Lizards, Lineage and Latitude: Behavioural Responses to Microclimate Vary Latitudinally and Show Limited Acclimatisation to a Common Environment After Two Years
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Translocation
2.2. Fieldwork
2.3. Environmental Data
2.4. Statistical Analysis
3. Results
3.1. Environmental Variables
3.2. Principal Component Analysis
3.3. Overview
3.4. Wild Lizards
3.5. Translocated Lizards
4. Discussion
4.1. Conditions for Reduced Approach Distance
4.2. Conditions for Surface Activity
4.3. Relative Humidity in the Burrow
4.4. Acclimatisation to Translocation
4.5. Lineage Response to Microclimate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Woods, H.A.; Dillon, M.E.; Pincebourde, S. The Roles of Microclimatic Diversity and of Behavior in Mediating the Responses of Ectotherms to Climate Change. J. Therm. Biol. 2015, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Marshall, K.E.; Sewell, M.A.; Levesque, D.L.; Willett, C.S.; Slotsbo, S.; Dong, Y.; Harley, C.D.G.; Marshall, D.J.; Helmuth, B.S.; et al. Can We Predict Ectotherm Responses to Climate Change Using Thermal Performance Curves and Body Temperatures? Ecol. Lett. 2016, 19, 1372–1385. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; Blackburn, T.M.; Chown, S.L. Climatic Predictors of Temperature Performance Curve Parameters in Ectotherms Imply Complex Responses to Climate Change. Am. Nat. 2011, 177, 738–751. [Google Scholar] [CrossRef]
- Kefford, B.J.; Ghalambor, C.K.; Dewenter, B.; Poff, N.L.; Hughes, J.; Reich, J.; Thompson, R. Acute, Diel, and Annual Temperature Variability and the Thermal Biology of Ectotherms. Glob. Change Biol. 2022, 28, 6872–6888. [Google Scholar] [CrossRef]
- Pintor, A.F.V.; Schwarzkopf, L.; Krockenberger, A.K. Hydroregulation in a Tropical Dry-Skinned Ectotherm. Oecologia 2016, 182, 925–931. [Google Scholar] [CrossRef]
- Smith, A.L.; Gardner, M.G.; Fenner, A.L.; Bull, C.M. Restricted Gene Flow in the Endangered Pygmy Bluetongue Lizard (Tiliqua Adelaidensis) in a Fragmented Agricultural Landscape. Wildl. Res. 2009, 36, 466. [Google Scholar] [CrossRef]
- Miller, K.A.; Miller, H.C.; Moore, J.A.; Mitchell, N.J.; Cree, A.; Allendorf, F.W.; Sarre, S.D.; Keall, S.N.; Nelson, N.J. Securing the Demographic and Genetic Future of Tuatara through Assisted Colonization: Assisted Colonization of Tuatara. Conserv. Biol. 2012, 26, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Ram, M.; et al. The Conservation Status of the World’s Reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Clusella-Trullas, S.; Chown, S.L. Lizard Thermal Trait Variation at Multiple Scales: A Review. J. Comp. Physiol. B 2014, 184, 5–21. [Google Scholar] [CrossRef]
- Delean, S.; Bull, C.M.; Brook, B.W.; Heard, L.M.B.; Fordham, D.A. Using Plant Distributions to Predict the Current and Future Range of a Rare Lizard. Divers. Distrib. 2013, 19, 1125–1137. [Google Scholar] [CrossRef]
- Urban, M.C.; Richardson, J.L.; Freidenfelds, N.A. Plasticity and Genetic Adaptation Mediate Amphibian and Reptile Responses to Climate Change. Evol. Appl. 2014, 7, 88–103. [Google Scholar] [CrossRef]
- Chapple, D.G.; Roll, U.; Böhm, M.; Aguilar, R.; Amey, A.P.; Austin, C.C.; Baling, M.; Barley, A.J.; Bates, M.F.; Bauer, A.M.; et al. Conservation Status of the World’s Skinks (Scincidae): Taxonomic and Geographic Patterns in Extinction Risk. Biol. Conserv. 2021, 257, 109101. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral Syndromes: An Ecological and Evolutionary Overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, Â.M.; Lloyd, P.; Bowie, R.C.K. A Tight Balance between Natural Selection and Gene Flow in a Southern African Arid-Zone Endemic Bird: Spatial Heterogeneous Environments and Gene Flow. Evolution 2011, 65, 3499–3514. [Google Scholar] [CrossRef]
- Pavlova, A.; Amos, J.N.; Joseph, L.; Loynes, K.; Austin, J.J.; Keogh, J.S.; Stone, G.N.; Nicholls, J.A.; Sunnucks, P. Perched at the Mito-Nuclear Crossroads: Divergent Mitochondrial Lineages Correlate with Environment in the Face of Ongoing Nuclear Gene Flow in an Australian Bird. Evolution 2013, 67, 3412–3428. [Google Scholar] [CrossRef]
- Campbell-Staton, S.C.; Bare, A.; Losos, J.B.; Edwards, S.V.; Cheviron, Z.A. Physiological and Regulatory Underpinnings of Geographic Variation in Reptilian Cold Tolerance across a Latitudinal Cline. Mol. Ecol. 2018, 27, 2243–2255. [Google Scholar] [CrossRef]
- Trewartha, D.M.; Clayton, J.L.; Godfrey, S.S.; Gardner, M.G. Heat Water and Reptiles—Do the Hydro-thermal Properties of Animals at the Source Location Persist at the Translocation Site? Anim. Conserv. 2024, 28, 33–48. [Google Scholar] [CrossRef]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural Effects of Temperature on Ectothermic Animals: Unifying Thermal Physiology and Behavioural Plasticity: Effects of Temperature on Animal Behaviour. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Neilson, K.A. Evaporative Water Loss as a Restriction on Habitat Use in Endangered New Zealand Endemic Skinks. J. Herpetol. 2002, 36, 342–348. [Google Scholar] [CrossRef]
- Cowles, R.B.; Bogert, C.M. A Preliminary Study of the Thermal Requirements of Desert Reptiles. Q. Rev. Biol. 1945, 20, 170. [Google Scholar] [CrossRef]
- Stevenson, R.D. The Relative Importance of Behavioral and Physiological Adjustments Controlling Body Temperature in Terrestrial Ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic Niche Modelling: Combining Physiological and Spatial Data to Predict Species’ Ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef]
- Leal, M.; Gunderson, A.R. Rapid Change in the Thermal Tolerance of a Tropical Lizard. Am. Nat. 2012, 180, 815–822. [Google Scholar] [CrossRef]
- Sinervo, B.; Lara-Reséndiz, R.A.; Miles, D.B.; Lovich, J.E.; Rosen, P.C.; Gadsden, H.; Gaytán, G.C.; Tessaro, P.G.; Luja, V.H.; Huey, R.B.; et al. Climate Change and Collapsing Thermal Niches of Desert Reptiles and Amphibians: Assisted Migration and Acclimation Rescue from Extirpation. Sci. Total Environ. 2024, 908, 168431. [Google Scholar] [CrossRef]
- Wild, K.H.; Huey, R.B.; Pianka, E.R.; Clusella-Trullas, S.; Gilbert, A.L.; Miles, D.B.; Kearney, M.R. Climate Change and the Cost-of-Living Squeeze in Desert Lizards. Science 2025, 387, 303–309. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Diamond, S.E.; Buckley, L.B. Heat Stress and the Fitness Consequences of Climate Change for Terrestrial Ectotherms. Funct. Ecol. 2013, 27, 1415–1423. [Google Scholar] [CrossRef]
- Daltry, J.C.; Ross, T.; Thorpe, R.S.; Wuster, W. Evidence That Humidity Influences Snake Activity Patterns: A Field Study of the Malayan Pit Viper Calloselasma rhodostoma. Ecography 1998, 21, 25–34. [Google Scholar] [CrossRef]
- Kearney, M.; Munns, S.L.; Moore, D.; Malishev, M.; Bull, C.M. Field Tests of a General Ectotherm Niche Model Show How Water Can Limit Lizard Activity and Distribution. Ecol. Monogr. 2018, 88, 672–693. [Google Scholar] [CrossRef]
- Le Galliard, J.-F.; Rozen-Rechels, D.; Lecomte, A.; Demay, C.; Dupoué, A.; Meylan, S. Short-Term Changes in Air Humidity and Water Availability Weakly Constrain Thermoregulation in a Dry-Skinned Ectotherm. PLoS ONE 2021, 16, e0247514. [Google Scholar] [CrossRef]
- Stamps, J.A. Rainfall, Activity and Social Behaviour in the Lizard, Anolis aeneus. Anim. Behav. 1976, 24, 603–608. [Google Scholar] [CrossRef]
- Crowley, S.R. The Effect of Desiccation upon the Preferred Body Temperature and Activity Level of the Lizard Sceloporus Undulatus. Copeia 1987, 1987, 25. [Google Scholar] [CrossRef]
- Jones, S.M.; Waldschmidt, S.R.; Potvin, M.A. An Experimental Manipulation of Food and Water: Growth and Time-Space Utilization of Hatchling Lizards (Sceloporus undulatus). Oecologia 1987, 73, 53–59. [Google Scholar] [CrossRef]
- Lorenzon, P.; Clobert, J.; Oppliger, A.; John-Alder, H. Effect of Water Constraint on Growth Rate, Activity and Body Temperature of Yearling Common Lizard (Lacerta vivipara). Oecologia 1999, 118, 423–430. [Google Scholar] [CrossRef]
- Kerr, G.D.; Bull, C.M. Field Observations of Extended Locomotor Activity at Sub-Optimal Body Temperatures in a Diurnal Heliothermic Lizard (Tiliqua Rugosa). J. Zool. 2004, 264, 179–188. [Google Scholar] [CrossRef]
- Sannolo, M.; Carretero, M.A. Dehydration Constrains Thermoregulation and Space Use in Lizards. PLoS ONE 2019, 14, e0220384. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Rutschmann, A.; DupouÉ, A.; Blaimont, P.; Chauveau, V.; Miles, D.B.; Guillon, M.; Richard, M.; Badiane, A.; Meylan, S.; et al. Interaction of Hydric and Thermal Conditions Drive Geographic Variation in Thermoregulation in a Widespread Lizard. Ecol. Monogr. 2021, 91, e01440. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations, version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; ISBN 978-2-8317-1609-1.
- Germano, J.M.; Bishop, P.J. Suitability of Amphibians and Reptiles for Translocation. Conserv. Biol. 2009, 23, 7–15. [Google Scholar] [CrossRef]
- Besson, A.A.; Cree, A. Integrating Physiology into Conservation: An Approach to Help Guide Translocations of a Rare Reptile in a Warming Environment: Thermal Biology and Conservation of Tuatara. Anim. Conserv. 2011, 14, 28–37. [Google Scholar] [CrossRef]
- Chauvenet, A.L.M.; Ewen, J.G.; Armstrong, D.P.; Blackburn, T.M.; Pettorelli, N. Maximizing the Success of Assisted Colonizations: Maximizing the Success of Assisted Colonization. Anim. Conserv. 2013, 16, 161–169. [Google Scholar] [CrossRef]
- Batson, W.G.; Gordon, I.J.; Fletcher, D.B.; Manning, A.D. REVIEW: Translocation Tactics: A Framework to Support the IUCN Guidelines for Wildlife Translocations and Improve the Quality of Applied Methods. J. Appl. Ecol. 2015, 52, 1598–1607. [Google Scholar] [CrossRef]
- Besson, A.A.; Cree, A. A Cold-Adapted Reptile Becomes a More Effective Thermoregulator in a Thermally Challenging Environment. Oecologia 2010, 163, 571–581. [Google Scholar] [CrossRef]
- Jetz, W.; Ashton, K.G.; La Sorte, F.A. Phenotypic Population Divergence in Terrestrial Vertebrates at Macro Scales. Ecol. Lett. 2009, 12, 1137–1146. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ebrahimie, E.; Bull, C.M. Minimizing the Cost of Translocation Failure with Decision-Tree Models That Predict Species’ Behavioral Response in Translocation Sites: Species Behavior and Decision-Tree Models. Conserv. Biol. 2015, 29, 1208–1216. [Google Scholar] [CrossRef]
- Rummel, L.; Martínez–Abraín, A.; Mayol, J.; Ruiz-Olmo, J.; Mañas, F.; Jiménez, J.; Gómez, J.A.; Oro, D. Use of Wild–Caught Individuals as a Key Factor for Success in Vertebrate Translocations. Anim. Biodiv. Conserv. 2016, 39, 207–219. [Google Scholar] [CrossRef]
- Caldwell, A.J.; While, G.M.; Wapstra, E. Plasticity of Thermoregulatory Behaviour in Response to the Thermal Environment by Widespread and Alpine Reptile Species. Anim. Behav. 2017, 132, 217–227. [Google Scholar] [CrossRef]
- Fordham, D.A.; Watts, M.J.; Delean, S.; Brook, B.W.; Heard, L.M.B.; Bull, C.M. Managed Relocation as an Adaptation Strategy for Mitigating Climate Change Threats to the Persistence of an Endangered Lizard. Glob. Change Biol. 2012, 18, 2743–2755. [Google Scholar] [CrossRef]
- Bulova, S.J. Ecological Correlates of Population and Individual Variation in Antipredator Behavior of Two Species of Desert Lizards. Copeia 1994, 1994, 980. [Google Scholar] [CrossRef]
- Zani, P.A.; Tillman, J.L.; Scoular, K.M. Geographic Variation of Movement and Display Behavior of Side-Blotched Lizards (Uta stansburiana) Related to Predation Environment. J. Herpetol. 2013, 47, 85–92. [Google Scholar] [CrossRef]
- Monasterio, C.; Shoo, L.P.; Salvador, A.; Siliceo, I.; Díaz, J.A. Thermal Constraints on Embryonic Development as a Proximate Cause for Elevational Range Limits in Two Mediterranean Lacertid Lizards. Ecography 2011, 34, 1030–1039. [Google Scholar] [CrossRef]
- Ward-Fear, G.; Brown, G.P.; Pearson, D.J.; West, A.; Rollins, L.A.; Shine, R. The Ecological and Life History Correlates of Boldness in Free-Ranging Lizards. Ecosphere 2018, 9, e02125. [Google Scholar] [CrossRef]
- Ma, L.; Sun, B.; Cao, P.; Li, X.; Du, W. Phenotypic Plasticity May Help Lizards Cope with Increasingly Variable Temperatures. Oecologia 2018, 187, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Milne, T.; Bull, C.M. Burrow Choice by Individuals of Different Sizes in the Endangered Pygmy Blue Tongue Lizard Tiliqua adelaidensis. Biol. Conserv. 2000, 95, 295–301. [Google Scholar] [CrossRef]
- Souter, N.J.; Bull, C.M.; Lethbridge, M.R.; Hutchinson, M.N. Habitat Requirements of the Endangered Pygmy Bluetongue Lizard, Tiliqua adelaidensis. Biol. Conserv. 2007, 135, 33–45. [Google Scholar] [CrossRef]
- Pettigrew, M.; Bull, C.M. The Impact of Heavy Grazing on Burrow Choice in the Pygmy Bluetongue Lizard, Tiliqua adelaidensis. Wildl. Res. 2011, 38, 299. [Google Scholar] [CrossRef]
- Clayton, J.; Bull, C.M. The Impact of Sheep Grazing on Burrows for Pygmy Bluetongue Lizards and on Burrow Digging Spiders: Grazing Impact on Spider Burrow Dynamics. J. Zool. 2015, 297, 44–53. [Google Scholar] [CrossRef]
- Cooper, W. Pursuit Deterrence, Predation Risk, and Escape in the Lizard Callisaurus draconoides. Behav. Ecol. Sociobiol. 2011, 65, 1833. [Google Scholar] [CrossRef]
- Maxim Integrated. DS1923 iButton Hygrochron Temperature/Humidity Logger with 8KB Data-Log Memory|Maxim Integrated. Available online: https://www.maximintegrated.com/en/products/ibutton-one-wire/data-loggers/DS1923.html (accessed on 3 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Muff, S.; Nilsen, E.B.; O’Hara, R.B.; Nater, C.R. Rewriting Results Sections in the Language of Evidence. Trends Ecol. Evol. 2022, 37, 203–210. [Google Scholar] [CrossRef]
- Muff, S.; Nilsen, E.B.; O’Hara, R.B.; Nater, C.R. Response to ‘Why P Values Are Not Measures of Evidence’ by D. Lakens. Trends Ecol. Evol. 2022, 37, 291–292. [Google Scholar] [CrossRef]
- Grimm-Seyfarth, A.; Mihoub, J.; Gruber, B.; Henle, K. Some like It Hot: From Individual to Population Responses of an Arboreal Arid-zone Gecko to Local and Distant Climate. Ecol. Monogr. 2018, 88, 336–352. [Google Scholar] [CrossRef]
- Doody, J.S.; McGlashan, J.; Fryer, H.; Coleman, L.; James, H.; Soennichsen, K.; Rhind, D.; Clulow, S. Plasticity in Nest Site Choice Behavior in Response to Hydric Conditions in a Reptile. Sci. Rep. 2020, 10, 16048. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Ma, L.; Wang, Y.; Wu, D.; Du, W.; Sun, B. Temperate and Tropical Lizards Are Vulnerable to Climate Warming Due to Increased Water Loss and Heat Stress. Proc. R. Soc. B. 2022, 289, 20221074. [Google Scholar] [CrossRef]
- Pintor, A.F.V.; Schwarzkopf, L.; Krockenberger, A.K. Extensive Acclimation in Ectotherms Conceals Interspecific Variation in Thermal Tolerance Limits. PLoS ONE 2016, 11, e0150408. [Google Scholar] [CrossRef] [PubMed]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J. When Water Interacts with Temperature: Ecological and Evolutionary Implications of Thermo-hydroregulation in Terrestrial Ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, G.W. Specialists and Generalists in Changing Environments. I. Fitness Landscapes of Thermal Sensitivity. Am. Nat. 1995, 146, 252–270. [Google Scholar] [CrossRef]
- Cruz, F.B.; Fitzgerald, L.A.; Espinoza, R.E.; Schulte Ii, J.A. The Importance of Phylogenetic Scale in Tests of Bergmann’s and Rapoport’s Rules: Lessons from a Clade of South American Lizards: Bergmann’s and Rapoport’s Rules in Lizards. J. Evol. Biol. 2005, 18, 1559–1574. [Google Scholar] [CrossRef]
- Treilibs, C.E.; Pavey, C.R.; Raghu, S.; Bull, M.C. Weather Correlates of Temporal Activity Patterns in a Desert Lizard: Insights for Designing More Effective Surveys. J. Zool. 2016, 300, 281–290. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W.P. Mapping the Fundamental Niche: Physiology, Climate, and the Distribution of a Nocturnal Lizard. Ecology 2004, 85, 3119–3131. [Google Scholar] [CrossRef]
- Paget, S.; Gleiss, A.C.; Kuchling, G.; Mitchell, N.J. Activity of a Freshwater Turtle Varies across a Latitudinal Gradient: Implications for the Success of Assisted Colonisation. Funct. Ecol. 2023, 37, 1897–1909. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, J.A.; Merilä, J. Climate Change and Evolution: Disentangling Environmental and Genetic Responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Al-Sadoon, K.; Spellerberg, F. Comparison of Thermal Acclimation Effects on the Metabolism of Chalcides ocellatus (Desert Lizard) and Lacerta vivipara (Cool-Temperate Lizard). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1985, 81, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Ferri-Yáñez, F.; Bozinovic, F.; Marquet, P.A.; Valladares, F.; Chown, S.L. Heat Freezes Niche Evolution. Ecol. Lett. 2013, 16, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Herrando-Pérez, S.; Ferri-Yáñez, F.; Monasterio, C.; Beukema, W.; Gomes, V.; Belliure, J.; Chown, S.L.; Vieites, D.R.; Araújo, M.B. Intraspecific Variation in Lizard Heat Tolerance Alters Estimates of Climate Impact. J. Anim. Ecol. 2019, 88, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B.; Mendez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagran-Santa Cruz, M.; Lara-Reséndiz, R.; Martinez-Mendez, N.; Calderon-Espinosa, M.L.; Meza-Lazaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, J.A.M.; Jess, M.; Williams, S.E. Predicting Organismal Vulnerability to Climate Warming: Roles of Behaviour, Physiology and Adaptation. Philos. Trans. R. Soc. B 2012, 367, 1665–1679. [Google Scholar] [CrossRef]
- Muñoz, M.M.; Langham, G.M.; Brandley, M.C.; Rosauer, D.F.; Williams, S.E.; Moritz, C. Basking Behavior Predicts the Evolution of Heat Tolerance in Australian Rainforest Lizards: Physiological Evolution in Australian Skinks. Evolution 2016, 70, 2537–2549. [Google Scholar] [CrossRef]
- Campbell-Staton, S.C.; Edwards, S.V.; Losos, J.B. Climate-Mediated Adaptation after Mainland Colonization of an Ancestrally Subtropical Island Lizard, Anolis carolinensis. J. Evol. Biol. 2016, 29, 2168–2180. [Google Scholar] [CrossRef]
- Senior, A.F.; Atkins, Z.S.; Clemann, N.; Gardner, M.G.; Schroder, M.; While, G.M.; Wong, B.B.M.; Chapple, D.G. Variation in Thermal Biology of Three Closely Related Lizard Species along an Elevation Gradient. Biol. J. Linn. Soc. 2019, 127, 278–291. [Google Scholar] [CrossRef]
- Sinervo, B. Evolution of Thermal Physiology and Growth Rate between Populations of the Western Fence Lizard (Sceloporus occidentalis). Oecologia 1990, 83, 228–237. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Tang, X.-L.; Yue, F.; Chen, Z.; Li, R.-D.; Chen, Q. Effect of Gestation Temperature on Sexual and Morphological Phenotypes of Offspring in a Viviparous Lizard, Eremias multiocellata. J. Therm. Biol. 2010, 35, 129–133. [Google Scholar] [CrossRef]
- Tang, X.-L.; Yue, F.; Yan, X.-F.; Zhang, D.-J.; Xin, Y.; Wang, C.; Chen, Q. Effects of Gestation Temperature on Offspring Sex and Maternal Reproduction in a Viviparous Lizard (Eremias multiocellata) Living at High Altitude. J. Therm. Biol. 2012, 37, 438–444. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Eplanova, G.V.; Kotenko, T.I.; Amat, F.; Carretero, M.A.; Kuranova, V.N.; Bulakhova, N.A.; Zinenko, O.I.; Yakovlev, V.A. Geographic Variation of Life-History Traits in the Sand Lizard, Lacerta agilis: Testing Darwin’s Fecundity-Advantage Hypothesis. J. Evol. Biol. 2015, 28, 613–629. [Google Scholar] [CrossRef] [PubMed]
- While, G.M.; Williamson, J.; Prescott, G.; Horváthová, T.; Fresnillo, B.; Beeton, N.J.; Halliwell, B.; Michaelides, S.; Uller, T. Adaptive Responses to Cool Climate Promotes Persistence of a Non-Native Lizard. Proc. R. Soc. B 2015, 282, 20142638. [Google Scholar] [CrossRef]
- Shine, R.; Wapstra, E.; Olsson, M. Seasonal Shifts along the Oviparity-Viviparity Continuum in a Cold-Climate Lizard Population. J. Evol. Biol. 2018, 31, 4–13. [Google Scholar] [CrossRef]
- Smith, G.D.; Zani, P.A.; French, S.S. Life-history Differences across Latitude in Common Side-blotched Lizards (Uta stansburiana). Ecol. Evol. 2019, 9, 5743–5751. [Google Scholar] [CrossRef]
- Llewelyn, J.; Macdonald, S.L.; Moritz, C.; Martins, F.; Hatcher, A.; Phillips, B.L. Adjusting to Climate: Acclimation, Adaptation and Developmental Plasticity in Physiological Traits of a Tropical Rainforest Lizard. Integr. Zool. 2018, 13, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Cadby, C.D.; Jones, S.M.; Wapstra, E. Geographical Differences in Maternal Basking Behaviour and Offspring Growth Rate in a Climatically Widespread Viviparous Reptile. J. Exp. Biol. 2014, 217, 1175–1179. [Google Scholar] [CrossRef]
- Niewiarowski, P.H.; Roosenburg, W. Reciprocal Transplant Reveals Sources of Variation in Growth Rates of the Lizard Sceloporus undulatus. Ecology 1993, 74, 1992–2002. [Google Scholar] [CrossRef]
- McDonald, S.; Schwanz, L.E. Thermal Parental Effects on Offspring Behaviour and Their Fitness Consequences. Anim. Behav. 2018, 135, 45–55. [Google Scholar] [CrossRef]
- Frankham, R. Genetic Rescue Benefits Persist to at Least the F3 Generation, Based on a Meta-Analysis. Biol. Conserv. 2016, 195, 33–36. [Google Scholar] [CrossRef]
- Paranjpe, D.A.; Bastiaans, E.; Patten, A.; Cooper, R.D.; Sinervo, B. Evidence of Maternal Effects on Temperature Preference in Side-Blotched Lizards: Implications for Evolutionary Response to Climate Change. Ecol. Evol. 2013, 3, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Artacho, P.; Saravia, J.; Perret, S.; Bartheld, J.L.; Le Galliard, J.-F. Geographic Variation and Acclimation Effects on Thermoregulation Behavior in the Widespread Lizard Liolaemus pictus. J. Therm. Biol. 2017, 63, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Shine, R.; Brown, G.P. Adapting to the Unpredictable: Reproductive Biology of Vertebrates in the Australian Wet–Dry Tropics. Philos. Trans. R. Soc. B 2008, 363, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, C.A.; Christian, K.A.; James, C.D.; Morton, S.R. Seven Lizard Species and a Blind Snake: Activity, Body Condition and Growth of Desert Herpetofauna in Relation to Rainfall. Aust. J. Zool. 2010, 58, 273. [Google Scholar] [CrossRef]
- Treilibs, C.E.; Pavey, C.R.; Gardner, M.G.; Ansari, M.H.; Bull, C.M. Spatial Dynamics and Burrow Occupancy in a Desert Lizard Floodplain Specialist, Liopholis slateri. J. Arid Environ. 2019, 167, 8–17. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Bradshaw, C.J.A.; Dickman, C.R.; Hobbs, R.; Johnson, C.N.; Johnston, E.L.; Laurance, W.F.; Lindenmayer, D.; McCarthy, M.A.; Nimmo, D.G.; et al. Continental-Scale Governance and the Hastening of Loss of Australia’s Biodiversity: Editorial. Conserv. Biol. 2013, 27, 1133–1135. [Google Scholar] [CrossRef]
- Geyle, H.M.; Tingley, R.; Amey, A.P.; Cogger, H.; Couper, P.J.; Cowan, M.; Craig, M.D.; Doughty, P.; Driscoll, D.A.; Ellis, R.J.; et al. Reptiles on the Brink: Identifying the Australian Terrestrial Snake and Lizard Species Most at Risk of Extinction. Pac. Conserv. Biol. 2020, 27, 3–12. [Google Scholar] [CrossRef]
- Germano, J.; Ewen, J.G.; Mushinsky, H.; McCoy, E.; Ortiz-Catedral, L. Moving towards Greater Success in Translocations: Recent Advances from the Herpetofauna: Greater Success in Translocations. Anim. Conserv. 2014, 17, 1–3. [Google Scholar] [CrossRef]

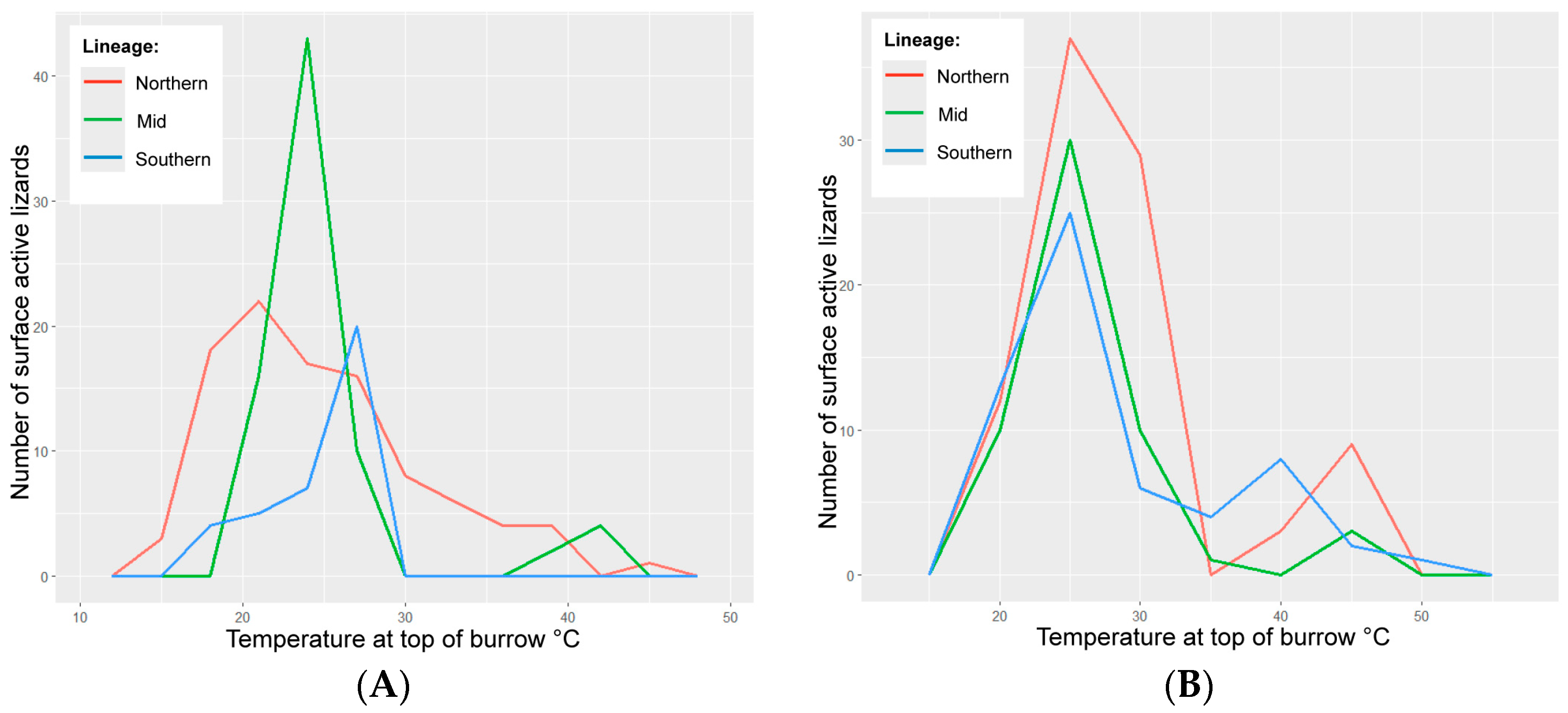
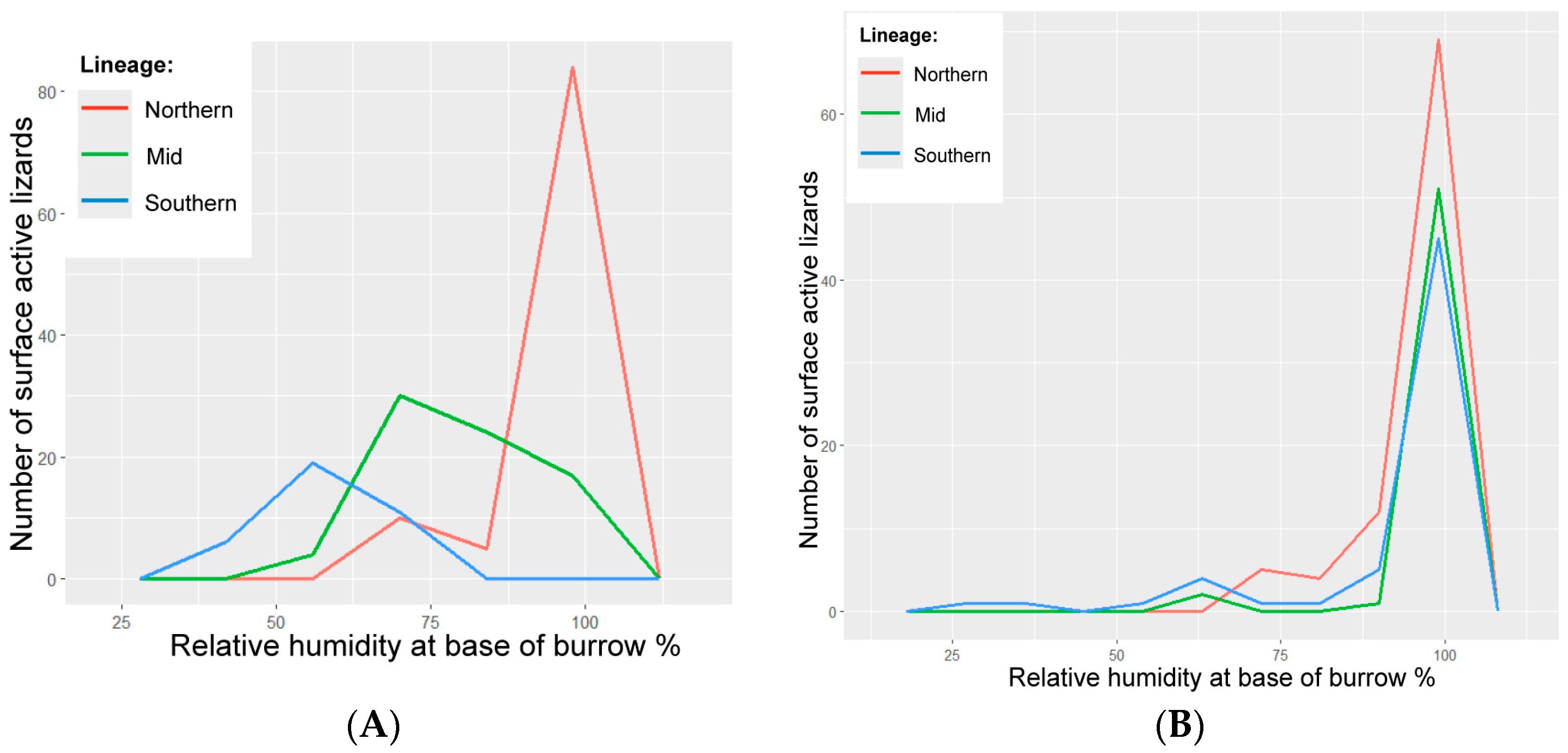


| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Mean ambient temperature | 0.36 | −0.50 | −0.09 |
| Mean ambient relative humidity | −0.36 | 0.46 | 0.13 |
| Mean top-of-burrow temperature | 0.42 | −0.18 | 0.00 |
| Mean top-of-burrow relative humidity | −0.40 | −0.01 | 0.02 |
| Mean mid-burrow temperature | 0.40 | 0.30 | 0.15 |
| Mean mid-burrow relative humidity | −0.32 | −0.44 | 0.16 |
| Mean base-of-burrow temperature | 0.36 | 0.44 | 0.22 |
| Mean base-of-burrow humidity | 0.00 | −0.19 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trewartha, D.M.; Godfrey, S.S.; Gardner, M.G. Lizards, Lineage and Latitude: Behavioural Responses to Microclimate Vary Latitudinally and Show Limited Acclimatisation to a Common Environment After Two Years. Biology 2025, 14, 622. https://doi.org/10.3390/biology14060622
Trewartha DM, Godfrey SS, Gardner MG. Lizards, Lineage and Latitude: Behavioural Responses to Microclimate Vary Latitudinally and Show Limited Acclimatisation to a Common Environment After Two Years. Biology. 2025; 14(6):622. https://doi.org/10.3390/biology14060622
Chicago/Turabian StyleTrewartha, Deanne M., Stephanie S. Godfrey, and Michael G. Gardner. 2025. "Lizards, Lineage and Latitude: Behavioural Responses to Microclimate Vary Latitudinally and Show Limited Acclimatisation to a Common Environment After Two Years" Biology 14, no. 6: 622. https://doi.org/10.3390/biology14060622
APA StyleTrewartha, D. M., Godfrey, S. S., & Gardner, M. G. (2025). Lizards, Lineage and Latitude: Behavioural Responses to Microclimate Vary Latitudinally and Show Limited Acclimatisation to a Common Environment After Two Years. Biology, 14(6), 622. https://doi.org/10.3390/biology14060622





