Epigenetics in Plant Response to Climate Change
Simple Summary
Abstract
1. Introduction: Climate Change, Plant Adaptation Challenges, and the Epigenetic Dimension
2. Core Epigenetic Mechanism
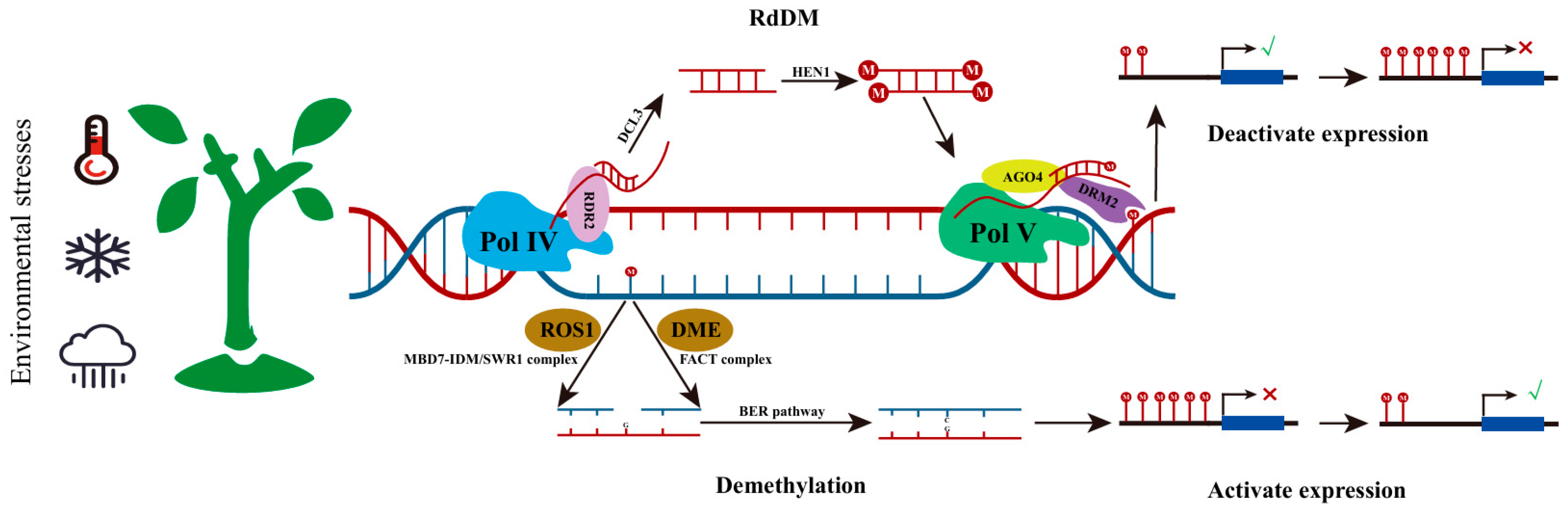
2.1. DNA Methylation
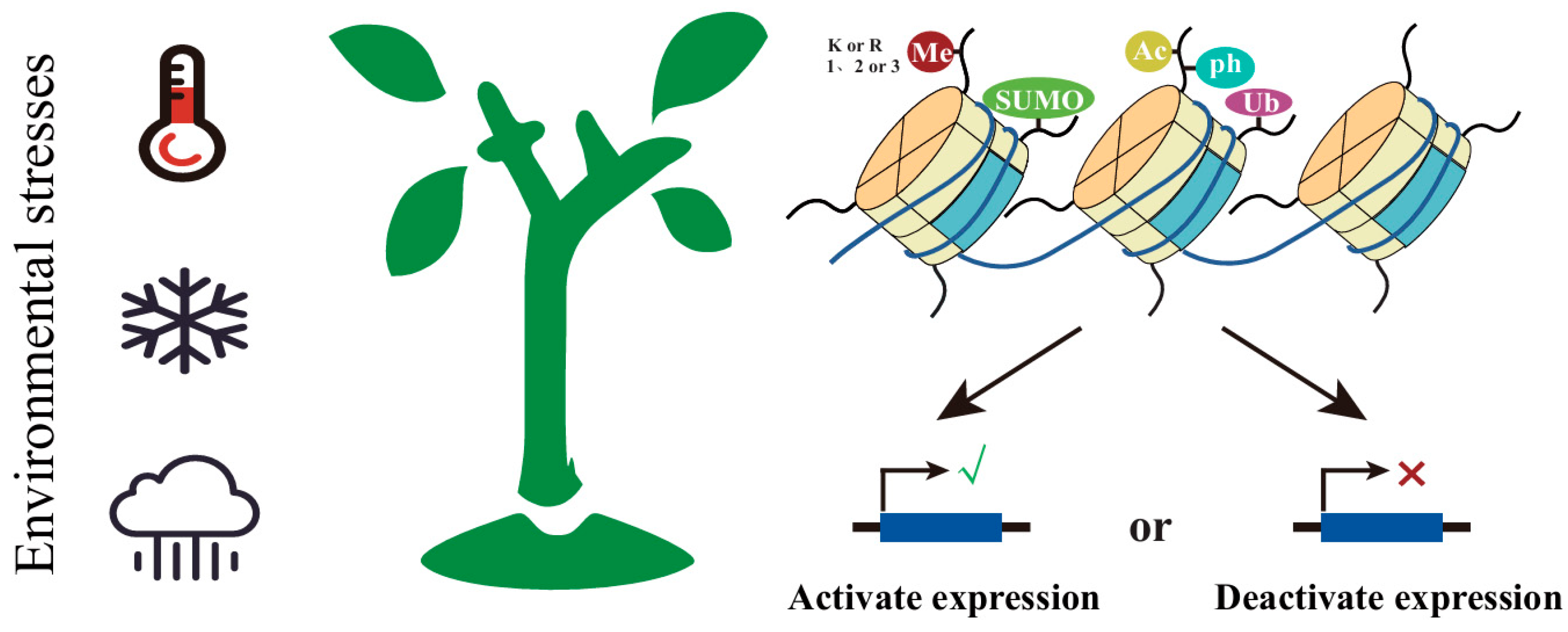
2.2. Histone Modification
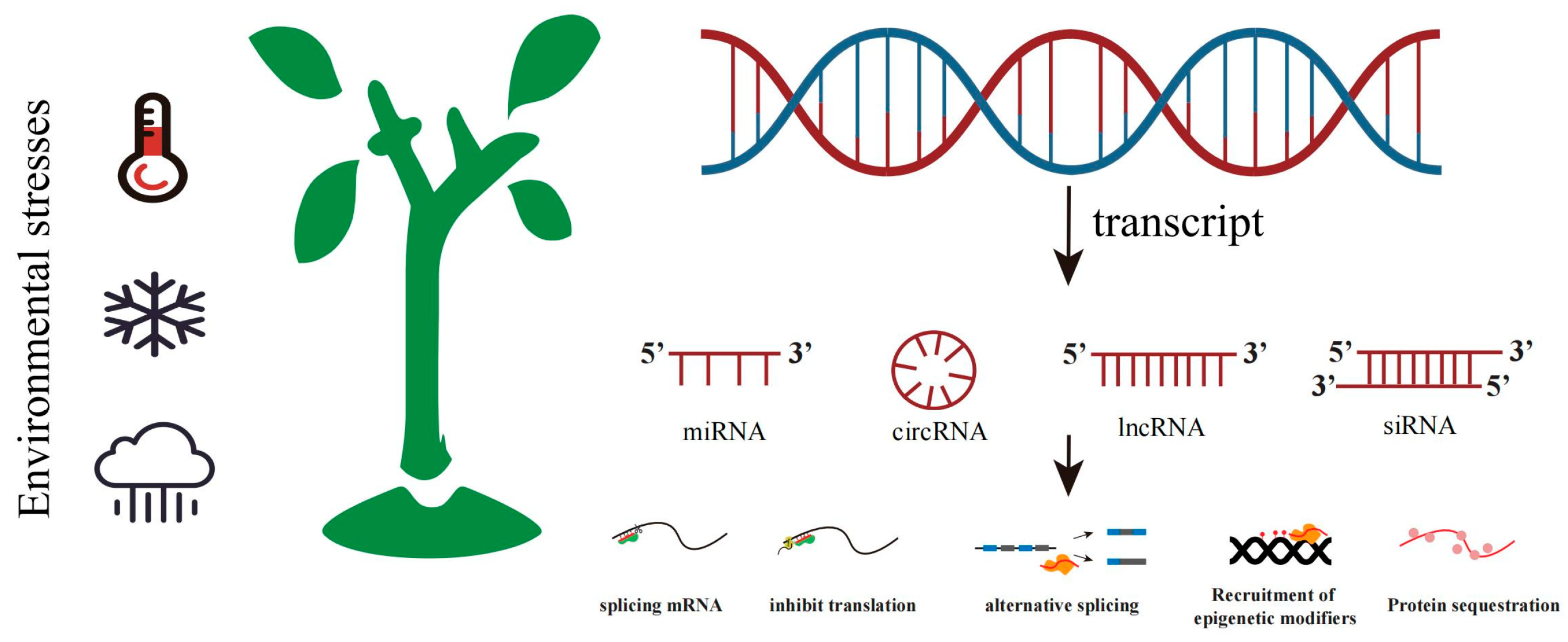
2.3. Non-Coding RNA
3. The Role of Epigenetic Modification in Plants’ Adaptation to Climate Changes
3.1. The Role of DNA Methylation in Plants’ Adaptation to Climate Changes
3.2. The Role of Histone Modification in Plants’ Adaptation to Climate Changes
3.3. Non-Coding RNA Adaptation to Climate Change
4. Epigenetic Stress Memory and Transgenerational Inheritance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2020, 375, 20190104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Q.; Wang, J.; Liang, Y.; Zwiers, F.W.; Zhang, X.; Li, T. Constraining projected changes in rare intense precipitation events across global land regions. Geophys. Res. Lett. 2024, 51, e2023GL105605. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; He, X. Probabilistic assessment of global drought recovery and its response to precipitation changes. Geophys. Res. Lett. 2024, 51, e2023GL106067. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Yang, S.; Wu, K. Role of epigenetic modifications in plant responses to environmental stresses. In Nuclear Functions in Plant Transcription, Signaling and Development; Springer: New York, NY, USA, 2015; pp. 81–92. [Google Scholar]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Balbus, J.M.; Boxall, A.B.; Fenske, R.A.; McKone, T.E.; Zeise, L. Implications of global climate change for the assessment and management of human health risks of chemicals in the natural environment. Environ. Toxicol. Chem. 2013, 32, 62–78. [Google Scholar] [CrossRef]
- Paltseva, A.A.; Neaman, A. An emerging frontier: Metal (loid) soil pollution threat under global climate change. Toxicol. Chem. 2020, 39, 1653–1654. [Google Scholar] [CrossRef]
- Antoniadis, V.; Alloway, B.J. Availability of Cd, Ni and Zn to ryegrass in sewage sludge-treated soils at different temperatures. Water Air Soil Pollut. 2001, 132, 201–204. [Google Scholar] [CrossRef]
- Meyer, P. Epigenetic variation and environmental change. J. Exp. Bot. 2015, 66, 3541–3548. [Google Scholar] [CrossRef]
- Kumar, M.; Rani, K. Epigenomics in stress tolerance of plants under the climate change. Mol. Biol. Rep. 2023, 50, 6201–6216. [Google Scholar] [CrossRef]
- Ma, L.; Xing, L.; Li, Z.; Jiang, D. Epigenetic control of plant abiotic stress responses. J. Genet. Genom. 2024, 52, 129–144. [Google Scholar] [CrossRef]
- Borevitz, J. Utilizing genomics to understand and respond to global climate change. Genome Biol. 2021, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, J.C.; Spurgin, L.G.; Laine, V.N.; Bosse, M.; Great Tit HapMap Consortium; Groenen, M.A.M.; van Oers, K.; Sheldon, B.C.; Visser, M.E.; Slate, J. The genomics of adaptation to climate in European great tit (Parus major) populations. Evol. Lett. 2023, 8, 18–28. [Google Scholar] [CrossRef] [PubMed]
- McCaw, B.A.; Stevenson, T.J.; Lancaster, L.T. Epigenetic responses to temperature and climate. Integr. Comp. Biol. 2020, 60, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ epigenetic mechanisms and abiotic stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef]
- Cao, S.; Wang, L.; Han, T.; Ye, W.; Liu, Y.; Sun, Y.; Moose, S.P.; Song, Q.; Chen, Z.J. Small RNAs mediate transgenerational inheritance of genome-wide trans-acting epialleles in maize. Genome Biol. 2022, 23, 53. [Google Scholar] [CrossRef]
- Calarco, J.P.; Borges, F.; Donoghue, M.T.; Van Ex, F.; Jullien, P.E.; Lopes, T.; Gardner, R.; Berger, F.; Feijó, J.A.; Becker, J.D.; et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 2012, 151, 194–205. [Google Scholar] [CrossRef]
- Holliday, R.; Pugh, J.E. DNA Modification Mechanisms and Gene Activity During Development: Developmental clocks may depend on the enzymic modification of specific bases in repeated DNA sequences. Science 1975, 187, 226–232. [Google Scholar] [CrossRef]
- Riggs, A.D. X inactivation, differentiation, and DNA methylation. Cytogenet. Genome Res. 1975, 14, 9–25. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef]
- Law, J.A.; Du, J.; Hale, C.J.; Feng, S.; Krajewski, K.; Palanca, A.M.S.; Strahl, B.D.; Patel, D.J.; Jacobsen, S.E. Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature 2013, 498, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.-Y.; Zeng, L.; Tanaka, K.; Zhang, C.-J.; Ma, J.; Bai, G.; Wang, P.; Zhang, S.-W.; Liu, Z.-W. DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV. Proc. Natl. Acad. Sci. USA 2013, 110, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Johansen, L.K.; Gustafson, A.M.; Kasschau, K.D.; Lellis, A.D.; Zilberman, D.; Jacobsen, S.E.; Carrington, J.C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004, 2, e104. [Google Scholar] [CrossRef] [PubMed]
- Haag, J.R.; Ream, T.S.; Marasco, M.; Nicora, C.D.; Norbeck, A.D.; Pasa-Tolic, L.; Pikaard, C.S. In vitro transcription activities of Pol IV, Pol V, and RDR2 reveal coupling of Pol IV and RDR2 for dsRNA synthesis in plant RNA silencing. Mol. Cell 2012, 48, 811–818. [Google Scholar] [CrossRef]
- Qi, Y.; Denli, A.M.; Hannon, G.J. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol. Cell 2005, 19, 421–428. [Google Scholar] [CrossRef]
- Zilberman, D.; Cao, X.; Jacobsen, S.E. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 2003, 299, 716–719. [Google Scholar] [CrossRef]
- Li, C.F.; Pontes, O.; El-Shami, M.; Henderson, I.R.; Bernatavichute, Y.V.; Chan, S.W.-L.; Lagrange, T.; Pikaard, C.S.; Jacobsen, S.E. An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 2006, 126, 93–106. [Google Scholar] [CrossRef]
- Qi, Y.; He, X.; Wang, X.-J.; Kohany, O.; Jurka, J.; Hannon, G.J. Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature 2006, 443, 1008–1012. [Google Scholar] [CrossRef]
- Wierzbicki, A.T.; Haag, J.R.; Pikaard, C.S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 2008, 135, 635–648. [Google Scholar] [CrossRef]
- Zhong, X.; Du, J.; Hale, C.J.; Gallego-Bartolome, J.; Feng, S.; Vashisht, A.A.; Chory, J.; Wohlschlegel, J.A.; Patel, D.J.; Jacobsen, S.E. Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 2014, 157, 1050–1060. [Google Scholar] [CrossRef]
- Liu, W.; Duttke, S.H.; Hetzel, J.; Groth, M.; Feng, S.; Gallego-Bartolome, J.; Zhong, Z.; Kuo, H.Y.; Wang, Z.; Zhai, J. RNA-directed DNA methylation involves co-transcriptional small-RNA-guided slicing of polymerase V transcripts in Arabidopsis. Nat. Plants 2018, 4, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; McCue, A.D.; Panda, K.; Fultz, D.; DeFraia, C.; Thomas, E.N.; Slotkin, R.K. The initiation of epigenetic silencing of active transposable elements is triggered by RDR6 and 21–22 nucleotide small interfering RNAs. Plant Physiol. 2013, 162, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Kankel, M.W.; Ramsey, D.E.; Stokes, T.L.; Flowers, S.K.; Haag, J.R.; Jeddeloh, J.A.; Riddle, N.C.; Verbsky, M.L.; Richards, E.J. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 2003, 163, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Do, T.; Du, J.; Zhong, X.; Feng, S.; Johnson, L.; Patel, D.J.; Jacobsen, S.E. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nat. Struct. Mol. Biol. 2014, 21, 64–72. [Google Scholar] [CrossRef]
- Lindroth, A.M.; Cao, X.; Jackson, J.P.; Zilberman, D.; McCallum, C.M.; Henikoff, S.; Jacobsen, S.E. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 2001, 292, 2077–2080. [Google Scholar] [CrossRef]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532. [Google Scholar] [CrossRef]
- Miura, A.; Nakamura, M.; Inagaki, S.; Kobayashi, A.; Saze, H.; Kakutani, T. An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J. 2009, 28, 1078–1086. [Google Scholar] [CrossRef]
- Ebbs, M.L.; Bender, J. Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 2006, 18, 1166–1176. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Zemach, A.; Kim, M.Y.; Hsieh, P.-H.; Coleman-Derr, D.; Eshed-Williams, L.; Thao, K.; Harmer, S.L.; Zilberman, D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 2013, 153, 193–205. [Google Scholar] [CrossRef]
- Stroud, H.; Greenberg, M.V.; Feng, S.; Bernatavichute, Y.V.; Jacobsen, S.E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 2013, 152, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kapoor, A.; Sridhar, V.V.; Agius, F.; Zhu, J.-K. The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr. Biol. 2007, 17, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.-K. Active DNA demethylation in plants and animals. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl. Acad. Sci. USA 2012, 109, E2183–E2191. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, L.; Cui, X.; Bao, S.; Geng, Y.; Yu, G.; Liang, F.; Xie, S.; Lu, T.; Gu, X.; et al. DNA N6-adenine methylation in Arabidopsis thaliana. Dev. Cell 2018, 45, 406–416. [Google Scholar] [CrossRef]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Mozgova, I.; Köhler, C.; Hennig, L. Keeping the gate closed: Functions of the polycomb repressive complex PRC 2 in development. Plant J. Cell Mol. Biol. 2015, 83, 121–132. [Google Scholar] [CrossRef]
- Lu, F.; Li, G.; Cui, X.; Liu, C.; Wang, X.J.; Cao, X. Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J. Integr. Plant Biol. 2008, 50, 886–896. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, H. Evolutionary history of histone demethylase families: Distinct evolutionary patterns suggest functional divergence. BMC Evol. Biol. 2008, 8, 294. [Google Scholar] [CrossRef]
- Martignago, D.; Bernardini, B.; Polticelli, F.; Salvi, D.; Cona, A.; Angelini, R.; Tavladoraki, P. The four FAD-dependent histone demethylases of Arabidopsis are differently involved in the control of flowering time. Front. Plant Sci. 2019, 10, 669. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, J.K.; Prasad, M.J.C.; Sciences, M.L. Histone acetylation dynamics regulating plant development and stress responses. Cell. Mol. Life Sci. CMLS 2021, 78, 4467–4486. [Google Scholar] [CrossRef]
- Gao, S.; Zeng, X.; Wang, J.; Xu, Y.; Yu, C.; Huang, Y.; Wang, F.; Wu, K.; Yang, S. Arabidopsis SUMO E3 ligase SIZ1 interacts with HDA6 and negatively regulates HDA6 function during flowering. Cells 2021, 10, 3001. [Google Scholar] [CrossRef]
- Keren, I.; Citovsky, V. Activation of gene expression by histone deubiquitinase OTLD1. Epigenetics 2017, 12, 584–590. [Google Scholar] [CrossRef]
- Demidov, D.; Hesse, S.; Tewes, A.; Rutten, T.; Fuchs, J.; Karimi Ashtiyani, R.; Lein, S.; Fischer, A.; Reuter, G.; Houben, A. Aurora1 phosphorylation activity on histone H3 and its cross-talk with other post-translational histone modifications in Arabidopsis. Plant J. 2009, 59, 221–230. [Google Scholar] [CrossRef]
- Zhang, P.; Dai, M. CircRNA: A rising star in plant biology. J. Genet. Genom. 2022, 49, 1081–1092. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.-Y.; Jia, C.-L.; Shi, W.-G.; Deng, S.-R.; Luo, Z.-B. Identification and functional prediction of poplar root circRNAs involved in treatment with different forms of nitrogen. Front. Plant Sci. 2022, 13, 941380. [Google Scholar] [CrossRef]
- Song, R.; Ma, S.; Xu, J.; Ren, X.; Guo, P.; Liu, H.; Li, P.; Yin, F.; Liu, M.; Wang, Q. A novel polypeptide encoded by the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR. Mol. Cancer 2023, 22, 16. [Google Scholar] [CrossRef]
- Liu, J.; Jung, C.; Xu, J.; Wang, H.; Deng, S.; Bernad, L.; Arenas-Huertero, C.; Chua, N.-H. Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 2012, 24, 4333–4345. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, homeostasis, and degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Panchal, A.; Maurya, J.; Seni, S.; Singh, R.K.; Prasad, M. An insight into the roles of regulatory ncRNAs in plants: An abiotic stress and developmental perspective. Plant Physiol. Biochem. PPB 2023, 201, 107823. [Google Scholar] [CrossRef]
- Wang, W.-S.; Pan, Y.-J.; Zhao, X.-Q.; Dwivedi, D.; Zhu, L.-H.; Ali, J.; Fu, B.-Y.; Li, Z.-K. Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 1951–1960. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Pu, X.; Lv, H.; Liu, Y.; Ma, H.; Wu, F.; Wang, Q.; Feng, X.; Liu, T. Maize DNA methylation in response to drought stress is involved in target gene expression and alternative splicing. Int. J. Mol. Sci. 2021, 22, 8285. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, L.; Huang, R.; Song, F.; Li, L.; Li, P.; Fang, Y.; Lu, W.; Lv, C.; Quan, M. Local diversity of drought resistance and resilience in Populus tomentosa correlates with the variation of DNA methylation. Plant Cell Environ. 2023, 46, 479–497. [Google Scholar] [CrossRef]
- Chwialkowska, K.; Nowakowska, U.; Mroziewicz, A.; Szarejko, I.; Kwasniewski, M. Water-deficiency conditions differently modulate the methylome of roots and leaves in barley (Hordeum vulgare L.). J. Exp. Bot. 2016, 67, 1109–1121. [Google Scholar] [CrossRef]
- Fei, Y.; Xue, Y.; Du, P.; Yang, S.; Deng, X. Expression analysis and promoter methylation under osmotic and salinity stress of TaGAPC1 in wheat (Triticum aestivum L). Protoplasma 2017, 254, 987–996. [Google Scholar] [CrossRef]
- Zi, N.; Ren, W.; Guo, H.; Yuan, F.; Liu, Y.; Fry, E. DNA methylation participates in drought stress memory and response to drought in Medicago ruthenica. Genes 2024, 15, 1286. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, X.; Zi, N.; Ren, W.; Yin, J.; Yuan, T.; Wang, M.; Yuan, F.; Liu, Y. Drought stress memory enhances the tolerance of alfalfa Medicago sativa L. in response to a subsequent drought: A physiological and omics perspective. Environ. Exp. Bot. 2025, 230, 106088. [Google Scholar] [CrossRef]
- Prasad, M.; Shetty, P.; Pal, A.K.; Rigó, G.; Kant, K.; Zsigmond, L.; Nagy, I.; Shivaprasad, P.; Szabados, L. Transcriptional and epigenomic changes in response to PEG-triggered osmotic stress in rapeseed (Brassica napus L.). J. Exp. Bot. 2025, eraf123. [Google Scholar] [CrossRef]
- Fan, M.; Shibata, H. Simulation of watershed hydrology and stream water quality under land use and climate change scenarios in Teshio River watershed, northern Japan. Ecol. Indic. 2015, 50, 79–89. [Google Scholar] [CrossRef]
- Xia, X.; Wu, Q.; Mou, X.; Lai, Y. Potential impacts of climate change on the water quality of different water bodies. J. Environ. Inf. 2015, 25, 85–98. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Huang, R.; Yang, Z.; Fan, W.; Huang, L.; Yang, J.; Chen, W. Epigenetic modification of a pectin methylesterase gene activates apoplastic iron reutilization in tomato roots. Plant Physiol. 2024, 195, 2339–2353. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, H.; Wei, M.; Tang, Y.; Xia, Y.; Chen, Y.; Shen, Z.; Chen, C. Reactive oxygen species partly mediate DNA methylation in responses to different heavy metals in pokeweed. Front. Plant Sci. 2022, 13, 845108. [Google Scholar] [CrossRef]
- Luo, D.; Lu, H.; Wang, C.; Mubeen, S.; Cao, S.; Yue, J.; Pan, J.; Wu, X.; Wu, Q.; Zhang, H. Physiological and DNA methylation analysis provides epigenetic insights into kenaf cadmium tolerance heterosis. Plant Sci. 2023, 331, 111663. [Google Scholar] [CrossRef]
- Galati, S.; Gullì, M.; Giannelli, G.; Furini, A.; DalCorso, G.; Fragni, R.; Buschini, A.; Visioli, G. Heavy metals modulate DNA compaction and methylation at CpG sites in the metal hyperaccumulator Arabidopsis halleri. Environ. Mol. Mutagen. 2021, 62, 133–142. [Google Scholar] [CrossRef]
- Shafiq, S.; Zeb, Q.; Ali, A.; Sajjad, Y.; Nazir, R.; Widemann, E.; Liu, L. Lead, cadmium and zinc phytotoxicity alter DNA methylation levels to confer heavy metal tolerance in wheat. Int. J. Mol. Sci. 2019, 20, 4676. [Google Scholar] [CrossRef]
- Cong, W.; Li, N.; Miao, Y.; Huang, Y.; Zhao, W.; Kang, Y.; Zhang, B.; Wang, J.; Zhang, J.; Lv, Y. DNA hypomethylation-associated transcriptional rewiring enables resistance to heavy metal mercury (Hg) stress in rice. J. Hazard. Mater. 2024, 461, 132649. [Google Scholar] [CrossRef]
- Tan, J.; Fahad, M.; Zhang, L.; Wu, L.; Wu, X. Microrchidia OsMORC6 Positively Regulates Cadmium Tolerance and Uptake by Mediating DNA Methylation in Rice. Rice 2025, 18, 25. [Google Scholar] [CrossRef]
- Lancíková, V.; Kačírová, J.; Hricová, A. Identification and gene expression analysis of cytosine-5 DNA methyltransferase and demethylase genes in Amaranthus cruentus L. under heavy metal stress. Front. Plant Sci. 2023, 13, 1092067. [Google Scholar] [CrossRef]
- Farahani, F.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O.; Haghighat, S. Nitric oxide and ascorbic acid confer cadmium (Cd) tolerance by improving plant terpenoid metabolism and epigenetically modifying DNA methylation. Environ. Pollut. 2024, 362, 124917. [Google Scholar] [CrossRef]
- Yung, W.S.; Wang, Q.; Chan, L.Y.; Wang, Z.; Huang, M.; Li, M.W.; Wong, F.L.; Lam, H.M. DNA Hypomethylation Is One of the Epigenetic Mechanisms Involved in Salt-Stress Priming in Soybean Seedlings. Plant Cell Environ. 2024. [Google Scholar] [CrossRef]
- Al-Lawati, A.; Al-Bahry, S.; Victor, R.; Al-Lawati, A.H.; Yaish, M.W. Salt stress alters DNA methylation levels in alfalfa (Medicago spp). Genet. Mol. Res. 2016, 15, 15018299. [Google Scholar] [CrossRef]
- Skorupa, M.; Szczepanek, J.; Mazur, J.; Domagalski, K.; Tretyn, A.; Tyburski, J. Salt stress and salt shock differently affect DNA methylation in salt-responsive genes in sugar beet and its wild, halophytic ancestor. PLoS ONE 2021, 16, e0251675. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Peng, Y.; Lu, Z.; Zheng, M.; Wang, Z.; Liu, J.; He, Y.; Luo, J. ZmKTF1 promotes salt tolerance by mediating RNA-directed DNA methylation in maize. New Phytol. 2025, 245, 200–214. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Wang, T.T.; Duan, Y.Y.; Ren, J.; Gao, H.; Fan, Y.J.; Xia, Q.M.; Cao, H.X.; Xie, K.D. Polyploidization leads to salt stress resilience via ethylene signaling in citrus plants. New Phytol. 2025, 246, 176–191. [Google Scholar] [CrossRef]
- Lu, X.; Cui, J.; Qi, J.; Li, S.; Yu, W.; Li, C. The strigolactones-mediated DNA demethylation activates the phosphoinositide pathway in response to salt stress. Int. J. Biol. Macromol. 2025, 301, 139954. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Z.; Feng, L.; Wang, C.; Liu, C.; Li, A.; Yao, K.; Liao, W. The response of DNA methyltransferase and demethylase genes to abiotic stresses in tomato seedling. Plant Physiol. Biochem. 2024, 217, 109276. [Google Scholar] [CrossRef]
- Chen, B.; Saltveit, M.E.; Beckles, D.M. Chilling-stress modifies DNA methylation level in cucumber (Cucumis sativus L.) seedling radicle to regulate elongation rate. Sci. Hortic. 2019, 252, 14–19. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Z.; Zhang, S.; Wang, Y.; Xia, X.; Jiang, Y.; Gull, S.; Chen, L.; Guo, H.; Wu, T.; et al. Methylation and expression of rice NLR genes after low temperature stress. Gene 2022, 845, 146830. [Google Scholar] [CrossRef]
- Chen, B.; Guo, Y.; Zhang, X.; Wang, L.; Cao, L.; Zhang, T.; Zhang, Z.; Zhou, W.; Xie, L.; Wang, J.; et al. Climate-responsive DNA methylation is involved in the biosynthesis of lignin in birch. Front. Plant Sci. 2022, 13, 1090967. [Google Scholar] [CrossRef]
- Van Antro, M.; Prelovsek, S.; Ivanovic, S.; Gawehns, F.; Wagemaker, N.C.; Mysara, M.; Horemans, N.; Vergeer, P.; Verhoeven, K.J. DNA methylation in clonal duckweed (Lemna minor L.) lineages reflects current and historical environmental exposures. Mol. Ecol. 2023, 32, 428–443. [Google Scholar] [CrossRef]
- Li, B.; Yang, C.; An, B.; Wang, H.; Albaqami, M.; Abou-Elwafa, S.F.; Xu, L.; Xu, Y. Comparative transcriptomic and epigenetic analyses reveal conserved and divergent regulatory pathways in barley response to temperature stresses. Physiol. Plant. 2022, 174, e13727. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Yang, X.; Gu, X.; Chen, J.; Shi, T. 6mA DNA methylation on genes in plants is associated with gene complexity, expression and duplication. Plants 2023, 12, 1949. [Google Scholar] [CrossRef]
- Mao, F.; Xie, H.; Shi, Y.; Jiang, S.; Wang, S.; Wu, Y. The Global Changes of N6-methyldeoxyadenosine in Response to Low Temperature in Arabidopsis thaliana and Rice. Plants 2023, 12, 2373. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, X.; Liu, Q.; Li, J.; Wang, W.; Ma, X.; Zhu, B.; Li, S.; Gong, S.; Tian, J.; et al. A DNA adenine demethylase impairs PRC2-mediated repression of genes marked by a specific chromatin signature. Genome Biol. 2023, 24, 198. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, C.; Liu, H.; Zhou, Q.; Liu, Q.; Guo, Y.; Peng, T.; Song, J.; Zhang, J.; Chen, L.; et al. Identification and analysis of adenine N 6-methylation sites in the rice genome. Nat. Plants 2018, 4, 554–563. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Z.; Cui, X.; Ji, C.; Li, Y.; Zhang, P.; Liu, J.; Riaz, A.; Yao, P.; Liu, M.; et al. N6-methyladenine DNA methylation in Japonica and Indica rice genomes and its association with gene expression, plant development, and stress responses. Mol. Plant 2018, 11, 1492–1508. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, A.; Jin, J.B.; Zhao, B.; Wang, T.J.; Wu, Y.; Wang, S.; Liu, Y.; Wang, J.; Guo, P. Arabidopsis histone H3K4 demethylase JMJ 17 functions in dehydration stress response. New Phytol. 2019, 223, 1372–1387. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Cheong, J.-J. The AtMYB44 promoter is accessible to signals that induce different chromatin modifications for gene transcription. Plant Physiol. Biochem. 2018, 130, 14–19. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Jung, C.; Cheong, J.-J. Chromatin remodeling for the transcription of type 2C protein phosphatase genes in response to salt stress. Plant Physiol. Biochem. 2019, 141, 325–331. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, P.; Jing, H.; Zhou, X.F.; Zhao, B.; Li, Y.; Jin, J.B. JMJ27-mediated histone H3K9 demethylation positively regulates drought-stress responses in Arabidopsis. New Phytol. 2021, 232, 221–236. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Zhang, Q.; Zheng, Q.; Yao, H.; Gu, X.; Liu, D.; Tian, X.; Wang, X.; Li, Y.; et al. H3K36 demethylase JMJ710 negatively regulates drought tolerance by suppressing MYB48-1 expression in rice. Plant Physiol. 2022, 189, 1050–1064. [Google Scholar] [CrossRef]
- Zong, W.; Yang, J.; Fu, J.; Xiong, L. Synergistic regulation of drought-responsive genes by transcription factor OsbZIP23 and histone modification in rice. J. Integr. Plant Biol. 2020, 62, 723–729. [Google Scholar] [CrossRef]
- González, R.M.; Ricardi, M.M.; Iusem, N.D. Epigenetic marks in an adaptive water stress-responsive gene in tomato roots under normal and drought conditions. Epigenetics 2013, 8, 864–872. [Google Scholar] [CrossRef]
- González, R.M.; Ricardi, M.M.; Iusem, N.D. Atypical epigenetic mark in an atypical location: Cytosine methylation at asymmetric (CNN) sites within the body of a non-repetitive tomato gene. BMC Plant Biol. 2011, 11, 94. [Google Scholar] [CrossRef]
- Sani, E.; Herzyk, P.; Perrella, G.; Colot, V.; Amtmann, A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013, 14, 1–24. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Xue, S.; Quan, T.; Cui, D.; Han, L.; Cong, W.; Li, M.; Yun, D.J.; Liu, B.; et al. SET DOMAIN GROUP 721 protein functions in saline–alkaline stress tolerance in the model rice variety Kitaake. Plant Biotechnol. J. 2021, 19, 2576–2588. [Google Scholar] [CrossRef]
- Zhu, N.; Cheng, S.; Liu, X.; Du, H.; Dai, M.; Zhou, D.-X.; Yang, W.; Zhao, Y. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci. 2015, 236, 146–156. [Google Scholar] [CrossRef]
- Guo, P.; Chong, L.; Jiao, Z.; Xu, R.; Niu, Q.; Zhu, Y. Salt stress activates the CDK8-AHL10-SUVH2/9 module to dynamically regulate salt tolerance in Arabidopsis. Nat. Commun. 2025, 16, 2454. [Google Scholar] [CrossRef]
- Folsom, J.J.; Begcy, K.; Hao, X.; Wang, D.; Walia, H. Rice fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol. 2014, 165, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, M.; Shi, B.; Zhu, K.; Chen, J.; Xu, S.; Bie, X.; Zhang, X.; Lin, X.; Xiao, J. Distinct roles of H3K27me3 and H3K36me3 in vernalization response, maintenance, and resetting in winter wheat. Sci. China Life Sci. 2024, 67, 2251–2266. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, W.; Fan, C.; Sun, J.; Yuan, G.; Guo, Y.; Yu, X.; Chang, Y.; Liu, J.; Wang, C. Telo boxes within the AGAMOUS second intron recruit histone 3 lysine 27 methylation to increase petal number in rose (Rosa chinensis) in response to low temperatures. Plant J. 2024, 118, 1486–1499. [Google Scholar] [CrossRef]
- Wang, Z.; Zang, C.; Cui, K.; Schones, D.E.; Barski, A.; Peng, W.; Zhao, K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 2009, 138, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.Y.; Kim, M.-J.; Jeong, S.; Moon, S.Y.; Kim, J.S.; Jeon, J.; Lee, B.; Lee, M.R.; Kim, J. HIGH PLOIDY2-mediated SUMOylation of transcription factor ARR1 controls two-component signaling in Arabidopsis. Plant Cell 2024, 36, 3521–3542. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, Z.; Jin, L.; Qin, T.; Zhan, C.; Huang, J. Histone deacetylase OsHDA716 represses rice chilling tolerance by deacetylating OsbZIP46 to reduce its transactivation function and protein stability. Plant Cell 2024, 36, 1913–1936. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; He, D.; Zhou, Y.; Ni, H.; Tian, D.; Chang, G.; Jing, Y.; Lin, R.; Huang, J.; et al. AGAMOUS-LIKE67 cooperates with the histone mark reader EBS to modulate seed germination under high temperature. Plant Physiol. 2020, 184, 529–545. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, L.; Zheng, X.; Gautam, M.; Yue, M.; Hou, J.; Chen, Z.; Wang, P.; Li, L. Dynamic changes in histone modification are associated with upregulation of Hsf and rRNA genes during heat stress in maize seedlings. Protoplasma 2019, 256, 1245–1256. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, M.; Zheng, X.; Gautam, M.; He, S.; Li, L. The role of promoter-associated histone acetylation of Haem Oxygenase-1 (HO-1) and Giberellic Acid-Stimulated Like-1 (GSL-1) genes in heat-induced lateral root primordium inhibition in maize. Front. Plant Sci. 2018, 9, 1520. [Google Scholar] [CrossRef]
- Yang, F.; Sun, Y.; Du, X.; Chu, Z.; Zhong, X.; Chen, X. Plant-specific histone deacetylases associate with ARGONAUTE4 to promote heterochromatin stabilization and plant heat tolerance. New Phytol. 2023, 238, 252–269. [Google Scholar] [CrossRef]
- Hu, Z.; Song, N.; Zheng, M.; Liu, X.; Liu, Z.; Xing, J.; Ma, J.; Guo, W.; Yao, Y.; Peng, H.; et al. Histone acetyltransferase GCN 5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J. 2015, 84, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Yang, Y.; Feng, H.; Pan, Z.; Shen, W.H.; Zhu, Y.; Dong, A. Histone chaperone ASF1 is involved in gene transcription activation in response to heat stress in Arabidopsis thaliana. Plant Cell Environ. 2014, 37, 2128–2138. [Google Scholar] [CrossRef]
- Zhou, N.; Li, C.; Xie, W.; Liang, N.; Wang, J.; Wang, B.; Wu, J.; Shen, W.-H.; Liu, B.; Dong, A. Histone methylation readers MRG1/2 interact with PIF4 to promote thermomorphogenesis in Arabidopsis. Cell Rep. 2024, 43, 113726. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Lin, J.; Liu, X.; Wang, Z.; Xin, M.; Yao, Y.; Peng, H.; Zhou, D.X.; Ni, Z.; et al. Histone acetyltransferase GCN 5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 2019, 97, 587–602. [Google Scholar] [CrossRef]
- Li, H.; Yan, S.; Zhao, L.; Tan, J.; Zhang, Q.; Gao, F.; Wang, P.; Hou, H.; Li, L. Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, S.; Ozdemir, F.; Güler, A.; Bor, M. Histone acetylation influences the transcriptional activation of POX in Beta vulgaris L. and Beta maritima L. under salt stress. Plant Physiol. Biochem. 2016, 100, 37–46. [Google Scholar] [CrossRef]
- Liu, K.; Chen, J.; Sun, S.; Chen, X.; Zhao, X.; Hu, Y.; Qi, G.; Li, X.; Xu, B.; Miao, J.; et al. Histone deacetylase OsHDA706 increases salt tolerance via H4K5/K8 deacetylation of OsPP2C49 in rice. J. Integr. Plant Biol. 2023, 65, 1394–1407. [Google Scholar] [CrossRef]
- Gandhivel, V.H.-S.; Sotelo-Parrilla, P.; Raju, S.; Jha, S.; Gireesh, A.; Harshith, C.Y.; Gut, F.; Vinothkumar, K.R.; Berger, F.; Jeyaprakash, A.A.; et al. An Oryza-specific histone H4 variant predisposes H4 lysine 5 acetylation to modulate salt stress responses. Nat. Plants 2025, 11, 790–807. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, J.; Song, Y.; Chen, N.; Ni, B.; Zhang, J.; He, C. Histone H3K9 acetylation modulates gene expression of key enzymes in the flavonoid and abscisic acid pathways and enhances drought resistance of sea buckthorn. Physiol. Plant. 2023, 175, e13936. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Wang, S.; Wang, C.; Guo, M.; Song, Y.; Guo, J.; Yan, J.; Ma, F.; Guan, Q.; et al. HISTONE DEACETYLASE 6 interaction with ABSCISIC ACID-INSENSITIVE 5 decreases apple drought tolerance. Plant Physiol. 2023, 193, 2711–2733. [Google Scholar] [CrossRef]
- Żabka, A.; Gocek, N.; Winnicki, K.; Szczeblewski, P.; Laskowski, T.; Polit, J.T. Changes in epigenetic patterns related to DNA replication in Vicia faba root meristem cells under cadmium-induced stress conditions. Cells 2021, 10, 3409. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, M.; Xiao, X.; Yin, F.; Yao, Y.; Sui, M.; Hu, Y.; Xiang, Y.; Wang, L. Regulation of reactive oxygen molecules in pakchoi by histone acetylation modifications under Cd stress. PLoS ONE 2024, 19, e0314043. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Liao, J.; Liu, X.; Zhou, Y.; Qian, Y. Genome-wide identification of maize protein arginine methyltransferase genes and functional analysis of ZmPRMT1 reveal essential roles in Arabidopsis flowering regulation and abiotic stress tolerance. Int. J. Mol. Sci. 2022, 23, 12793. [Google Scholar] [CrossRef]
- Wang, Z.; Casas-Mollano, J.A.; Xu, J.; Riethoven, J.-J.M.; Zhang, C.; Cerutti, H. Osmotic stress induces phosphorylation of histone H3 at threonine 3 in pericentromeric regions of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 8487–8492. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Q.; Sun, Y.; Li, Y. Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ. 2017, 40, 1512–1530. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Li, X.; Li, Y. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. New Phytol. 2020, 227, 455–472. [Google Scholar] [CrossRef]
- Ma, S.; Tang, N.; Li, X.; Xie, Y.; Xiang, D.; Fu, J.; Shen, J.; Yang, J.; Tu, H.; Li, X.; et al. Reversible histone H2B monoubiquitination fine-tunes abscisic acid signaling and drought response in rice. Mol. Plant 2019, 12, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, Z.; Kong, D.; Duan, M.; Luo, L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010, 61, 4157–4168. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Wang, G.; Zhang, P.; Fu, J.; Wang, Z.; Wei, L.; Wang, T. Transcriptional regulatory networks in response to drought stress and rewatering in maize (Zea mays L.). Mol. Genet. Genom. MGG 2021, 296, 1203–1219. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Ci, D.; Song, Y.; Tian, M.; Zhang, D. Methylation of miRNA genes in the response to temperature stress in Populus simonii. Front. Plant Sci. 2015, 6, 921. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Abe, M. Regulation of reproductive development by non-coding RNA in Arabidopsis: To flower or not to flower. J. Plant Res. 2012, 125, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Moison, M.; Pacheco, J.M.; Lucero, L.; Fonouni-Farde, C.; Rodríguez-Melo, J.; Mansilla, N.; Christ, A.; Bazin, J.; Benhamed, M.; Ibañez, F.; et al. The lncRNA APOLO interacts with the transcription factor WRKY42 to trigger root hair cell expansion in response to cold. Mol. Plant 2021, 14, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Miskevish, F.; Lodeyro, A.; Ponso, M.A.; Bouzo, C.; Meeley, R.; Timmermans, M.C.; Dotto, M. Maize mutants in miR394-regulated genes show improved drought tolerance. Physiol. Plant. 2025, 177, e70155. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, K.; Long, W.; Zhao, L.; Li, W.; Xue, X.; Han, S. Cytosolic and Nucleosolic Calcium-Regulated Long Non-Coding RNAs and Their Target Protein-Coding Genes in Response to Hyperosmolarity and Salt Stresses in Arabidopsis thaliana. Int. J. Mol. Sci. 2025, 26, 2086. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Niu, F.; Sun, X.; Hu, Z.; Gao, F.; Zhang, H.; Jiang, Q. Overexpression of lncRNA77580 regulates drought and salinity stress responses in soybean. Plants 2023, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Hyun, T.K. Identification and functional prediction of drought-responsive long non-coding RNA in tomato. Agronomy 2019, 9, 629. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Deng, F.; Wang, W.; Cheng, Y.; Song, L.; Hu, M.; Shen, J.; Xu, Q.; Shen, F. The long non-coding RNA lncRNA973 is involved in cotton response to salt stress. BMC Plant Biol. 2019, 19, 459. [Google Scholar] [CrossRef]
- Chen, L.; Shi, S.; Jiang, N.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Peng, X.; Xu, J.; Chen, Y.; Yu, Q.; et al. Genome-wide analysis of long non-coding RNAs affecting roots development at an early stage in the rice response to cadmium stress. BMC Genom. 2018, 19, 460. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, K.; Sun, W.; Yang, T.; Wu, T.; Song, T.; Zhang, J.; Yao, Y.; Tian, J. A long noncoding RNA functions in high-light-induced anthocyanin accumulation in apple by activating ethylene synthesis. Plant Physiol. 2022, 189, 66–83. [Google Scholar] [CrossRef]
- Kiger, N.M.; Schroeder, S.J. SVALKA: A Long Noncoding Cis-Natural Antisense RNA That Plays a Role in the Regulation of the Cold Response of Arabidopsis thaliana. Non-Coding RNA 2024, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Zhang, H.; Wang, J.; Zinta, G.; Xie, S.; Zhu, W.; Nie, W.-F. Genome-wide identification of circular RNAs in response to low-temperature stress in tomato leaves. Front. Genet. 2020, 11, 591806. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Gao, S.; Zhang, H.; Li, B.; Zhong, H.; Wang, Y.; Hu, H.; Zhang, H.; Luo, B.; Zhang, X.; et al. Identification and characterization of circRNAs in maize seedlings under deficient nitrogen. Plant Biol. 2021, 23, 850–860. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Liu, H.; Liang, Z.; Zhang, M.; Zou, C.; Yuan, G.; Gao, S.; Pan, G.; Shen, Y.; et al. A combination of a genome-wide association study and a transcriptome analysis reveals circRNAs as new regulators involved in the response to salt stress in maize. Int. J. Mol. Sci. 2022, 23, 9755. [Google Scholar] [CrossRef]
- Yao, Y.; Ni, Z.; Peng, H.; Sun, F.; Xin, M.; Sunkar, R.; Zhu, J.-K.; Sun, Q. Non-coding small RNAs responsive to abiotic stress in wheat (Triticum aestivum L.). Funct. Integr. Genom. 2010, 10, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. Heat-induced tas1 target1 mediates thermotolerance via heat stress transcription factor A1a–directed pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Long, P.; Wang, C.; Liu, P.; Hou, F.; Zhang, M.; Zou, C.; Huang, Y.; Ma, L.; et al. A phased small interfering RNA-derived pathway mediates lead stress tolerance in maize. Plant Physiol. 2024, 196, 1163–1179. [Google Scholar] [CrossRef]
- Bäurle, I.; Trindade, I. Chromatin regulation of somatic abiotic stress memory. J. Exp. Bot. 2020, 71, 5269–5279. [Google Scholar] [CrossRef]
- Kovalchuk, I. Role of epigenetic factors in response to stress and establishment of somatic memory of stress exposure in plants. Plants 2023, 12, 3667. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; Vetukuri, R.R.; Testillano, P.S. Epigenetics for crop adaptation to climate change. Physiol. Plant. 2022, 174, e13835. [Google Scholar] [CrossRef]
- Pelayo, M.A.; Morishita, F.; Sawada, H.; Matsushita, K.; Iimura, H.; He, Z.; Looi, L.S.; Katagiri, N.; Nagamori, A.; Suzuki, A.; et al. AGAMOUS regulates various target genes via cell cycle–coupled H3K27me3 dilution in floral meristems and stamens. Plant Cell 2023, 35, 2821–2847. [Google Scholar] [CrossRef]
- Sedaghatmehr, M.; Thirumalaikumar, V.P.; Kamranfar, I.; Marmagne, A.; Masclaux-Daubresse, C.; Balazadeh, S. A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ. 2019, 42, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.M.; Almeida, L.A.d.H.; Santana-Vieira, D.D.S.; Freschi, L.; Ferreira, C.F.; Soares Filho, W.d.S.; Costa, M.G.C.; Micheli, F.; Coelho Filho, M.A.; Gesteira, A.d.S. Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci. Rep. 2017, 7, 13684. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, K.; Sun, Y.; Chen, B.; Pan, Z.; Wang, Z.; Pang, B.; He, S.; Miao, Y.; Du, X. Physiological and transcriptional analyses reveal formation of memory under recurring drought stresses in seedlings of cotton (Gossypium hirsutum). Plant Sci. 2024, 338, 111920. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Guedes, F.A.; Nobres, P.; Ferreira, D.C.R.; Menezes-Silva, P.E.; Ribeiro-Alves, M.; Correa, R.L.; DaMatta, F.M.; Alves-Ferreira, M. Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environ. Exp. Bot. 2018, 147, 220–233. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Transgenerational effects of water-deficit and heat stress on germination and seedling vigour—New insights from durum wheat microRNAs. Plants 2020, 9, 189. [Google Scholar] [CrossRef]
- Li, P.; Yang, H.; Wang, L.; Liu, H.; Huo, H.; Zhang, C.; Liu, A.; Zhu, A.; Hu, J.; Lin, Y.; et al. Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, G.; Peng, X.; Sun, F.; Liu, S.; Xi, Y. Long non-coding RNAs of switchgrass (Panicum virgatum L.) in multiple dehydration stresses. BMC Plant Biol. 2018, 18, 79. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Xiong, Y.; Moshood, A.Y.; Cadenas-Pliego, G.; Zhang, H.; Hu, J. Mechanisms of plant epigenetic regulation in response to plant stress: Recent discoveries and implications. Plants 2024, 13, 163. [Google Scholar] [CrossRef]
- Hewezi, T. Editorial: Epigenetic regulation of plant development and stress responses. Plant Cell Rep. 2018, 37, 1–2. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Huang, H.; Zhang, G.; He, L.; Pang, J.; Lozano-Durán, R.; Lang, Z.; Zhu, J.-K. Epigenetic memory marks determine epiallele stability at loci targeted by de novo DNA methylation. Nat. Plants 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Jullien, P.E.; Kinoshita, T.; Ohad, N.; Berger, F. Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 2006, 18, 1360–1372. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, Y. The functions of E (Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 2004, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-F.; Ibarra, C.A.; Silva, P.; Zemach, A.; Eshed-Williams, L.; Fischer, R.L.; Zilberman, D. Genome-wide demethylation of Arabidopsis endosperm. Science 2009, 324, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.K. Epigenetic gene regulation in plants and its potential applications in crop improvement. Nat. Rev. Mol. Cell Biol. 2025, 26, 51–67. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef]
- Liu, J.; Feng, L.; Gu, X.; Deng, X.; Qiu, Q.; Li, Q.; Zhang, Y.; Wang, M.; Deng, Y.; Wang, E. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019, 29, 379–390. [Google Scholar] [CrossRef]
- Morgado, L.; Preite, V.; Oplaat, C.; Anava, S.; Ferreira de Carvalho, J.; Rechavi, O.; Johannes, F.; Verhoeven, K.J.F. Small RNAs reflect grandparental environments in apomictic dandelion. Mol. Biol. Evol. 2017, 34, 2035–2040. [Google Scholar] [CrossRef]
| Species | Epigenetic Modifications | Stress | Key Proteins Involved | Reference |
|---|---|---|---|---|
| Arabidopsis thaliana | CG methylation | Cd | [77] | |
| Arabidopsis thaliana | 6mA | Col stress | [96] | |
| Arabidopsis thaliana | H3K4me3 | drought | OST1 | [100] |
| Arabidopsis thaliana | H3K4me3 | osmotic stress | AtMYB44, ABI1, ABI2, HAI1 | [101,102] |
| Arabidopsis thaliana | H3K9me2 | salt stress | salt-responsive genes | [111] |
| Arabidopsis thaliana | H3 acetylation | cold stress | ARR1 | [116] |
| Arabidopsis thaliana | H4K5ac | heat stress | SOM | [118] |
| Arabidopsis thaliana | Histone hyperacetylation | heat stress | HD2B, HD2C | [121] |
| Arabidopsis thaliana | H3K9ac, H3K14ac | heat stress | HSFA3, UVH6 | [122] |
| Arabidopsis thaliana | H3K56ac | heat stress | HsfA2, Hsa32 | [123] |
| Arabidopsis thaliana | H3K9ac, H3K14ac | high salt stress | CTL-1, PGX3/MYB54 | [125] |
| Arabidopsis thaliana | H3T3ph | osmotic stress | Knob region | [135] |
| Arabidopsis thaliana | H2Bub1 | salt stress | [136] | |
| Arabidopsis thaliana | H2Bub1 | salt stress | MYB42, MPK4 | [137] |
| Arabidopsis thaliana | COOLAIR, COLDAIR | low-temperature stress | FLC | [143] |
| Arabidopsis thaliana | APOLO | cold stress | RHD6 | [144] |
| Arabidopsis thaliana | SVALKA | low-temperature stress | CBF1 | [152] |
| Arabidopsis thaliana | TAS1 tasiRNAs | heat stress | HTT1, HTT2 | [157] |
| Beta vulgaris | H3K9ac, H3K27ac | salt stress | POX | [127] |
| Beta vulgaris L. | DNA methylation | salt stress | [85] | |
| Betula platyphylla Suk. | DNA methylation | heat stress | BpNST1/2, BpSND1 | [92] |
| Brassica napus | DNA methylation | PEG | BnP5CSA | [70] |
| Citrus | DNA methylation | salt stress | ACO1 | [87] |
| Cucumis sativus | DNA methylation | low-temperature stress | [90] | |
| Glycine max | DNA methylation | salt stress | [83] | |
| Glycine max | H3K4me3/H3K4me2 | salt stress | [83] | |
| Glycine max | lncRNA77580 | drought | [147] | |
| Gossypium hirsutum | lncRNA-973 | salt stress | [149] | |
| Hibiscus cannabinus L. | Cd | Cd | NPF2.7 | [76] |
| Hordeum vulgare | DNA methylation | water stress | [66] | |
| Hordeum vulgare | DNA demrthylation | heat stress | [94] | |
| Hordeum vulgare | DNA methylation | mild low-temperature stress | [94] | |
| Hordeum vulgare | H3K9ac, H3K4me3 | heat stress | [94] | |
| Lemna minor | CG and CHG methylation | heat stress | [93] | |
| Malus pumila Mill | Histone deacetylation | drought | [131] | |
| Malus pumila Mill | miR156, SPL13 | salt stress | MdWRKY100 | [141] |
| Malus pumila Mill | MdLNC610 | strong light | [151] | |
| Medicago ruthenica | DNA methylation | drought | [68] | |
| Medicago sativa | DNA methylation | drought | [69] | |
| Medicago sativa | DNA methylation | salt stress | [84] | |
| Oryza sativa | DNA methylation | drought | [63] | |
| Oryza sativa | DNA methylation | Hg | mercury resistance-related genes | [79] |
| Oryza sativa | DNA methylation | Cd | genes related to the plant cell wall and oxidative stress | [80] |
| Oryza sativa | CG methylation | low-temperature stress | NLR | [91] |
| Oryza sativa | 6mA | heat stress | [99] | |
| Oryza sativa | 6mA | salt stress | [99] | |
| Oryza sativa | 6mA | cold stress | [96] | |
| Oryza sativa | H3K36me3 | drought | MYB48-1 | [104] |
| Oryza sativa | H3K4me3/H3K27me3 | drought | dehydrin gene cluster | [105] |
| Oryza sativa | H3K34me3 | Saline–alkali stress | OsHKT1;5 | [109] |
| Oryza sativa | H3K27me3 | high salt stress | OsMYB91 | [110] |
| Oryza sativa | H3K27me3 | heat stress | OsMADS82, OsMADS87, AGL36 | [112] |
| Oryza sativa | deacetylase | cold stress | OsbZIP46 | [117] |
| Oryza sativa | H4K5 deacetylation, H4K8 deacetylation | salt stress | OsPP2C49 | [128] |
| Oryza sativa | H2Bub1 | drought | OsbZIP46, RAB21 | [138] |
| Pakchoi | Histone acetylation | Cd | [133] | |
| Phytolacca americana | DNA methylation | Mn/Cd | [75] | |
| Populus tomentosa | non-CG methylation | drought | [65] | |
| Rosa chinensis | H3K27me3 | low-temperature stress | RcAG | [114] |
| Sea buckthorn | H3K9ac | drought | [130] | |
| Solanum lycopersicum | CG methylation | Fe | SlPME53 | [74] |
| Solanum lycopersicum | DNA methylation | salt stress | SlDML1, SlDML3, SlDML4, SlHKT1, SlNHX1, SlSOS1 | [89] |
| Solanum lycopersicum | H3K9me2 | drought | Asr2 | [106] |
| Solanum lycopersicum | H3K27me3 | drought | Asr1 | [107] |
| Solanum lycopersicum | lncRNA535 | drought | [148] | |
| Triticum aestivum | DNA methylation | osmotic stress and salt stress | TaGAPC1 | [67] |
| Triticum aestivum | CG methylation | Pb/Cd/Zn | metal detoxification transporters | [78] |
| Triticum aestivum | H3K36me3 | cold stress | VRN1 | [113] |
| Triticum aestivum | nat-siRNA005047_0654_1904.1 | low-temperature stress | [156] | |
| Vicia faba | H3K56ac | Cd | [132] | |
| Zataria multiflora | DNA methylation | Cd | [82] | |
| Zea mays | DNA methylation | water stress | [64] | |
| Zea mays | H3K9ac | heat stress | ZmHsf-01, ZmHsf-15 | [119] |
| Zea mays | H3K9ac/H3K5ac | high salt stress | [126] | |
| Zea mays | zma-mir167e, zma- miR167j, zma-mir167f, miR5072, zma-mir529, miR397, miR6214 | drought | [140] | |
| Zea mays | tasiARF | Pb | [158] | |
| Zea mays | CHH methylation | salt stress | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Wang, M.; Wang, L.; Liu, Y.; Tian, Z.; Xie, L.; Wang, Y. Epigenetics in Plant Response to Climate Change. Biology 2025, 14, 631. https://doi.org/10.3390/biology14060631
Zhou W, Wang M, Wang L, Liu Y, Tian Z, Xie L, Wang Y. Epigenetics in Plant Response to Climate Change. Biology. 2025; 14(6):631. https://doi.org/10.3390/biology14060631
Chicago/Turabian StyleZhou, Wei, Min Wang, Lishan Wang, Yinghui Liu, Zaimin Tian, Linan Xie, and Yu Wang. 2025. "Epigenetics in Plant Response to Climate Change" Biology 14, no. 6: 631. https://doi.org/10.3390/biology14060631
APA StyleZhou, W., Wang, M., Wang, L., Liu, Y., Tian, Z., Xie, L., & Wang, Y. (2025). Epigenetics in Plant Response to Climate Change. Biology, 14(6), 631. https://doi.org/10.3390/biology14060631






