated region (DMR) in its promoter to regulate parent-of-origin expression [12], unlike mice, equine Igf2r lacks promoter DMRs, suggesting lineage-specific adaptation [35]. Interestingly, all three species—mouse, horse, and human—share a conserved DMR within intron 2. However, humans show a critical divergence: despite the persistence of this intronic DMR, Igf2r lost its imprinted status and switched to biallelic expression [35]. This juxtaposition highlights a dual evolutionary trajectory: the conservation of regulatory architecture and species-specific adaptations, reflecting both functional constraint and lineage-specific innovation in genomic imprinting mechanisms.
Specific DNA sequences contribute to the cis inheritance of epigenetic modifications and gene expression [36,37]. Our data indicate that the CpGI2 DNA methylation pattern is inherited in-cis, and even in hybrid cells, and parental alleles can maintain a species-specific DNA methylation status. This is in stark contrast to the imprinting DMR, which is typically reset during germ cell development [38]. The stability of DNA methylation cis-inheritance suggests that DNA methylation is closely linked to SNVs and DNA motifs [5,39,40]. This mechanism may represent a primitive but evolutionarily conserved strategy to maintain parental epigenetic memory. It is worth noting that the strong negative correlation between CpGI2 DNA methylation and Igf2r expression in the spleen, lung, and kidney highlights its regulatory role, while the weaker correlation in the muscle and heart may reflect tissue-specific differences in enhancers or co-regulatory factors [41]. Future studies mapping enhancer landscapes across tissues will clarify how cis-regulatory elements interact with CpGI2 methylation to fine-tune dosage [42].
While our hybrid model provides unique insights, several limitations warrant attention. First, the absence of Airn in equines does not exclude the involvement of other lncRNAs. Second, the functional causality between CpGI2 DNA methylation and Igf2r expression remains correlative. CRISPR-mediated DNA methylation editing or allele-specific knockout experiments are needed to provide direct evidence [43]. Comparative analyses of DNA methylation and gene expression patterns across species may yield more robust conclusions when validated within the same tissue type of a single species, minimizing the confounding variables inherent in inter-species comparisons. Despite these limitations, our work establishes equine hybrids as a powerful model for studying cis-acting epigenetic inheritance.
5. Conclusions
In this study, we investigated the mechanisms by which DNA methylation in the promoter and intron 2 of Igf2r regulates its expression in equids. Through comparative epigenetic analyses of horses, donkeys, and their hybrids (mules and hinnies), we obtained the following results: (1) A conserved maternal allele-specific DMR in Igf2r intron 2 was identified in horses, but it was not associated with imprinted expression patterns. (2) There was no DMR in the Igf2r promoter region, but the DNA methylation of CpG island 2 (CpGI2) showed cis-acting inheritance, and the parental methylation pattern was retained in hybrids. (3) CpGI2 methylation was strongly negatively correlated with Igf2r expression in spleen, lung, and kidney tissues (R2 > 0.85, p < 0.001), suggesting a regulatory role. These findings broaden our comprehension of epigenetic diversity across mammals and underscore the utility of equine inbreeding models for investigating genomic imprinting mechanisms. Future research combining multi-omics strategies and functional assays will further clarify how sequence-specific DNA methylation influences developmental pathways and evolutionary adaptations in hybrids and beyond.
Author Contributions
Conceptualization, X.W. and G.B.; methodology, X.W.; software, X.W.; validation, X.W., Y.S. and H.R.; formal analysis, G.B.; investigation, Y.S.; resources, M.Y.; data curation, X.W.; writing—original draft preparation, X.W.; writing—review and editing, G.B.; visualization, X.W.; supervision, G.B.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Health Science and Technology Project of Shandong Province, grant number [202402010752], and the Jining Medical University Scientific Research Start-Up Funds for Young Doctors, grant number [6001/601004001].
Institutional Review Board Statement
All experiments and methods involving animals in this study were conducted in accordance with the guidelines and regulations of Jining Medical University, and all procedures were approved and authorized by the Animal Care and Use Committee of Jining Medical University (JNMC-2024-DW-057) (approval date on 8 March 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge staff members who helped with sampling and the anonymous reviewers for their comments on this work.
Conflicts of Interest
Yingchao Shen was employed by the company Anchee (Shandong) Animal Nutrition Research Academy Co., Ltd., Jinan, Shandong. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Schuff, M.; Strong, A.D.; Welborn, L.K.; Ziermann-Canabarro, J.M. Imprinting as Basis for Complex Evolutionary Novelties in Eutherians. Biology 2024, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.P.; Bartolomei, M.S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382. [Google Scholar] [CrossRef] [PubMed]
- Surani, M.A.; Barton, S.C.; Norris, M.L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 1984, 308, 548–550. [Google Scholar] [CrossRef]
- Barton, S.C.; Surani, M.A.; Norris, M.L. Role of paternal and maternal genomes in mouse development. Nature 1984, 311, 374–376. [Google Scholar] [CrossRef]
- Qu, W.; Hashimoto, S.; Shimada, A.; Nakatani, Y.; Ichikawa, K.; Saito, T.L.; Ogoshi, K.; Matsushima, K.; Suzuki, Y.; Sugano, S.; et al. Genome-wide genetic variations are highly correlated with proximal DNA methylation patterns. Genome Res. 2012, 22, 1419–1425. [Google Scholar] [CrossRef]
- Yang, Y.L.; Fan, X.H.; Yan, J.Y.; Chen, M.Y.; Zhu, M.; Tang, Y.J.; Liu, S.Y.; Tang, Z.L. A comprehensive epigenome atlas reveals DNA methylation regulating skeletal muscle development. Nucleic Acids Res. 2021, 49, 1313–1329. [Google Scholar] [CrossRef]
- Vuu, Y.M.; Roberts, C.T.; Rastegar, M. MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism. Int. J. Mol. Sci. 2023, 24, 4218. [Google Scholar] [CrossRef]
- Hughes, J.; Surakhy, M.; Can, S.; Ducker, M.; Davies, N.; Szele, F.; Bühnemann, C.; Carter, E.; Trikin, R.; Crump, M.P.; et al. Maternal transmission of an Igf2r domain 11: IGF2 binding mutant allele (Igf2rI1565A) results in partial lethality, overgrowth and intestinal adenoma progression. Sci. Rep. 2019, 9, 11388. [Google Scholar] [CrossRef]
- Sandovici, I.; Georgopoulou, A.; Pérez-García, V.; Hufnagel, A.; López-Tello, J.; Lam, B.Y.H.; Schiefer, S.N.; Gaudreau, C.; Santos, F.; Hoelle, K.; et al. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev. Cell 2022, 57, 63–79.e8. [Google Scholar] [CrossRef]
- Wang, X.S.; Asgenbaatar, N.; Shen, Y.C.; Yi, M.N.; Zhao, B.L.; Ren, H.; Davshilt, T.; Ulaangerel, T.; Wang, M.; Burenbaatar, A.; et al. Lower expression of the equine maternally imprinted gene IGF2R is related to the slow proliferation of hinny embryonic fibroblast. Mol. Biol. Rep. 2023, 50, 185–192. [Google Scholar] [CrossRef]
- Gicquel, C.; Weiss, J.; Amiel, J.; Gaston, V.; Le Bouc, Y.; Scott, C.D. Epigenetic abnormalities of the mannose-6-phosphate/ IGF2 receptor gene are uncommon in human overgrowth syndromes. J. Med. Genet. 2004, 41, e4. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Jirtle, R.L.; Hoffman, A.R. Cross-species clues of an epigenetic imprinting regulatory code for the IGF2R gene. Cytogenet. Genome Res. 2006, 113, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wutz, A.; Smrzka, O.W.; Schweifer, N.; Schellander, K.; Wagner, E.F.; Barlow, D.P. Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature 1997, 389, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, F.; Zwart, R.; Barlow, D.P. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature 2002, 415, 810–813. [Google Scholar] [CrossRef]
- Wutz, A.; Smrzka, O.W.; Barlow, D.P. Making sense of imprinting the mouse and human IGF2R loci. Novartis Found. Symp. 1998, 214, 251–259; discussion 260–263. [Google Scholar] [CrossRef]
- Yotova, I.Y.; Vlatkovic, I.M.; Pauler, F.M.; Warczok, K.E.; Ambros, P.F.; Oshimura, M.; Theussl, H.C.; Gessler, M.; Wagner, E.F.; Barlow, D.P. Identification of the human homolog of the imprinted mouse Air non-coding RNA. Genomics 2008, 92, 464–473. [Google Scholar] [CrossRef]
- Dini, P.; Kalbfleisch, T.; Uribe-Salazar, J.M.; Carossino, M.; Ali, H.E.; Loux, S.C.; Esteller-Vico, A.; Norris, J.K.; Anand, L.; Scoggin, K.E.; et al. Parental bias in expression and interaction of genes in the equine placenta. Proc. Natl. Acad. Sci. USA 2021, 118, e2006474118. [Google Scholar] [CrossRef]
- Wang, X.; Miller, D.C.; Harman, R.; Antczak, D.F.; Clark, A.G. Paternally expressed genes predominate in the placenta. Proc. Natl. Acad. Sci. USA 2013, 110, 10705–10710. [Google Scholar] [CrossRef]
- Wang, X.S.; Bou, G.; Zhang, X.Z.; Tao, L.; Shen, Y.C.; Na, R.G.; Liu, G.Q.; Ren, H.; Ren, X.J.; Song, L.J.; et al. A Fast PCR Test for the Simultaneous Identification of Species and Gender in Horses, Donkeys, Mules and Hinnies. J. Equine Vet. Sci. 2021, 102, 103458. [Google Scholar] [CrossRef]
- Feinstein, S.I.; Miller, D.A.; Ehrlich, M.; Gehrke, C.W.; Eden, L.B.; Miller, O.J. DNA methylation is not increased in mouse-human somatic cell hybrids. Biochim. Biophys. Acta 1985, 824, 336–340. [Google Scholar] [CrossRef]
- Scott, E.Y.; Mansour, T.; Bellone, R.R.; Brown, C.T.; Mienaltowski, M.J.; Penedo, M.C.; Ross, P.J.; Valberg, S.J.; Murray, J.D.; Finno, C.J. Identification of long non-coding RNA in the horse transcriptome. BMC Genom. 2017, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Eichten, S.R.; Hermanson, P.J.; Springer, N.M. Inheritance Patterns and Stability of DNA Methylation Variation in Maize Near-Isogenic Lines. Genetics 2014, 196, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Kayashima, T.; Soejima, H.; Kinoshita, A.; Yoshiura, K.; Matsumoto, N.; Ohta, T.; Urano, T.; Masuzaki, H.; Ishimaru, T.; et al. Neuron-specific relaxation of Igf2r imprinting is associated with neuron-specific histone modifications and lack of its antisense transcript Air. Hum. Mol. Genet. 2005, 14, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Pervjakova, N.; Kasela, S.; Morris, A.P.; Kals, M.; Metspalu, A.; Lindgren, C.M.; Salumets, A.; Mägi, R. Imprinted genes and imprinting control regions show predominant intermediate methylation in adult somatic tissues. Epigenomics 2016, 8, 789–799. [Google Scholar] [CrossRef]
- Sharp, A.J.; Stathaki, E.; Migliavacca, E.; Brahmachary, M.; Montgomery, S.B.; Dupre, Y.; Antonarakis, S.E. DNA methylation profiles of human active and inactive X chromosomes. Genome Res. 2011, 21, 1592–1600. [Google Scholar] [CrossRef]
- Vilain, A.; Bernardino, J.; Gerbault-Seureau, M.; Vogt, N.; Niveleau, A.; Lefrançois, D.; Malfoy, B.; Dutrillaux, B. DNA methylation and chromosome instability in lymphoblastoid cell lines. Cytogenet. Cell Genet. 2000, 90, 93–101. [Google Scholar] [CrossRef]
- Santoro, F.; Mayer, D.; Klement, R.M.; Warczok, K.E.; Stukalov, A.; Barlow, D.P.; Pauler, F.M. Imprinted Igf2r silencing depends on continuous Airn lncRNA expression and is not restricted to a developmental window. Development 2013, 140, 1184–1195. [Google Scholar] [CrossRef]
- Springer, M.S.; Foley, N.M.; Brady, P.L.; Gatesy, J.; Murphy, W.J. Evolutionary Models for the Diversification of Placental Mammals Across the KPg Boundary. Front. Genet. 2019, 10, 1241. [Google Scholar] [CrossRef]
- Ge, D.Y.; Feijó, A.; Wen, Z.X.; Abramov, A.V.; Lu, L.; Cheng, J.L.; Pan, S.K.; Ye, S.C.; Xia, L.; Jiang, X.L.; et al. Demographic History and Genomic Response to Environmental Changes in a Rapid Radiation of Wild Rats. Mol. Biol. Evol. 2021, 38, 1905–1923. [Google Scholar] [CrossRef]
- Marra, N.J.; Richards, V.P.; Early, A.; Bogdanowicz, S.M.; Bitar, P.D.P.; Stanhope, M.J.; Shivji, M.S. Comparative transcriptomics of elasmobranchs and teleosts highlight important processes in adaptive immunity and regional endothermy. BMC Genom. 2017, 18, 87. [Google Scholar] [CrossRef]
- Vrana, P.B.; Guan, X.J.; Ingram, R.S.; Tilghman, S.M. Genomic imprinting is disrupted in interspecific Peromyscus hybrids. Nat. Genet. 1998, 20, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Parameswaran, S.; Cai, L.; Elston, S.; Pham, C.; Barski, A.; Weirauch, M.T.; Ji, H. TET1 regulates responses to house dust mite by altering chromatin accessibility, DNA methylation, and gene expression in airway epithelial cells. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Cao, S.; Chen, K.; Lu, K.; Chen, S.; Zhang, X.; Shen, C.; Zhu, S.; Niu, Y.; Fan, L.; Chen, Z.J.; et al. Asymmetric variation in DNA methylation during domestication and de-domestication of rice. Plant Cell 2023, 35, 3429–3443. [Google Scholar] [CrossRef]
- Cusack, M.; King, H.W.; Spingardi, P.; Kessler, B.M.; Klose, R.J.; Kriaucionis, S. Distinct contributions of DNA methylation and histone acetylation to the genomic occupancy of transcription factors. Genome Res. 2020, 30, 1393–1406. [Google Scholar] [CrossRef]
- Kalscheuer, V.M.; Mariman, E.C.; Schepens, M.T.; Rehder, H.; Ropers, H.H. The insulin-like growth factor type-2 receptor gene is imprinted in the mouse but not in humans. Nat. Genet. 1993, 5, 74–78. [Google Scholar] [CrossRef]
- Wang, X.Y.; Moazed, D. DNA sequence-dependent epigenetic inheritance of gene silencing and histone H3K9 methylation. Science 2017, 356, 88–91. [Google Scholar] [CrossRef]
- Xiong, X.; Geden, C.J.; Tan, Y.J.; Zhang, Y.; Zhang, D.P.; Werren, J.H.; Wang, X. Genome Structure, Evolution, and Host Shift of. Biol. Basel 2024, 13, 952. [Google Scholar] [CrossRef]
- Lee, J.; Inoue, K.; Ono, R.; Ogonuki, N.; Kohda, T.; Kaneko-Ishino, T.; Ogura, A.; Ishino, F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development 2002, 129, 1807–1817. [Google Scholar] [CrossRef]
- Castora, F.J.; Arnheim, N.; Simpson, M.V. Mitochondrial DNA polymorphism: Evidence that variants detected by restriction enzymes differ in nucleotide sequence rather than in methylation. Proc. Natl. Acad. Sci. USA 1980, 77, 6415–6419. [Google Scholar] [CrossRef]
- de Oliveira, N.F.P.; Persuhn, D.C.; Dos Santos, M. Can Global DNA Methylation Be Influenced by Polymorphisms in Genes Involved in Epigenetic Mechanisms? A Review. Genes 2024, 15, 1504. [Google Scholar] [CrossRef]
- Kieffer-Kwon, K.R.; Tang, Z.H.; Mathe, E.; Qian, J.S.; Sung, M.H.; Li, G.L.; Resch, W.; Baek, S.; Pruett, N.; Grontved, L.; et al. Interactome Maps of Mouse Gene Regulatory Domains Reveal Basic Principles of Transcriptional Regulation. Cell 2013, 155, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Zhang, C.R.; Bi, X.M.; Xu, J.K.; Guo, S.N.; Li, P.H.; Shen, Y.T.; Cai, J.L.; Zhang, N.H.; Tian, G.H.; et al. Mapping enhancer and chromatin accessibility landscapes charts the regulatory network of Alzheimer’s disease. Comput. Biol. Med. 2024, 168, 107802. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, S.X. CRISPR/dCas9-Tet1-Mediated DNA Methylation Editing. Bio-Protocol 2024, 14, e4976. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).