Acneiform Eruptions Caused by Lithium Treatment May Be Related to Demodex Mites (Prostigmata: Demodecidae): A Hypothesis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
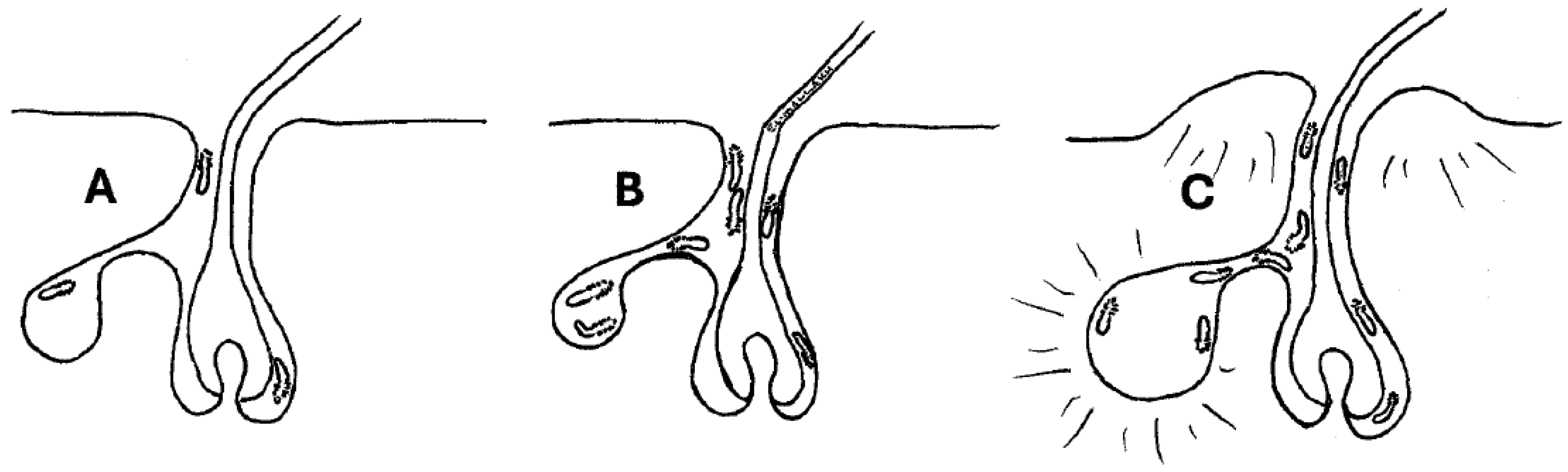
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372, Erratum in: Lancet 2012, 379, 314. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Manzano Marín, A.; Reyes-Prieto, M.; Ribeiro Antunes, C.S.; Ashworth, V.; Goselle, O.N.; Jan, A.A.A.; Moya, A.; Latorre, A.; Perotti, M.A.; et al. Human follicular mites: Ectoparasites becoming symbionts. Mol. Biol. Evol. 2022, 39, msac125. [Google Scholar] [CrossRef] [PubMed]
- Clanner-Engelshofen, B.M.; Ruzicka, T.; Reinholz, M. Efficient isolation and observation of the most complex human commensal, Demodex spp. Exp. Appl. Acarol. 2018, 76, 71–80. [Google Scholar] [CrossRef]
- Hecht, I.; Melzer-Golik, A.; Sadi Szyper, N.; Kaiserman, I. Permethrin cream for the treatment of Demodex blepharitis. Cornea 2019, 38, 1513–1518. [Google Scholar] [CrossRef]
- Aktaş Karabay, E.; Aksu Çerman, A. Demodex folliculorum infestations in common facial dermatoses: Acne vulgaris, rosacea, seborrheic dermatitis. An. Bras. Dermatol. 2020, 95, 187–193. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Shen, W.W.; D’Ocon, P.; Romero, A.; Álamo, C. A history of the pharmacological treatment of bipolar disorder. Int. J. Mol. Sci. 2018, 19, 2143. [Google Scholar] [CrossRef]
- Yeung, C.K.; Chan, H.H. Cutaneous adverse effects of lithium: Epidemiology and management. Am. J. Clin. Dermatol. 2004, 5, 3–8. [Google Scholar] [CrossRef]
- Chan, H.H.; Wing, Y.; Su, R.; Van Krevel, C.; Lee, S. A control study of the cutaneous side effects of chronic lithium therapy. J. Affect. Disord. 2000, 57, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.K.; Yeu, E.; Banett, M.; Rapuano, C.J.; Dhaliwal, D.K.; Nichols, K.K.; Karpecki, P.; Hah, F.S.; Chan, A.; Mun, J.; et al. Demodex blepharitis: A comprehensive review of the disease, current management, and emerging therapies. Eye Contact Lens 2023, 49, 311–318. [Google Scholar] [CrossRef]
- Erbağci, Z.; Ozgöztaşi, O. The significance of Demodex folliculorum density in rosacea. Int. J. Dermatol. 1998, 37, 421–425. [Google Scholar] [CrossRef]
- Fromstein, R.; Harthan, J.S.; Patel, J.; Opitz, D.L. Demodex blepharitis: Clinical perspectives. Clin. Optom. (Auckl.) 2018, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.G.; Oakley, C.L.; Tan, A.; Vote, B.J. Demodex species in human ocular disease: New clinicopathological aspects. Int. Ophthalmol. 2017, 37, 303–312. [Google Scholar] [CrossRef] [PubMed]
- English, F.P.; Nutting, W.B. Demodicosis of ophthalmic concern. Am. J. Ophthalmol. 1981, 91, 362–372. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Tseng, S.C. Pathogenic role of Demodex mites in blepharitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 505–510. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Guo, N.; Xun, M.; Xu, J.R.; Wang, M.; Wang, D.L. Sociodemographic characteristics and risk factor analysis of Demodex infestation (Acari: Demodicidae). J. Zhejiang Univ. Sci. B 2011, 12, 998–1007. [Google Scholar] [CrossRef]
- Akçınar, U.G.; Ünal, E.; Doğruman, A.F. Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Postepy Dermatol. Alergol. 2018, 35, 174–181. [Google Scholar] [CrossRef]
- Erdal, B.; Albayrak, H. Investigation of the prevalence of Demodex spp. in Dermatological Diseases. Turkiye Parazitol. Derg. 2022, 46, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Gómez, W.; Guevara-Sánchez, E.; Guevara-Vásquez, G.; Mera-Villasis, K.; Munayco, C.V. Association between Demodex infestation and severe acne vulgaris: A cross-sectional study of 168 patients. Actas Dermosifiliogr. 2022, 113, 758–764. [Google Scholar] [CrossRef]
- Chang, Y.S.; Huang, Y.C. Role of Demodex mite infestation in rosacea: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 441–447.e6. [Google Scholar] [CrossRef]
- Paichitrojjan, A.; Chalermachai, T. The association between acne vulgaris with nonspecific facial dermatitis, and Demodex mite presence. Clin. Cosmet. Investig. Dermatol. 2024, 17, 137–146. [Google Scholar] [CrossRef]
- Signore, R.J. A pilot study of 5 percent permethrin cream versus 0.75 percent metronidazole gel in acne rosacea. Cutis 1995, 56, 177–179. [Google Scholar] [PubMed]
- Kocak, M.; Yagli, S.; Vahapoğlu, G.; Ekşioğlu, M. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea: A randomized double-blind placebo-controlled study. Dermatol. 2002, 205, 265–270. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Hu, L.; Wu, L.P.; Ma, J.X. A meta-analysis of association between acne vulgaris and Demodex infestation. J. Zhejiang Univ. Sci. B 2012, 13, 192–202. [Google Scholar] [CrossRef]
- Rather, P.A.; Hassan, I. Human demodex mite: The versatile mite of dermatological importance. Indian. J. Dermatol. 2014, 59, 60–66. [Google Scholar] [CrossRef]
- Vashisht, D.; Singh, J.; Baveja, S.; Tiwari, R.; Bhatnagar, A. Unilateral demodicidosis of face mimicking Hansens Disease. Dermatol. Rep. 2016, 8, 6891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paichitrojjana, A. Demodicosis imitating acne vulgaris: A case report. Clin. Cosmet. Investig. Dermatol. 2022, 15, 497–501. [Google Scholar] [CrossRef]
- Paichitrojjana, A. Demodex: The worst enemies are the ones that used to be friends. Dermatol. Rep. 2022, 14, 9399. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Jefferson, J.W.; Greist, J.H.; Clagnaz, P.J.; Eischens, R.R.; Marten, W.C.; Evenson, M.A. Effect of strenuous exercise on serum lithium level in man. Am. J. Psychiatry 1982, 139, 1593–1595. [Google Scholar] [CrossRef]
- Loeffler, H.H.; Rode, B.M. The hydration structure of the lithium ion. J. Chem. Physics 2002, 11791, 110–117. [Google Scholar] [CrossRef]
- Rothstein, G.; Clarkson, D.R.; Larsen, W.; Grosser, B.I.; Athens, J.W. Effect of lithium on neutrophil mass and production. N. Engl. J. Med. 1978, 298, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Williams, C.C.; Preston, D. The use of lithium carbonate to reduce infection and leukopenia during systemic chemotherapy. N. Engl. J. Med. 1980, 302, 257–260. [Google Scholar] [CrossRef]
- Lyman, G.H.; Williams, C.C.; Preston, D.; Goldman, A.; Dinwoodie, W.R.; Saba, H.; Hartmann, R.; Jensen, R.; Shukovsky, L. Lithium carbonate in patients with small cell lung cancer receiving combination chemotherapy. Am. J. Med. 1981, 70, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Ziegelmann, B.; Abele, E.; Hannus, S.; Beitzinger, M.; Berg, S.; Rosenkranz, P. Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci. Rep. 2018, 8, 683. [Google Scholar] [CrossRef]
- Kolics, É.; Specziár, A.; Taller, J.; Klára Mátás, K.; Kolics, B. Lithium chloride outperformed oxalic acid sublimation in a preliminary experiment for Varroa mite control in pre-wintering honey bee colonies. Acta Veterin Hung. 2021, 68, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Kolics, É.; Sajtos, Z.; Mátyás, K.; Szepesi, K.; Solti, I.; Németh, G.; Taller J Baranyai, E.; Specziár, A.; Kolics, B. Changes in lithium levels in bees and their products following anti-Varroa treatment. Insects 2021, 12, 579. [Google Scholar] [CrossRef]
- Yuan, C.; Zheng, S.L.; Ma, Y.F.; Philippe, J.; Philippe, H. Cleanser use could decrease numbers of Demodex folliculorum in mild to moderate acne patients. Dermatoendocrinol 2017, 9, e1348444. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Chalermchai, T. Evaluating the efficacy of oral ivermectin on clinical symptoms and Demodex densities in patients with demodicosis. Drug Des. Devel Ther. 2024, 18, 5299–5306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mallakh, R.S.; Doroodgar, M.; Pahwa, M.; Elsayed, O.H.A. Acneiform Eruptions Caused by Lithium Treatment May Be Related to Demodex Mites (Prostigmata: Demodecidae): A Hypothesis. Biology 2025, 14, 605. https://doi.org/10.3390/biology14060605
El-Mallakh RS, Doroodgar M, Pahwa M, Elsayed OHA. Acneiform Eruptions Caused by Lithium Treatment May Be Related to Demodex Mites (Prostigmata: Demodecidae): A Hypothesis. Biology. 2025; 14(6):605. https://doi.org/10.3390/biology14060605
Chicago/Turabian StyleEl-Mallakh, Rif S., Masoud Doroodgar, Mehak Pahwa, and Omar H. A. Elsayed. 2025. "Acneiform Eruptions Caused by Lithium Treatment May Be Related to Demodex Mites (Prostigmata: Demodecidae): A Hypothesis" Biology 14, no. 6: 605. https://doi.org/10.3390/biology14060605
APA StyleEl-Mallakh, R. S., Doroodgar, M., Pahwa, M., & Elsayed, O. H. A. (2025). Acneiform Eruptions Caused by Lithium Treatment May Be Related to Demodex Mites (Prostigmata: Demodecidae): A Hypothesis. Biology, 14(6), 605. https://doi.org/10.3390/biology14060605





