Recent Advances on Chitosan-Based Thermosensitive Hydrogels for Skin Wound Treatment
Simple Summary
Abstract
1. Introduction
2. The Sol–Gel Phase-Transformation Mechanism of Thermosensitive Injectable Hydrogels
2.1. Molecular Level
2.2. Thermodynamic Angle
3. Wound-Repair Process
3.1. Hemostasis
3.2. Inflammation
3.3. Proliferation
3.4. Remodeling
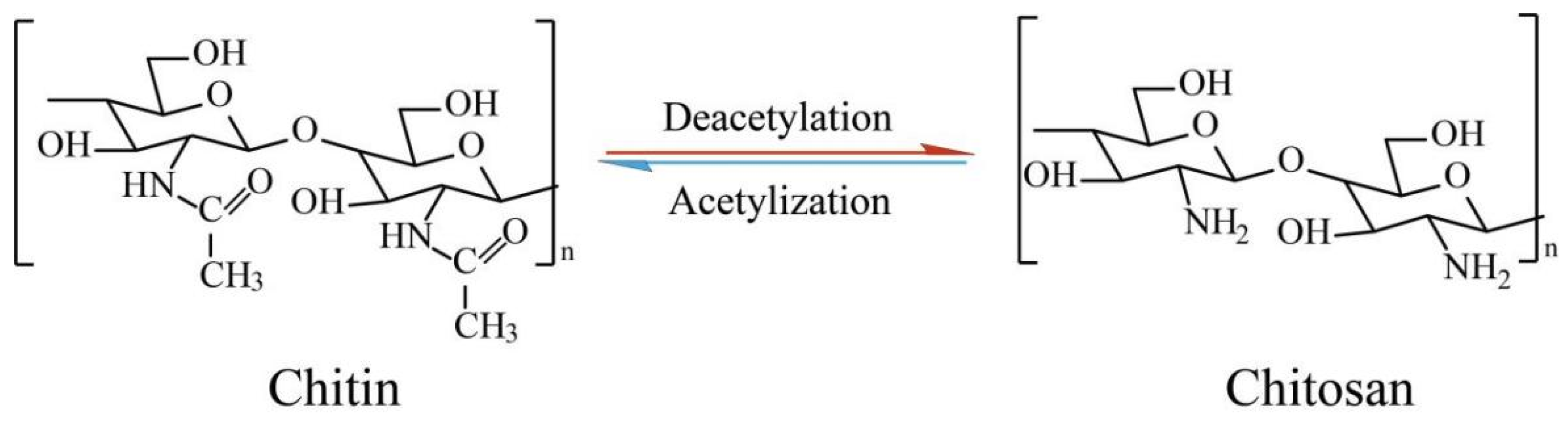
4. Chitosan-Based Thermosensitive Hydrogels
4.1. Chitosan–Sodium Glycerophosphate Thermosensitive Gel
4.2. Hydroxybutyl Chitosan Hydrogel
4.3. Chitosan/Polysol-Polymer Hydrogel
4.4. Chitosan/Amphiphilic Polymer Hydrogel
4.5. Chitosan/Alkaline Inorganic Salt Hydrogels
5. The Application of Chitosan-Based Thermosensitive Hydrogels in the Treatment of Different Types of Wounds
5.1. Infected Wounds
5.2. Burn Wounds
5.3. Diabetic Wounds
5.4. Surgical Wounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bi, M.L.; Qin, Y.H.; Wang, L.R.; Zhang, J. The protective role of resveratrol in diabetic wound healing. Phytother. Res. 2023, 37, 5193–5204. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Huang, X.H.; Lin, L.C.; Jiao, Y.P.; Zhou, C.R.; Liu, Z.H. Polyphenol and Cu2+ surface-modified chitin sponge synergizes with antibacterial, antioxidant and pro-vascularization activities for effective scarless regeneration of burned skin. Chem. Eng. J. 2021, 419, 129488. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound dressing modifications for accelerated healing of infected wounds. Int. J. Mol. Sci. 2023, 24, 7193. [Google Scholar] [CrossRef]
- Guo, J.; Shu, X.; Deng, H.; Zhang, J.; Wang, Y.; Meng, G.; He, J.; Wu, F. Stiff and tough hydrogels prepared through integration of ionic cross-linking and enzymatic mineralization. Acta. Biomater. 2022, 149, 220–232. [Google Scholar] [CrossRef]
- Pu, X.; Tong, L.; Wang, X.; Liu, Q.; Chen, M.; Li, X.; Lu, G.; Lan, W.; Li, Q.; Liang, J.; et al. Bioinspired hydrogel anchoring 3DP GelMA/HAP scaffolds accelerates bone reconstruction. ACS Appl. Mater. Interfaces 2022, 14, 20591–20602. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, F.; He, Y.; Wang, F.; Xia, D.; Liu, Y. Multifunctional hydrogel with photothermal ROS scavenging and antibacterial activity accelerates diabetic wound healing. Adv. Healthc. Mater. 2025, 14, 199–217. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Ouyang, Y.L.; Fan, H.W.; Zhang, L.Y.; Wang, S.G.; Zeng, Y.B.; Hu, L.H.; Zhao, J.L. Molybdesum selenide-based platelet-rich plasma containing carboxymethyl chitosan/polyvinyl pyrrolidone composite antioxidant hydrogels dressing promotes the wound healing. J. Nanobiotechnol. 2024, 22, 217. [Google Scholar] [CrossRef]
- Cao, B.; Wang, C.J.; Guo, P.Q.; Zhang, Q.; Wang, C.T.; Sun, H.H.; Wen, H.Y.; Chen, X.; Wang, Y.R.; Wang, Y.; et al. Photo-crosslinked enhanced double-network hydrogels based on modified gelatin and oxidized sodium alginate for diabetic wound healing. Int. J. Biol. Macromol. 2023, 245, 125528. [Google Scholar] [CrossRef]
- Basit, A.; Yu, H.J.; Wang, L.; Uddin, M.A.; Wang, Y.; Awan, K.M.; Keshta, B.E.; Malik, M.O. Recent advances in wet surface tissue adhesive hydrogels for wound treatment. Eur. Polym. J. 2024, 216, 113260. [Google Scholar] [CrossRef]
- Yang, M.W.; Fei, X.; Tian, J.; Xu, L.Q.; Wang, Y.; Li, Y. A starch-regulated adhesive hydrogel dressing with controllable separation properties for painless dressing change. J. Mater. Chem. B 2022, 10, 6026–6037. [Google Scholar] [CrossRef]
- Li, R.Z.; Zhou, C.; Chen, J.; Luo, H.T.; Li, R.Y.; Chen, D.Y.; Zou, X.N.; Wang, W.C. Synergistic osteogenic and angiogenic effects of kp and qk peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioact. Mater. 2022, 18, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Sun, L.; Gu, Z.Y.; Li, W.Y.; Guo, L.L.; Ma, S.B.; Guo, L.; Zhang, W.W.; Han, B.Q.; Chang, J. N-carboxymethyl chitosan/sodium alginate composite hydrogel loading plasmid DNA as a promising gene activated matrix for in-situ burn wound treatment. Bioact. Mater. 2022, 15, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Q.; Ma, B.; Hua, S.Y.; Ping, R.; Ding, L.; Tian, B.R.; Zhang, X. Chitosan-based injectable hydrogel with multifunction for wound healing: A critical review. Carbohydr. Polym. 2024, 333, 121952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, Q.; Zheng, N.; Wang, R.; Visentin, S.; He, L.; Liu, S. Plant polyphenol-based injectable hydrogels: Advances and biomedical applications. Adv. Healthc. Mater. 2025, 14, e2500445. [Google Scholar] [CrossRef]
- Qureshi, A.T.; Afrin, S.; Asim, S.; Rizwan, M. Imine crosslinked, injectable, and self-healing fucoidan hydrogel with immunomodulatory properties. Adv. Healthc. Mater. 2025, e2405260. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.B.; Liu, Z.J.; Wang, X. Advances of stimulus-responsive hydrogels for bone defects repair in tissue engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef]
- Peng, S.; Niu, S.; Gao, Q.; Song, R.; Wang, Z.; Luo, Z.; Zhang, X.; Qin, X. Hydroxypropyl chitosan/ε-poly-L-lysine based injectable and self-healing hydrogels with antimicrobial and hemostatic activity for wound repair. Carbohyd. Polym. 2025, 337, 122135. [Google Scholar] [CrossRef]
- Gu, Z.; He, Y.; Xiang, H.; Qin, Q.; Cao, X.; Jiang, K.; Zhang, H.; Li, Y. Self-healing injectable multifunctional hydrogels for intervertebral disc disease. Mater. Today Bio 2025, 32, 101655. [Google Scholar] [CrossRef]
- Skok, K.; Duh, M.; Stozer, A.; Markota, A.; Gosak, M. Thermoregulation: A journey from physiology to computational models and the intensive care unit. Wires. Mech. Dis. 2021, 13, 1513. [Google Scholar] [CrossRef]
- Zhao, B.F.; Zhuang, Y.P.; Liu, Z.M.; Mao, J.Y.; Qian, S.T.; Zhao, Q.Y.; Lu, B.L.; Mao, X.Y.; Zhang, L.C.; Zhang, Y.G.; et al. Regulated extravascular microenvironment via reversible thermosensitive hydrogel for inhibiting calcium influx and vasospasm. Bioact. Mater. 2023, 21, 422–435. [Google Scholar] [CrossRef]
- Gu, R.T.; Zhou, H.Q.; Zhang, Z.R.; Lv, Y.; Pan, Y.S.; Li, Q.Q.; Shi, C.F.; Wang, Y.H.; Wei, L.L. Research progress related to thermosensitive hydrogel dressings in wound healing: A review. Nanoscale Adv. 2023, 5, 6017–6037. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.R.; Cheng, Y.; Wang, R.R.; Zhang, T.; Zhang, H.; Li, J.C.; Song, S.H.; Zheng, A.P. Thermosensitive hydrogels and advances in their application in disease therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.P.; Mandsberg, N.K.; Levkin, P.A.; Hsu, S.H. UV-Induced photopatterning of the thermoresponsive properties of a poly(ethylene glycol) methylether acrylate-co-poly opropylacrylamide) hydrogel. Small Struct. 2025, 2400560. [Google Scholar] [CrossRef]
- Gul, K.; Gan, R.Y.; Sun, C.X.; Jiao, G.; Wu, D.T.; Li, H.B.; Kenaan, A.; Corke, H.; Fang, Y.P. Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels. Crit. Rev. Food Sci. Nut. 2022, 62, 3817–3832. [Google Scholar] [CrossRef]
- Saravanan, V.; Davoodbasha, M.; Rajesh, A.; Nooruddin, T.; Lee, S.Y.; Kim, J.W. Extraction and characterization of chitosan from shell of borassus flabellifer and their antibacterial and antioxidant applications. Int. J. Biol. Macromol. 2023, 253, 126592. [Google Scholar] [CrossRef]
- Alkandari, M.; Barai, P.; Atia, G.A.N.; Mohamed, S.Z.; Ghobashy, M.M.; Shalaby, H.K.; Foda, T.; Rabbee, M.F.; Mallick, S.; Barai, H.R.; et al. Bioactive functionalized chitosan thermo-responsive hydrogels as promising platforms for therapeutic, regenerative oral, and maxillofacial applications. Biotechnol. J. 2025, 20, e202400653. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Lim, C.C.; Owh, C.; Wong, J.H.M.; Ow, V.; Sim, B.; Boo, Y.J.; Yew, M.P.Y.; Leow, Y.H.; Guo, L.F.; et al. PEG-free pH-responsive thermogels containing amphiphilic polycationic polyethylenimine copolymers. Macromolecules 2023, 56, 9368–9378. [Google Scholar] [CrossRef]
- Song, H.Y.; Xing, L.; Liu, W.T.; Wang, X.; Hou, Z.S.; Wang, Y.; Zhang, Z.H.; Li, Y.M.; Li, T.D.; Wang, X.L.; et al. Biomimetic and multifunctional hemostatic hydrogel with rapid thermoresponsive gelation and robust wet adhesion for emergency hemostasis: A rational design based on photo-cross-linking coordinated hydrophilic-hydrophobic balance strategies. Biomacromolecules 2023, 24, 3327–3344. [Google Scholar] [CrossRef]
- Xing, Z.Y.; Lu, H.B.; Fu, Y.Q. Local conservation law of rubber elasticity in hydrogel networks undergoing microphase separation and toughening. Polymer 2021, 222, 123656. [Google Scholar] [CrossRef]
- Hahn, L.; Zorn, T.; Kehrein, J.; Kielholz, T.; Ziegler, A.L.; Forster, S.; Sochor, B.; Lisitsyna, E.S.; Durandin, N.A.; Laaksonen, T.; et al. Unraveling an alternative mechanism in polymer self-assemblies: An order-order transition with unusual molecular interactions between hydrophilic and hydrophobic polymer blocks. ACS Nano 2023, 17, 6932–6942. [Google Scholar] [CrossRef]
- Seo, B.B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.E.; Song, H.R.; Kim, Y.M.; Song, S.C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- He, J.Q.; Zhou, Q.; Ge, Z.Q.; Jiang, S.F.; Li, J.H.; Feng, W.; Yang, H.Y. pH-gated switch of LCST-UCST phase transition of hydrogels. Adv. Funct. Mater. 2024, 34, 2404341. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.J.; Chen, C.W.; Dong, C.D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.T.; Zhu, J.L.; Li, J. Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Mahmood, A.; Tezcan, F.; Kardas, G. Thermal decomposition of sol-gel derived Zn0.8Ga0.2O precursor-gel: A kinetic, thermodynamic, and DFT studies. Acta. Mater. 2018, 146, 152–159. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, T.; Seo, M.; Kim, S.Y. Synthesis and thermo-responsive behavior of poly (N-isopropylacrylamide)-b-poly (N-vinylisobutyramide) diblock copolymer. Polymers 2024, 16, 830. [Google Scholar] [CrossRef]
- Loh, W.W.; Lin, Q.; Lim, C.C.; Guo, L.; Tang, Y.K.; Loh, X.J.; Lim, J.Y.C. Hofmeister effects of anions on self-assembled thermogels. Mater. Today Chem. 2022, 23, 100674. [Google Scholar] [CrossRef]
- Hanyková, L.; Krakovsky, I.; Sestáková, E.; Stastná, J.; Labuta, J. Poly(N,N′-Diethylacrylamide)-based thermoresponsive hydrogels with double network structure. Polymers 2020, 12, 2502. [Google Scholar] [CrossRef]
- Forero-Martinez, N.C.; Cortes-Huerto, R.; Benedetto, A.; Ballone, P. Thermoresponsive lonic liquid/water mixtures: From nanostructuring to phase separation. Molecules 2022, 27, 1647. [Google Scholar] [CrossRef]
- Quoika, P.K.; Podewitz, M.; Wang, Y.; Kamenik, A.S.; Loeffler, J.R.; Liedl, K.R. Thermosensitive hydration of four acrylamide-based polymers in coil and globule conformations. J. Phys. Chem. B 2020, 124, 9745–9756. [Google Scholar] [CrossRef]
- Liu, H.; Sun, W.; Cai, W.; Luo, K.; Lu, C.; Jin, A.; Zhang, J.; Liu, Y. Current status, challenges, and prospects of artificial intelligence applications in wound repair theranostics. Theranostics 2025, 15, 1662–1688. [Google Scholar] [CrossRef] [PubMed]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Risman, R.A.; Belcher, H.A.; Ramanujam, R.K.; Weisel, J.W.; Hudson, N.E.; Tutwiler, V. Comprehensive analysis of the role of fibrinogen and thrombin in clot formation and structure for plasma and purified fibrinogen. Biomolecules 2024, 14, 230. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, S.Y.; Tian, X.Y.; Guang, S.Y.; Xu, H.Y. Fabrication of microspheres containing coagulation factors by reverse microemulsion method for rapid hemostasis and wound healing. Colloids Surf. B Biointerfaces 2022, 218, 112742. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Gao, X.; Guo, X.; Hou, S.; Shi, J.; Lv, Q. What else should hemostatic materials do beyond hemostasis: A review. Mater. Today Bio 2024, 25, 101008. [Google Scholar] [CrossRef]
- Beudert, M.; Gutmann, M.; Lühmann, T.; Meinel, L. Fibrin sealants: Challenges and solutions. ACS Biomater. Sci. Eng. 2022, 8, 2220–2231. [Google Scholar] [CrossRef]
- Xu, K.G.; Wu, X.Z.; Zhang, X.Y.; Xing, M. Bridging wounds: Tissue adhesives’ essential mechanisms, synthesis and characterization, bioinspired adhesives and future perspectives. Burn. Trauma 2022, 10, tkac033. [Google Scholar] [CrossRef]
- Grosjean, M.; Girard, E.; Bethry, A.; Chagnon, G.; Garric, X.; Nottelet, B. Degradable bioadhesives based on star PEG-PLA hydrogels for soft tissue applications. Biomacromolecules 2022, 24, 4430–4443. [Google Scholar] [CrossRef]
- Caballero-Sánchez, N.; Alonso-Alonso, S.; Nagy, L. Regenerative inflammation: When immune cells help to re-build tissues. FEBS J. 2024, 291, 1597–1614. [Google Scholar] [CrossRef]
- Matar, D.Y.; Ng, B.; Darwish, O.; Wu, M.F.; Orgill, D.P.; Panayi, A.C. Skin inflammation with a focus on wound healing. Adv. Wound Care 2023, 12, 269–287. [Google Scholar] [CrossRef]
- Liang, X.; Ding, L.; Ma, J.; Li, J.; Cao, L.; Liu, H.; Teng, M.; Li, Z.; Peng, Y.; Chen, H.; et al. Enhanced mechanical strength and sustained drug release in carrier-free silver-coordinated anthraquinone natural antibacterial anti-inflammatory hydrogel for infectious wound healing. Adv. Healthc. Mater. 2024, 13, e2400841. [Google Scholar] [CrossRef] [PubMed]
- Sunniyoshi, H.; Nakao, S.; Endo, H.; Yanagawa, T.; Nakano, Y.; Okamura, Y.; Kawaguchi, A.T.; Inagaki, Y. A novel composite biomaterial made of jellyfish and porcine collagens accelerates dermal wound healing by enhancing reepithelization and granulation tissue formation in mice. Adv. Wound Care 2020, 9, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, Y.; Pei, J.; Sun, P.; Wang, Y. Ganoderma lucidum Polysaccharide/carboxymethyl Chitosan Hydrogels Modulate Macrophage Polarization for Wound Healing. Biomacromolecules 2025, 26, 2675–2689. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.E.; Park, K.D.; Park, K.M. Bioadhesives and bioactive hydrogels for wound management. J. Control. Release 2025, 379, 285–302. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, J.; Gao, Y.; Fan, Y.; Chen, B.; Su, J.; Li, H. Multifunctional nanocomposite hydrogels: An effective approach to promote diabetic wound healing. Biomed. Mater. 2025, 20, 032006. [Google Scholar] [CrossRef]
- Haririan, Y.; Asefnejad, A. Biopolymer hydrogels and synergistic blends for tailored wound healing. Int. J. Biol. Macromol. 2024, 279, 135519. [Google Scholar] [CrossRef]
- Chang, L.; Du, H.; Xu, F.; Xu, C.; Liu, H. Hydrogel-enabled mechanically active wound dressings. Trends Biotechnol. 2024, 42, 31–42. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, S.; Deng, Y.; Xie, M.; Zhao, M.; Sun, T.; Yu, C.; Zhong, Y.; Guo, R.; Cheng, K.; et al. Construction of multifunctional hydrogel with metal-polyphenol capsules for infected full-thickness skin wound healing. Bioact. Mater. 2023, 24, 69–80. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Sauter, M.; Luebken, A.; Rawish, E.; Szepanowski, R.; Von Esebeck, J.; Sun, Y.; Emami, H.; Meusel, M.; et al. Platelets regulate ischemia-induced revascularization and angiogenesis by secretion of growth factor-modulating factors. Blood Adv. 2023, 7, 6411–6427. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, Z.; Wang, Y.Y.; Wang, Q.Q.; Yao, M.H.; Zhao, L.; Shi, J.J.; Guan, F.X.; Ma, S.S. PDGF-BB/SA/Dex injectable hydrogels accelerate BMSC-mediated functional full thickness skin wound repair by promoting angiogenesis. J. Mater. Chem. B 2021, 9, 6176–6189. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliver. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.-J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Ouyang, Q.Q.; Hu, Z.; Lu, S.T.; Quan, W.Y.; Li, P.W.; Chen, Y.; Li, S.D. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lang, C.; Ding, Y.P.; Sun, S.Y.; Sun, G.W. Chitosan with enhanced deprotonation for accelerated thermosensitive gelation with β-glycerophosphate. Eur. Polym. J. 2023, 196, 112229. [Google Scholar] [CrossRef]
- Filion, D.; Buschmann, M.D. Chitosan-glycerol-phosphate (GP) gels release freely diffusible GP and possess titratable fixed charge. Carbohyd. Polym. 2013, 98, 813–819. [Google Scholar] [CrossRef]
- Kleine-Brueggeney, H.; Zorzi, G.K.; Fecker, T.; El Gueddari, N.E.; Moerschbacher, B.M.; Goycoolea, F.M. A rational approach towards the design of chitosan-based nanoparticles obtained by ionotropic gelation. Colloids Surf. B Biointerfaces 2015, 135, 99–108. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Dang, Q.F.; Yan, J.Q.; Li, J.J.; Cheng, X.J.; Liu, C.S.; Chen, X.G. Controlled gelation temperature, pore diameter and degradation of a highly porous chitosan-based hydrogel. Carbohyd. Polym. 2011, 83, 171–178. [Google Scholar] [CrossRef]
- Sun, M.J.; Wang, T.; Pang, J.H.; Chen, X.G.; Liu, Y. Hydroxybutyl chitosan centered biocomposites for potential curative applications: A critical review. Biomacromolecules 2020, 21, 1351–1367. [Google Scholar] [CrossRef]
- Li, L.; Xiang, F.T.; Chen, A.Q.; Liu, Y. Preparation and sustained-release mechanism of hydroxybutyl chitosan/graphene oxide temperature-sensitive hypoglycaemic subcutaneous implants. Colloid. Surface B 2024, 236, 113801. [Google Scholar] [CrossRef]
- Hu, S.H.; Bi, S.C.; Yan, D.; Zhou, Z.Z.; Sun, G.H.; Cheng, X.J.; Chen, X.G. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohyd. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.H.; Feng, C.; Jiang, C.Q.; Zhang, T.T.; Bao, Z.X.; Zuo, Y.J.; Kong, M.; Cheng, X.J.; Liu, Y.; Chen, X.G. Thermo-responsive hydroxybutyl chitosan hydrogel as artery intervention embolic agent for hemorrhage control. Int. J. Biol. Macromol. 2017, 105, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, P.; Liu, Y.; Hou, D.; Chen, Y.; Cheng, L.; Xue, Y.; Liu, J. Revolutionizing pressure ulcer regeneration: Unleashing the potential of extracellular matrix-derived temperature-sensitive injectable antioxidant hydrogel for superior stem cell therapy. Biomaterials 2025, 314, 122880. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Wang, J.X.; Luo, Y.; Li, C.L.; Sun, Y.A.; Wang, K.Y.; Deng, G.Y.; Zhao, L.J.; Yuan, C.P.; Lu, J.; et al. A pH-responsive ZC-QPP hydrogel for synergistic antibacterial and antioxidant treatment to enhance wound healing. J. Mater. Chem. B 2023, 11, 9300–9310. [Google Scholar] [CrossRef]
- Deng, L.D.; Qi, H.Y.; Yao, C.M.; Feng, M.H.; Dong, A.J. Investigation on the properties of methoxy poly(ethylene glycol)/chitosan graft co-polymers. J. Biomat. Sci.-Polym. E. 2007, 18, 1575–1589. [Google Scholar] [CrossRef]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M.Q. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef]
- Stoica, A.E.; Albulet, D.; Birca, A.C.; Iordache, F.; Ficai, A.; Grumezescu, A.M.; Vasile, B.S.; Andronescu, E.; Marinescu, F.; Holban, A.M. Electrospun nanofibrous mesh based on PVA, chitosan, and usnic acid for applications in wound healing. Int. J. Mol. Sci. 2023, 24, 11037. [Google Scholar] [CrossRef]
- Qi, B.W.; Yu, A.X.; Zhu, S.B.; Chen, B.A.; Li, Y. The preparation and cytocompatibility of injectable thermosensitive chitosan/poly(vinyl alcohol) hydrogel. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2010, 30, 89–93. [Google Scholar] [CrossRef]
- Tang, Y.F.; Du, Y.M.; Li, Y.; Wang, X.Y.; Hu, X.W. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery. J. Biomed. Mater. Res. A 2009, 91, 953–963. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhai, W.; Xu, J.; Hou, Z.; She, P.; Li, X.; Ma, X.; Wang, X.; Liu, W. Preparation of an antibacterial, injectable, thermosensitive, and physically cross-linked hemostatic hydrogel based on quaternized linetype poly(sopropylacrylamide). J. Mater. Chem. B 2025, 13, 4447–4462. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Navid, S.; Salami, M.A.; Ghasemi, Y.; Heidari, R.; Haghdel, M.; Zhang, H.; Samadi, A. Antibacterial and thermosensitive chitosan-g-poly(N-isopropylacrylamide) copolymer hydrogel containing tannic acid: An injectable therapy for bleeding control. Int. J. Biol. Macromol. 2025, 308, 142326. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of stable cross-linked multilayers based on thermo-responsive PNIPAM-grafted-chitosan/heparin to tailor their physiochemical properties and biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef] [PubMed]
- Mfoafo, K.; Kwon, Y.; Omidi, Y.; Omidian, H. Contemporary applications of thermogelling PEO-PPO-PEO triblock copolymers. J. Drug Deliv. Sci. Technol. 2022, 70, 103182. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki-Jirsaraei, N.; Simchi, A.; Mohandes, F.; Emami, Z.; Fassina, L.; Naro, F.; Conti, B.; Barbagallo, F. An electroconductive, thermosensitive, and injectable chitosan/pluronic/gold-decorated cellulose nanofiber hydrogel as an efficient carrier for regeneration of cardiac tissue. Materials 2022, 15, 5122. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Q.; Liu, W.; Zhang, S.; Wang, N.; Chai, G.; Wang, Y.; Sun, S.; Zheng, R.; Zhao, Y.; et al. A poloxamer 407/chitosan-based thermosensitive hydrogel dressing for diabetic wound healing via oxygen production and dihydromyricetin release. Int. J. Biol. Macromol. 2024, 263, 130256. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Veláquez-Ordoñz, C.; Cervantes-Uc, J.M.; Rodríguez-Rodríguez, R. Water behavior, thermal, structural, and viscoelastic properties of physically cross-linked chitosan hydrogels produced by NaHCO3 as a crosslinking agent. J. Mater. Sci. 2023, 58, 6025–6037. [Google Scholar] [CrossRef]
- Liu, L.; Tang, X.M.; Wang, Y.Y.; Guo, S.R. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int. J. Pharmaceut. 2011, 414, 6–15. [Google Scholar] [CrossRef]
- Casettari, L.; Cespi, M.; Palmieri, G.F.; Bonacucina, G. Characterization of the interaction between chitosan and inorganic sodium phosphates by means of rheological and optical microscopy studies. Carbohyd. Polym. 2013, 91, 597–602. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Singer, A.J. Healing mechanisms in cutaneous wounds: Tipping the balance. Tissue Eng. Part B Rev. 2022, 28, 1151–1167. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical applications. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Chen, Z.P.; Hong, Z.P.; Zhang, L.Y.; Liang, X.X.; Li, Y.; Duan, X.J.; Luo, H.S.; Peng, J.P.; Guo, J.W. Injectable thermo-sensitive and wide-crack self-healing hydrogel loaded with antibacterial anti-inflammatory dipotassium glycyrrhizate for full-thickness skin wound repair. Acta Biomater. 2022, 143, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.K.; Chen, J.B.; Gao, L.J.; Chen, X.; Lin, L.J.; Wei, X.; Liu, Y.; Cheng, H. Injectable photothermal PDA/chitosan/β-glycerophosphate thermosensitive hydrogels for antibacterial and wound healing promotion. Macromol. Biosci. 2024, 24, e2400080. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Liu, X.L.; Peng, X.J.; Zheng, Y.A.; Cheng, Z.Q.; Sun, S.W.; Ding, Q.T.; Liu, W.C.; Ding, C.B. A poloxamer/hyaluronic acid/chitosan-based thermosensitive hydrogel that releases dihydromyricetin to promote wound healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Bian, S.Q.; Li, Z.Q.; Zhang, Z.Y.; Liu, Y.; Zhai, X.Y.; Pan, H.B.; Zhao, X.L. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Woung, L.C.; Yen, J.-C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohyd. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef]
- Dang, L.H.; Nguyen, T.H.; Tran, H.L.B.; Doan, V.N.; Tran, N.Q. Injectable nanocurcumin-formulated chitosan-g-pluronic hydrogel exhibiting a great potential for burn treatment. J. Healthc. Eng. 2018, 2018, 5754890. [Google Scholar] [CrossRef]
- Zhou, P.P.; Li, X.; Zhang, B.; Shi, Q.; Li, D.; Ju, X.L. A human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/β-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. Biomed Res. Int. 2019, 2019, 5768285. [Google Scholar] [CrossRef]
- Bai, D.M.; Cheng, H.X.; Mei, J.M.; Tian, G.Q.; Wang, Q.Q.; Yu, S.M.; Gao, J.; Zhong, Y.H.; Xin, H.B.; Wang, X.L. Rapid formed temperature-sensitive hydrogel for the multi-effective wound healing of deep second-degree burn with shikonin based scar prevention. Biomater. Adv. 2024, 160, 213851. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Duan, Z.G.; Zhao, J.; Fu, R.Z.; Zhu, C.H.; Fan, D.D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Mu, Y.Z.; Hu, Y.; Liu, J.H.; Su, C.; Sun, X.J.; Chen, X.G.; Jia, N.; Feng, C. Zinc-mineralized diatom biosilica/hydroxybutyl chitosan composite hydrogel for diabetic chronic wound healing. J. Colloid Interf. Sci. 2024, 656, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, M.; Tang, Y.; Yang, J.L.; Wang, J.; Zhu, Y.Q.; Liang, H.T.; Lin, Q.R.; Cheng, Y.P.; Yang, X.Y.; et al. Temperature-sensitive hydrogel dressing loaded with nicotinamide mononucleotide accelerating wound healing in diabetic mice. Biomed. Pharmacother. 2023, 167, 115431. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.L.; Fang, Y.F.; Luo, M.C.; Wang, L.Y.; Huang, J.; Dai, G.; Liu, K.; Wu, J.; Du, J.H. Deferoxamine-loaded injectable chitosan-grafted chlorogenic acid/oxidized hyaluronic acid hybrid hydrogel with antibacterial, anti-inflammatory, and angiogenesis-promoting properties for diabetic wound repair. ACS Appl. Mater. Interfaces 2024, 16, 28209–28221. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, Q.C.; Zhang, M.; Lv, X.T.; Li, Z.H.; Mohammadniaei, M.; Zhou, N.L.; Sun, Y. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohyd. Polym. 2021, 265, 118065. [Google Scholar] [CrossRef]
- Ni, P.X.Z.; Li, R.P.; Ye, S.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.J.; Zhang, X.D. Lactobionic acid-modified chitosan thermosensitive hydrogels that lift lesions and promote repair in endoscopic submucosal dissection. Carbohyd. Polym. 2021, 263, 118001. [Google Scholar] [CrossRef]
- Zhang, E.S.; Guo, Q.; Ji, F.; Tian, X.L.; Cui, J.; Song, Y.H.; Sun, H.; Li, J.J.; Yao, F.L. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef]
- Chang, R.; Zhao, D.H.; Zhang, C.; Liu, K.Y.; He, Y.M.; Guan, F.X.; Yao, M.H. PMN-incorporated multifunctional chitosan hydrogel for postoperative synergistic photothermal melanoma therapy and skin regeneration. Int. J. Biol. Macromol. 2023, 253, 126854. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, W.; Wu, J.; Huang, J.; Wang, W.; Peng, W.; Chen, J.; Bo, R.; Liu, M.; Li, J. Polyvinyl alcohol/chitosan hydrogel based on deep eutectic solvent for promoting methicillin-resistant Staphylococcus aureus-infected wound healing. Int. J. Biol. Macromol. 2025, 297, 139916. [Google Scholar] [CrossRef]
- Rong, X.; Gao, G.; Lou, J.; Wang, X.; Han, W. Double-network hydrogel dressing regulated by cationic polymer-grafted bacterial cellulose for promote rapid healing of infected wounds. Carbohyd. Polym. 2025, 353, 123257. [Google Scholar] [CrossRef]
- Park, S.G.; Li, M.X.; Cho, W.K.; Joung, Y.K.; Huh, K.M. Thermosensitive gallic acid-conjugated hexanoyl glycol chitosan as a novel wound healing biomaterial. Carbohyd. Polym. 2021, 260, 117808. [Google Scholar] [CrossRef]
- Hamed, R.; Sawalha, S.; Assali, M.; Shqair, R.A.; Al-Qadi, A.; Hussein, A.; Alkowni, R.; Jodeh, S. Visible light-driven ZnO nanoparticles/carbon nanodots hybrid for broad-spectrum antimicrobial activity. Surf. Interfaces 2023, 38, 102760. [Google Scholar] [CrossRef]
- Feng, H.M.; Wang, W.; Wang, T.; Pu, Y.A.; Ma, C.C.; Chen, S.G. Interfacial regulation of bioi@bi2s3/mxene heterostructures for enhanced photothermal and photodynamic therapy in antibacterial applications. Acta Biomater. 2023, 171, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ying, Z.R.; Luo, X.; Tan, S.; Liu, X.H.; Zhao, X.Y.; He, S.S.; Chen, F.; Kulak, A.I.; Lu, B.Q. Gallic acid-modified bioglass with combined photothermal and antibacterial effects for the regeneration of infected diabetic wound. Compos. Part B Eng. 2023, 257, 110668. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Shen, J.; Zhou, N.; Li, Y.; Tang, B.Z.; Zhang, M. Athermosensitive hydrogel with efficient NIR photothermal conversion as injectable wound dressing for accelerating skin wound healing. Adv. Funct. Mater. 2024, 34, 2312374. [Google Scholar] [CrossRef]
- Lan, J.Z.; Shi, L.X.; Xiao, W.Y.; Zhang, X.B.; Wang, S.T. A rapid self-pumping organohydrogel dressing with hydrophilic fractal microchannels to promote burn wound healing. Adv. Mater. 2023, 35, 2301765. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Khodadadi Yazdi, M.; Reza Saeb, M.; Bączek, T.; Farokhi, M. Green and sustainable hydrogels based on quaternized chitosan to enhance wound healing. Chem. Eng. J. 2024, 492, 152288. [Google Scholar] [CrossRef]
- Fathi, A.; Gholami, M.; Motasadizadeh, H.; Malek-Khatabi, A.; Sedghi, R.; Dinarvand, R. Thermoresponsive in situ forming and self-healing double-network hydrogels as injectable dressings for silymarin/levofloxacin delivery for treatment of third-degree burn wounds. Carbohyd. Polym. 2024, 331, 121856. [Google Scholar] [CrossRef]
- Lv, X.; Li, H.; Chen, Y.; Wang, Y.; Chi, J.; Wang, S.; Yang, Y.; Han, B.; Jiang, Z. Crocin-1 laden thermosensitive chitosan-based hydrogel with smart anti-inflammatory performance for severe full-thickness burn wound therapeutics. Carbohyd. Polym. 2024, 345, 122603. [Google Scholar] [CrossRef]
- Vanderpant, N.; Ward, E.; Farrell, E.; Theodoraki, A. Insulin for people with type 2 diabetes mellitus. BMJ 2024, 386, e078015. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Liu, Y.; Duan, P.; Liu, C.; Liu, K.; Yu, Y.; Liang, X.; Dai, H.; Yu, A. An injectable and adaptable system for the sustained release of hydrogen sulfide for targeted diabetic wound therapy by improving the microenvironment of inflammation regulation and angiogenesis. Acta Biomater. 2025, 196, 364–379. [Google Scholar] [CrossRef]
- Huang, K.; Mi, B.; Xiong, Y.; Fu, Z.; Zhou, W.; Liu, W.; Liu, G.; Dai, G. Angiogenesis during diabetic wound repair: From mechanism to therapy opportunity. Burns Trauma 2025, 13, 052. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Tian, P.F.; Hao, L.Z.; Hu, C.W.; Liu, B.; Meng, F.Z.; Yi, X.; Pan, X.H.; Hu, X.H.; Wang, H.; et al. Antioxidative bioactive glass reinforced injectable hydrogel with reactive oxygen species scavenging capacity for diabetic wounds treatment. Chem. Eng. J. 2024, 481, 148768. [Google Scholar] [CrossRef]
- Su, D.D.; Tsai, H.I.; Xu, Z.X.; Yan, F.X.; Wu, Y.Y.; Xiao, Y.M.; Liu, X.Y.; Wu, Y.P.; Parvanian, S.; Zhu, W.S.; et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J. Extracell. Vesicles 2020, 9, 1709262. [Google Scholar] [CrossRef]
- Azlan, A.; Katas, H.; Habideen, N.H.; Busra, M.F.M. Dual-action of thermoresponsive gels containing DsiRNA-loaded gold nanoparticles for diabetic wound therapy: Characterization, in vitro safety and healing efficacy. Saudi Pharm. J. 2020, 28, 1420–1430. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.Y.; Feng, Z.X.; Zhang, L.Y.; Zhong, Y.F.; Jin, M.Y.; Xu, F.W.; Feng, C.Y.; Du, Y.Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Li, F.; Li, B.; Zhang, M.; Li, W.; Zhuge, P.; Yao, J.; Zhang, Y.; Chen, S.; et al. Thermosensitive hydrogel integrated with bimetallic nano-enzymes for modulating the microenvironment in diabetic wound beds. Adv. Sci. 2024, 12, 2411575. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Annabi, N.; Khademhosseini, A. Surgical sealants and high strength adhesives. Mater. Today 2015, 18, 176–177. [Google Scholar] [CrossRef]
- Ni, P.X.Z.; Ye, S.; Li, R.P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.J.; Zhang, X.D. Chitosan thermosensitive hydrogels based on lyophilizate powders demonstrate significant potential for clinical use in endoscopic submucosal dissection procedures. Int. J. Biol. Macromol. 2021, 184, 593–603. [Google Scholar] [CrossRef]
- Abdollahi, A.; Malek-Khatabi, A.; Razavi, M.S.; Sheikhi, M.; Abbaspour, K.; Rezagholi, Z.; Atashi, A.; Rahimzadegan, M.; Sadeghi, M.; Javar, H.A. The recent advancement in the chitosan-based thermosensitive hydrogel for tissue regeneration. J. Drug Deliv. Sci. Technol. 2023, 86, 104627. [Google Scholar] [CrossRef]
- Weng, H.J.; Jia, W.B.; Li, M.; Chen, Z.G. New injectable chitosan-hyaluronic acid based hydrogels for hemostasis and wound healing. Carbohyd. Polym. 2022, 294, 119767. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.F.; Zhou, J.N.; Zhang, B.C.; Zhang, L.H.; Gou, D.X. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef]
- Guo, W.; Ding, X.Y.; Zhang, H.; Liu, Z.Z.; Han, Y.T.; Wei, Q.Q.; Okoro, O.V.; Shavandi, A.; Nie, L. Recent advances of chitosan-based hydrogels for skin-wound dressings. Gels 2024, 10, 175. [Google Scholar] [CrossRef]












| Application | Name of Dressing | Composition | Tsol-Gel | Gelation Times | Mechanical Strength | Healing Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Infected wound | DG-loaded HP hydrogels | Dipotassium glycyrrhizinate (DG), hydroxypropyl chitosan/N-isopropylacrylamide | 18.5–23.7 °C | / | Tensile stress: 0.021 MPa | Mouse full-thickness skin defect model: 99.5% average would healing rate at day 14 | [92] |
| Polydopamine-loaded hydrogels | Polydopamine, chitosan/β-glycerophosphate | 37 °C | / | / | Mouse infection wound model: almost complete healing at day 12 | [93] | |
| Dihydromyricetin-loaded hydrogels | Dihydromyricetin, poloxamer/chitosan/hyaluronic acid/ | 37 °C | 0.5 ± 0.2 min | / | Mouse infection wound model: almost complete healing at day 15 | [94] | |
| Bioactive glass-loaded hydrogels | Bioactive glass, quaternized chitosan/PLEL | 32.6 °C | / | Adhesion strength: 16.98 ± 0.84 KPa | Practical laceration model: the wound closure reached nearly 99.40% at day 10 | [95] | |
| Burn wounds | FA-loaded hydrogels | Ferulic acid (FA), chitosan/ gelatin/glycerol phosphate | 37 °C | / | / | Rabbits model of corneal alkali burn: mild corneal hyperplasia at 24 h | [96] |
| Nanocurcumin-loaded hydrogels | Nanocurcumin(nCur), chitosan/g-pluronic | 35 °C | / | / | Second-degree burn model: complete healing at day 14 | [97] | |
| MSC-conditioned medium-loaded hydrogels | MSC-conditioned medium (MSC-CM), chitosan/collagen/β-glycerophosphate | 37 °C | 10 min | / | Third-degree burn model: complete healing at day 14 | [98] | |
| Mesoporous carbon nanospheres (MCNs), NO, Sodium nitroprusside (SNP)-loaded hydrogels | MCNs, NO, SNP/chitosan β- glycerophosphate | 37 °C | / | / | Rats deep second-degree scald infected model: almost complete healing at day 15 | [99] | |
| Diabetic wounds | Insulin and celecoxib-loaded hydrogels | Insulin (INS), celecoxib, polyvinyl alcohol/chitosan/gelatin/phenylboric acid | 37 °C | Within 3 s | Adhesive strength 39.36 ± 6.58 kPa | Diabetic rat wound model: the wound-healing rate is 96.68 ± 2.04% on day 14 | [100] |
| Zinc-mineralized-loaded hydrogels | Zinc-mineralized (ZnDBs), Hydroxybutyl chitosan (HBC) | 22.2 °C | / | 222.51 ± 19.98 Pa | Diabetic rat wound model: the wound-healing rate is 95.33 ± 0.12% on day 14 | [101] | |
| Nicotinamide mononucleotide-loaded hydrogels | Nicotinamide mononucleotide (NMN), Poluronic F127/Pluronic F68/chitosan | 37 °C | 80 s | G′: 10 KPa G″: 1 KPa | Diabetic rat wound model: complete healing on the 14th day | [102] | |
| Chlorogenic acid and deferoxamine-loaded hydrogels | Chlorogenic acid (CGA), deferoxamine (DFO)/chitosan/oxidized hyaluronic acid | RT | / | / | Diabetic rat wound model: the wound-healing rate is 96.5 ± 1.5% on day 14 | [103] | |
| Surgical wounds | Black phosphate nanosheets and copper nanoparticles-loaded hydrogels | Black phosphate nanosheets (BPNSs), copper nanoparticles (CuNPs)/chitosan | 37 °C | / | / | Mouse infection wound model: almost complete healing at day 10 | [104] |
| Lactobionic acid-modified chitosan-loaded hydrogel | Lactobionic acid-modified chitosan/chitosan β-glycerophosphate | 37 °C | Within 5 min | / | / | [105] | |
| Galactose modified xyloglucan-loaded hydrogels | Galactose modified xyloglucan (mXG)/hydroxybutyl chitosan | 31 °C | 20~60 s | / | Rats full-thickness skin defect model: the wound-healing rate is close to 95% on day 14 | [106] | |
| Tannic acid capped gold nanoparticles-loaded hydrogel | Tannic acid capped gold nanoparticles, carboxymethyl chitosan, oxidized fucoidan | 37 °C | 4.4~5.3 min | Adhesion data: 6.2~9.2 KPa | Mouse infection wound model: the wound-healing rate is 95.8% on day 14 | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, L.; Wu, E.; Li, X.; Rao, Y.; Zhu, C. Recent Advances on Chitosan-Based Thermosensitive Hydrogels for Skin Wound Treatment. Biology 2025, 14, 619. https://doi.org/10.3390/biology14060619
Wang J, Huang L, Wu E, Li X, Rao Y, Zhu C. Recent Advances on Chitosan-Based Thermosensitive Hydrogels for Skin Wound Treatment. Biology. 2025; 14(6):619. https://doi.org/10.3390/biology14060619
Chicago/Turabian StyleWang, Jin, Lianghui Huang, Enguang Wu, Xiao Li, Yi Rao, and Caiqing Zhu. 2025. "Recent Advances on Chitosan-Based Thermosensitive Hydrogels for Skin Wound Treatment" Biology 14, no. 6: 619. https://doi.org/10.3390/biology14060619
APA StyleWang, J., Huang, L., Wu, E., Li, X., Rao, Y., & Zhu, C. (2025). Recent Advances on Chitosan-Based Thermosensitive Hydrogels for Skin Wound Treatment. Biology, 14(6), 619. https://doi.org/10.3390/biology14060619






