Simple Summary
Nutrition during pregnancy not only affects the health of cows but also plays a critical role in shaping the future of their calves. In beef cattle, what a cow eats while pregnant can influence the development of muscle, fat, and immune systems in her unborn calf. This review examines how cows with excess nutrients (overfeeding) or specific supplements such as protein, vitamins, or minerals during pregnancy impact the growth and health of their calves after birth. We explain how these feeding strategies can alter how genes are turned on or off before birth, which may lead to better meat quality or healthier animals. However, overfeeding can sometimes lead to unwanted effects like too much fat or lower meat tenderness. Recent research also highlights important differences between male and female calves, impacts on the calf gut microbiome, breed-specific responses, and advanced molecular analyses that can improve nutritional strategies. Feeding the right nutrients at the right time can help farmers raise stronger, healthier, and more productive animals, benefiting farmers, scientists, and anyone interested in animal welfare and sustainable meat production.
Abstract
Maternal overnutrition and targeted supplements during pregnancy strongly affect fetal development in beef cattle, influencing gene expression, tissue development, and productivity after birth. As modern feeding practices often result in cows receiving energy and protein above requirements, understanding the balance between adequate nutrition and overconditioning is critical for sustainable beef production. This review synthesizes findings from recent studies on maternal overnutrition and supplementation, focusing on macronutrients (energy, protein, methionine) and key micronutrients (e.g., selenium, zinc). It evaluates the timing and impact of supplementation during different gestational stages, with emphasis on fetal muscle and adipose tissue development, immune function, and metabolic programming. The role of epigenetic mechanisms, such as DNA methylation and non-coding RNAs, is also discussed in relation to maternal dietary inputs. Mid-gestation supplementation promotes muscle growth by activating muscle-specific genes, whereas late-gestation diets enhance marbling and carcass traits. However, maternal overnutrition may impair mitochondrial efficiency, encourage fat deposition over muscle, and promote collagen synthesis, reducing meat tenderness. Recent evidence highlights sex-specific fetal programming differences, the significant impact of maternal diets on offspring gut microbiomes, and breed-specific nutritional responses, and multi-OMICs integration reveals metabolic reprogramming mechanisms. Targeted trace mineral and methionine supplementation enhance antioxidant capacity, immune function, and reproductive performance. Precision feeding strategies aligned with gestational requirements improve feed efficiency and minimize overfeeding risks. Early interventions, including protein and vitamin supplementation, optimize placental function and fetal development, supporting stronger postnatal growth, immunity, and fertility. Balancing nutritional adequacy without excessive feeding supports animal welfare, profitability, and sustainability in beef cattle systems.
1. Introduction
Maternal nutrition during gestation has a strong effect on fetal development and subsequent performance in beef cattle [1,2]. Adequate nutrient supply is vital for developing key physiological systems in the fetus such as muscle growth, adipogenesis, immune responsiveness, and metabolic regulation in the developing fetus [3,4,5,6]. These lasting phenotypic effects are encapsulated by the concept of fetal programming, wherein the maternal environment drives enduring structural and functional changes in offspring [7,8]. Such effects often arise through epigenetic mechanisms including DNA methylation, histone modifications, and non-coding RNA regulation, which mediate transcriptional and metabolic pathways implicated in lifelong productivity [9,10].
Historically, research attention in ruminant systems has focused predominantly on maternal undernutrition and its detrimental consequences for progeny [4,11,12]. However, contemporary beef production practices, characterized by generous dietary planes or ad libitum feeding of energy- and protein-rich rations, have introduced a contrasting but equally problematic scenario: MO [13,14]. Under these high-intake conditions, pregnant cows often exceed optimal body condition scores, leading to impaired placental function, disturbed nutrient partitioning, and potential metabolic disorders that can negatively impact calf growth and carcass quality [15,16]. Therefore, managing MO is essential for maintaining productivity, as excessive in utero nutrient exposure may predispose offspring to unwanted fat deposition, reduced meat tenderness, and suboptimal feed efficiency [13,14].
Advances in precision feeding aim to match nutrient intake with pregnancy needs to avoid deficiencies or excesses [17,18]. Indeed, research suggests that carefully timed and moderate supplementation can improve outcomes such as fetal muscle fiber formation, marbling, and immune capacity [6,19]. Nonetheless, if nutrition during pregnancy is too low or too high, it may induce irreversible developmental changes that impair postnatal growth and carcass traits [3,4,20]. Recent research has shown how maternal diets, especially those with excess nutrients, alter fetal gene expression, impacting muscle growth, fat development, and lifelong metabolic health [21]. While certain micronutrients (e.g., zinc, Se, copper) also affect immune function and oxidative balance [22,23,24], the main concern in MO situations is exceeding energy or protein requirements, which promotes overconditioning in pregnant cows.
In this context, the present review focuses on MO in beef cattle, synthesizing current knowledge on how excessive dietary intake during gestation shapes fetal programming, affects metabolic health, and ultimately influences offspring growth, carcass characteristics, and overall performance. Understanding these mechanisms helps producers improve feeding strategies to avoid overnutrition and apply targeted interventions for more sustainable and profitable beef production.
2. Fetal Development Under MO
MO in beef cattle influences fetal development through alterations in placental function, critical windows of organogenesis, and key epigenetic mechanisms governing tissue differentiation. These processes have been well studied under maternal nutrient restriction. However, recent evidence shows that excess energy or protein intake can also disrupt normal fetal programming [2,4,14]. This section discusses the critical periods during which fetal tissues are most susceptible to overfeeding, explores how the placenta adapts (or fails to adapt) to excess maternal nutrients, and examines implications for muscle growth and long-term offspring performance.
2.1. Critical Windows in Fetal Development
Embryonic and fetal stages, from early embryogenesis to late gestation, are critical for forming cellular, metabolic, hormonal, and organ systems in beef cattle [11]. During these stages, skeletal muscle, adipose tissue, immune organs, and the placenta develop. Each of these tissues is highly sensitive to maternal nutritional imbalances, whether due to undernutrition or MO [2,11]. The principle of developmental programming posits that maternal conditions during pregnancy induce permanent structural and functional changes in offspring tissues, exerting lifelong effects on metabolism, growth, and health [2,11].
The timing of organ and tissue formation is critical for fetal development. During the first trimester, organogenesis encompasses the formation of vital organs such as the heart, brain, liver, and muscles [25]. By around 50 days post-conception (dpc), organogenesis is largely complete [26,27]. After organogenesis, fetal development involves rapid growth and depends heavily on placental nutrient delivery [28]. If maternal nutrition is inadequate or excessive during this stage, it can harm placental function, causing restricted or excessive fetal growth, both affecting long-term metabolism and growth (Figure 1) [29,30].
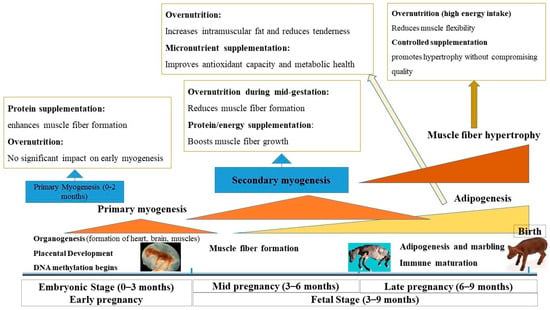
Figure 1.
Critical windows of fetal development and the impact of maternal nutrition during pregnancy. This timeline illustrates key stages of embryonic and fetal development, highlighting periods of primary and secondary myogenesis, adipogenesis, and muscle fiber hypertrophy. MO and supplementation at different stages affect muscle and fat development. Early protein supplementation enhances muscle fiber formation, while MO in late gestation promotes intramuscular fat deposition and marbling. Controlled supplementation ensures optimal growth, balancing muscle development and fat accumulation.
2.2. Importance of Placental Development
The placenta is pivotal in mediating fetal nutrient and oxygen supply, directly influencing fetal growth outcomes [31,32,33]. When maternal nutrition is compromised—whether by deficiency or overfeeding—placental vascularization and nutrient transport efficiency may be disturbed [8,34,35]. In cases of undernutrition, inadequate placental development often results in IUGR and lower birth weights [8,34,35]. Conversely, MO can also disrupt normal placental function through altered metabolic environments and endocrine signaling [36].
The placenta may sometimes compensate for nutrient shortfalls by adjusting its structure and vascular function [37]. However, these adaptations may fail if maternal diets remain unbalanced for prolonged periods. Severe or prolonged nutrient restriction leads to impaired organogenesis and diminished muscle fiber formation [3,8]. At the same time, excess maternal nutrition may boost placental nutrient transfer beyond fetal needs. This can lead to fat accumulation in organs like the heart and muscle, which may reduce carcass quality and increase the risk of metabolic disorders later in life [38,39].
2.3. Nutritional Programming and Muscle Development
Muscle development in beef cattle primarily occurs in utero, underscoring the significance of maternal diet for lifelong growth and performance [40]. Mid-gestation (second trimester) is particularly critical for myogenesis, during which primary and secondary muscle fibers form [3,40]. Nutritional restriction in this window can irreversibly reduce the number of muscle fibers, thus limiting skeletal muscle growth potential [3,4] (Figure 1). On the other hand, MO may increase intramuscular fat deposition, potentially improving tenderness, flavor, and juiciness. However, this advantage may raise consumer health concerns if it also alters the fatty acid profile unfavorably [41].
Evidence suggests that optimal maternal nutrition during late gestation supports higher birth weights, enhanced muscle fiber hyperplasia, and increased adipocyte formation, which collectively bolster postnatal growth and meat quality [4,42]. Specific interventions, such as methionine supplementation, can induce epigenetic modifications (e.g., DNA methylation, alternative splicing). These changes improve muscle glycolysis, contraction efficiency, and cytoskeletal organization, key traits for feed efficiency and meat quality (Figure 1) [42,43,44]. In contrast, nutrient restriction during gestation compromises fetal muscle fiber counts, ultimately diminishing growth performance and feed efficiency in calves [45,46].
Recent research has shown that maternal feeding strategies, ranging from no protein–energy supplementation to full supplementation, differently shape nitrogen metabolism and protein retention. These differences influence offspring growth trajectories [47].
While calves can partially regain muscle mass after birth, the reduction in muscle fiber number due to mid-gestation nutrient restriction cannot be fully reversed [3,40,48]. Therefore, maintaining balanced maternal nutrition, avoiding both under- and overfeeding, is essential for optimal muscle development and robust postnatal performance in beef cattle [4,40,45].
2.4. Developmental Programming and Long-Term Growth
The developmental origins of health and disease framework posits that prenatal conditions, particularly maternal nutrition, have enduring effects on offspring growth, health, and overall productivity. The concept of fetal programming, introduced by Barker [7], underlines that poor gestational nutrition, whether deficient or excessive, can cause permanent changes in offspring metabolism, organ function, and disease susceptibility. In beef cattle, fetal programming is most evident in postnatal growth and carcass traits. Nutrient imbalances during key gestational periods often result in reduced growth, lower carcass quality, and impaired reproduction [8,49].
Although maternal undernutrition has historically received greater attention [3,4,42], growing evidence suggests that MO also disrupts normal developmental pathways, potentially leading to increased birth weight, disproportionate fat deposition, and decreased feed efficiency in offspring [50,51]. Studies consistently show that nutrient restriction during early to mid-gestation reduces fetal muscle fiber formation, slows growth rates, and impairs carcass quality [3,52]. These deficits are irreversible because muscle fiber numbers are set prenatally and cannot be increased after birth [3,40]. Moreover, placental function can be compromised by nutrient shortfalls, reducing fetal nutrient transfer [8,34]. Conversely, excessive maternal nutrient intake may enhance placental transport beyond fetal needs. This can lead to greater fat accumulation in the fetus and raise the risk of postnatal metabolic issues [13,14].
Research on strategic protein–energy supplementation underscores the critical role of gestational timing in shaping offspring performance. Late-gestation supplementation improves amino acid metabolism in both dams and fetuses. It elevates the plasma levels of taurine, glutamic acid, and histidine, key metabolites for fetal muscle development, metabolic programming, and postnatal growth efficiency [6,53,54,55]. Furthermore, this supplementation modulates pathways tied to histidine and beta-alanine metabolism, implying long-term benefits for feed efficiency and carcass attributes [55]. Polizel, et al. [56] further support these findings by demonstrating persistent changes in plasma metabolome profiles. These changes, especially in arginine biosynthesis and histidine metabolism, appeared during the rearing and finishing phases, reinforcing the idea that prenatal nutrition strategies can have lasting effects.
Likewise, mineral supplementation, especially Se and other trace minerals, facilitates robust immune function and uterine health during mid-to-late gestation. Organic Se supplementation, in particular, can reinforce immune responsiveness and reproductive success [57]. Nevertheless, the timing of such interventions remains pivotal: nutrient deficits early in gestation can induce excessive fat deposition, lower muscle mass, and diminish growth efficiency [58]. Similar caution applies when excess maternal nutrients are supplied early in pregnancy, as they may set the stage for offspring adiposity and associated metabolic drawbacks [13,14].
3. Effect of Maternal Feed Supplementation on Maternal Physiology
3.1. Impact on Body Weight, Body Condition, and Energy Balance
Maternal feed supplementation during pregnancy significantly influences BW and BCS, especially in the final trimester [8,13,14]. For instance, protein supplementation helps maintain BCS around 5.1, preventing declines seen in non-supplemented cows. It can also increase maternal BW at weaning while enhancing cyclicity at the start of fixed-time artificial insemination protocols [59]. Diets with 33% CP, 150% of requirement feeding, or corn supplementation at 0.2% BW have been shown to improve BW and BCS. These strategies enhance energy balance, reduce fat mobilization, and support better nutrient partitioning [53,60,61,62].
Mid-gestation protein supplementation (3.5 g/kg BW) can enhance BCS, weight gain, and DMI. It also improves digestibility and microbial protein synthesis, contributing to improved placental blood flow [53,63,64]. Sustained protein–energy supplementation throughout gestation may increase BW by 25–30 kg compared to mineral-only diets. This highlights the need for sufficient nutrient provision to preserve maternal energy reserves and support reproductive success [65]. A plant-based protein supplement given periconception has not consistently altered BW or BCS [64]. However, offspring from RPM dams show better feed efficiency and post-weaning growth, suggesting long-term metabolic benefits [66].
However, supplementation results are not always consistent across reproductive metrics. Some trials report no marked difference in pregnancy rates, even when BCS and weaning weights improve [59,67]. Overfeeding in the third trimester (100% or 150% of requirements) enhances BW and BCS at calving. However, it does not always translate into higher pregnancy rates [68,69]. RUP feeding during late gestation boosts ADG and final BW, but it does not clearly enhance fertility [54]. Micronutrient supplementation (e.g., copper, Se, zinc) may not significantly affect BW or BCS. However, it can improve mineral status and enhance placental nutrient transfer [70]. Fat supplementation strategies, such as high-fat bakery waste or specific oils, have also been used to maintain maternal weight and BCS through calving [71,72].
Energy balance is central to these outcomes. Severe nutrient restriction between day 30 and 190 of gestation reduces BW, BCS, and plasma glucose concentrations [73,74,75], while moderately overfeeding (125% total digestible nutrients, TDN) allows cows to gain weight without necessarily altering postpartum BCS [73,74,75]. MO at 120% ME decreases maternal fat mobilization pre-calving and stabilizes BCS, potentially aiding postpartum recovery [14]. HFAT supplementation may also improve fertility by increasing the number of cows that calve within the first 21 days of the breeding season [71]. Overall, balancing energy intake to align with fetal demands and avoiding extremes of under- or overfeeding remains vital for sustained maternal health and optimal reproductive outcomes.
3.2. Effects on Reproductive Function and Metabolic Parameters
While the goal of maternal supplementation is to enhance reproductive performance, its effect on actual fertility outcomes can be complex. Some studies report that protein supplementation elevates BCS and BW but does not significantly improve pregnancy rates relative to controls [59,76]. Similar patterns hold for enhanced follicular growth or cyclicity; these physiological indicators do not always culminate in higher conception rates [76,77]. Periconception RPM supplementation likewise did not improve pregnancy outcomes [66]. One recent report found that continuous protein–energy feeding (0.3% BW) elevated BW and rump fat thickness but conferred minimal benefits in reproductive traits, likely due to compensatory growth that offset early advantages [78].
Although better BCS and metabolism aid postpartum recovery, too much protein may impair pregnancy outcomes. This occurs due to ammonia and homocysteine toxicity, disrupted uteroplacental flow, and lower progesterone levels [79]. This underlines the importance of balancing rather than simply maximizing protein supplementation.
Nevertheless, specific interventions during late gestation do appear beneficial. Cows receiving 100% or 150% of requirements often show quicker follicular recovery and increased ovulation rates by 21 days postpartum, with some improvements in subsequent breeding seasons, particularly at the higher supplementation rate [68,80]. Even if reproductive outcomes do not improve immediately, these interventions can stabilize metabolism and improve nutrient use. For instance, RUP-based diets improve maternal protein synthesis and energy metabolism, whereas control diets yield higher methionine levels that may favor antioxidant capacity [54]. In addition, supplemented cows generally exhibit more stable glucose regulation and lower lipolysis (as indicated by reduced NEFA and triglyceride levels), minimizing the excessive mobilization of maternal reserves [61,81].
Similarly, vitamin and mineral supplementation early in gestation can augment amino acid concentrations in fetal fluids, enhancing nutrient transport [82]. A balanced maternal energy status throughout pregnancy provides a more favorable metabolic environment for both dam and fetus [83]. While these enhancements in metabolic indicators do not invariably translate into higher pregnancy percentages, they reinforce dams’ overall physiological resilience, a critical factor for subsequent conception and calving intervals.
3.3. Long-Term Economic and Production Benefits
In addition to physiological and performance improvements, maternal supplementation can yield economic advantages by increasing weaning weights, enhancing reproduction, and ultimately boosting farm profitability. For instance, cows fed 150% of their nutritional requirements during the third trimester produced heavier calves. This results in higher sale values and greater returns across subsequent reproductive seasons [68,80]. However, the net benefit depends on feed costs, calf prices, and breed-specific factors. For example, in Hanwoo cows, no significant performance differences were found between calves from supplemented and control dams [61].
Producers must weigh feed expenses and logistical efforts against potential returns like heavier weaning weights, shorter postpartum intervals, or improved cow longevity. Supplementation can reduce postpartum anestrus and lower the number of open cows, improving herd efficiency [84]. However, if it only sustains overconditioned cows without boosting fertility or calf performance, returns may be limited. This highlights the value of precision feeding, carefully aligning rations with gestational needs to control costs and improve reproductive and growth outcomes.
In situations where supplementation does elevate weaning weights and maternal BCS, producers may offset higher feed expenditures through increased income at sale [85]. Partial budget analyses that consider feed costs, labor, and market trends help assess whether gains in calf weight or fertility justify the added cost. If feed costs are moderate or if heavier calves command a premium, supplementation often delivers a favorable return on investment [85,86].
Post-calving management also affects profitability. For example, cows grazing in subirrigated meadows show better BW and BCS than those fed hay, resulting in heavier weaning weights [59]. Similarly, early-life strategies like creep feeding can enhance the benefits of maternal supplementation. Creep-fed heifers may initially exhibit higher body weight and backfat, although post-weaning growth often levels out. However, they tend to reach first-service pregnancy earlier, reducing long-term costs by lowering the number of open cows [87].
When choosing supplementation strategies, producers should weigh feed costs against potential gains in fertility, reduced postpartum anestrus, and heavier market calves [88]. When well-timed nutrient support ensures good maternal BCS, higher productivity, and fewer culls, the investment is often justified. But if overfeeding fails to produce matching gains in offspring, costs may exceed revenue [89]. Aligning gestational nutrition with postnatal management is essential for achieving productivity and profitability. This reinforces the role of precision feeding and cost–benefit analysis in modern beef systems.
4. Epigenetics and Nutritional Interventions
4.1. Epigenetic Mechanisms and Metabolic Imprinting
Epigenetics refers to heritable modifications in gene expression that do not alter the DNA sequence itself [90]. These changes including DNA methylation, histone modifications, and non-coding RNAs regulate gene activity and influence offspring growth, metabolism, and health. In beef cattle, metabolic imprinting denotes early-life nutritional interventions that induce enduring changes in muscle and adipose development, ultimately impacting carcass quality and reproductive traits [91,92].
4.1.1. DNA Methylation
DNA methylation typically involves the addition or removal of methyl groups at cytosine–phosphate–guanine (CpG) sites, thereby modulating transcription. MO can disrupt methylation patterns in fetal tissues, affecting key genes involved in myogenesis, adipogenesis, and insulin signaling [13,52].
In some cases, the hypermethylation of muscle-regulatory genes may suppress myofiber formation, whereas hypomethylation in adipogenic genes can elevate intramuscular fat [41,93].
Early-weaned calves on high-concentrate diets also display specific DNA methylation changes that reinforce marbling [94]. These epigenetic adaptations persist into the finishing phase, underscoring the importance of balanced maternal and early-life nutrition [21,95]. Importantly, DNA methylation changes are not limited to muscle or adipose tissue. For example, restricting maternal nutrient intake (60% requirements) in Wagyu cattle significantly altered fetal thymus methylation, impairing immune signaling pathways (Ras, MAPK, cAMP) [96]. Maternal methionine supplementation in early gestation altered over 28,000 cytosine methylation sites in fetal and postnatal muscle. These changes affected genes related to contraction, PI3K signaling, mitochondrial function, and ROS homeostasis [21].
A recent multi-omics study found that small differences in maternal weight gain (0.28 vs. 0.79 kg/day) during early gestation changed fetal liver DNA methylation in beef calves. This affected key metabolic pathways such as lipid metabolism, Wnt signaling, and PI3K-Akt signaling [97]. These changes occurred in parallel with shifts in miRNA profiles, particularly the upregulation of miR-206, suggesting the coordinated epigenetic programming of hepatic development.
Taken together, these findings illustrate how maternal nutrition reshapes DNA methylation profiles across multiple organ systems, with long-lasting consequences for growth and health.
4.1.2. Histone Modifications
Histone proteins organize DNA; chemical marks (e.g., acetylation, methylation, phosphorylation) on histone tails remodel chromatin structure. Open chromatin typically promotes gene transcription, whereas condensed chromatin restricts it. High maternal energy intake may cause histone modifications in fetal precursors, promoting fat cell formation over muscle development [13,19,93].
Direct bovine data are still emerging, but parallel research in sheep and rodents demonstrates that excess maternal nutrients elevate histone marks on adipogenic genes such as PPARG and C/EBPα, while downregulating those tied to muscle growth [98,99,100]. This histone-driven epigenetic reprogramming likely explains why offspring from overfed dams exhibit higher intramuscular fat but fewer muscle fibers.
4.1.3. Non-Coding RNAs (miRNAs and lncRNAs)
ncRNAs, including miRNAs and lncRNAs, operate post-transcriptionally, shaping metabolic and developmental pathways [92]. MO can alter circulating miRNA profiles, which can affect fetal cell proliferation and growth [101,102]. For example, the upregulation of pro-adipogenic miRNAs (e.g., miR-103, miR-143) boosts adipocyte formation, while the downregulation of muscle-specific miRNAs hinders myogenesis [103].
Recent work also highlights the role of lncRNAs in mediating prenatal programming. In a 2 × 2 factorial study with Angus-cross heifers, vitamin/mineral-supplemented diets (at 110% requirements) and different target weight gains significantly changed fetal liver lncRNA expression at day 83, regulating fatty acid oxidation, glycolysis, amino acid metabolism, and mineral transport [92]. Another study found that prenatal protein–energy supplementation changed over 1800 lncRNAs in the muscle tissue of offspring aged 15–22 months. These were linked to Wnt signaling, angiogenesis, and adipocyte development [104]. These observations reinforce the notion that maternal diet and especially overfeeding can durably shape postnatal muscle and metabolic phenotypes via lncRNA-based regulation.
4.2. Transgenerational and Multigenerational Epigenetic Effects
Epigenetic modifications including DNA methylation, histone modifications, and non-coding RNAs can be transmitted across generations, influencing traits such as growth, feed efficiency, and reproductive capacity [10]. These are classified as multigenerational, when both the fetus (F1) and its germ cells (F2) are directly exposed during gestation, and transgenerational, where changes appear in F3 or later generations without direct exposure [105,106].
Recent work in beef cattle indicates the potential for these transgenerational effects. A multigenerational Zebu study found that good maternal conditions in previous generations improved F3 offspring traits. Reproductive traits were most affected, with up to 52.9% of variation explained by historical maternal environment [106]. Nutritional interventions during pregnancy may thus alter DNA methylation patterns in fetal tissues, and in some cases, these changes persist for multiple generations [107]. Although data in cattle are still emerging, findings in other livestock suggest that strategic gestational nutrition could help improve long-term productivity through epigenetic mechanisms.
miRNAs further mediate these heritable processes, regulating mRNAs essential for muscle development, fat deposition, and overall growth [9,108]. MO frequently upregulates pro-adipogenic miRNAs, elevating fat accrual, while nutrient restriction can downregulate miRNAs that favor muscle development [94,101]. By continuously shaping gene expression, these small RNAs may help transmit metabolic or compositional traits across generations.
While maternal influences dominate most epigenetic literature, paternal diet is increasingly recognized. In sheep, F0 rams supplemented with methionine from weaning to puberty exhibited persistent changes in sperm DNA methylation that were passed to F1 and F2 generations, affecting over 100 cytosines [109]. These epigenetic marks were associated with growth and reproductive phenotypes, such as altered scrotal circumference and loin muscle depth, in unexposed offspring [109]. Another study found that 216 differentially methylated regions and 824 differentially methylated cytosine sites emerged in F0 sperm following prepubertal dietary methionine supplementation, targeting genes implicated in puberty onset (e.g., DAZAP1, CHD7) and testis development (TAB1, MTMR2, CELSR1). As a result, F1 progeny had lower pubertal weight and smaller testes, highlighting paternal influence on epigenetic inheritance [110].
Collectively, these studies highlight that environmentally induced epimutations arising from either maternal or paternal nutritional exposure can escape traditional epigenetic “erasure” during gametogenesis, influencing traits across multiple generations. By combining nutritional strategies (e.g., avoiding MO or underfeeding), genetic selection, and an understanding of epigenetic inheritance, producers can optimize muscle growth, fat development, and reproductive traits in beef cattle [41]. MO can enhance marbling but also increase collagen, which reduces tenderness. Its timing and intensity must be managed carefully to maximize benefits and avoid negative effects.
5. Impact of MO on Fetal Gene Expression
The effects of MO on fetal gene expression are well-documented, but a mechanistic synthesis is still needed to understand how these prenatal changes affect postnatal physiology. MO during gestation alters fetal gene expression in skeletal muscle, adipose, connective tissue, and energy metabolism (Table 1). These changes shape growth potential, carcass traits, metabolic efficiency, and long-term health.
5.1. Impacts on Muscle Development
MO and nutritional interventions affect myogenesis by altering gene expression and epigenetic regulation during gestation. Mid-gestation protein supplementation affects more than 300 genes. It upregulates muscle markers (e.g., LOC107131843, KRT8/18/19) and downregulates mitochondrial genes (e.g., AK9, LOC510904, CEACAM19), shifting fetal energy use toward fatty acids [111,112]. Methionine supplementation changes DNA methylation and splicing, reducing MYF5 expression and altering histone modifiers like ASH1L and NSD1. These effects link one-carbon metabolism to muscle development [43,52,113,114,115].
In late gestation, higher protein intake upregulates p70S6k and GSK3B mRNA, promoting muscle growth without triggering catabolic markers (MuRF1, Atrogin-1) [53]. In contrast, excess energy intake downregulates oxidative and myogenic genes (e.g., PPARGC1A, THBS1), impairing myogenesis and mitochondrial function [116]. PUFA supplementation upregulates MYH7 and C/EBPβ in fetal muscle, improving lipid metabolism [117].
Overfeeding promotes glycolytic (type 2x) fibers and increases fat and collagen expression, even when MyoD and MyoG levels remain stable [13,118]. RPF supplementation in late pregnancy shifts the balance toward glycolytic fibers [119]. Mid-gestation protein–energy supplementation (e.g., soybean meal) enhances fetal muscle growth by upregulating IGF1 and IGF2 [120]. Se supplementation affects contractile genes like MYOG and MYH1. However, Se intake in early gestation may temporarily reduce their expression and slightly affect birth weight [121,122].
5.2. Regulation of Adipogenesis and Fat Deposition
Maternal nutrition during pregnancy influences fetal fat development by altering gene networks and epigenetic regulators that direct cell fate toward muscle or fat. Late-gestation PUFA supplementation upregulates MYH7 and C/EBPβ while repressing MYF5 in muscle precursors and decreases SCD in adipose tissue, indicative of leaner growth [117]. Protein–energy supplementation similarly promotes the expression of SPRING1 and FBXW8, increasing backfat thickness [65,123]. Conversely, MO elevates PPARG and ZFP423, tipping the balance toward adipogenesis at the expense of myogenesis (Figure 2). Fetuses from nutrient-restricted dams show higher UCP1 and PGC1α, indicating BAT development. Overfed dams produce fetuses with elevated LEP, CEBPA, and PPARG, typical of WAT [102]. Figure 3 summarizes how MO, micronutrient supplementation, and epigenetic mechanisms (e.g., miRNA regulation) influence fat accumulation and marbling.
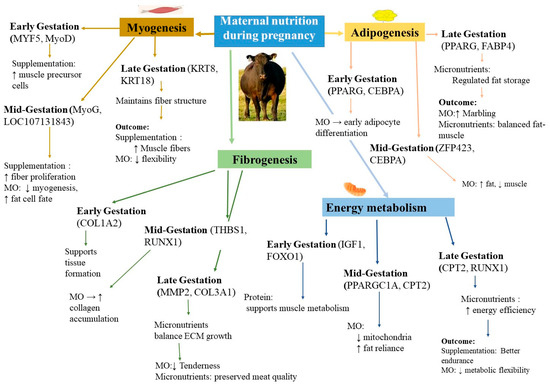
Figure 2.
Maternal nutrition reprograms key fetal pathways. Gold (myogenesis), orange (adipogenesis), green (fibrogenesis), and blue (energy metabolism) arrows indicate major developmental axes. Upward (↑) and downward (↓) arrows show increased or decreased activity under protein/micronutrient supplementation or MO, respectively. Icons denote the maternal plane (cow), muscle fiber development (muscle icon), fat deposition (fat droplet), collagen deposition (collagen icon), and mitochondrial/energy pathways (mitochondrion). Abbreviations: MYF5 (myogenic factor 5), MyoD (myoblast determination protein 1), KRT8/18 (keratins 8 and 18), PPARG (peroxisome proliferator-activated receptor γ), FABP4 (fatty acid binding protein 4), ZFP423 (zinc finger protein 423), COL1A2/COL3A1 (collagen types I α2/III α1), THBS1 (thrombospondin 1), RUNX1 (runt-related transcription factor 1), MMP2 (matrix metalloproteinase 2), IGF1 (insulin-like growth factor 1), FOXO1 (forkhead box O1), PPARGC1A (PPAR γ coactivator 1 α), CPT2 (carnitine palmitoyltransferase 2).
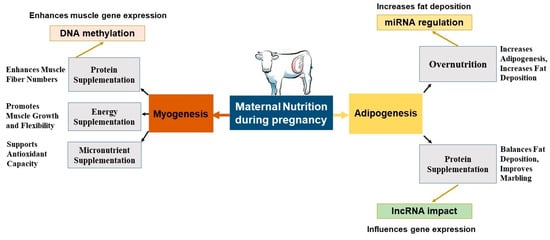
Figure 3.
Maternal nutrition during pregnancy differentially programs myogenesis and adipogenesis. The blue central box denotes the maternal dietary plane. The orange pathway (left) traces myogenesis: protein, energy, and micronutrient supplementation increase muscle-fiber number, growth flexibility, and antioxidant capacity; epigenetic activation via DNA methylation and lncRNA networks further upregulates myogenic genes. The yellow pathway (right) traces adipogenesis: MO or targeted micronutrients upregulate adipocyte differentiation and fat deposition through miRNA regulation and lncRNA-mediated gene control; a balanced micronutrient supply can instead improve marbling while limiting excess fat. Arrow thickness indicates the direction of regulation: upward arrows = stimulation; downward arrows = repression. Abbreviations: MO, MO; lncRNA, long non-coding RNA; miRNA, microRNA.
Changes in miRNAs (e.g., miR-103, miR-107) and β-catenin downregulation promote adipocyte differentiation [3,13,94,124,125,126]. High RUP intake also increases ZFP423, PPARG, and C/EBPα, encouraging intramuscular fat deposition [54].
Specific nutrients fine-tune these effects. RPF lowers PPARα, promoting subcutaneous fat, while vitamin A activates RARβ, DLK1, and PPARG in intramuscular cells without affecting subcutaneous fat [119,127]. These gestational diets coordinate the balance of muscle and fat development, strongly affecting carcass quality, metabolic health, and production efficiency [128,129].
5.3. Fibrogenesis and Connective Tissue Formation
MO profoundly alters fetal ECM integrity by affecting collagen and adhesion gene networks. Overfeeding during mid-to-late gestation downregulates ECM regulators (THBS1, CD44, CNN1, JUND) and upregulates MMP2, a matrix-degrading enzyme. This shift may weaken muscle by disrupting cytoskeletal organization [116]. MO also enhances fibrogenesis by increasing the expression of COL3A1, FN1, and TGF-β, which promote collagen cross-linking and connective tissue stiffness [13,119,130]. Histological analyses corroborate these transcriptomic changes, revealing elevated type III collagen deposition in fetal muscle under excessive maternal nutrition [13,131,132]. Nutritional interventions can influence these outcomes. PUFA supplementation upregulates COL1A2, COL8A1, and FBN3 in muscle, reinforcing ECM structure [117]. Methionine supplementation reduces histone methylation and suppresses fibrogenic regulators like ASH1L and NSD1, limiting excess collagen synthesis [115]. In nutrient-restricted conditions, genes like PCDH19 are upregulated to stabilize the ECM [133,134]. In contrast, imbalanced diets increase COL1A1 and LOX in subcutaneous fat, connecting fat growth to fibrotic remodeling [54,120,131,135].
5.4. Energy Metabolism
MO or restriction alters fetal energy metabolism by reprogramming genes related to mitochondria, glucose use, and fat oxidation. Excess maternal energy often reduces PPARGC1A and other oxidative genes (e.g., THBS1, CD44, RUNX1). This shifts fetal muscle toward fatty acid oxidation instead of glucose use [116,121]. Our previous study showed that ADCY6 is upregulated, heightening ATP-to-cAMP conversion as an adaptive response to nutrient excess [116]. As shown in Figure 2, MO suppresses PPARGC1A, impairing mitochondrial function and reducing oxidative capacity in muscle tissues.
Nutritional supplements may either reduce or worsen these metabolic shifts. Methionine supplementation improves ATP production and redox status. It also lowers CBS expression, influencing one-carbon metabolism and the methylation of metabolic genes [100,113,115]. Moderate protein–energy supplementation increases PITPNA and CDK5R1, supporting mitochondrial growth and insulin signaling in fetal tissues [53,121]. Se activates PRKAG3, enhancing AMPK pathways that promote energy efficiency. However, early excess Se may suppress FOXO1 and FOXO3, showing how these pathways are sensitive to timing [121,132].
Maternal energy intake shapes fetal fat metabolism. Overfeeding raises FASN and CPT2, boosting fat production and oxidation [18,119,136]. In contrast, restriction increases FAS and PREF-1, pushing progenitors toward fat cell development [18,129]. Epigenetic mechanisms also control energy use. Methylation at PCDH19 and shifts in miRNAs (e.g., miR-15b, miR-27b) adjust the balance between oxidation and fat storage [102,134].
These gene and epigenetic changes reveal how maternal diets reprogram the fetus. They lead to lifelong effects on feed efficiency, metabolic health, and productivity.
5.5. Long-Term Consequences of MO-Induced Fetal Gene Expression
Although MO-induced transcriptional and epigenetic changes are well established [3], their true impact lies in how they influence the animal throughout life and even across generations [93]. First, deficits in primary and secondary myogenesis (e.g., reduced MYF5 and PPARGC1A activity) [129,137] limit muscle fiber number and alter fiber-type composition. This results in lower lean mass, slower growth, and reduced feed efficiency across the lifespan [3,13,93,116]. Second, the overactivation of adipogenic genes (PPARG, ZFP423, C/EBPβ) leads to more intramuscular fat and improved marbling [13,94]. However, it also increases collagen cross-linking (COL3A1, FN1), which can reduce meat tenderness and affect consumer preference [13,118,130]. Third, changes in mitochondrial (THBS1, RUNX1, ADCY6) and insulin-signaling genes (PITPNA, CDK5R1) create a metabolic profile with reduced oxidative capacity and disrupted glucose–lipid balance. These animals may have lower resilience to nutritional or environmental stress and a higher risk of metabolic disorders [98,121,134].
MO affects more than just growth and carcass traits. Changes in immune and endocrine gene networks, shaped by interventions like trace minerals and methionine, have been associated with differences in disease resistance, vaccine response, and reproductive milestones such as age at puberty and conception rates [65,138,139]. Emerging evidence shows that epigenetic marks such as DNA methylation, histone changes, and ncRNA profiles can persist into the F₂ and F₃ generations. These heritable changes affect growth, fertility, and carcass traits in animals that were never directly exposed to the original maternal nutrition [105,108].
These findings highlight that MO-induced fetal programming is not merely a short-term effect. It plays a major role in lifetime productivity, animal health, and performance across generations of beef cattle.

Table 1.
Differential expression of genes (or regulatory RNAs) in bovine fetuses and postnatal calves in response to specific maternal nutritional interventions, with inferred functional roles and phenotypic outcomes.
Table 1.
Differential expression of genes (or regulatory RNAs) in bovine fetuses and postnatal calves in response to specific maternal nutritional interventions, with inferred functional roles and phenotypic outcomes.
| Gene(s) | Function | Nutritional Status | Stage | Expression Change | Impact | References |
|---|---|---|---|---|---|---|
| LOC107131843 | Insulin receptor signaling (Akt3-mTOR pathway) | Prot. Sup. | Postnatal | ↑ | Promotes muscle hypertrophy | [111] |
| KRT8, KRT18, KRT19 | Apoptosis regulation and muscle fiber structure | ↑ | Balances protein synthesis and degradation, promoting muscle development | |||
| CLSTN3 | Cell adhesion | ↑ | Enhances muscle integrity | |||
| ANGPTL4 | Lipid metabolism | ↑ | Supports lipid metabolism and energy regulation | |||
| KCNH3 | Potassium ion transport | ↑ | Enhances muscle function | |||
| ENSBTAG00000055143 | Mitochondrial energy metabolism | ↑ | Improves energy efficiency and muscle development | |||
| AK9 | Nucleobase metabolism | ↓ | Reduces metabolic activity in muscle | |||
| LOC510904 | AA transport | ↓ | Limits nutrient transport | |||
| CEACAM19, ENSBTAG00000032057 | ATP synthesis and energy production | ↓ | Alters mitochondrial function | |||
| ALKBH1 | DNA methylation (N6-methyladenine regulation) | ↑ | Promotes muscle proliferation while reducing differentiation | |||
| MYF5 | Myogenic regulatory factor (muscle fiber formation) | Energy + Prot. Sup. PUFA/MPN | Postnatal | ↓ | Reduces muscle fiber differentiation. 2- PUFA supplementation may preserve muscle development by maintaining MYF5 expression | [94,115] |
| MYH7 | Muscle fiber development | PUFA | Postnatal | ↑ | Increased muscle development from birth to weaning | [117] |
| Zfp423 | Regulates early preadipocyte commitment | MO, PUFA, RUP | Fetal and postnatal | ↑↓ | Promotes adipogenesis and fat deposition via PPARγ activation | [13,54,117] |
| PPARG | Adipogenesis and lipid metabolism | MO, MPN, PUFA, RUP | Fetal and postnatal | ↑- | Increases fat accumulation, marbling, and adipogenic programming no significant difference (PUFA) | [13,54,94,117,120,124,140] |
| AGRP | Appetite regulation (appetite-stimulating) | A high-protein or nutrient-rich diet | Postnatal | ↑ | Increases appetite and DMI | [141] |
| C/EBPα | Adipocyte differentiation and fat deposition | MO, MPN, PUFA, RUP, low-energy diet | Fetal and postnatal | ↑ | 1. Increases adipogenesis and fat accumulation in muscle through interaction with PPARγ (MO). 2. Enhances adipogenic programming and fat deposition during fetal development (MPN). | [13,54,94,117,120,124,129] |
| PSPH, PSAT1, PHGDH | Enzymes in serine biosynthesis pathway | Met Sup. | Postnatal | ↓ | Reduced serine synthesis and AA metabolism | [115] |
| SLC7A1, SLC7A5, SLC3A2 | AA transporters | ↓ | Limited AA transport and reduced mTOR signaling | |||
| CBS | Enzyme in the transsulfuration pathway | ↓ | Decreased transsulfuration pathway; potential methionine remethylation | |||
| DNMT3a | DNA methyltransferase (de novo methylation) | Energy + Prot. Sup. | ↓ | Possible effects on epigenetic regulation and gene expression | ||
| TGFBR2, SMAD2, SMAD4 | TGF-β signaling pathway | ↓ | Suppression of muscle growth, increased fibroblast activity | |||
| Wnt-related (e.g., FZD7, APC) | Wnt/β-catenin pathway | ↓ | Reduced myogenesis, favoring adipogenesis | |||
| MTHFD2, ALDH1L2, MTHFD1L | Mitochondrial folate metabolism | ↓ | Impacts one-carbon metabolism and methyl donor availability | |||
| β-catenin | Suppresses adipogenesis (Wnt/β-catenin pathway) | MO/Met Sup. | Fetal and postnatal | ↓ | Favors adipocyte differentiation over myogenesis | [13,94,113] |
| PCDH19 | Protocadherin; involved in immunity | Low maternal diet | Postnatal | ↑ | Regulates energy metabolism and growth, linked with feed efficiency and metabolic weight | [134] |
| GSTM1/2 | Glutathione S-transferase; antioxidant activity | Prot. En. Sup. | ↑ | Regulates oxidative stress and detoxification | ||
| CAST | Calpastatin; inhibits calpain enzymes | Prot. En. Sup | ↑ | Regulates muscle protein degradation, impacting meat quality | ||
| μ-Calpain | Protein turnover and muscle fusion | HE diet | Fetal | ↑ | May indicate enhanced myoblast fusion and larger muscle fiber potential | [129] |
| PAX3, GLI | Essential for skeletal myogenesis | Met Sup. | Postnatal | ↓ | Reduces myocyte development | [113] |
| TGF-β | Regulates fibrogenesis and collagen deposition | MO, MPN, High-RUP diet | Fetal and postnatal | ↑ | Increases muscle fibrosis and collagen cross-linking | [13,54,94] |
| COL3A1 | Collagen type III; fibrosis | MO | Fetal | ↑ | Promotes connective tissue formation and fibrosis | [13] |
| FABP4 | Fatty acid metabolism | MPN | Postnatal | ↑ | Supports fat accumulation during fetal development | [117,124] |
| FASN | Fatty acid synthesis | MPN, PUFA | Fetal and postnatal | ↑ | Increases marbling in muscle | [94,117,124,140] |
| IGF2R | Insulin-like growth factor 2 receptor, regulates cell differentiation and apoptosis | HSD | Postnatal | ↑ | Facilitates IGF2 degradation to prevent fetal overgrowth | [95] |
| MEG8 | Non-coding RNA associated with muscle growth | ↑ | Possibly involved in fetal programming and muscle development | |||
| DNMT3a | DNA methyltransferase, involved in epigenetic regulation | ↑ | Modulates DNA methylation and epigenetic programming | |||
| PDGFRα | Marker of fibro-adipogenic progenitor cells | High-RUP diet | Postnatal | ↑ | Enhances adipogenic and fibrogenic potential | [54] |
| SCD | Stearoyl-CoA desaturase, regulates fat composition | MPN, PUFA | Postnatal | ↑↓ | Increases (MPN) and decreases (PUFA) marbling in muscle | [94,117,124] |
| PPARGC1A | Mitochondrial biogenesis and energy metabolism | MO | Postnatal | ↓ | Reduces muscle oxidative capacity, suppresses energy metabolism | [116] |
| miR-103, miR-107 | Regulate lipid metabolism (target CAV1) | MPN | Postnatal | ↑ | Modulate insulin sensitivity | [124] |
| miR-27a/b, miR-130a | Suppress adipogenesis (target PPARG, C/EBPα) | ↓ | Facilitate fat accumulation | |||
| miR-143 | Promotes adipocyte differentiation (target DLK1) | ↑ | Enhances adipogenesis | |||
| ADCY6 | Converts ATP to cAMP | MO | Postnatal | ↑ | Adapts energy metabolism | [116] |
| COL1A1, COL1A2 | Collagen organization and cross-linking | MO/Met Sup. | Postnatal | ↑ | Promotes fibrosis and connective tissue development | [113,135] |
| HSPA6, HSPA1A | Cellular stress response | MO | Postnatal | ↑ | Protects cells from metabolic stress | [135] |
| NME1, MAPK4 | Cell proliferation and differentiation | LPN | Postnatal | ↓ | Reduces muscle development | [94] |
| SCUBE1, CARD14 | Immune signaling and inflammation | ↑ | Induces inflammatory response | |||
| PREF-1 | Inhibits adipocyte differentiation | HE maternal diet | Fetal | ↑ | Delays adipogenesis | [129] |
| IGF-II | Promotes muscle growth | Tendency ↑ | Promotes fetal muscle development, though without observable fiber differences | |||
| AGR | Appetite stimulation | Periconception (HPeri/LPost) | Postnatal | ↑ | Increases feed intake | [141] |
| FGF2, PPARα | Regulate muscle hypertrophy and metabolism | Mid-gestation supplementation | Postnatal | ↑ | Enhances muscle growth and energy metabolism | [120] |
| MYF3, MYF6, MYH1, MYH3 | Muscle structure and contraction | Se Sup. (TR1/TR3) | Postnatal | ↓ (TR1)/↑ (TR3) | Affects muscle development depending on trimester | [121] |
| MYOG | Myogenin; regulates muscle differentiation | Maternal PS/Se Sup., Low-energy diet | Fetal and postnatal | ↑ | Promotes muscle fiber differentiation and regulates hypertrophy and atrophy | [121,129,134] |
| ABCA6, ABCB11, SLC27A6, SLC2A2, SLC2A4 | Nutrient transport and metabolism | Vit/Min Sup. | Fetal | ↑ | Enhances metabolic activity | [140] |
| MT1A, MT1E, MT2A | Metallothioneins involved in metal ion binding | ↑ | Enhances metal ion homeostasis | |||
| PPARD | Regulates fatty acid oxidation | ↓ | Reduces fat oxidation | |||
| FADS2, LPL | Lipid synthesis and metabolism | ↓ | Reduces fat storage |
↑/↓—up- or downregulation of transcript or protein abundance; AA—amino acid; AGR—agouti-related peptide; AKT3—RAC-α serine/threonine kinase 3; ALKBH1—AlkB homolog 1; APC—adenomatous polyposis coli; bta-miR-…—bovine micro-RNA; CAST—calpastatin; CPT2—carnitine-palmitoyl-transferase 2; DNMT3a—DNA-(cytosine-5)-methyl-transferase 3α; FABP4—fatty-acid-binding protein 4; FADS2—fatty-acid desaturase 2; FASN—fatty-acid synthase; FOXO1—forkhead box O1; GSTM—glutathione-S-transferase mu; IGF—insulin-like growth factor; KRT—keratin; lncRNA—long non-coding RNA; MAPK4—mitogen-activated protein kinase 4; MMP2—matrix metalloproteinase 2; MYF/MYH/MYOG—myogenic regulatory or myosin heavy-chain genes; PPARG/PPARGC1A/PPARD—PPAR γ, its co-activator 1α, and PPAR δ; PTPRC—protein-tyrosine-phosphatase receptor-type C; RUNX1—runt-related transcription factor 1; SCD—stearoyl-CoA desaturase; SMAD—mothers-against-decapentaplegic homologue; TGF-β/TGFBR2—transforming-growth-factor-β and its receptor; WNT/FZD7/β-catenin—canonical Wnt/β-catenin signaling components; ZFP423—zinc-finger protein 423; LPN—low plane of nutrition; MPN—medium plane; HPN—high plane; MO—maternal overnutrition; PUFA—poly-unsaturated fatty-acid supplementation; RUP—rumen-undegradable protein; Prot. Sup./Prot. En. Sup.—protein or protein-plus-energy supplementation; Met Sup.—rumen-protected methionine; Vit/Min Sup.—vitamin and/or mineral supplementation; Se Sup.—selenium supplementation (TR1 = first trimester, TR3 = third trimester); HSD/LHD—high- or low-starch diet; HPeri/LPost—high nutrition periconception, low post-conception.
6. Effect of Maternal Nutrition During Pregnancy on Postnatal Outcomes and Production Traits
Maternal nutrition during gestation strongly influences postnatal growth, health, and productivity in beef cattle. Both over- and underfeeding relative to recommended energy or protein requirements can cause lasting changes in growth, immunity, reproduction, carcass traits, and metabolic resilience. While earlier sections focused on in utero mechanisms like epigenetic and gene expression changes, this section emphasizes the observed phenotypic and performance outcomes.
6.1. Growth Performance and Development
Numerous studies demonstrate that maternal energy and protein intake significantly affect calf birth weights and subsequent growth. For instance, a high-energy (HE) diet during the final 45 days of gestation can boost birth weight and enhance neonatal immune and antioxidant capacity [138]. Similarly, Wilson et al. [75] reported that higher prepartum energy intake correlates with increased calf birth weights, underscoring the importance of energy-rich diets in late gestation. When RPM was provided near conception, offspring showed higher birth weights and improved postnatal ADG. This suggests that early gestational nutrition enhances growth efficiency [66]. These phenotypic outcomes are linked to gestational transcriptional changes. Reduced MYF5 and increased PPARG and ZFP423 expression shape muscle fiber development and fat cell formation.
In addition to late-gestation feeding, periconception and mid-gestation dietary strategies have yielded both transient and persistent effects on calf muscle development. Periconception protein supplementation increases the longissimus dorsi area at birth, likely through better nutrient partitioning toward muscles [141]. In contrast, Costa, et al. [142] found that mid-gestation protein restriction reduced offspring muscle fiber number, a deficit still evident at 450 days. However, changes in fiber-type composition (e.g., MHC2X) were observed only at 30 days and not maintained through finishing. Conversely, high-level MO during late gestation does not always yield long-term benefits. Several studies report no significant differences in birth, weaning, or carcass traits between supplemented and control groups [6,14,68,74,118]. Likewise, Lawson [69] reported that late-gestation protein or methionine changes had little impact on pre-weaning growth. In contrast, postnatal management (e.g., grazing vs. drylot) had a greater effect on calf performance and health. These mixed results suggest that the effectiveness of gestational nutritional programming depends heavily on when and how it is applied and on the conditions after birth.
Nevertheless, protein supplementation remains consistently beneficial. Heifer calves from supplemented dams exhibited higher 205-day weaning weights and body weights at both pre-breeding and pregnancy diagnosis, likely reflecting improved fetal muscle development from enhanced AA availability [12,143]. Cracco et al. [78] similarly observed only a trend toward heavier weaning weights in calves from fully supplemented dams, with no significant differences by 12–22 months of age. Similarly, Nascimento et al. [64] reported that mid-gestation protein supplementation increased birth and weaning weights, enhanced skeletal growth, and enlarged muscle fibers. These effects varied by sex.
Increasing maternal CP intake by 5% raised birth weights and improved ADG, ultimately increasing carcass weights and FCR in male progeny [144]. However, both low and excessively high protein intake can impair fetal muscle development and calf growth [79].
Early- to mid-gestation supplementation can similarly impact growth. Cracco et al. [78] found that prenatal protein–energy supplementation increased weaning weights but had little effect on ribeye area or fat thickness. This suggests that compensatory growth may reduce long-term advantages. Similarly, Tanner et al. [62] reported that corn supplementation during mid-to-late gestation produced heavier calves at 21 days postpartum, although it did not affect birth or final weaning weights. Other studies have also reported a minimal impact of gestational supplementation on birth or weaning weight [59]. In another trial, LFAT supplementation outperformed high-fat or no-supplement strategies, producing the highest pre-weaning weights and ADG [71]. Such findings underscore the nuanced interplay of timing, nutrient type, and maternal body condition in shaping calf growth trajectories. The overall benefits of protein and energy supplementation for growth performance are summarized in Figure 4.
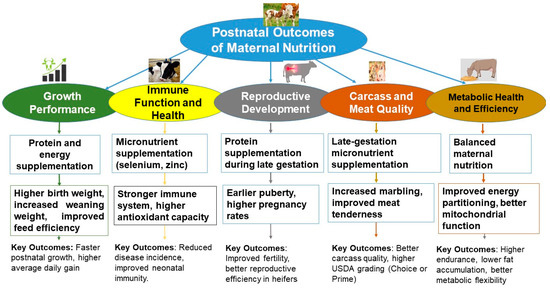
Figure 4.
Postnatal outcomes linked to specific maternal nutrition strategies. The central blue ellipse denotes the overarching theme of Postnatal Outcomes of Maternal Nutrition. The five colored ovals highlight major performance domains: green = Growth Performance; yellow = Immune Function and Health; dark grey = Reproductive Development; orange = Carcass and Meat Quality; and brown = Metabolic Health and Efficiency. Under each domain, the grey rectangles list representative gestational interventions (e.g., protein-plus-energy, trace-mineral or micronutrient supplementation, balanced diets), and the white rectangles below summarize the principal offspring responses. Upward arrows indicate the direction of improvement. Key reported benefits include higher birth and weaning weights, stronger neonatal immunity, earlier puberty and improved fertility, superior marbling and tenderness (higher USDA grading), and enhanced energy partitioning with better mitochondrial efficiency. Abbreviation: USDA, United States Department of Agriculture.
6.2. Immune Function and Health
Appropriate maternal nutrition fosters offspring’s immune competence and overall health in beef cattle. For example, calves from dams fed HE diets during late gestation had higher levels of interleukin-2 and -4, showing stronger neonatal immunity. They also had greater antioxidant capacity and superoxide dismutase activity [138]. Methionine supplementation improved calf immune responses during rearing, even though birth weights were unchanged. This suggests that methionine supports immune priming and metabolic programming more than fetal growth [66].
Trace mineral supplementation also contributes to healthier calves. Calves from dams supplemented with AAC had fewer cases of BRD and heavier weaning and slaughter weights, showing a link between immune function and growth [145]. Stephenson, et al. [146] reported that supplementing cows with 133% of NASEM-recommended copper, zinc, and manganese, especially in chelated form, improved colostrum yield, mineral transfer, and calf liver mineral status. These benefits boosted antioxidant activity and potentially enhanced immune resilience. Supplementing Cu, Mn, and Zn in hydroxychloride form reduced serum haptoglobin levels, suggesting improved immune resilience at weaning [147]. Prenatal VTM supplementation improved gut microbial diversity, reduced pro-inflammatory cytokines, and increased IP-10 levels, promoting immune priming more than birth weight gains [139]. Self-fed RUP supplementation lowered respiratory disease rates and feedlot mortality [148]. HFAT feeding also improved immune status, raising antibody titers and reducing cortisol levels in calves [71]. These effects of micronutrient supplementation on immune health are summarized in Figure 4.
6.3. Reproductive Development
Maternal nutrition during gestation also shapes reproductive efficiency in offspring. Post-weaning feedlot supplementation accelerates puberty in heifers. More heifers reached puberty by 18 months compared to those on pasture-only diets, likely due to increased gonadotropin secretion [76,149]. Bulls from FP-supplemented dams showed a trend toward more sperm defects, possibly due to excess scrotal fat and impaired thermoregulation [65].
Targeted protein supplementation in late gestation raises pregnancy rates and increases the number of heifers calving in the first 21 days. This suggests improved fetal reproductive and endocrine development [12,150]. However, regional forage conditions may limit effects. For example, cows grazing on endophyte-infected fescue showed no improvement in offspring reproduction, even with supplementation [151]. These reproductive outcomes are summarized in Figure 4.
Micronutrients also affect puberty onset. Heifers born to AAC-supplemented dams reached puberty sooner than those from non-supplemented groups [145]. These findings highlight the long-term value of trace mineral supplementation during gestation. By supporting endocrine and gonadal development, maternal nutrition can significantly affect fertility and lifetime productivity in the next generation of beef cattle.
6.4. Carcass Traits and Meat Quality
Maternal nutrition during gestation can strongly influence carcass traits and final meat quality in progeny [23,24]. Protein supplementation is often linked to better marbling scores and a higher percentage of carcasses graded USDA Choice or above. This reflects fetal programming that enhances intramuscular fat deposition [152]. These effects are summarized in Figure 4.
However, results vary: some studies report no significant effect on marbling, even when carcass weight rises [144], or minimal changes in the ribeye area and tenderness [153].
In other scenarios, MO has been shown to increase intramuscular fat, contributing to enhanced marbling in some cases. As explained in Section 5.3, overfeeding can also activate genes related to collagen formation. If collagen persists after birth, it may decrease meat tenderness. Thus, the net effect of MO on meat quality depends on balancing favorable fat infiltration against potential collagen cross-linking [13,20,23].
Micronutrient supplementation further influences carcass outcomes. For example, vitamin A during late gestation may heighten intramuscular fat deposition but can coincide with reduced tenderness if dosing is not carefully managed [127]. Meanwhile, RPF supplementation can lower overall growth rates but need not diminish tenderness or cooking losses. Maternal supplementation with different trace mineral sources (hydroxychloride, sulfate-based, organic) did not significantly influence offspring carcass characteristics, including marbling and ribeye area, indicating limited effects of mineral source on meat quality traits [147]. Producers aiming for premium carcass attributes may thus choose to tailor maternal diets for moderate muscle and marbling gains while preventing the overfeeding scenarios (i.e., >110% of requirements) that risk excessive collagen buildup.
6.5. Metabolic and Physiological Development
Maternal diets also influence the metabolic and physiological development of calves. For example, supplementation during mid-to-late gestation promotes gastrointestinal development. It increases rumen papillae length and villi size, which enhances nutrient absorption and structural growth [61]. RPM supplementation near conception helps steer nutrients toward muscle rather than fat, fostering steady gain through the rearing phase [66].
Additionally, metabolomic profiles in dams and calves shift in response to enhanced gestational feeding, especially for amino acid metabolism and energy pathways. Research suggests that protein–energy supplementation can raise levels of carnosine and alanine in offspring, while OCM supplementation elevates maternal folate and vitamin B12, improving fetal nutrient transfer and long-term metabolic health [55,154]. Early vitamin–mineral supplementation can likewise bolster antioxidant capacity and immune function, emphasizing metabolic resilience over immediate growth [70,82].
Late-gestation methionine supplementation specifically increases fetal growth and postnatal ADG, presumably by channeling nutrients more efficiently toward muscle [155]. These findings show that maternal nutrition not only affects birth weight but also shapes long-term metabolism, health, and growth in offspring. These metabolic improvements are summarized in Figure 4.
6.6. Influence of Fetal Sex on MO Outcomes
MO programs male and female offspring in markedly different ways. In female progeny, Nellore heifers born to dams under no, late-gestation (PELT), or whole-gestation (PEWG) protein–energy supplementation showed only modest early weight-trajectory differences and ultimately converged in average daily gain, ultrasound traits (ribeye area, backfat, rump fat), and age at puberty; genome-wide scans detected a single treatment-specific SNP per group, indicating minimal long-term divergence [123]. Late-gestation protein supplementation further improved heifer weaning weight, pregnancy rate, and calving age [12], whereas, in well-managed systems, varying maternal protein had no effect on heifer growth or reproduction [151]. By contrast, male calves exhibit deeper, often “silent” molecular programming despite similar growth metrics. Male progeny show greater weaning-weight sensitivity under undernutrition and steeper growth gains under adequate nutrition [156], and protein-supplementation × sex interactions affect digestibility, TDN intake, and rumination time; males increase nutrient use while females maintain feed efficiency under restriction [157]. Male fetuses of overfed dams express higher myogenic (MYOD1), adipogenic (PPARG, CEBPA, ZNF423), and fibrogenic (COL1A1, FN1) markers and possess more myocytes than females [158] and consistently outperform females in birth weight, ADG, and frame size regardless of late-gestation energy intake [14]. Maternal fat supplementation increases birth weight in males only [72]; first-trimester protein restriction boosts post-weaning weight in males but reduces it in females [159]; late-gestation Ca salts of soybean oil enhance steer ADG and carcass weight without affecting heifers [160], and methionine supplementation preferentially elevates male birth weight, hip width, and plasma amino acids [155]. Bulls from dams receiving full-gestation supplementation tend to show more sperm defects. They also exhibit SNP changes in insulin, lipid metabolism, androgen biosynthesis, and apoptosis pathways. These are accompanied by shifts in liver metabolites such as citrulline, sphingomyelins, and dicarboxylcarnitines during rearing, which normalize by finishing [56]. Complementing these findings, Hanwoo bull calves from mid-to-late-gestation overfed dams downregulate PPARGC1A, THBS1, and CD44 and upregulate ADCY6 and select lncRNAs in round and sirloin muscle, revealing profound transcriptomic remodeling despite unchanged growth performance [116].
These sex-stratified molecular and phenotypic footprints underscore the critical need to compare male and female offspring directly and to integrate genomics, transcriptomics, metabolomics, and epigenomics in fetal programming research, thereby enabling precision-feeding strategies optimized for both sexes.
6.7. Impact of Maternal Nutrition on Offspring Rumen Microbiome and Gut Flora
Maternal diet helps seed the calf gut microbiome. At birth, microbes in the hindgut originate from the umbilical cord (23.8%), placenta (15.6%), colostrum (14.4%), amniotic fluid (11.2%), and feces (10.5%) [161]. Gestational energy or supplement shifts maternal and offspring rumen taxa, Fibrobacter, Prevotella, and Ruminococcus, altering SCFA profiles (e.g., propionate) that influence fetal immunity and metabolism [162,163]. Probiotics and direct-fed microbes during gestation can similarly reshape maternal communities and, in turn, the calf’s early gut flora [164,165].
Late-gestation undernutrition reduces placental microbial diversity, whereas mineral supplementation increases fetal gut microbiota richness, implicating the placenta in vertical transfer [166,167]. Key rumen colonizers, including Fibrobacter succinogenes, Ruminococcus flavefaciens, Methanobrevibacter, and Prevotella, appear within minutes of birth in rumen fluid and meconium, priming fermentation and immune development [168,169].
Protein–energy programming enhances rumen papillae counts and alters cecal crypt depth in Nellore bulls, correlating with shifts in Bacteroidales BS11 and goblet cell numbers—evidence of microbiome–epithelium co-programming [170,171]. Vitamin–mineral supplementation during gestation increases calf rumen diversity and reduces Escherichia–Shigella [139,172], while dry-period concentrate feeding lowers enteric infections without altering colostral IgG [173]. Conversely, high-energy late-gestation diets reduce colostral IgG [174], whereas fish oil and linseed improve colostrum immunoglobulin content and early microbial establishment [175].
Protein–energy supplementation throughout gestation programs the offspring’s rumen and fecal microbiome. It enhances glycerophospholipid and PUFA pathways, in contrast to the methane- and amino acid-focused profiles in non-supplemented calves. Correlations between Saccharofermentans and malonylcarnitine support coordinated fermentation shifts [176]. In dairy calves, over 50% of meconium microbiota trace back to the maternal rumen, rich in Prevotella, Succiniclasticum, and Butyrivibrio, transmitting enhanced carbohydrate and lipid metabolism and reduced antimicrobial-resistance genes, whereas maternal contact alone yields only transient effects on the calf gut by four weeks. The authors of [177,178] found that although maternal contact influences calf oral microbiota transiently, diet and environment dominate gut microbiome maturation by four weeks of age.
These studies demonstrate that maternal nutrition programs the calf’s gut ecosystem via vertical microbial transfer, maternal microbiome modulation, and prenatal interventions. Future fetal programming research should integrate 16S rRNA sequencing, shotgun metagenomics, and metabolomics of calf rumen and gut contents to fully elucidate microbe-mediated developmental programming pathways.
6.8. Controversies and Breed-Specific Responses
Despite the general trends summarized above, responses to gestational supplementation are far from uniform (Table 2). For example, mid-gestation protein–energy inputs improved birth and weaning weights and enlarged muscle fibers in Nellore calves [64], whereas a comparable late-gestation protein or methionine boost produced little advantage over controls once postnatal management was standardized [69]. Likewise, raising total nutrients to ≈150% of requirements in the third trimester increased calf weight in British breeds [68,80] but not in Hanwoo newborn calves [61,135]. Also, similar energy surpluses showed no consistent benefit for calf growth or carcass traits in other trials [5,13,74,118]. The form of protein also matters: RUP feeding elevated dam BW and ADG but did not translate into better fertility [53].

Table 2.
Conflicting outcomes of major gestational nutrition strategies and the moderating role of breed genetics in beef-cattle fetal programming studies.
Genetic background influences outcomes. Angus-based cattle tend to respond to moderate MO with increased marbling. In contrast, Hanwoo and Wagyu, already marbling-oriented, are more likely to upregulate fibrogenic genes like COL3A1 and FN1 under high energy intake, which may reduce tenderness [60,103]. Bos indicus types show still different patterns: in Nellore, full-gestation programming drove immune-related hepatic networks and rumen-papillae expansion without the pronounced marbling response seen in Angus contemporaries [55,171], and a multigenerational Zebu dataset attributes up to 53% of the variation in reproductive traits to the historical maternal environment [105].
Collectively, these contradictions highlight that timing, nutrient form, postnatal management, and breed biology interact to determine whether a given gestational strategy proves advantageous or counter-productive, underscoring the need for precision, breed-specific feeding guidelines.
7. OMICs Integration Reveals Metabolic Programming Under MO
Integrative multi-omics studies have shown that MO reprograms offspring metabolism at every regulatory level. In fetal liver, muscle, and cerebrum, RNA-Seq and co-expression networks revealed that restricting versus meeting 100% of maternal energy requirements rewires glycolysis, lipid metabolism, and neurotransmission pathways by day 50 of gestation [121]. When combined with metabolomic and epigenomic profiles related to RFI, efficient (low-RFI) offspring display muscle-promoting metabolites; nutrient-sensitive regulation of IGF1, PPARG, and MEF2A; and tissue-specific methylation shifts at IGF2, H19, and PCDH19 [134].
In Nelore bulls, WGCNA of liver transcriptome and metabolome from non-supplemented (NP), partially programmed (PP), and fully programmed (FP) dams revealed NP enrichment in epigenetic regulation and oxidative metabolism modules (e.g., SLC6A14, glutamate), PP signatures in amino acid and lipid metabolism (ODC1, arginine), and FP activation of immune-related networks (PTPRC, SLC12A8) [176]. Additionally, integrated analysis of plasma metabolome along with both rumen and fecal microbiome data indicated that FP calves maintain elevated glycerophospholipid and PUFA pathways, suggesting lasting shifts in membrane signaling and energy metabolism. Conversely, NP calves showed enrichment in amino acid metabolism, nitrogen cycling, and methane-related pathways. FP animals also demonstrated the activation of RNA-degradation and LPS-biosynthesis pathways, highlighting significant host–microbe regulatory interactions [176].
Early-gestation maternal gain further reprograms the fetal liver via coordinated epigenetic and transcriptional changes: moderate gain alters methylation at WNT7B and POU2F2, upregulates bta-miR-206 (targeting morphogenesis and ion-transport genes), and converges on PI3K-Akt, MAPK, Wnt, and mTOR nutrient-sensing pathways [97]. Prenatal protein–energy supplementation likewise reshapes maternal and calf plasma metabolomes, elevating glutamate, alanine, and phosphatidylcholines and enriching histidine and beta-alanine metabolism, suggesting persistent effects on membrane integrity, immune signaling, and transgenerational programming despite minimal overt phenotype [55].
Together, these OMICs-based insights demonstrate that MO remodels host and microbial metabolic networks across epigenetic marks, transcripts, proteins, and metabolites. Incorporating such integrated approaches in fetal programming research will deepen mechanistic understanding and enable precision-feeding strategies to optimize lifelong productivity in beef cattle.
8. Nutritional Strategies and Recommendations
Preventing MO in pregnant beef cows involves meeting fetal developmental demands without exceeding the recommended energy intake. Studies consistently show that feeding beyond ~110–115% of NRC guidelines in late gestation can raise intramuscular and subcutaneous fat deposition, alongside elevated collagen expression, thus compromising offspring feed efficiency and meat tenderness [13,14,118]. Consequently, producers should aim to keep maternal BCS near 5.0–5.5 at calving, reducing the risk of metabolic disorders and difficult births associated with overconditioned cows.
Mid-gestation is especially critical for fetal muscle fiber development yet allows moderate weight gains if dams are under their target BCS [3,120,142]. During this period, supplementation should largely address protein shortfalls while holding TDN around 100–110% of NRC recommendations. For example, if forage quality is marginal, offering a protein supplement at 0.2–0.3% of cow body weight can avert undernutrition without drifting into overfeeding. Monitoring forage availability, adjusting rations weekly, and periodically scoring BCS all help maintain the balance between adequate fetal nutrition and preventing MO. Moreover, nutritional strategies should account for sex-specific differences in fetal programming. Male calves exhibit pronounced sensitivity to nutritional variations, displaying deeper molecular programming with significant muscle and adipose development changes [14,155,156]. Females, in contrast, often present more subtle, yet important, growth and reproductive responses, highlighting the need for targeted nutritional management to optimize outcomes in both sexes [12,123,157].
By late gestation, fetal nutrient demands escalate. Yet, surpassing ~110–115% TDN in this phase can push cows into overconditioning. If BCS is already ≥5.5, producers should moderate any energy-dense feeds (e.g., high-corn diets) and instead rely on forage-based or moderate-concentrate rations aligned with TDN targets. Where additional protein is indicated, focusing on RUP or rumen-protected methionine supports fetal muscle growth without excessively boosting maternal fat [66,155]. Stabilizing or gradually increasing BCS if cows are <5.0 is the goal; however, pushing them above 5.5 fosters detrimental adiposity.
Micronutrient supplementation remains essential to strengthen fetal immune and antioxidant systems, yet these interventions generally do not risk MO. Because minerals and vitamins carry negligible caloric content, modest surpluses rarely spur overconditioning [70,145]. For instance, Se, zinc, copper, and manganese can be given at or slightly above recommended levels to support offspring’s immune function. Recent data suggest that hydroxychloride trace minerals (zinc, copper, and manganese) may slightly enhance calf immunity; however, carcass traits, including marbling and ribeye area, were unaffected by mineral source, emphasizing balanced mineral nutrition as key regardless of supplementation form [147]. The careful use of vitamin A in late gestation may increase intramuscular fat, though high doses could raise collagen deposition if not monitored [127]. By contrast, vitamin D aids fetal bone and muscle development, reinforcing skeletal strength without contributing extra dietary energy.
Emerging evidence also underscores the benefits of managing maternal diets to positively influence offspring gut microbiota. Including probiotics or direct-fed microbial supplements during gestation can reshape maternal rumen communities, promoting beneficial gut flora in calves, thereby enhancing early immune and metabolic development [162,164,165,171,176].
A central consideration is cost–benefit analysis. Producers must weigh whether the expense of additional feed or specialized supplements realistically translates to improvements in calf growth or carcass returns. Evidence suggests that overshooting nutrient requirements does not always yield proportional performance gains, potentially undermining profitability [89]. Thus, precision feeding through total mixed rations or targeted supplementation helps producers avoid inadvertently exceeding energy needs and incurring feed costs that fail to pay off in calf performance.
Practical precision approaches, including electronic feeders, let managers adapt daily intakes based on real-time BCS measurements, preventing cows from drifting into overconditioning late in gestation. Such dynamic fine-tuning is especially valuable if animals are predisposed (genetically or otherwise) to accumulate fat rapidly.
Recognizing breed-specific nutritional responses is critical. Angus cattle may benefit from moderate MO through enhanced marbling [75,124]. Conversely, breeds genetically predisposed to marbling such as Hanwoo and Wagyu risk increased fibrogenic gene expression (e.g., COL3A1, FN1) and decreased meat tenderness under similar nutritional conditions, thus necessitating careful dietary adjustments [60,103,116].
Finally, leveraging advanced OMICs tools (genomics, transcriptomics, and metabolomics) can refine precision feeding practices. These technologies help identify optimal nutritional interventions based on specific metabolic, genetic, and microbiome profiles, enhancing productivity and economic returns [55,97,121,134].
9. Conclusions
Maternal nutrition during gestation exerts a profound influence on fetal programming, postnatal performance, and sustainability in beef production. In particular, MO feeding above ~110–115% of the recommended energy can impair mitochondrial function, shift muscle development toward excess fat and collagen deposition, and diminish carcass quality. Epigenetic mechanisms such as DNA methylation, histone modifications, and microRNA regulation underlie these nutrient-driven changes by modulating fetal gene expression in muscle, adipose, and immune tissues.
Nevertheless, targeted supplementation including micronutrients (Se, zinc, copper) and amino acids (e.g., methionine), can reinforce antioxidant defenses, immune responses, and reproductive outcomes without risking overconditioning when applied judiciously. Integrating precision feeding strategies that match nutrient levels to each gestational phase, along with regular BCS, remains central to controlling MO. This approach ensures healthy fetal development, maximizes postnatal growth and carcass merit, and improves economic returns in beef operations.
Recent research underscores critical considerations, such as sex-specific programming differences, the significant impact of maternal nutrition on offspring rumen microbiota, and the varied nutritional responses among cattle breeds. These findings highlight the necessity for targeted nutritional management that considers offspring sex, microbiome modulation strategies, and breed-specific dietary recommendations to enhance overall effectiveness.
Future research should further dissect breed-specific vulnerabilities to overfeeding and the multigenerational impacts of maternal diets, particularly through advanced epigenetic profiling. Refined feeding strategies that incorporate real-time energy monitoring and cost–benefit analyses will help producers balance productivity, animal welfare, and long-term sustainability under evolving market and environmental pressures.
Author Contributions
B.S. conceptualized the review, supervised, conducted data curation, performed formal analysis, and handled methodology and visualization. He also wrote the original and final draft of the manuscript. The manuscript was reviewed by Y.-C.B., G.-S.J., S.-J.M., S.J., K.-H.U., S.-S.J. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute of Animal Science (NIAS), Rural Development Administration (RDA), Republic of Korea, under Project No. RS-2021-RD010016. Additional support was provided by the 2025 RDA Fellowship Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in this study.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Amino acid |
| AAC | Organic-complexed trace minerals |
| ADCY6 | Adenylyl cyclase 6 |
| ADG | Average daily gain |
| AGR | Agouti-related peptide |
| AMPK | AMP-activated protein kinase |
| APC | Adenomatous polyposis coli (Wnt co-factor) |
| BAT | Brown adipose tissue |
| BCS | Body-condition score |
| BRD | Bovine respiratory disease |
| BW | Body weight |
| CBS | Cystathionine β-synthase |
| CP | Crude protein |
| CPT2 | Carnitine-palmitoyl-transferase 2 |
| DMI | Dry-matter intake |
| ECM | Extracellular matrix |
| FASN | Fatty-acid synthase |
| FCR | Feed-conversion ratio |
| FGF2 | Fibroblast growth factor 2 |
| FN1 | Fibronectin 1 |
| FOXO1/3 | Forkhead box O1/O3 |
| HCW | Hot-carcass weight |
| HE | High-energy (diet) |
| HFAT | High-fat bakery-waste diet |
| HPN | High plane of nutrition |
| HSD | High-starch diet |
| IgG | Immunoglobulin G |
| IUGR | Intrauterine growth retardation |
| JAK-STAT | Janus-kinase/signal-transducer-and-activator-of-transcription pathway |
| lncRNA | Long non-coding RNA |
| LFAT | Low-fat bakery-waste diet |
| LOX | Lysyl oxidase |
| LPN | Low plane of nutrition |
| ME | Metabolizable energy |
| miRNA | Micro-RNA |
| MO | Maternal overnutrition |
| mTOR | Mechanistic target of rapamycin |
| MPN | Medium plane of nutrition |
| NRC | National Research Council |
| OCM | One-carbon metabolites |
| PUFA | Poly-unsaturated fatty acid |
| RFI | Residual feed intake |
| RPF | Rumen-protected fat |
| RPM | Rumen-protected methionine |
| RUP | Rumen-undegradable protein |
| SCFA | Short-chain fatty acid |
| Se | Selenium |
| TDN | Total digestible nutrients |
| VTM | Vitamin–mineral supplement |
| WAT | White adipose tissue |
| WGCNA | Weighted gene-co-expression network analysis |
| ZFP423 | Zinc-finger protein 423 |
References
- Bazer, F.W.; Spencer, T.E.; Wu, G.; Cudd, T.A.; Meininger, C.J. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Greenwood, P.L. Prenatal origins of postnatal variation in growth, development and productivity of ruminants. Anim. Prod. Sci. 2016, 56, 1217–1232. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.; Zhu, M.; Ford, S.; Nathanielsz, P. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef] [PubMed]
- Funston, R.N.; Larson, D.M.; Vonnahme, K. Effects of maternal nutrition on conceptus growth and offspring performance: Implications for beef cattle production. J. Anim. Sci. 2010, 88, E205–E215. [Google Scholar] [CrossRef]
- Govoni, K.E.; Reed, S.A.; Zinn, S.A. Cell biology symposium: Metabolic responses to stress: From animal to cell: Poor maternal nutrition during gestation: Effects on offspring whole-body and tissue-specific metabolism in livestock species. J. Anim. Sci. 2019, 97, 3142–3152. [Google Scholar] [CrossRef]
- Moriel, P.; Palmer, E.A.; Harvey, K.M.; Cooke, R. Improving beef progeny performance through developmental programming. Front. Anim. Sci. 2021, 2, 728635. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental programming of fetal growth and development. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar] [CrossRef]
- Funston, R.; Summers, A. Epigenetics: Setting up lifetime production of beef cows by managing nutrition. Annu. Rev. Anim. Biosci. 2013, 1, 339–363. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Zhao, X. Epigenetic marks: Regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front. Genet. 2015, 6, 302. [Google Scholar] [CrossRef]
- Greenwood, P.; Clayton, E.; Bell, A. Developmental programming and beef production. Anim. Front. 2017, 7, 38–47. [Google Scholar] [CrossRef][Green Version]
- Martin, J.L.; Vonnahme, K.A.; Adams, D.; Lardy, G.; Funston, R.N. Effects of dam nutrition on growth and reproductive performance of heifer calves. J. Anim. Sci. 2007, 85, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Gionbelli, M.; Paulino, P.; Serão, N.; Nascimento, C.; Botelho, M.; Martins, T.; Filho, S.; Dodson, M.; Guimarães, S. Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Croft, E. The Effects of Maternal Late Gestation Metabolizable Energy Intake and Parity on Beef Cow-Calf Performance and Metabolism; University of Guelph: Guelph, ON, Canada, 2022. [Google Scholar]
- Radunz, A. Impact of Maternal Nutrition on Calf Performance. In Proceedings of the 2016 Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 15–17 February 2016; p. 47. [Google Scholar]
- Zago, D.; Canozzi, M.; Barcellos, J. Pregnant cow nutrition and its effects on foetal weight–a meta-analysis. J. Agr. Sci. 2019, 157, 83–95. [Google Scholar] [CrossRef]
- González, L.; Kyriazakis, I.; Tedeschi, L. Precision nutrition of ruminants: Approaches, challenges and potential gains. Animal 2018, 12, s246–s261. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Bell, A.W.; Vercoe, P.E.; Viljoen, G.J. Managing the Prenatal Environment to Enhance Livestock Productivity; Springer: New York, NY, USA, 2010. [Google Scholar]
- Zhao, L.; Liu, X.; Gomez, N.A.; Gao, Y.; Son, J.S.; Chae, S.A.; Zhu, M.-J.; Du, M. Stage-specific nutritional management and developmental programming to optimize meat production. J. Anim. Sci. Biotechnol. 2023, 14, 2. [Google Scholar] [CrossRef]
- Greenwood, P.; Cafe, L. Prenatal and pre-weaning growth and nutrition of cattle: Long-term consequences for beef production. Animal 2007, 1, 1283–1296. [Google Scholar] [CrossRef]
- Amorín, R.; Liu, L.; Moriel, P.; DiLorenzo, N.; Lancaster, P.A.; Peñagaricano, F. Maternal diet induces persistent DNA methylation changes in the muscle of beef calves. Sci. Rep. 2023, 13, 1587. [Google Scholar] [CrossRef]
- Van Emon, M.; Sanford, C.; McCoski, S. Impacts of bovine trace mineral supplementation on maternal and offspring production and health. Animals 2020, 10, 2404. [Google Scholar] [CrossRef]
- Blair, A.D.; Gubbels, E.R.; Block, J.J.; Olson, K.C.; Grubbs, J.K.; Underwood, K.R.; Grubbs, J.K. Maternal nutrition and meat quality of progeny. Meat Muscle Biol. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Funston, R.; Summers, A.; Roberts, A. Alpharma Beef Cattle Nutrition Symposium: Implications of nutritional management for beef cow-calf systems. J. Anim. Sci. 2012, 90, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A. Metabolic Programming and Energy Metabolism During Gestation, Lactation and Growth in Small Ruminants. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2007. [Google Scholar]
- Noah, T.K.; Donahue, B.; Shroyer, N.F. Intestinal development and differentiation. Exp. Cell Res. 2011, 317, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Crouse, M.S.P. Maternal Nutrition, One-Carbon Metabolites, and Programming of Fetal Development During Early Gestation; North Dakota State University: Fargo, ND, USA, 2019. [Google Scholar]
- Redifer, C.A.; Duncan, N.B.; Meyer, A.M. Factors affecting placental size in beef cattle: Maternal and fetal influences. Theriogenology 2021, 174, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, C. Nutritional effects on fetal development during gestation in ruminants. Niger. J. Anim. Prod. 2020, 47, 245–256. [Google Scholar] [CrossRef]
- Toschi, P.; Baratta, M. Ruminant placental adaptation in early maternal undernutrition: An Overview. Front. Vet. Sci. 2021, 8, 755034. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Utero-placental vascular development and placental function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006, 572, 51–58. [Google Scholar] [CrossRef]
- Garnica, A.D.; Chan, W.-Y. The role of the placenta in fetal nutrition and growth. J. Am. Coll. Nutr. 1996, 15, 206–222. [Google Scholar] [CrossRef]
- Oattes, J.L.; Shao, T.; Henley, P.A.; Shike, D.W. Fetal programming effects of early weaning on subsequent parity calf performance. Transl. Anim. Sci. 2021, 5, txab049. [Google Scholar] [CrossRef]
- Vonnahme, K. How the maternal environment impacts fetal and placental development: Implications for livestock production. Anim. Reprod. 2018, 9, 789–797. [Google Scholar]
- Velazquez, M. Impact of maternal malnutrition during the periconceptional period on mammalian preimplantation embryo development. Domest. Anim. Endocrinol. 2015, 51, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, D.; Mulliniks, J.T.; Funston, R.N. Developmental programming in a beef production system. Vet. Clin. N. America. Food Anim. Pract. 2019, 35, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Powell, T.L.; Jansson, T. The Role of Placental Nutrient Sensing in Maternal-Fetal Resource Allocation1. Biol. Reprod. 2014, 91, 121798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Q.; Halderson, S.J.; Arriola Apelo, S.I.; Jones, A.K.; Pillai, S.M.; Hoffman, M.L.; Reed, S.; Govoni, K.E.; Zinn, S.A. Maternal overnutrition during gestation in sheep alters autophagy associated pathways in offspring heart. Front. Genet. 2022, 12, 742704. [Google Scholar] [CrossRef]
- Gardner, J. Effects of Gestational Dietary Intake on calf Growth and Early Feedlot Performance of Offspring. Master’s Thesis, Utah State University, Logan, UT, USA, 2016. [Google Scholar]
- Tan, Z.; Jiang, H. Molecular and Cellular Mechanisms of Intramuscular Fat Development and Growth in Cattle. Int. J. Mol. Sci. 2024, 25, 2520. [Google Scholar] [CrossRef]
- Klein, J.L.; Adams, S.M.; Moura, A.F.d.; Borchate, D.; Antunes, D.P.; Maidana, F.M.; Cardoso, G.d.S.; Brondani, I.L.; Gindri, R.G. Effects of nutrition in the final third of gestation of beef cows on progeny development. Rev. Mex. Cienc. Pecu. 2022, 13, 658–673. [Google Scholar]
- Liu, L.; Amorín, R.; Moriel, P.; DiLorenzo, N.; Lancaster, P.A.; Peñagaricano, F. Maternal methionine supplementation during gestation alters alternative splicing and DNA methylation in bovine skeletal muscle. BMC Genom. 2021, 22, 780. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.-H.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; Baik, M. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Austral J. Anim. Sci. 2018, 31, 1043. [Google Scholar] [CrossRef]
- Rainforth, M. The effect of maternal nutritional restriction on fetal development and performance of offspring in beef cattle. Alberta Acad. Rev. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Long, J.M.; Trubenbach, L.A.; Hobbs, K.C.; Poletti, A.E.; Steinhauser, C.B.; Pryor, J.H.; Long, C.R.; Wickersham, T.A.; Sawyer, J.E.; Miller, R.K. Maternal nutrient restriction in late pregnancy programs postnatal metabolism and pituitary development in beef heifers. PLoS ONE 2021, 16, e0249924. [Google Scholar] [CrossRef]
- Meciano, G.d.P.; Schalch Junior, F.J.; Polizel, G.H.G.; Fernandes, A.C.; Cracco, R.C.; Saran Netto, A.; Gomes, R.d.C.; Cônsolo, N.R.B.; Santana, M.H.d.A. Maternal Nutrition Affects Nitrogen Isotopic Signature in Blood Plasma of Beef Cattle Dams and Their Offspring. Metabolites 2022, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Camacho, L.; Ebarb, S.; Swanson, K.; Vonnahme, K.; Stelzleni, A.; Johnson, S. Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth. J. Anim. Sci. 2013, 91, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Hess, B. Maternal plane of nutrition: Impacts on fetal outcomes and postnatal offspring responses. In Proceedings of the Proceedings 4th Grazing Livestock Nutrition Conference, Estes Park, CO, USA, 9–10 July 2010; Hess, B.W., DelCurto, T., Bowman, J.G., Waterman, R.C., Eds.; Western Section, American Society of Animal Science: Champaign, IL, USA, 2010; pp. 104–122. [Google Scholar]
- George, L.A.; Uthlaut, A.B.; Long, N.M.; Zhang, L.; Ma, Y.; Smith, D.T.; Nathanielsz, P.W.; Ford, S.P. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod. Biol. Endocrinol. 2010, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wu, Z.; Dai, Z.; Wang, X.; Li, J.; Wang, B.; Wu, G. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J. Anim. Sci. Biotechnol. 2017, 8, 42. [Google Scholar] [CrossRef]
- Paradis, F.; Wood, K.M.; Swanson, K.C.; Miller, S.P.; McBride, B.W.; Fitzsimmons, C. Maternal nutrient restriction in mid-to-late gestation influences fetal mRNA expression in muscle tissues in beef cattle. BMC Genom. 2017, 18, 632. [Google Scholar] [CrossRef]
- Lopes, R.; Sampaio, C.; Trece, A.; Teixeira, P.; Gionbelli, T.; Santos, L.; Costa, T.; Duarte, M.; Gionbelli, M. Impacts of protein supplementation during late gestation of beef cows on maternal skeletal muscle and liver tissues metabolism. Animal 2020, 14, 1867–1875. [Google Scholar] [CrossRef]
- Costa, T.; Lourenço, P.; Souza, R.; Lopes, M.; Araújo, R.; Santos, M.; Luciano, L.; Massensini, J.; Chalfun, L.; Rennó, L. Ruminal undegradable protein enriched diet during late gestation of beef cows affects maternal metabolism and offspring’s skeletal muscle development. Anim. Feed Sci. Technol. 2022, 291, 115400. [Google Scholar] [CrossRef]
- Schalch Junior, F.J.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Fernandes, A.C.; Mortari, I.; Pires, P.R.L.; Fukumasu, H.; Santana, M.H.d.A.; Saran Netto, A. Prenatal supplementation in beef cattle and its effects on plasma metabolome of dams and calves. Metabolites 2022, 12, 347. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Fernandes, A.C.; Furlan, É.; Prati, B.C.T.; Ferraz, J.B.S.; Santana, M.H.d.A. Impacts of Different Prenatal Supplementation Strategies on the Plasma Metabolome of Bulls in the Rearing and Finishing Phase. Metabolites 2023, 13, 259. [Google Scholar] [CrossRef]
- Thatcher, W.; Santos, J.E.; Staples, C.R. Dietary manipulations to improve embryonic survival in cattle. Theriogenology 2011, 76, 1619–1631. [Google Scholar] [CrossRef]
- Long, N.; Tousley, C.; Underwood, K.; Paisley, S.; Means, W.; Hess, B.; Du, M.; Ford, S. Effects of early-to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle. J. Anim. Sci. 2012, 90, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Stalker, L.A.; Adams, D.C.; Klopfenstein, T.J.; Feuz, D.M.; Funston, R.N. Effects of pre-and postpartum nutrition on reproduction in spring calving cows and calf feedlot performance. J. Anim. Sci. 2006, 84, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Lansford, A.; Tibbitts, B.; Funston, R. Effects of maternal late-gestation nutrition on dam and subsequent progeny growth and performance of beef cattle. App. Anim. Sci. 2021, 37, 479–489. [Google Scholar] [CrossRef]
- Park, M.S.; Shokrollahi, B.; Kim, U.H.; Won, J.I.; Cho, S.-H.; Jin, S.; Kang, S.S.; Moon, S.J.; Um, K.-H.; Jang, K.S. Effects of mid-to-late prepartum feed supplementation in Hanwoo beef cows on their performance, blood metabolites, and the carcass characteristics and metabolites of their neonatal calves. Front. Vet. Sci. 2023, 10, 1287119. [Google Scholar] [CrossRef]
- Tanner, A.; Bauer, M.; Kennedy, V.; Keomanivong, F.; Kirsch, J.; Reynolds, L.; Stokka, G.; Rodas-Gonzalez, A.; Ward, A.; Dahlen, C. Influence of corn supplementation to beef cows during mid-to late-gestation: Maternal feed intake, body condition, plasma metabolites, and calf growth. Livest. Sci. 2020, 240, 104142. [Google Scholar] [CrossRef]
- Meneses, J.A.; Nascimento, K.B.; Galvão, M.C.; Moreira, G.M.; Chalfun, L.H.L.; Souza, S.P.d.; Ramírez-Zamudio, G.D.; Ladeira, M.M.; Duarte, M.S.; Casagrande, D.R. Protein supplementation during mid-gestation affects maternal voluntary feed intake, performance, digestibility, and uterine blood flow of beef cows. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1678–1691. [Google Scholar] [CrossRef]
- Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.; Ramírez-Zamudio, G.D.; Pereira, D.G.; Paulino, P.V.; Casagrande, D.R.; Gionbelli, T.R.; Ladeira, M.M.; Duarte, M.S. Maternal protein supplementation during mid-gestation improves offspring performance and metabolism in beef cows. J. Anim. Sci. 2024, 102, skae058. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Espigolan, R.; Fantinato-Neto, P.; de Francisco Strefezzi, R.; Rangel, R.B.; de Carli, C.; Fernandes, A.C.; Dias, E.F.F.; Cracco, R.C.; de Almeida Santana, M.H. Different prenatal supplementation strategies and its impacts on reproductive and nutrigenetics assessments of bulls in finishing phase. Vet. Res. Commun. 2023, 47, 457–471. [Google Scholar] [CrossRef]
- Silva, G.; Chalk, C.; Ranches, J.; Schulmeister, T.; Henry, D.; DiLorenzo, N.; Arthington, J.; Moriel, P.; Lancaster, P. Effect of rumen-protected methionine supplementation to beef cows during the periconception period on performance of cows, calves, and subsequent offspring. Animal 2021, 15, 100055. [Google Scholar] [CrossRef]
- Richards, M.; Spitzer, J.; Warner, M. Effect of varying levels of postpartum nutrition and body condition at calving on subsequent reproductive performance in beef cattle. J. Anim. Sci. 1986, 62, 300–306. [Google Scholar] [CrossRef]
- Klein, J.; Adams, S.; De Moura, A.; Alves Filho, D.; Maidana, F.; Brondani, I.; Cocco, J.; Rodrigues, L.; Pizzuti, L.; Da Silva, M. Productive performance of beef cows subjected to different nutritional levels in the third trimester of gestation. Animal 2021, 15, 100089. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K. Exploring Relationships Between Cow-Calf Management Systems as Affected by Protein Level and Methionine Supplementation in Late Gestation on Cow-Calf Performance Prior to Weaning. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2020. [Google Scholar]
- Hurlbert, J.L.; Baumgaertner, F.; Menezes, A.C.B.; Bochantin, K.A.; Diniz, W.J.; Underdahl, S.R.; Dorsam, S.T.; Kirsch, J.D.; Sedivec, K.K.; Dahlen, C.R. Supplementing vitamins and minerals to beef heifers during gestation: Impacts on mineral status in the dam and offspring, and growth and physiological responses of female offspring from birth to puberty. J. Anim. Sci. 2024, 102, skae002. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, V.S.; Silva, J.V.L.; Palmer, E.; Ranches, J.; Bittar, J.H.; Santos, G.C.; Pickett, A.; Cooke, R.F.; Vendramini, J.M.; Moriel, P. Bakery waste supplementation to late gestating Bos indicus-influenced beef cows successfully impacted offspring postnatal performance. J. Anim. Sci. 2023, 101, skad244. [Google Scholar] [CrossRef] [PubMed]
- Cornand, E.E. Level of Supplemental Fat in Pregnant Beef Cow Diets During Mid-to-Late Gestation: Effects on Performance of the Dam and Progeny from Birth to Weaning. Master’s Thesis, University of Saskatchewan Saskatoon, Saskatoon, SK, Canada, 2024. [Google Scholar]
- Taylor, R.; LeMaster, C.; Mangrum, K.; Ricks, R.; Long, N. Effects of maternal nutrient restriction during early or mid-gestation without realimentation on maternal physiology and foetal growth and development in beef cattle. Animal 2018, 12, 312–321. [Google Scholar] [CrossRef]
- Long, N.; Vonnahme, K.; Hess, B.; Nathanielsz, P.; Ford, S. Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. J. Anim. Sci. 2009, 87, 1950–1959. [Google Scholar] [CrossRef]
- Wilson, T.; Faulkner, D.; Shike, D. Influence of prepartum dietary energy on beef cow performance and calf growth and carcass characteristics. Livest. Sci. 2016, 184, 21–27. [Google Scholar] [CrossRef]
- Nepomuceno, D.D.; Pires, A.V.; Junior, M.V.F.; Biehl, M.V.; Gonçalves, J.R.; Moreira, E.M.; Day, M.L. Effect of pre-partum dam supplementation, creep-feeding and post-weaning feedlot on age at puberty in Nellore heifers. Livest. Sci. 2017, 195, 58–62. [Google Scholar] [CrossRef]
- Funston, R.N.; Martin, J.L.; Adams, D.; Larson, D. Winter grazing system and supplementation of beef cows during late gestation influence heifer progeny. J. Anim. Sci. 2010, 88, 4094–4101. [Google Scholar] [CrossRef]
- Cracco, R.C.; Ruy, I.M.; Polizel, G.H.G.; Fernandes, A.C.; Furlan, É.; Baldin, G.C.; Santos, G.E.C.; Santana, M.H.d.A. Evaluation of Maternal Nutrition Effects in the Lifelong Performance of Male Beef Cattle Offspring. Vet. Sci. 2023, 10, 443. [Google Scholar] [CrossRef]
- Herring, C.M.; Bazer, F.W.; Johnson, G.A.; Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 2018, 243, 525–533. [Google Scholar] [CrossRef]
- Vaz, R.Z.; Restle, J.; Pacheco, P.S.; Vaz, F.N.; Alves Filho, D.C.; Brondani, I.L.; Pascoal, L.L.; Argenta, F.M. Produtividade e eficiência de produção de vacas de diferentes grupos genéticos submetidas a pastagens cultivadas no pré ou pós-parto. Semina Ciênc. Agrár. 2014, 35, 2697–2707. [Google Scholar] [CrossRef][Green Version]
- Janovick, N.; Boisclair, Y.; Drackley, J. Prepartum dietary energy intake affects metabolism and health during the periparturient period in primiparous and multiparous Holstein cows. J. Dairy Sci. 2011, 94, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.C.B.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation affect concentrations of amino acids in maternal serum and allantoic fluid of beef heifers. J. Anim. Sci. 2021, 99, skab024. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, Z.; Beauchemin, K.; Yang, W.; Tang, S.; Zhou, C.; Han, X.; Wang, M.; Kang, J.; Tan, Z. Effect of protein or energy restriction during late gestation on hormonal and metabolic status in pregnant goats and postnatal male offspring. Animal 2015, 9, 1843–1851. [Google Scholar] [CrossRef]
- Catussi, B.L.C.; Da Silva, L.G.; Júnior, F.J.S.; Auder, R.M.S.A.; Gómez, J.F.M.; Mingoti, R.D.; Morgulis, S.C.F.; Baruselli, P.S. Prepartum and/or postpartum supplementation with monensin-molasses multinutrient blocks to optimize fertility and calf performance in primiparous beef cows. Animal Biosci. 2022, 35, 1675. [Google Scholar]
- Luebbe, K.M.; Stalker, L.A.; Klopfenstein, T.J.; Funston, R.N. Influence of weaning date and late gestation supplementation on beef system productivity I: Animal performance. Transl. Anim. Sci. 2019, 3, 1492–1501. [Google Scholar] [CrossRef]
- Moriel, P.; Vedovatto, M.; Izquierdo, V.; Palmer, E.; Vendramini, J. Maternal prepartum supplementation of protein and energy and body condition score modulated the performance of Bos indicus-influenced cow-calf pairs. Anim. Reprod. Sci. 2024, 262, 107433. [Google Scholar] [CrossRef]
- Catussi, B.L.C.; Ferreira, J.R.; Lo Turco, E.G.; Morgulis, S.C.F.; Baruselli, P.S. Metabolic imprinting in beef calves supplemented with creep feeding on performance, reproductive efficiency and metabolome profile. Sci. Rep. 2024, 14, 9702. [Google Scholar] [CrossRef]
- Lamb, G.C.; Dahlen, C.; Maddox, M. What Is the Economic Impact of Infertility in Beef Cattle; Florida Cooperative Extension Service, Animal Science Department, Univeristy of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Terry, S.A.; Basarab, J.A.; Guan, L.L.; McAllister, T.A. Strategies to improve the efficiency of beef cattle production. Canad. J. Anim. Sci. 2020, 101, 1–19. [Google Scholar] [CrossRef]
- Abebe, B.K.; Wang, J.; Guo, Y.; Wang, H.; Li, A.; Zan, L. A review of the role of epigenetic studies for intramuscular fat deposition in beef cattle. Gene 2024, 908, 148295. [Google Scholar] [CrossRef]
- Scheffler, J.; McCann, M.; Greiner, S.; Jiang, H.; Hanigan, M.; Bridges, G.; Lake, S.; Gerrard, D. Early metabolic imprinting events increase marbling scores in fed cattle. J. Anim. Sci. 2014, 92, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Ward, A.K.; McCarthy, K.L.; Borowicz, P.P.; Reynolds, L.P.; Caton, J.S.; Dahlen, C.R.; Diniz, W.J. lncRNA-gene network analysis reveals the effects of early maternal nutrition on mineral homeostasis and energy metabolism in the fetal liver transcriptome of beef heifers. J. Nutr. Biochem. 2024, 132, 109691. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.C. Fetal Programming and Molecular Characterization of the Skeletal Muscle Development in Ruminants. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2022. [Google Scholar]
- Moisá, S.J.; Shike, D.W.; Shoup, L.; Rodriguez-Zas, S.L.; Loor, J.J. Maternal plane of nutrition during late gestation and weaning age alter Angus× Simmental offspring longissimus muscle transcriptome and intramuscular fat. PLoS ONE 2015, 10, e0131478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, X.; Radunz, A.; Khatib, H. Maternal nutrition during pregnancy is associated with differential expression of imprinted genes and DNA methyltranfereases in muscle of beef cattle offspring. J. Anim. Sci. 2015, 93, 35–40. [Google Scholar] [CrossRef]
- Phomvisith, O.; Muroya, S.; Otomaru, K.; Oshima, K.; Oshima, I.; Nishino, D.; Haginouchi, T.; Gotoh, T. Maternal Undernutrition Affects Fetal Thymus DNA Methylation, Gene Expression, and, Thereby, Metabolism and Immunopoiesis in Wagyu (Japanese Black) Cattle. Int. J. Mol. Sci. 2024, 25, 9242. [Google Scholar] [CrossRef]
- Anas, M.; Ward, A.K.; McCarthy, K.L.; Borowicz, P.P.; Reynolds, L.P.; Caton, J.S.; Dahlen, C.R.; Diniz, W.J. Intergenerational effects of maternal rate of body weight gain on the multi-omics hepatic profiles of bovine fetuses. Gene 2025, 936, 149082. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Liang, J.-F.; Rogers, C.J.; Zhao, J.-X.; Zhu, M.-J.; Du, M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 2013, 62, 3727–3735. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91. [Google Scholar] [CrossRef]
- Peñagaricano, F.; Wang, X.; Rosa, G.J.; Radunz, A.E.; Khatib, H. Maternal nutrition induces gene expression changes in fetal muscle and adipose tissues in sheep. BMC Genom. 2014, 15, 1034. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Y.; Zhao, J.-X.; Rogers, C.J.; Zhu, M.-J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity downregulates microRNA let-7g expression, a possible mechanism for enhanced adipogenesis during ovine fetal skeletal muscle development. Int. J. Obes. 2013, 37, 568–575. [Google Scholar] [CrossRef]
- Zhang, Y.; Otomaru, K.; Oshima, K.; Goto, Y.; Oshima, I.; Muroya, S.; Sano, M.; Roh, S.; Gotoh, T. Maternal nutrition during gestation alters histochemical properties, and mRNA and microRNA expression in adipose tissue of wagyu fetuses. Front. Endocrinol. 2022, 12, 797680. [Google Scholar] [CrossRef] [PubMed]
- Shintani-Ishida, K.; Tsurumi, R.; Ikegaya, H. Decrease in the expression of muscle-specific miRNAs, miR-133a and miR-1, in myoblasts with replicative senescence. PLoS ONE 2023, 18, e0280527. [Google Scholar] [CrossRef] [PubMed]
- Cracco, R.C.; Alexandre, P.A.; Polizel, G.H.G.; Fernandes, A.C.; de Almeida Santana, M.H. Evaluation of muscle long non-coding RNA profile during rearing and finishing phase of bulls subjected to different prenatal nutritional strategies. Animals 2024, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.M.; Karagas, M.R.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E. Environmental exposures influence multigenerational epigenetic transmission. Clin. Epigenet. 2024, 16, 145. [Google Scholar] [CrossRef]
- Santana, M.L.; Bignardi, A.B.; Pereira, R.J.; Sterman Ferraz, J.B.; Eler, J.P. Transgenerational effects of the maternal gestational environment on the post-natal performance of beef cattle: A reaction norm approach. J. Anim. Breed. Genet. 2025, 142, 24–42. [Google Scholar] [CrossRef]
- Vonnahme, K.A.; Lemley, C.O. Programming the offspring through altered uteroplacental hemodynamics: How maternal environment impacts uterine and umbilical blood flow in cattle, sheep and pigs. Reprod. Fert. Develop. 2011, 24, 97–104. [Google Scholar] [CrossRef]
- Sufianov, A.; Beilerli, A.; Kudriashov, V.; Ilyasova, T.; Liang, Y.; Mukhamedzyanov, A.; Bessonova, M.; Mashkin, A.; Beylerli, O. The role of long non-coding RNAs in the development of adipose cells. Non-Cod RNA Res. 2023, 8, 255–262. [Google Scholar] [CrossRef]
- Braz, C.U.; Taylor, T.; Namous, H.; Townsend, J.; Crenshaw, T.; Khatib, H. Paternal diet induces transgenerational epigenetic inheritance of DNA methylation signatures and phenotypes in sheep model. PNAS Nexus 2022, 1, pgac040. [Google Scholar] [CrossRef]
- Gross, N.; Taylor, T.; Crenshaw, T.; Khatib, H. The intergenerational impacts of paternal diet on DNA methylation and offspring phenotypes in sheep. Front. Genet. 2020, 11, 597943. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Costa, T.C.; Sanglard, L.P.; Nascimento, K.B.; Meneses, J.A.; Galvão, M.C.; Serão, N.V.; Duarte, M.S.; Gionbelli, M.P. Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 2022, 263, 104995. [Google Scholar] [CrossRef]
- Long, Y.C.; Cheng, Z.; Copps, K.D.; White, M.F. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell. Biol. 2011, 31, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Amorín, R.; Moriel, P.; DiLorenzo, N.; Lancaster, P.A.; Peñagaricano, F. Differential network analysis of bovine muscle reveals changes in gene coexpression patterns in response to changes in maternal nutrition. BMC Genom. 2020, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kim, Y.; Gewirtz, A.D.; Jo, B.; Gao, C.; McDowell, I.C.; Engelhardt, B.E.; Battle, A.; Aguet, F.; Ardlie, K.G. Co-expression networks reveal the tissue-specific regulation of transcription and splicing. Genom. Res. 2017, 27, 1843–1858. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.A.; Peñagaricano, F.; Vedovatto, M.; Oliveira, R.A.; Field, S.L.; Laporta, J.; Moriel, P. Effects of maternal gestational diet, with or without methionine, on muscle transcriptome of Bos indicus-influenced beef calves following a vaccine-induced immunological challenge. PLoS ONE 2021, 16, e0253810. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Park, M.; Baek, Y.-C.; Jin, S.; Jang, G.-S.; Moon, S.-J.; Um, K.-H.; Jang, S.-S.; Lee, H.-J. Differential gene expression in neonatal calf muscle tissues from Hanwoo cows overfed during mid to late pregnancy period. Sci. Rep. 2024, 14, 23298. [Google Scholar] [CrossRef]
- Shao, T.; Ireland, F.A.; McCann, J.C.; Shike, D.W. Effects of supplements differing in fatty acid profile to late gestational beef cows on cow performance, calf growth performance, and mRNA expression of genes associated with myogenesis and adipogenesis. J. Anim. Sci. Biotechnol. 2021, 12, 67. [Google Scholar] [CrossRef]
- McCann, J.; Wilson, T.; Guan, L.; Shike, D.; Loor, J. 129 Maternal plane of nutrition during mid-gestation affects the skeletal muscle transcriptome in beef cattle progeny. J. Anim. Sci. 2017, 95, 63–64. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Zamudio, G.D.; da Cruz, W.F.; Schoonmaker, J.P.; de Resende, F.D.; Siqueira, G.R.; Neto, O.R.M.; Gionbelli, T.R.; Teixeira, P.D.; Rodrigues, L.M.; Gionbelli, M.P. Effect of rumen-protected fat on performance, carcass characteristics and beef quality of the progeny from Nellore cows fed by different planes of nutrition during gestation. Livest. Sci. 2022, 258, 104851. [Google Scholar] [CrossRef]
- Marquez, D.; Paulino, M.; Rennó, L.; Villadiego, F.; Ortega, R.; Moreno, D.; Martins, L.; De Almeida, D.; Gionbelli, M.; Manso, M. Supplementation of grazing beef cows during gestation as a strategy to improve skeletal muscle development of the offspring. Animal 2017, 11, 2184–2192. [Google Scholar] [CrossRef]
- Diniz, W.J.; Bobe, G.; Klopfenstein, J.J.; Gultekin, Y.; Davis, T.Z.; Ward, A.K.; Hall, J.A. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes 2021, 12, 1884. [Google Scholar] [CrossRef]
- Gao, J.; Nie, W.; Wang, F.; Guo, Y. Maternal selenium supplementation enhanced skeletal muscle development through increasing protein synthesis and SelW mRNA levels of their offspring. Biol. Trace Elem. Res. 2018, 186, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cracco, R.C.; Bussiman, F.d.O.; Polizel, G.H.G.; Furlan, É.; Garcia, N.P.; Poit, D.A.S.; Pugliesi, G.; Santana, M.H.d.A. Effects of maternal nutrition on female offspring weight gain and sexual development. Front. Genet. 2021, 12, 737382. [Google Scholar] [CrossRef] [PubMed]
- Moisá, S.J.; Shike, D.W.; Shoup, L.; Loor, J.J. Maternal plane of nutrition during late-gestation and weaning age alter steer calf longissimus muscle adipogenic microRNA and target gene expression. Lipids 2016, 51, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gao, Z.; Alarcon, R.M.; Ye, J.; Yun, Z. A role of miR-27 in the regulation of adipogenesis. FEBS 2009, 276, 2348–2358. [Google Scholar] [CrossRef]
- Tong, J.F.; Yan, X.; Zhu, M.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity downregulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E917–E924. [Google Scholar] [CrossRef]
- Dean, S.; Gomes, M.; Silva, W.; Steele, M.; Wood, K.; Du, M.; Costa, T.; Serão, N.; Gionbelli, M.; Duarte, M. Vitamin A–Enriched Diet at Late Gestation Affects Intramuscular Fat Deposition in Beef Offspring. Meat Muscle Biol. 2024, 8, 17646. [Google Scholar]
- Du, M. Prenatal development of muscle and adipose and connective tissues and its impact on meat quality. Meat Muscle Biol. 2023, 7, 16230. [Google Scholar] [CrossRef]
- Jennings, T.; Gonda, M.; Underwood, K.; Wertz-Lutz, A.; Blair, A. The influence of maternal nutrition on expression of genes responsible for adipogenesis and myogenesis in the bovine fetus. Animal 2016, 10, 1697–1705. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.-X.; Yan, X.; Zhu, M.-J.; Long, N.M.; McCormick, R.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring. PLoS ONE 2012, 7, e31691. [Google Scholar] [CrossRef]
- Bonnet, M.; Cassar-Malek, I.; Chilliard, Y.; Picard, B. Ontogenesis of muscle and adipose tissues and their interactions in ruminants and other species. Animal 2010, 4, 1093–1109. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Zhao, Q.; Zhan, T.; Li, Y.; Sun, W.; Li, S.; Sun, D.; Si, X.; Yu, X. Targeted metabolomics analysis reveals that dietary supranutritional selenium regulates sugar and acylcarnitine metabolism homeostasis in pig liver. J. Nutr. 2020, 150, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Paradis, F.; Yue, S.; Grant, J.; Stothard, P.; Basarab, J.; Fitzsimmons, C. Transcriptomic analysis by RNA sequencing reveals that hepatic interferon-induced genes may be associated with feed efficiency in beef heifers. J. Anim. Sci. 2015, 93, 3331–3341. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Devos, J.; Wishart, D.S.; Li, C.; Colazo, M.; Kastelic, J.; Thundathil, J.; Fitzsimmons, C. Impact of prenatal maternal nutrition and parental residual feed intake (RFI) on mRNA abundance of metabolic drivers of growth and development in young Angus bulls. Livest. Sci. 2021, 243, 104365. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Lee, H.-J.; Baek, Y.C.; Jin, S.; Jang, G.-S.; Moon, S.J.; Um, K.-H.; Jang, S.S.; Park, M.S. Transcriptomic Analysis of Newborn Hanwoo Calves: Effects of Maternal Overnutrition during Mid-to Late Pregnancy on Subcutaneous Adipose Tissue and Liver. Genes 2024, 15, 704. [Google Scholar] [CrossRef]
- Sul, H.S.; Latasa, M.-J.; Moon, Y.; Kim, K.-H. Regulation of the fatty acid synthase promoter by insulin. J. Nutr. 2000, 130, 315S–320S. [Google Scholar] [CrossRef]
- Du, M.; Yan, X.; Tong, J.F.; Zhao, J.; Zhu, M.J. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol. Reprod. 2010, 82, 4–12. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effect of prepartum dietary energy density on beef cow energy metabolites, and birth weight and antioxidative capabilities of neonatal calves. Sci. Rep. 2022, 12, 4828. [Google Scholar] [CrossRef]
- Luecke, S.M.; Holman, D.B.; Schmidt, K.N.; Gzyl, K.E.; Hurlbert, J.L.; Menezes, A.C.B.; Bochantin, K.A.; Kirsch, J.D.; Baumgaertner, F.; Sedivec, K.K. Whole-body microbiota of newborn calves and their response to prenatal vitamin and mineral supplementation. Front. Microbiol. 2023, 14, 1207601. [Google Scholar] [CrossRef]
- Diniz, W.J.; Ward, A.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Reynolds, L.P.; Borowicz, P.P.; Sedivec, K.K.; Kirsch, J.D.; Dorsam, S.T. Periconceptual maternal nutrition affects fetal liver programming of energy-and lipid-related genes. Animals 2023, 13, 600. [Google Scholar] [CrossRef]
- Copping, K.J.; Callaghan, M.J.; Geesink, G.H.; Gugusheff, J.R.; McMillen, I.C.; Rodgers, R.J.; Muhlhausler, B.S.; Vithayathil, M.A.; Perry, V.E. Periconception and first trimester diet modifies appetite, hypothalamic gene expression, and carcass traits in bulls. Front. Genet. 2021, 12, 720242. [Google Scholar] [CrossRef]
- Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.d.S. Skeletal muscle development in postnatal beef cattle resulting from maternal protein restriction during mid-gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Beaty, J.; Cochran, R.; Lintzenich, B.; Vanzant, E.; Morrill, J.; Brandt, R., Jr.; Johnson, D. Effect of frequency of supplementation and protein concentration in supplements on performance and digestion characteristics of beef cattle consuming low-quality forages. J. Anim. Sci. 1994, 72, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Son, G.H.; Kwon, E.G.; Chung, K.Y.; Jang, S.S.; Kim, U.H.; Song, J.Y.; Lee, H.J.; Park, B.K. Intramuscular fat formation in fetuses and the effect of increased protein intake during pregnancy in Hanwoo cattle. J. Anim. Sci. Technol. 2023, 65, 818. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.M.; Cooke, R.F.; Marques, R.d.S. Supplementing trace minerals to beef cows during gestation to enhance productive and health responses of the offspring. Animals 2021, 11, 1159. [Google Scholar] [CrossRef]
- Stephenson, E.L.; Rathert-Williams, A.R.; Kenny, A.L.; Nagy, D.W.; Shoemake, B.M.; McFadden, T.B.; Tucker, H.A.; Meyer, A.M. Effects of copper, zinc, and manganese source and inclusion during late gestation on beef cow–calf performance, mineral transfer, and metabolism. Transl. Anim. Sci. 2023, 7, txad097. [Google Scholar] [CrossRef]
- Cruz, V.A.; Marques, R.S.; Kvamme, K.; Limede, A.C.; Cidrini, F.A.A.; Cidrini, I.A.; dos Santos Nascimento, K.; Mackey, S.J.; Cooke, R.F.; Farmer, C. Effects of maternal Cu, Mn, and Zn supplementation from different sources on physiological and productive responses of cows and their offspring. J. Anim. Sci. 2025, 103, skae391. [Google Scholar] [CrossRef]
- Mulliniks, J.; Mathis, C.; Cox, S.; Petersen, M. Supplementation strategy during late gestation alters steer progeny health in the feedlot without affecting cow performance. Anim. Feed. Sci. Technol. 2013, 185, 126–132. [Google Scholar] [CrossRef]
- Roa, J.; García-Galiano, D.; Castellano, J.M.; Gaytan, F.; Pinilla, L.; Tena-Sempere, M. Metabolic control of puberty onset: New players, new mechanisms. Mole. Cell. Endocrinol. 2010, 324, 87–94. [Google Scholar] [CrossRef]
- Rhind, S.M.; Rae, M.T.; Brooks, A.N. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 2001, 122, 205–214. [Google Scholar] [CrossRef]
- Shoup, L.M.; Ireland, F.A.; Shike, D.W. Effects of dam prepartum supplement level on performance and reproduction of heifer progeny. Ital. J. Anim. Sci. 2017, 16, 75–81. [Google Scholar] [CrossRef]
- Summers, A.; Ramsay, K.; Funston, R.N. Case Study: The effects of maternal nutrition on steer progeny performance. Prof. Anim. Sci. 2011, 27, 251–256. [Google Scholar] [CrossRef]
- Christofaro Fernandes, A.; Beline, M.; Polizel, G.H.G.; Cavalcante Cracco, R.; Ferreira Dias, E.F.; Furlan, É.; da Luz e Silva, S.; de Almeida Santana, M.H. Fetal programming and its effects on meat quality of Nellore bulls. Vete. Sci. 2023, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Syring, J.G.; Crouse, M.S.; Entzie, Y.L.; King, L.E.; Hirchert, M.R.; Ward, A.K.; Reynolds, L.P.; Borowicz, P.P.; Dahlen, C.R.; Caton, J.S. One-carbon metabolite supplementation increases vitamin B12, folate, and methionine cycle metabolites in beef heifers and fetuses in an energy dependent manner at day 63 of gestation. J. Anim. Sci. 2024, 102, skae202. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.S.; Batistel, F.; Abdelmegeid, M.K.; Lascano, G.; Parys, C.; Helmbrecht, A.; Trevisi, E.; Loor, J.J. Maternal supply of methionine during late-pregnancy enhances rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotechnol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Barcelos, S.d.S.; Nascimento, K.B.; Silva, T.E.d.; Mezzomo, R.; Alves, K.S.; de Souza Duarte, M.; Gionbelli, M.P. The effects of prenatal diet on calf performance and perspectives for fetal programming studies: A meta-analytical investigation. Animals 2022, 12, 2145. [Google Scholar] [CrossRef]
- Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Moreira, G.M.; Ramírez-Zamudio, G.D.; Souza, S.P.d.; Prezotto, L.D.; Chalfun, L.H.L.; Duarte, M.d.S.; Casagrande, D.R. Effects of maternal protein supplementation at mid-gestation of cows on intake, digestibility, and feeding behavior of the offspring. Animals 2022, 12, 2865. [Google Scholar] [CrossRef]
- Gionbelli, T.; Veloso, C.; Rotta, P.; Valadares Filho, S.; Carvalho, B.C.; Marcondes, M.; Cunha, C.S.; Novaes, M.; Prezotto, L.D.; Duarte, M. Foetal development of skeletal muscle in bovines as a function of maternal nutrition, foetal sex and gestational age. J. Anim. Physiol. Anim. Nutr. 2018, 102, 545–556. [Google Scholar] [CrossRef]
- Micke, G.; Sullivan, T.; Gatford, K.; Owens, J.; Perry, V. Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Scie. 2010, 121, 208–217. [Google Scholar] [CrossRef]
- Brandão, A.P.; Cooke, R.F.; Schubach, K.M.; Rett, B.; Souza, O.A.; Schachtschneider, C.L.; Perry, G.A.; Arispe, S.A.; Jump, D.B.; Pohler, K.G. Supplementing Ca salts of soybean oil to late-gestating beef cows: Impacts on performance and physiological responses of the offspring. J. Anim. Sci. 2020, 98, skaa247. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, M.; Loor, J.J.; Elolimy, A.; Li, L.; Xu, C.; Wang, W.; Yin, S.; Qu, Y. Analysis of cow-calf microbiome transfer routes and microbiome diversity in the newborn Holstein dairy calf hindgut. Front. Nutr. 2021, 8, 736270. [Google Scholar] [CrossRef]
- Amat, S.; Dahlen, C.R.; Swanson, K.C.; Ward, A.K.; Reynolds, L.P.; Caton, J.S. Bovine animal model for studying the maternal microbiome, in utero microbial colonization and their role in offspring development and fetal programming. Front. Microbiol. 2022, 13, 854453. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microb. Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cheng, G.; Xu, Y.; Wang, Y.; Xia, Q.; Hu, S. Rumen microbiota distribution analyzed by high-throughput sequencing after oral doxycycline administration in beef cattle. Front. Vet. Sci. 2020, 7, 251. [Google Scholar] [CrossRef]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infec. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- Hummel, G.L.; Austin, K.; Cunningham-Hollinger, H.C. Comparing the maternal-fetal microbiome of humans and cattle: A translational assessment of the reproductive, placental, and fetal gut microbiomes. Biol. Reprod. 2022, 107, 371–381. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Abecia, L.; Newbold, C.J. Manipulating rumen microbiome and fermentation through interventions during early life: A review. Front. Microbiol. 2015, 6, 1133. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genom. Biol. 2019, 20, 172. [Google Scholar] [CrossRef]
- Dias, E.F.F.; de Carvalho, F.E.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Furlan, É.; Fernandes, A.C.; Schalch Júnior, F.J.; Santos, G.E.C.; Ferraz, J.B.S.; Santana, M.H.d.A. Fetal Programming Influence on Microbiome Diversity and Ruminal and Cecal Epithelium in Beef Cattle. Animals 2024, 14, 870. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G. New developments in goblet cell mucus secretion and function. Mucos Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Diddeniya, G.; Ghaffari, M.H.; Hernandez-Sanabria, E.; Guan, L.L.; Malmuthuge, N. Invited review: Impact of maternal health and nutrition on the microbiome and immune development of neonatal calves. J. Dairy Sci. 2024, 107, 7504–7519. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Ashfield, A.; Earley, B.; Welsh, M.; Gordon, A.; McGee, M.; Morrison, S. Effect of concentrate supplementation during the dry period on colostrum quality and effect of colostrum feeding regimen on passive transfer of immunity, calf health, and performance. J. Dairy Sci. 2017, 100, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Chen, Y.; Liang, G.; Goonewardene, L.A.; Guan, L.L. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. J. Dairy Sci. 2015, 98, 8044–8053. [Google Scholar] [CrossRef] [PubMed]
- Grodkowska, K.; Gołębiewski, M.; Slósarz, J.; Sakowski, T.; Puppel, K. The effect of supplementation using a mixture of fish oil and linseed on the level of immunomodulatory components in bovine colostrum. Molecules 2023, 28, 2154. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Diniz, W.J.; Cesar, A.S.M.; Ramírez-Zamudio, G.D.; Cánovas, A.; Dias, E.F.F.; Fernandes, A.C.; Prati, B.C.T.; Furlan, É.; Pombo, G.d.V. Impacts of prenatal nutrition on metabolic pathways in beef cattle: An integrative approach using metabolomics and metagenomics. BMC Genom. 2025, 26, 359. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, S.; Gao, D.; Xu, Y.; Jiang, W.; Hou, G.; Li, S.; Zhao, X.; Chen, T.; Li, S. Maternal gastrointestinal microbiome shapes gut microbial function and resistome of newborns in a cow-to-calf model. Microbiome 2024, 12, 216. [Google Scholar] [CrossRef]
- Barden, M.; Richards-Rios, P.; Ganda, E.; Lenzi, L.; Eccles, R.; Neary, J.; Oultram, J.; Oikonomou, G. Maternal influences on oral and faecal microbiota maturation in neonatal calves in beef and dairy production systems. Anim. Microbiom. 2020, 2, 31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).