Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Materials
2.3. Experimental Design and Management
2.4. Collection and Preservation of Samples
2.5. Assessment of Antioxidant Enzymes Activity
2.6. Assessment of Intestinal Enzymes Activity
2.7. Real-Time PCR Analysis of Antioxidant and Digestive-Related Genes Expression Patterns
2.8. Intestinal Microbial Community Analysis
2.9. Statistical Analysis
3. Results
3.1. Activity of Antioxidant Enzymes in Hemolymph
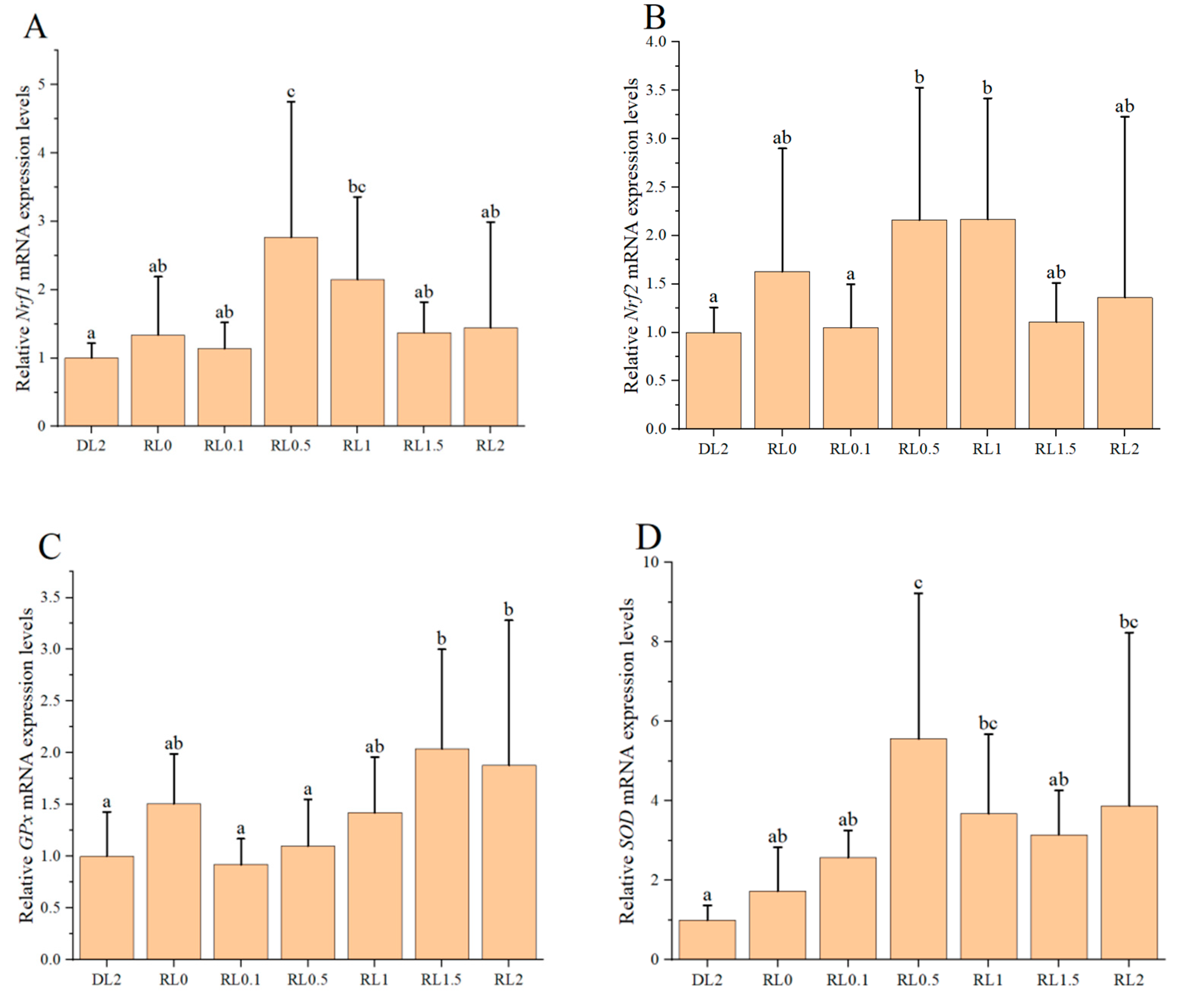
3.2. Relative Expression Levels of Antioxidant Genes in the Hemocytes
3.3. Activity of Intestinal Digestive Enzymes
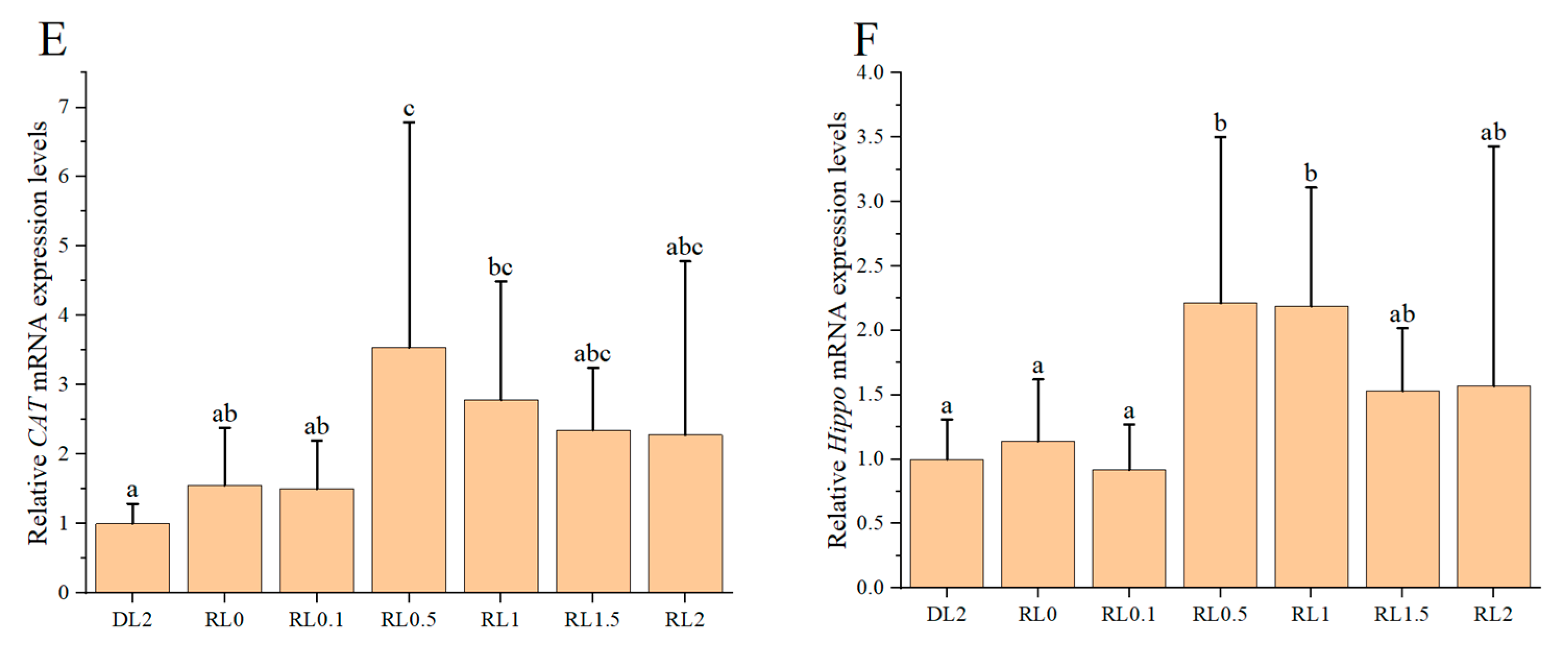
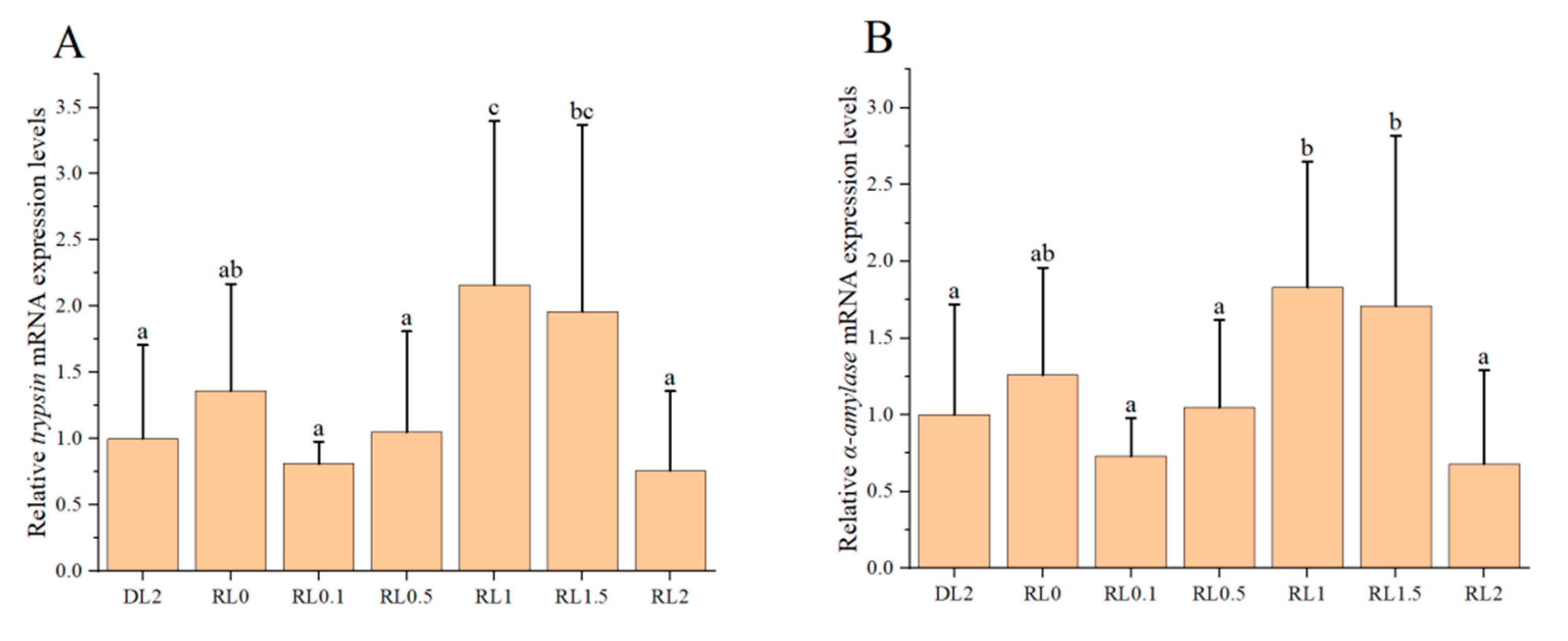
3.4. Relative Expression Levels of Intestinal Digestive Enzyme Genes
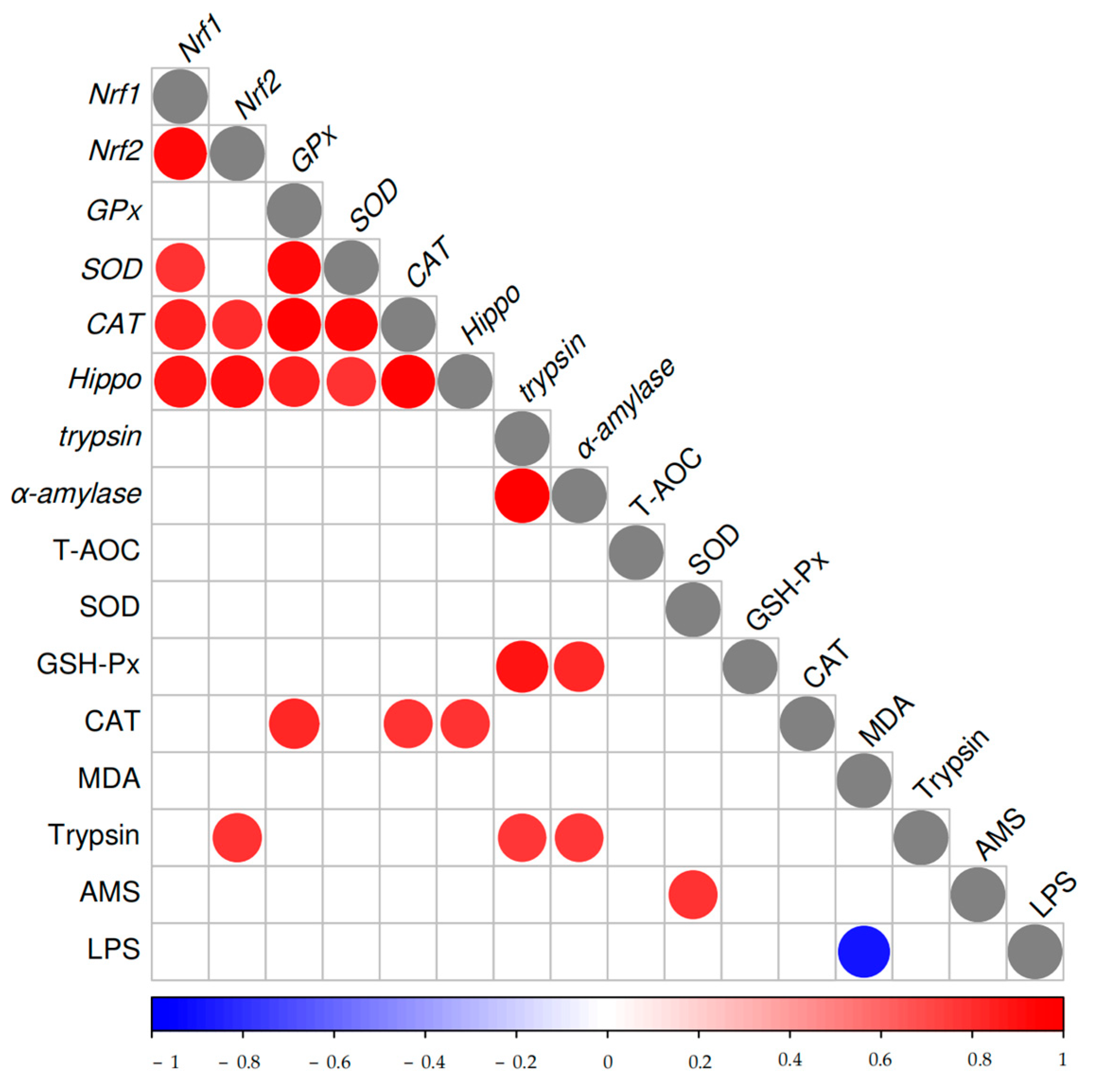
3.5. Analysis of Data by Means of Pearson Correlation
3.6. Intestinal Microbiota Community Changes
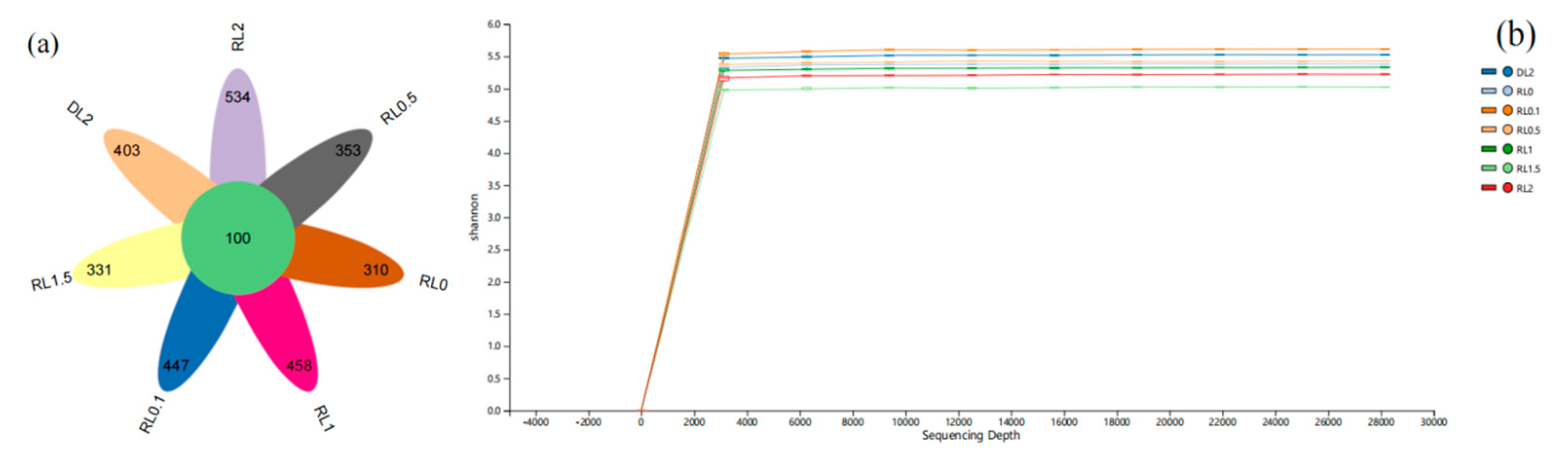
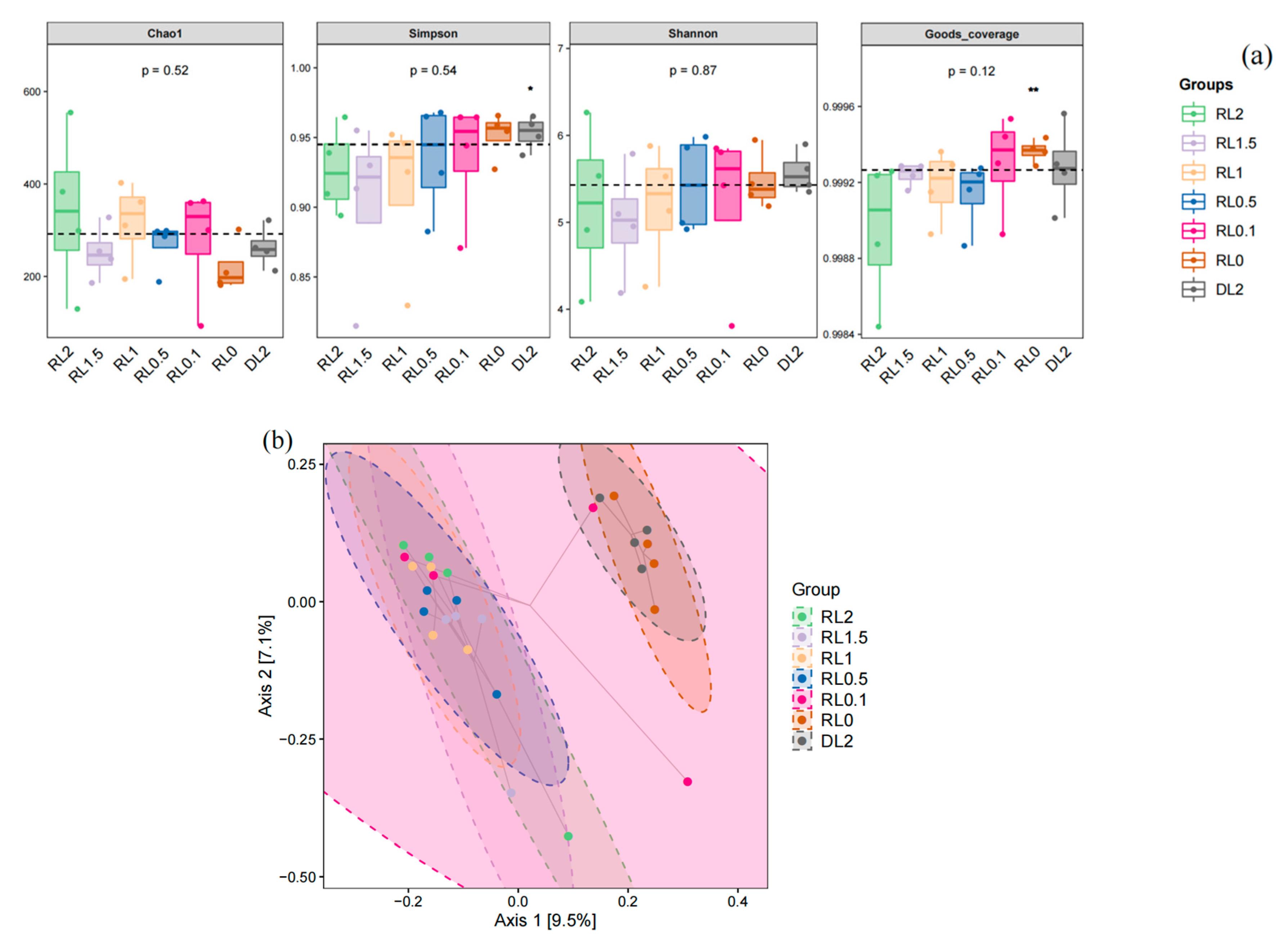
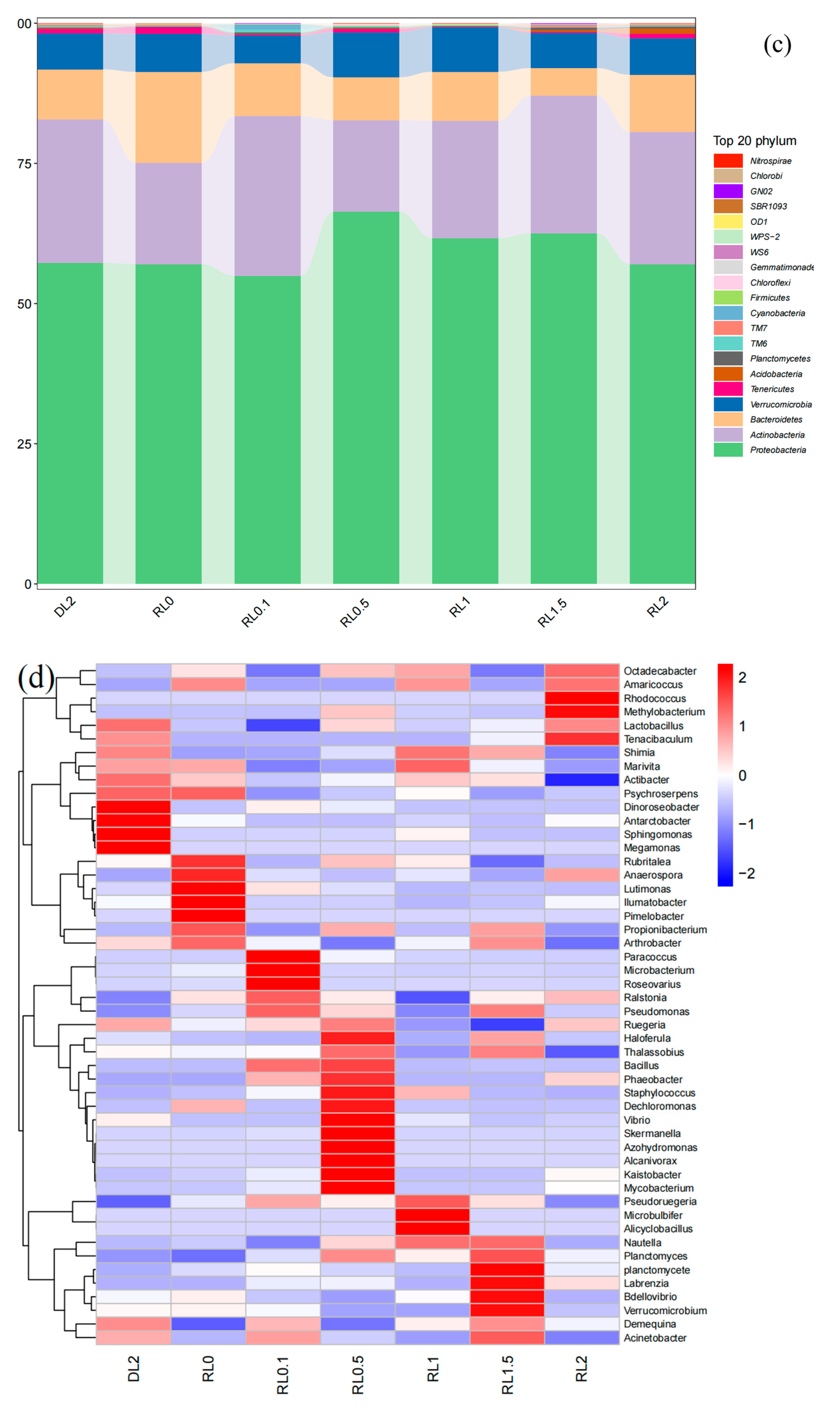
3.6.1. Microbiota Diversity and Composition Changes
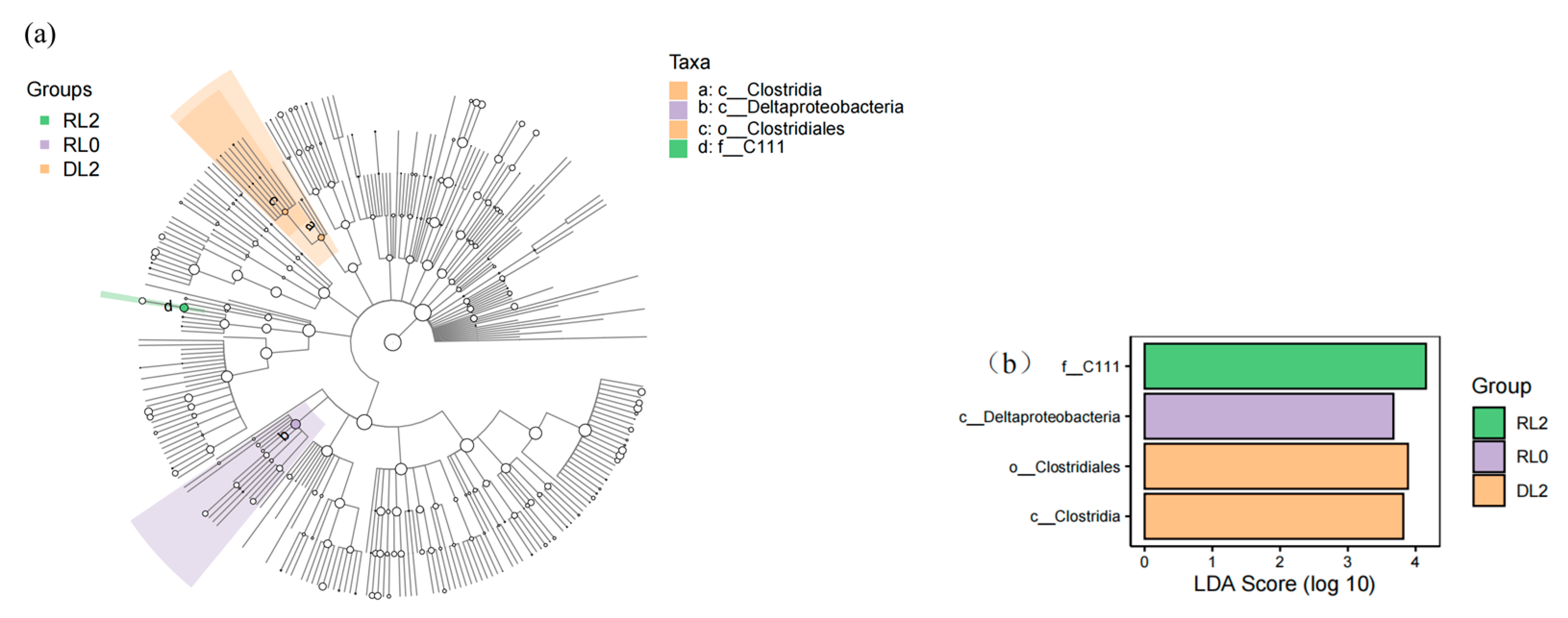
3.6.2. Variations in the Phenotypes of Intestinal Bacteria
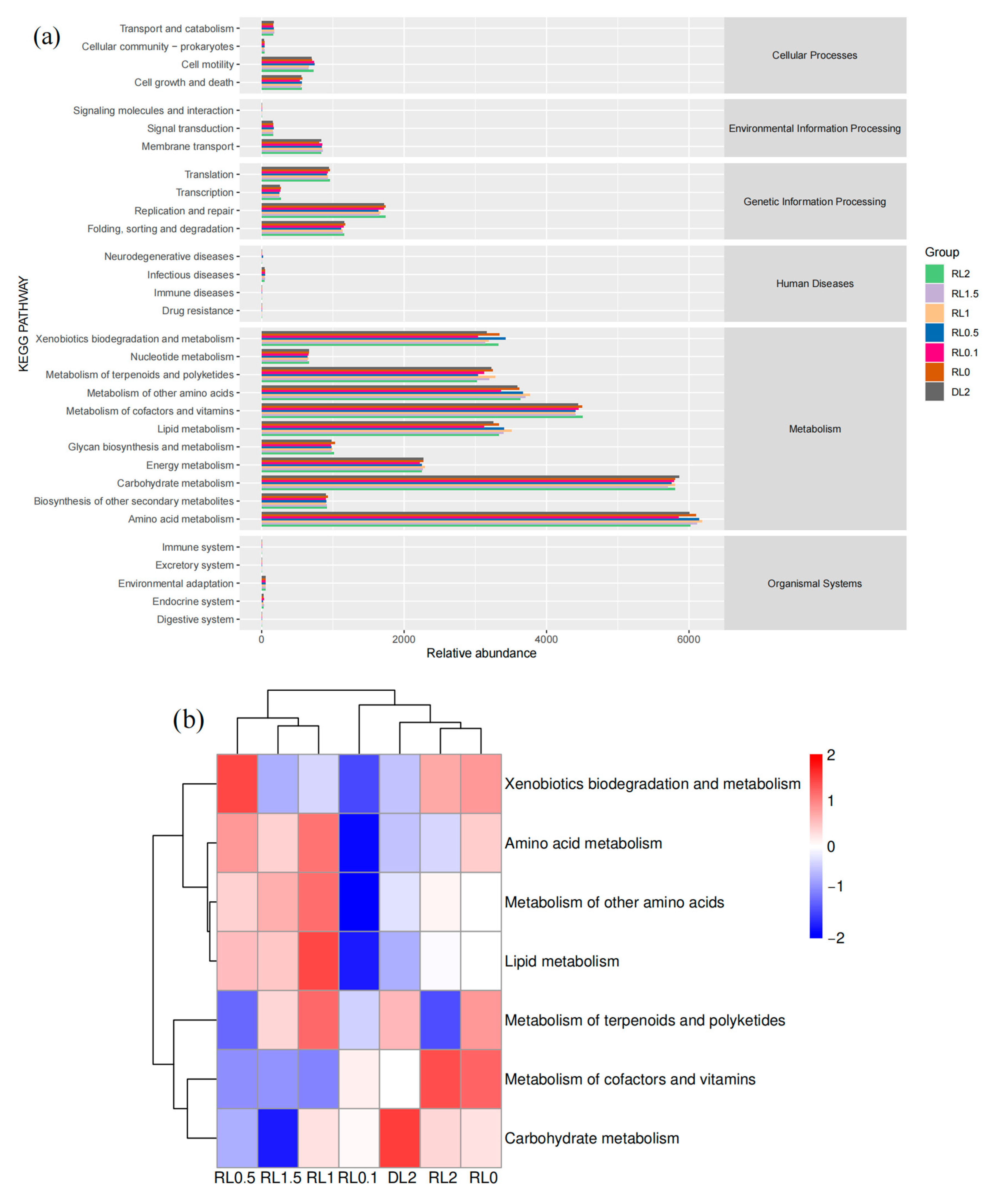
3.6.3. Prediction of the Functional Abundance of Intestinal Microbiome
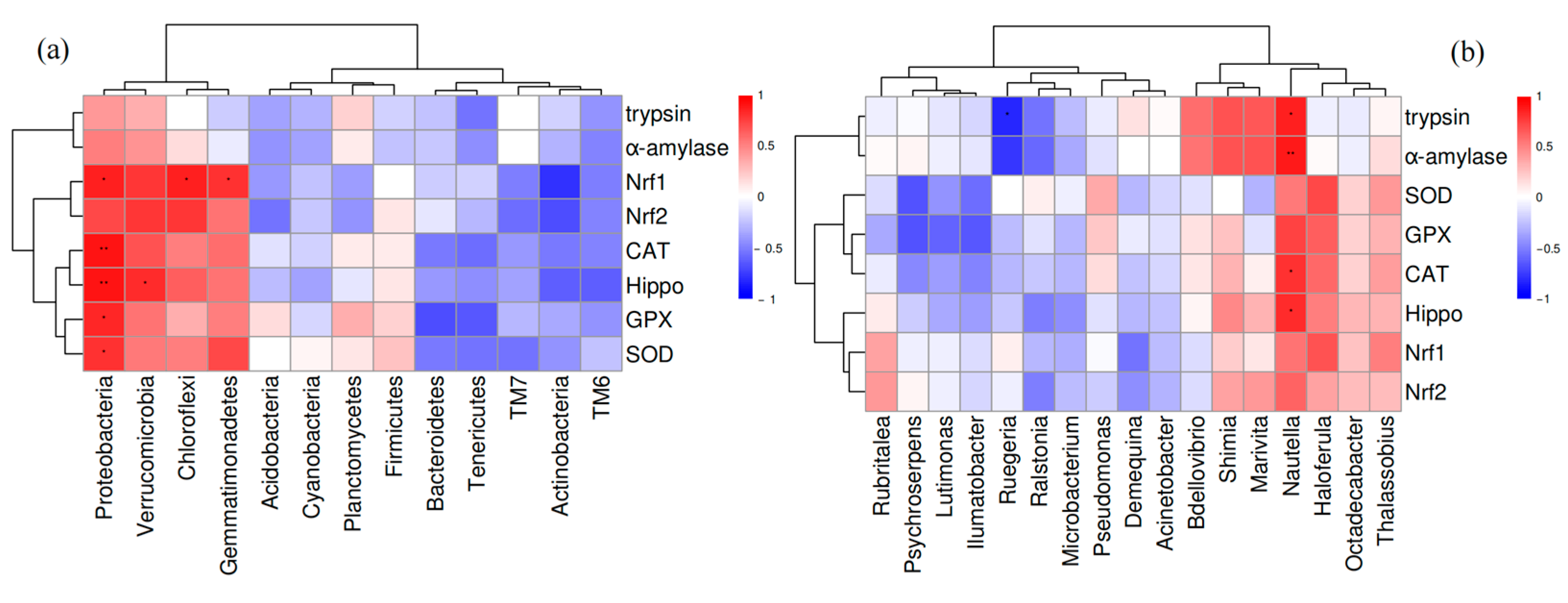
3.6.4. The Relationship Between Intestinal Microbiota and Host Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Gao, S. Current Status of Industrialized Aquaculture in China: A Review. Environ. Sci. Pollut. Res. 2023, 30, 32278–32287. [Google Scholar] [CrossRef] [PubMed]
- N’Souvi, K.; Sun, C.; Che, B.; Vodounon, A. Shrimp Industry in China: Overview of the Trends in the Production, Imports and Exports during the Last Two Decades, Challenges, and Outlook. Front. Sustain. Food Syst. 2024, 7, 1287034. [Google Scholar] [CrossRef]
- Liao, I.C.; Chien, Y.-H. The Pacific White Shrimp, Litopenaeus vannamei, in Asia: The World’s Most Widely Cultured Alien Crustacean. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 489–519. ISBN 978-94-007-0590-6. [Google Scholar]
- Zhang, C.; Guo, C.-Y.; Shu, K.-H.; Xu, S.-L.; Wang, D.-L. Comparative Analysis of the Growth Performance, Vitality, Body Chemical Composition and Economic Efficiency of the Main Cultivated Strains of Pacific White Shrimp (Litopenaeus vannamei) in Coastal Areas of China. Aquaculture 2024, 587, 740856. [Google Scholar] [CrossRef]
- Nakano, K.; Ashida, K. Role of Some Hormones in Protein Sparing Action of Dietary Fat. Agric. Biol. Chem. 1975, 39, 1233–1238. [Google Scholar] [CrossRef][Green Version]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effect of Dietary Protein and Lipid Level on Growth, Feed Utilization, and Muscle Composition in Golden Mandarin Fish Siniperca Scherzeri. Fish. Aquat. Sci. 2017, 20, 7. [Google Scholar] [CrossRef]
- Ma, J.; Kong, L.; Zhou, S.; Lin, H.; Lin, Y.; Qin, H.; Long, Z.; Liu, L.; Huang, Z.; Li, Z. Effect of Supplementation of Chlorogenic Acid to High-Fat Diet on Growth, Lipid Metabolism, Intestinal and Hepatic Histology, and Gut Microbiota of Spotted Sea Bass (Lateolabrax maculatus). Metabolites 2023, 13, 1067. [Google Scholar] [CrossRef]
- Gong, H.; Lawrence, A.L.; Jiang, D.-H.; Gatlin, D.M. Lipid Nutrition of Juvenile Litopenaeus vannamei. Aquaculture 2000, 190, 325–342. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, M.; Feng, Y.; Dong, Q.; Cui, M. Lysophospholipids and Their G-Coupled Protein Signaling in Alzheimer’s Disease: From Physiological Performance to Pathological Impairment. Front. Mol. Neurosci. 2020, 13, 58. [Google Scholar] [CrossRef]
- Heringdorf, D.M. zu Lysophospholipids. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 710–716. ISBN 978-3-540-38918-7. [Google Scholar]
- Sedghi, M.; Javanmard, F.; Amoozmehr, A.; Zamany, S.; Mohammadi, I.; Kim, W.; Choppa, V.S.R. Lysophospholipid Supplementation in Broiler Breeders’ Diet Benefits Offspring’s Productive Performance, Blood Parameters, and Hepatic β-Oxidation Genes. Animals 2024, 14, 3066. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, H.; Zhao, Y.; Wang, R.; Li, G.; Zhang, G.; Zhang, Y. Effects of Dietary Lysophospholipid Inclusion on the Growth Performance, Nutrient Digestibility, Nitrogen Utilization, and Blood Metabolites of Finishing Beef Cattle. Antioxidants 2022, 11, 1486. [Google Scholar] [CrossRef]
- Grove, S.S.; Dall, J.; Madsen, J.G. The Effect of Lysophospholipids and Sex on Growth Performance and Small Intestine Morphology in Weanling Pigs, 7–30 Kg. Animals 2024, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Z.; Sun, Y.; Wang, S.; Huang, B.; Wang, J. Effects of Dietary Lysolecithin (LPC) on Growth, Apparent Digestibility of Nutrient and Lipid Metabolism in Juvenile Turbot Scophthalmus Maximus L. Aquac. Fish. 2019, 4, 61–66. [Google Scholar] [CrossRef]
- Liu, G.; Ma, S.; Chen, F.; Gao, W.; Zhang, W.; Mai, K. Effects of Dietary Lysolecithin on Growth Performance, Feed Utilization, Intestinal Morphology and Metabolic Responses of Channel Catfish (Ictalurus punctatus). Aquacult Nutr. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- Weng, M.; Zhang, W.; Zhang, Z.; Tang, Y.; Lai, W.; Dan, Z.; Liu, Y.; Zheng, J.; Gao, S.; Mai, K.; et al. Effects of Dietary Lysolecithin on Growth Performance, Serum Biochemical Indexes, Antioxidant Capacity, Lipid Metabolism and Inflammation-Related Genes Expression of Juvenile Large Yellow Croaker (Larimichthys crocea). Fish Shellfish. Immunol. 2022, 128, 50–59. [Google Scholar] [CrossRef]
- Taghavizadeh, M.; Hosseini Shekarabi, S.P.; Mehrgan, M.S.; Islami, H.R. Efficacy of Dietary Lysophospholipids (LipidolTM) on Growth Performance, Serum Immuno-Biochemical Parameters, and the Expression of Immune and Antioxidant-Related Genes in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2020, 525, 735315. [Google Scholar] [CrossRef]
- Yan, M.; Wang, W.; Huang, X.; Wang, X.; Wang, Y. Interactive Effects of Dietary Cholesterol and Phospholipids on the Growth Performance, Expression of Immune-Related Genes and Resistance against Vibrio Alginolyticus in White Shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2020, 97, 100–107. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Dietary Optimal Reduced Glutathione Improves Innate Immunity, Oxidative Stress Resistance and Detoxification Function of Grass Carp (Ctenopharyngodon idella) against Microcystin-LR. Aquaculture 2019, 498, 594–605. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−LiangMethod. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Tan, P.; Xu, D.; Wang, L.; Ding, Z.; Shao, Q. Yarrowia Lipolytica as a Promising Protein Source for Pacific White Shrimp (Litopenaeus vannamei) Diet: Impact on Growth Performance, Metabolism, Antioxidant Capacity, and Apparent Digestibility. Front. Mar. Sci. 2024, 11, 1370371. [Google Scholar] [CrossRef]
- Qiao, X.-L.; Liang, Q.-J.; Liu, Y.; Wang, W.-N. A Novel Kelch-Like-1 Is Involved in Antioxidant Response by Regulating Antioxidant Enzyme System in Penaeus Vannamei. Genes 2020, 11, 1077. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhu, B.; Yun, J.; Yang, Y.; Tian, J.; Xu, W.; Du, X.; Zhao, Y.; Li, Y. Comparison of Antioxidant Capacity and Immune Response between Low Salinity Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Litopenaeus vannamei). Aquacult Int. 2024, 32, 1879–1894. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, W.; Zhao, R.; Gu, C.; Li, H.; Wang, L.; Wan, X. Effects of Cold Stress on the Hemolymph of the Pacific White Shrimp Penaeus Vannamei. Fishes 2024, 9, 36. [Google Scholar] [CrossRef]
- Holme, M.-H.; Southgate, P.C.; Zeng, C. Assessment of Dietary Lecithin and Cholesterol Requirements of Mud Crab, Scylla Serrata, Megalopa Using Semi-Purified Microbound Diets. Aquac. Nutr. 2007, 13, 413–423. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Feng, W.; He, J.; Han, T.; Wang, J.; Wang, C. Dietary Phosphatidylcholine Improved the Survival, Growth Performance, Antioxidant, and Osmoregulation Ability of Early Juvenile Mud Crab Scylla Paramamosain. Aquaculture 2023, 563, 738899. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, M.; Hu, S.; Wang, M. Keap1-Nrf2 Pathway: A Key Mechanism in the Occurrence and Development of Cancer. Front. Oncol. 2024, 14, 1381467. [Google Scholar] [CrossRef]
- Wang, Z.; Aweya, J.J.; Yao, D.; Zheng, Z.; Wang, C.; Zhao, Y.; Li, S.; Zhang, Y. Taurine Metabolism Is Modulated in Vibrio-Infected Penaeus Vannamei to Shape Shrimp Antibacterial Response and Survival. Microbiome 2022, 10, 213. [Google Scholar] [CrossRef]
- Tian, W.; Rojo De La Vega, M.; Schmidlin, C.J.; Ooi, A.; Zhang, D.D. Kelch-like ECH-Associated Protein 1 (KEAP1) Differentially Regulates Nuclear Factor Erythroid-2–Related Factors 1 and 2 (NRF1 and NRF2). J. Biol. Chem. 2018, 293, 2029–2040. [Google Scholar] [CrossRef]
- Ibrahim, L.; Mesgarzadeh, J.; Xu, I.; Powers, E.T.; Wiseman, R.L.; Bollong, M.J. Defining the Functional Targets of Cap‘n’Collar Transcription Factors NRF1, NRF2, and NRF3. Antioxidants 2020, 9, 1025. [Google Scholar] [CrossRef]
- Sykiotis, G.P. Keap1/Nrf2 Signaling Pathway. Antioxidants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Palliyath, G.K.; Jangam, A.K.; Katneni, V.K.; Kaikkolante, N.; Panjan Nathamuni, S.; Jayaraman, R.; Jagabattula, S.; Moturi, M.; Shekhar, M.S. Meta-Analysis to Unravel Core Transcriptomic Responses in Penaeus Vannamei Exposed to Biotic and Abiotic Stresses. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Sangklai, N.; Supungul, P.; Jaroenlak, P.; Tassanakajon, A. Immune Signaling of Litopenaeus vannamei C-Type Lysozyme and Its Role during Microsporidian Enterocytozoon Hepatopenaei (EHP) Infection. PLoS Pathog. 2024, 20, e1012199. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma- and Garlic-Derived Hybrids. Front. Pharmacol. 2020, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, H.; Xu, X.; Saw, P.E.; Zhang, L. Nanocarrier-Mediated Antioxidant Delivery for Liver Diseases. Theranostics 2020, 10, 1262–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, Z.; Turchini, G.M.; Wang, X.; Fang, Z.; Chen, N.; Xie, R.; Zhang, H.; Li, S. Effects of Dietary Phospholipids on Growth Performance, Digestive Enzymes Activity and Intestinal Health of Largemouth Bass (Micropterus salmoides) Larvae. Front. Immunol. 2022, 12, 827946. [Google Scholar] [CrossRef]
- Amer, A.-R.; Eweedah, N.M.; Amer, A.A.; Gewaily, M.S.; Younis, N.A.; Ahmed, H.A.; Dawood, M.A.O. Dietary Effect of Soybean Lecithin on the Growth Performance, Digestive Enzyme Activity, Blood Biomarkers, and Antioxidative Status of Striped Catfish, Pangasianodon hypophthalmus. PLoS ONE 2023, 18, e0291954. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Shehata, A.M.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Selim, D.A.; Abdo, M.; Khafaga, A.F.; El-Tarabily, K.A.; El-Shall, N.A.; Abd El-Hack, M.E. A Review of Shrimp Aquaculture and Factors Affecting the Gut Microbiome. Aquacult Int. 2022, 30, 2847–2869. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut Microbiota of Marine Fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Bruno, A.; Sandionigi, A.; Panio, A.; Rimoldi, S.; Orizio, F.; Agostinetto, G.; Hasan, I.; Gasco, L.; Terova, G.; Labra, M. Aquaculture Ecosystem Microbiome at the Water-Fish Interface: The Case-Study of Rainbow Trout Fed with Tenebrio Molitor Novel Diets. BMC Microbiol. 2023, 23, 248. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Ding, X.; Xiong, D.; Zhang, J. Response of Intestine Microbiota, Digestion, and Immunity in Pacific White Shrimp Litopenaeus vannamei to Dietary Succinate. Aquaculture 2020, 517, 734762. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.; Wan, T.; Tang, L.; Sun, B.; Zhou, B.; Lam, J.C.W.; Lam, P.K.S.; Chen, L. Disturbances in Microbial and Metabolic Communication across the Gut–Liver Axis Induced by a Dioxin-like Pollutant: An Integrated Metagenomics and Metabolomics Analysis. Environ. Sci. Technol. 2021, 55, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Feldman, N.; Zarodkiewicz, P.; Shropshire, H.; Biamis, C.; El-Halfawy, O.M.; McCain, J.; Dezanet, C.; Décout, J.-L.; Chen, Y.; et al. An Evolutionary Conserved Detoxification System for Membrane Lipid-Derived Peroxyl Radicals in Gram-Negative Bacteria. PLoS Biol. 2022, 20, e3001610. [Google Scholar] [CrossRef]
- DeLong, E.F. Microbial Community Genomics in the Ocean. Nat. Rev. Microbiol. 2005, 3, 459–469. [Google Scholar] [CrossRef] [PubMed]
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Ingredients a | |||||||
| Fish meal | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean meal | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Wheat flour | 21.99 | 23.99 | 23.89 | 23.49 | 22.99 | 22.49 | 21.99 |
| Krill meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Peanut meal | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 |
| Brewer’s yeast | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Multi-minerals b | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Multi-vitamins c | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Soybean phospholipids | 2.00 | ||||||
| Lysophospholipids d | 0 | 0.1 | 0.5 | 1 | 1.5 | 2 | |
| Vc phosphate | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Sodium alginate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Y2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional Levels e | |||||||
| Moisture | 5.10 | 5.40 | 5.80 | 7.80 | 7.80 | 8.93 | 8.70 |
| Crude fat | 6.01 | 5.61 | 5.91 | 6.5 | 6.99 | 7.27 | 7.46 |
| Crude protein | 41.28 | 41.34 | 40.63 | 40.23 | 40.14 | 39.04 | 38.38 |
| Crude ash | 10.60 | 10.56 | 10.49 | 10.23 | 10.5 | 10.15 | 10.04 |
| cDNA | Forward Primer (5′~3′) | Reverse Primer (5′~3′) | Size of Production (bp) | GenBank Nos. |
|---|---|---|---|---|
| β-actin | CGAGGTATCCTCACCCTGAA | GTCATCTTCTCGCGGTTAGC | 176 | AF300705.2 |
| Antioxidant-Related Genes | ||||
| Nrf1 | TCCCAAGAGTGAGACAAAGATT | CACCAGTCTCAGAGTCGATA | 86 | XM_027363478.1 |
| Nrf2 | TCTTGTTGGTCCCTCGCTCCTC | TCACTGCTTGGGGTCATCCTTCC | 89 | XM_027367070.1 |
| GPx | GGCACCAGGAGAACACTAC | CGACTTTGCCGAACATAAC | 102 | AY973252.2 |
| SOD | GCAATGAATGCCCTTCTACC | CAGAGCCTTTCACTCCAACG | 199 | XM_027376216.1 |
| CAT | TACTGCAAGTTCCATTACAAGACG | GTAATTCTTTGGATTGCGGTCA | 285 | XM_027383088.1 |
| Hippo | TGAGCACAACCAAACCCACCATC | CATCGTCCGACTGTCCACTTCATC | 86 | MW415984.1 |
| Digestion-Related Genes | ||||
| trypsin | CGGAGAGCTGCCTTACCAG | TCGGGGTTGTTCATGTCCTC | 141 | PP817223.1 |
| α-amylase | CTCTGGTAGTGCTGTTGGCT | TGTCTTACGTGGGACTGGAAG | 116 | AJ133526.3 |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| T-AOC (U/mL) | 2.18 ± 0.30 a | 2.91 ± 0.27 ab | 4.46 ± 0.87 b | 3.39 ± 0.35 ab | 3.42 ± 1.59 ab | 3.77 ± 1.00 ab | 2.19 ± 0.11 a |
| SOD (U/mL) | 62.05 ± 8.52 a | 73.61 ± 2.66 b | 75.61 ± 6.61 b | 75.25 ± 3.83 b | 73.07 ± 0.47 b | 78.84 ± 6.72 b | 80.69 ± 5.68 b |
| GSH-Px (U/mL) | 144.62 ± 39.80 a | 174.07 ± 34.89 a | 189.44 ± 20.10 a | 172.81 ± 29.56 a | 298.52 ± 33.04 b | 276.56 ± 30.84 b | 186.16 ± 27.88 a |
| CAT (U/mL) | 8.33 ± 0.31 d | 5.62 ± 0.48 a | 6.74 ± 0.31 b | 9.99 ± 0.33 e | 9.56 ± 0.33 e | 9.69 ± 0.32 e | 7.39 ± 0.27 c |
| MDA (nmol/mL) | 18.65 ± 1.85 a | 16.55 ± 1.65 a | 15.64 ± 2.24 a | 28.42 ± 2.34 bc | 21.69 ± 5.90 ab | 27.55 ± 2.01 bc | 30.21 ± 7.49 c |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Trypsin | 66.63 ± 3.92 b | 91.79 ± 8.60 d | 81.75 ± 11.16 cd | 80.02 ± 1.95 cd | 123.35 ± 5.48 e | 70.48 ± 3.63 bc | 48.38 ± 7.62 a |
| AMS | 1.17 ± 0.26 a | 2.13 ± 0.04 bc | 1.90 ± 0.09 b | 2.61 ± 0.24 d | 2.58 ± 0.22 d | 2.24 ± 0.06 bcd | 2.50 ± 0.17 cd |
| LPS | 3.75 ± 0.52 cd | 3.62 ± 0.27 cd | 3.98 ± 0.21 d | 3.37 ± 0.18 abc | 3.45 ± 0.16 bc | 2.94 ± 0.20 a | 3.06 ± 0.10 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Wang, Y.; Liang, H.; Duan, Y.; Wang, J.; Zhou, C.; Huang, Z. Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei. Biology 2025, 14, 90. https://doi.org/10.3390/biology14010090
Yan H, Wang Y, Liang H, Duan Y, Wang J, Zhou C, Huang Z. Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei. Biology. 2025; 14(1):90. https://doi.org/10.3390/biology14010090
Chicago/Turabian StyleYan, Hailiang, Yun Wang, Hong Liang, Yafei Duan, Jun Wang, Chuanpeng Zhou, and Zhong Huang. 2025. "Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei" Biology 14, no. 1: 90. https://doi.org/10.3390/biology14010090
APA StyleYan, H., Wang, Y., Liang, H., Duan, Y., Wang, J., Zhou, C., & Huang, Z. (2025). Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei. Biology, 14(1), 90. https://doi.org/10.3390/biology14010090








