Differential Proneness to Obesity in Two Rat Strains with Diverse Immune Responses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Mass, Lee Obesity Index, and Abdominal and Thoracic Circumferences
2.3. Fasting Blood Glucose, Glucose Tolerance, and Insulin Resistance Test
2.4. Clinical Biochemistry
2.5. Coagulation Parameters
2.6. Oxidative Stress Measurement in Peripheral Blood
2.7. Isolation and Culture of Peripheral Blood Mononuclear and Polymorphonuclear Cells
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Bacterial Microbiota Analysis
2.10. Statistical Analysis
3. Results
3.1. Body Mass and Lee Obesity Index in DA and AO Rats
3.2. DA and AO Rats Differently Respond to Intraperitoneal Glucose and Insulin
3.3. Serum Lipid Levels in DA and AO Rats
3.4. Hematological Parameters in DA and AO Rats
3.5. DA and AO Rats Differ in Hemostasis
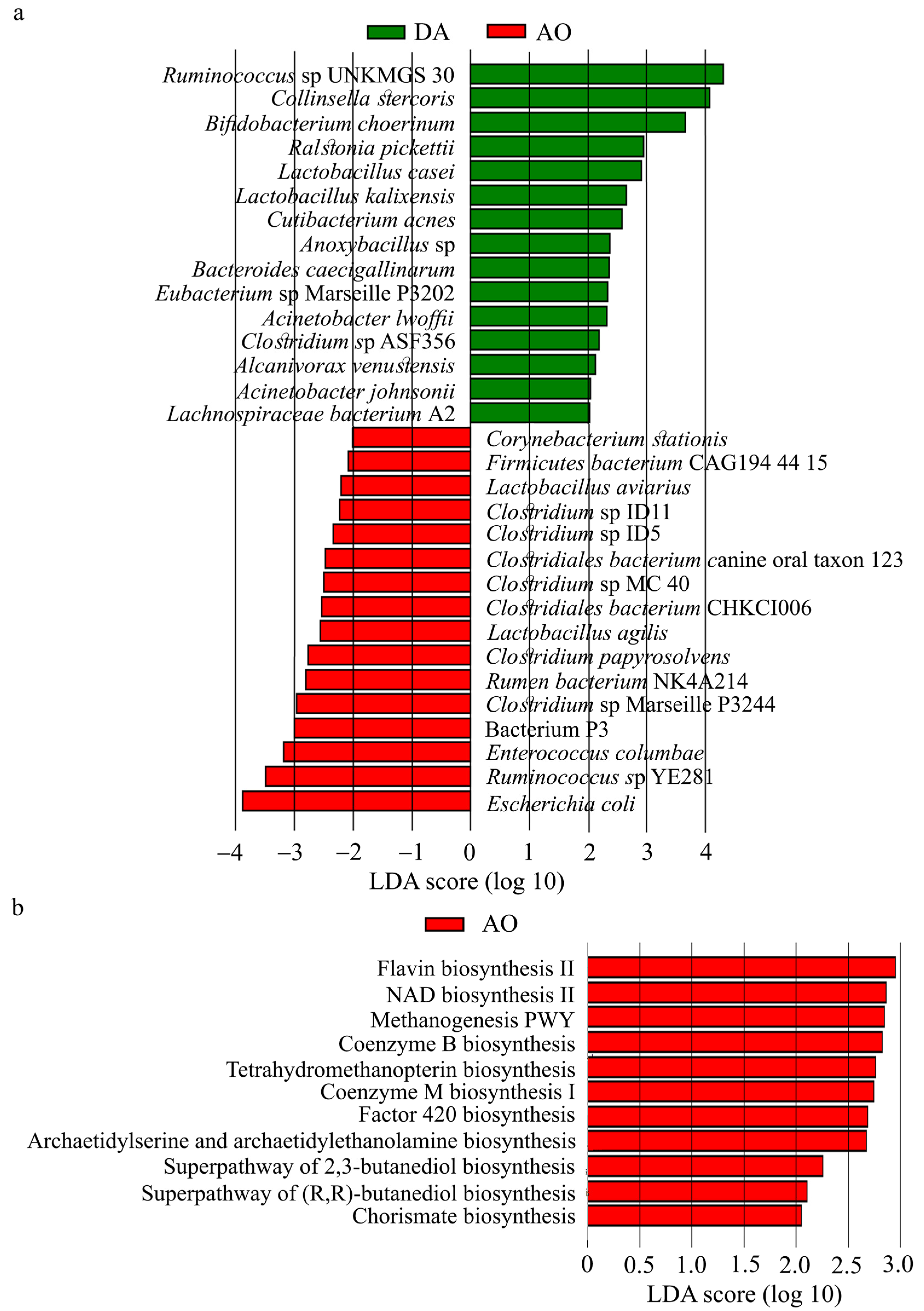
3.6. Gut Microbiota in DA and AO Rats
3.7. Oxidative Stress and Inflammation in Peripheral Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DA rats | rats of Dark Agouti rat strain |
| AO rats | rats of Albino Oxford rat strain |
| HDL | high-density lipoprotein cholesterol |
| LDL | low-density lipoprotein cholesterol |
| AC | abdominal circumference |
| TC | thoracic circumference |
| BMI | body mass index |
| MCV | mean corpuscular volume |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| GTT | glucose tolerance test |
| ITT | insulin tolerance test |
| PT | prothrombin time |
| PTT | partial thromboplastin time |
| PDW | platelet distribution width |
| PCT | plateletcrit. |
| SOD | superoxide dismutase |
| CAT | catalase |
| MDA | malondialdehyde |
| PBMC | peripheral blood mononuclear cells |
| PMN | polymorphonuclear cells |
| IL | interleukin |
| IFN-γ | interferon gamma |
| TNF | tumor necrosis factor |
| MHC II | major histocompatibility complex class II molecule |
| LPS | lipopolysaccharide |
| TLR | toll-like receptors |
References
- Noubiap, J.J.; Jobert, R.N., Jr.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ha, S.; Lau, H.C.H.; Yu, J. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin. Cancer Biol. 2023, 92, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic syndrome-related kidney injury: A review and update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Kwitek, A.E. Rat models of metabolic syndrome. Methods Mol. Biol. 2019, 2018, 269–285. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; Gonzáles-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Palma-Jacinto, J.A.; Santiago-Roque, I.; Arroyo Helguera, O.E. Hypercaloric cafeteria diet-induces obesity, dyslipidemia, insulin resistance, inflammation and oxidative stress in Wistar rats. J. Exp. Life Sci. 2023, 3, 17–23. [Google Scholar]
- Geerling, E.; Stone, E.T.; Steffen, T.L.; Hassert, M.; Brien, J.D.; Pinto, A.K. Obesity enhances disease severity in female mice following West Nile virus infection. Front. Immunol. 2021, 12, 739025. [Google Scholar] [CrossRef]
- Smith, A.G.; Sheridan, P.A.; Harp, J.B.; Beck, M.A. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J. Nutr. 2007, 137, 1236–1243. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.K.; Kim, D.J.; Nam, J.H.; Shim, S.M.; Choi, Y.K.; Lee, C.H.; Poo, H. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J. Infect. Dis. 2012, 205, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Otranto, M.; do Nascimento, A.P.; Monte-Alto-Costa, A. Insulin resistance impairs cutaneous wound healing in mice. Wound Rep. Regen. 2013, 21, 464–472. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.B.; Vogel, P.; Duan, S.; Govorkova, E.A.; Webby, R.J.; McCullers, J.A.; Schultz-Cherry, S. Impaired wound healing predispose obese mice to severe influenza virus infection. J. Infect. Dis. 2012, 205, 252–261. [Google Scholar] [CrossRef]
- Pérez-Echarri, N.; Pérez-Matute, P.; Marcos-Gómez, B.; Baena, M.J.; Marti, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: Effects of EPA ethyl ester treatment. Eur. J. Nutr. 2008, 47, 380–386. [Google Scholar] [CrossRef]
- Arnke, K.; Pfister, P.; Reid, G.; Vasella, M.; Ruhl, T.; Seitz, A.K.; Lindenblatt, N.; Cinelli, P.; Kim, B.S. Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice. Int. J. Mol. Sci. 2023, 24, 17299. [Google Scholar] [CrossRef]
- Ghanim, H.; Aljada, A.; Hofmeyer, D.; Syed, T.; Mohanty, P.; Dandona, P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004, 110, 1564–1571. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef]
- Reed, D.R.; Duke, F.F.; Ellis, H.K.; Rosazza, M.R.; Lawler, M.P.; Alarcon, L.K.; Tordoff, M.G. Body fat distribution and organ weights of 14 common strains and a 22-strain consomic panel of rats. Physiol. Behav. 2011, 103, 523–5299. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Barz, T.; Ekkernkamp, A.; Wilke, B.; Klöting, I.; Follak, N. Phenotypic and gene expression differences between DA, BN and WOKW Rats. PLoS ONE 2012, 7, e38981. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Kusnecov, A.W.; Riechman, S.E.; Rabin, B.S. Effect of a conditioned aversive stimulus on the immune response in three strains of rats. Psychoneuroendocrinology 1995, 20, 837–849. [Google Scholar] [CrossRef]
- Gustafsson, A.; Jonasson, S.; Sandstrom, T.; Lorentzen, J.C.; Bucht, A. Genetic variation influences immune response in sensitive rats following exposure to TiO2 nanoparticles. Toxicology 2014, 326, 74–85. [Google Scholar] [CrossRef]
- Griffin, A.C.; Whitacre, C.C. Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rat: Implications for rodent models of autoimmune disease. J. Neuroimmunol. 1991, 35, 53–64. [Google Scholar] [CrossRef]
- Knippels, L.M.J.; Penninks, A.H.; van Meeteren, M.; Houben, G.F. Humoral and cellular immune responses in different rat strains on oral exposure to ovalbumin. Food Chem. Toxicol. 1999, 37, 881–888. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and metabolic syndrome on immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Djedovic, N.; Jevtić, B.; Mansilla, M.J.; Petković, F.; Blaževski, J.; Timotijević, G.; Navarro-Barriuso, J.; Martinez-Caceres, E.; Mostarica Stojković, M.; Miljković, Đ. Comparison of dendritic cells obtained from autoimmunty-prone and resistant rats. Immunobiology 2019, 224, 470–476. [Google Scholar] [CrossRef]
- Nacka-Aleksić, M.; Stojanović, M.; Pilipović, I.; Stojić-Vukanić, Z.; Kosec, D.; Leposavić, G. Strain differences in thymic atrophy in rats immunized for EAE correlate with the clinical outcome of immunization. PLoS ONE 2018, 3, e0201848. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Laban, O.; Djurić, V.J.; Stanojević, S.; Miletić, T.; Kovačević-Jovanović, V.; Todorović, Č.; Radulović, J. Behavior and severity of adjuvant arthritis in four rat strains. Brain Behav. Immun. 2001, 15, 255–265. [Google Scholar] [CrossRef]
- Arsov, I.; Pravica, V.; Ejdus, L.; Badovinac, V.; Mostarica, M.; Lukić, M.L. Selection for susceptibility to experimental allergic encephalomyelitis also selects for high IFN-gamma production. Transplant. Proc. 1995, 27, 1537–1538. [Google Scholar] [PubMed]
- Miljkovic, D.; Stosic-Grujicic, S.; Markovic, M.; Momcilovic, M.; Ramic, Z.; Maksimovic-Ivanic, D.; Mijatovic, S.; Popadic, D.; Cvetkovic, I.; Mostarica-Stojkovic, M. Strain difference in susceptibility to experimental autoimmune encephalomyelitis between Albino Oxford and Dark Agouti rats correlates with disparity in production of IL-17, but not nitric oxide. J. Neurosci. Res. 2006, 84, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, S.; Lukić, J.; Soković, S.; Mihajlovic, S.; Mostarica Stojković, M.; Miljković, D.; Golić, N. Correlation of Gut Microbiota Composition with Resistance to Experimental Autoimmune Encephalomyelitis in Rats. Front. Microbiol. 2016, 7, 2005. [Google Scholar] [CrossRef] [PubMed]
- Popov Aleksandrov, A.; Mirkov, I.; Demenesku, J.; Ninkov, M.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Strain differences in contact hypersensitivity reaction to dinitrochlorobenzene (DNCB) in rats. Food Chem. Toxicol. 2015, 75, 94–103. [Google Scholar] [CrossRef]
- Stanojević, S.; Kuštrimović, N.; Mitić, K.; Vujić, V.; Dimitrijević, M. Role of Mast Cells and C-Sensory Fibers in Concanavalin A-Induced Paw Edema in Two Rat Strains. Inflammation 2015, 38, 1434–4149. [Google Scholar] [CrossRef]
- Tucovic, D.; Mirkov, I.; Kulas, J.; Zeljkovic, M.; Popovic, D.; Zolotarevski, L.; Djurdjic, S.; Mutic, J.; Kataranovski, M.; Popov Aleksandrov, A. Dermatotoxicity of oral cadmium is strain-dependent and related to differences in skin stress response and inflammatory/immune activity. Environ. Toxicol. Pharmacol. 2020, 75, 103326. [Google Scholar] [CrossRef]
- Mirkov, I.; Popov Aleksandrov, A.; Ninkov, M.; Mileusnic, D.; Demenesku, J.; Zolotarevski, L.; Subota, V.; Stefik, D.; Kataranovski, D.; Kataranovski, M. Strain differences in intestinal toxicity of warfarin in rats. Environ. Toxicol. Pharmacol. 2016, 48, 175–182. [Google Scholar] [CrossRef]
- Stanojević, S.; Kuštrimović, N.; Mitić, K.; Vujić, V.; Aleksić, I.; Dimitrijević, M. Peritoneal mast cell degranulation differently affected thioglycollate-induced macrophage phenotype and activity in Dark Agouti and Albino Oxford rats. Life Sci. 2013, 93, 564–572. [Google Scholar] [CrossRef]
- Miletić, T.; Kovačević-Jovanović, V.; Vujić, V.; Stanojević, S.; Mitić, K.; Lazarević-Macanović, M.; Dimitrijević, M. Reactive oxygen species (ROS), but not nitric oxide (NO), contribute to strain differences in the susceptibility to experimental arthritis in rats. Immunobiology 2007, 212, 95–105. [Google Scholar] [CrossRef]
- Blagojević, V.; Kovačević-Jovanović, V.; Ćuruvija, I.; Petrović, R.; Vujnović, I.; Vujić, V.; Stanojević, S. Rat strain differences in peritoneal immune cell response to selected gut microbiota: A crossroad between tolerance and autoimmunity? Life Sci. 2018, 197, 147–157. [Google Scholar] [CrossRef]
- Stanojević, S.; Blagojević, V.; Ćuruvija, I.; Vujić, V. Lactobacillus rhamnosus Affects Rat Peritoneal Cavity Cell Response to Stimulation with Gut Microbiota: Focus on the Host Innate Immunity. Inflammation 2021, 44, 2429–2447. [Google Scholar] [CrossRef] [PubMed]
- Grubić-Kezele, T.; Blagojević Zagorac, G.; Jakovac, H.; Domitrović, R.; Milin, C.; Radošević-Stašić, B. Hepatic expression of metallothionein I/II, glycoprotein 96, IL-6, and TGF-β in rat strains with different susceptibilities to experimental autoimmune encephalomyelitis. Clin. Dev. Immunol. 2013, 2013, 750406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van den Brandt, J.; Kovacs, P.; Klöting, I. Metabolic syndrome and aging in Wistar Ottawa Karlsburg W rats. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.D.; Sardi, G.L.; Reed, J.F., 3rd. Components of the metabolic syndrome differ between young and old adults in the US population. J. Clin. Hypertens. 2012, 14, 502–506. [Google Scholar] [CrossRef]
- Hattis, D.; Goble, R.; Chu, M. Age-related differences in susceptibility to carcinogenesis. II. Approaches for application and uncertainty analyses for individual genetically acting carcinogens. Environ. Health Perspect. 2005, 113, 509–516. [Google Scholar] [CrossRef][Green Version]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431–1445. [Google Scholar] [CrossRef]
- Finegood, D.T.; Scaglia, L.; Bonner-Weir, S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 1995, 44, 249–256. [Google Scholar] [CrossRef]
- Devaraj, S.; Rosenson, R.S.; Jialal, I. Metabolic syndrome: An appraisal of the pro-inflammatory and procoagulant status. Endocrinol. Metab. Clin. N. Am. 2004, 33, 431–453. [Google Scholar] [CrossRef]
- Leo, F.; Rossodivita, A.N.; Segni, C.D.; Raimondo, S.; Canichella, S.; Silvestrini, A.; Miggiano, G.A.; Meucci, E.; Mancini, A. Frailty of Obese Children: Evaluation of Plasma Antioxidant Capacity in Pediatric Obesity. Exp. Clin. Endocrinol. Diabetes. 2016, 124, 481–486. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, Z.V.; Ellis, G.S. Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. J. Appl. Physiol. 1991, 71, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kalkhoff, R.K. Sex steroid influence on triglyceride metabolism. J. Clin. Investig. 1975, 56, 888–896. [Google Scholar] [CrossRef]
- Nunes-Souza, V.; César-Gomes, C.J.; Da Fonseca, L.J.; Guedes Gda, S.; Smaniotto, S.; Rabelo, L.A. Aging Increases Susceptibility to High Fat Diet-Induced Metabolic Syndrome in C57BL/6 Mice: Improvement in Glycemic and Lipid Profile after Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 2016, 1987960. [Google Scholar] [CrossRef]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol. Content 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Friedwald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Pogorzelska, K.; Krętowska, A.; Krawczuk-Rybak, M.; Sawicka-Żukowska, M. Characteristics of platelet indices and their prognostic significance in selected medical condition—A systematic review. Adv. Med. Sci. 2020, 65, 310–315. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Beutler, E. (Ed.) Catalase. In Red Cell Metabolism: A Manual of Biochemical Methods; Grune and Stratton: New York, NY, USA, 1982; pp. 105–106. [Google Scholar]
- Villacara, A.; Kumami, K.; Yamamoto, T.; Mrsulja, B.B.; Spatz, M. Ischemic modification of cerebrocortical membranes: 5-hydroxytryptamine receptors, fluidity, and inducible in vitro lipid peroxidation. J. Neurochem. 1989, 53, 595–601. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- QIIME. 2021. Available online: http://qiime.org/scripts/assign_taxonomy.html (accessed on 15 July 2021).
- Max Plank Institute for Marine Microbiology and Jacobs University, Bremen, Germany. The SILVA Ribosomal RNA Database. 2021. Available online: http://www.arb-silva.de/ (accessed on 15 July 2021).
- The Huttenhower Lab, Harvard. LEfSe Platform from Galaxy. 2024. Available online: http://mbac.gmu.edu/mbac_wp/ (accessed on 10 February 2024).
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Chikopela, T.; Heimburger, D.C.; Kaluba, L.; Hamambulu, P.; Simfukwe, N.; Mutale, W.; Koethe, J.R.; Goma, F. Endothelial dysfunction and body mass index: Is there a role for plasma peroxynitrite? Beni Suef Univ. J. Basic. Appl. Sci. 2021, 10, 4. [Google Scholar] [CrossRef]
- Simson, E.L.; Gold, R.M. The Lee Obesity Index vindicated? Physiol. Behav. 1982, 29, 371–376. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Russo, I. The prothrombotic tendency in metabolic syndrome: Focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica 2012, 2012, 525374. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef]
- Popovic, D.; Kulas, J.; Tucovic, D.; Popov Aleksandrov, A.; Malesevic, A.; Glamoclija, J.; Brdaric, E.; Sokovic Bajic, S.; Golic, N.; Mirkov, I.; et al. Gut microbial dysbiosis occurring during pulmonary fungal infection in rats is linked to inflammation and depends on healthy microbiota composition. Microbiol. Spectr. 2023, 11, e0199023. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, A.P.A.; Cordeiro, G.S.; Santos, L.S.; Santo, D.A.E.; Perez, G.S.; Couto, R.D.; Machado, M.E.P.C.; Medeiros, J.M.B. Murinometric measurements and retroperitoneal adipose tissue in young rats exposed to the high-fat diet: Is there correlation? Braz. J. Biol. 2021, 81, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Auberval, N.; Dal, S.; Bietiger, W.; Pinget, M.; Jeandidier, N.; Maillard-Pedracini, E.; Schini-Kerth, V.; Sigrist, S. Metabolic and oxidative stress markers in Wistar rats after 2 months on a high-fat diet. Diabetol. Metab. Syndr. 2014, 6, 130. [Google Scholar] [CrossRef]
- Nobukata, H.; Ishikawa, T.; Obata, M.; Shibutani, Y. Age-related changes in coagulation, fibrinolysis, and platelet aggregation in male WBN/Kob rats. Thromb. Res. 2000, 98, 507–516. [Google Scholar] [CrossRef]
- Wanyonyi, S.; du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a food supplement prevents diet-induced metabolic syndrome in rats. Nutrients 2017, 9, 1261. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Xu, R.; Li, S.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PLoS ONE 2014, 9, e115148. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Wang, N.; Zhou, Q.; Lu, Y.; Qu, Z.; Wang, H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021, 9, 4266–4277. [Google Scholar] [CrossRef]
- Lechleitner, M. Obesity and the metabolic syndrome in the elderly—A mini-review. Gerontology 2008, 54, 253–259. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Raber, M.N. Coagulation tests. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, K.H., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; pp. 739–742. [Google Scholar]
- Tzur, I.; Barchel, D.; Izhakian, S.; Swarka, M.; Garach-Jehoshua, O.; Krutkina, E.; Plotnikov, G.; Gorelik, O. Platelet distribution width: A novel prognostic marker in an internal medicine ward. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Solá, E.; Navarro, S.; Medina, P.; Vayá, A.; Estellés, A.; Hernández-Mijares, A.; España, F. Activated protein C levels in obesity and weight loss influence. Thromb. Res. 2009, 123, 697–700. [Google Scholar] [CrossRef] [PubMed]
- El-Menyar, A.; Asim, M.; Al-Thani, H. Obesity paradox in patients with deep venous thrombosis. Clin. Appl. Throm Hemost. 2018, 24, 986–992. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Sharp, P.; Villano, J. The Laboratory Rat, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kuwabara, W.M.T.; Yokota, C.N.F.; Curi, R.; Alba-Loureiro, T.C. Obesity and Type 2 diabetes mellitus induce lipopolysaccharide tolerance in rat neutrophils. Sci. Rep. 2018, 8, 17534. [Google Scholar] [CrossRef]
- Stanojević, S.; Ćuruvija, I.; Blagojević, V.; Petrović, R.; Vujić, V.; Dimitrijević, M. Strain-dependent response to stimulation in middle-aged rat macrophages: A quest after a useful indicator of healthy aging. Exp. Gerontol. 2016, 85, 95–107. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stanojević, S.; Vujić, V.; Aleksić, I.; Pilipović, I.; Leposavić, G. Aging oppositely affects TNF-α and IL-10 production by macrophages from different rat strains. Biogerontology 2014, 15, 475–486. [Google Scholar] [CrossRef]
- Rose, R.; Banerjee, A.; Ramaiah, S.K. Characterization of a lipopolysaccharide mediated neutrophilic hepatitis model in Sprague Dawley rats. J. Appl. Toxicol. 2007, 27, 602–611. [Google Scholar] [CrossRef]
- Nicoară, D.M.; Munteanu, A.I.; Scutca, A.C.; Mang, N.; Juganaru, I.; Brad, G.F.; Mărginean, O. Assessing the Relationship between Systemic Immune-Inflammation Index and Metabolic Syndrome in Children with Obesity. Int. J. Mol. Sci. 2023, 24, 8414. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Hao, N.; Chen, Z.; Wei, L.; Tan, L.; Chen, Y.; Feng, H.; Chen, Q.; Zhu, G. Simvastatin Reduces Neutrophils Infiltration into Brain Parenchyma After Intracerebral Hemorrhage via Regulating Peripheral Neutrophils Apoptosis. Front. Neurosci. 2018, 12, 977. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Orbe-Orihuela, Y.C.; Díaz-Benítez, C.E.; Castañeda-Márquez, A.C.; Cornejo-Granados, F.; Ochoa-Leyva, A.; Sanchez-Flores, A.; Cruz, M.; Burguete-García, A.I.; Lagunas-Martínez, A. Alterations of the Gut Microbiome Associated to Methane Metabolism in Mexican Children with Obesity. Children 2022, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes. Nutr. 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Laverdure, R.; Mezouari, A.; Carson, M.A.; Basiliko, N.; Gagnon, J. A role for methanogens and methane in the regulation of GLP-1. Endocrinol. Diabetes Metab. 2017, 1, e00006. [Google Scholar] [CrossRef] [PubMed]
- Basseri, R.J.; Basseri, B.; Pimentel, M.; Chong, K.; Youdim, A.; Low, K.; Hwang, L.; Soffer, E.; Chang, C.; Mathur, R. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol. Hepatol. 2012, 8, 22–28. [Google Scholar]
- Medina, G.; Vera-Lastra, O.; Peralta-Amaro, A.L.; Jiménez-Arellano, M.P.; Saavedra, M.A.; Cruz-Domínguez, M.P.; Jara, L.J. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol. Res. 2018, 133, 277–288. [Google Scholar] [CrossRef]
- Chen, K.; Sun, W.; He, L.; Dong, W.; Zhang, D.; Zhang, T.; Zhang, H. Exploring the bidirectional relationship between metabolic syndrome and thyroid autoimmunity: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1325417. [Google Scholar] [CrossRef]
- Mostarica-Stojković, M.; Petrović, M.; Lukić, M.L. Resistance to the induction of EAE in AO rats: Its prevention by the pre-treatment with cyclophosphamide or low dose of irradiation. Clin. Exp. Immunol. 1982, 50, 311–317. [Google Scholar]
- Lukić, M.L.; Stosić-Grujicić, S.; Shahin, A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev. Immunol. 1998, 6, 119–128. [Google Scholar] [CrossRef]
- Djuretić, J.; Pilipović, I.; Stojić-Vukanić, Z.; Leposavić, G. Natural killer cells as participants in pathogenesis of rat experimental autoimmune encephalomyelitis (EAE): Lessons from research on rats with distinct age and strain. Cent. Eur. J. Immunol. 2019, 44, 337–356. [Google Scholar] [CrossRef]
- Miljković, D.; Stanojević, Z.; Momcilović, M.; Odoardi, F.; Flügel, A.; Mostarica-Stojković, M. CXCL12 expression within the CNS contributes to the resistance against experimental autoimmune encephalomyelitis in Albino Oxford rats. Immunobiology 2011, 216, 979–987. [Google Scholar] [CrossRef]
- Miletić, T.; Kovačević-Jovanović, V.; Stanojević, S.; Vujic, V.G.; Kosec, D.; Mitić, K.V.; Dimitrijević, M. Strain Differences and the Role for HSP47 and HSP70 in Adjuvant Arthritis in Rats. Scand. J. Immunol. 2006, 64, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Stojić-Vukanić, Z.; Pilipović, I.; Vujnović, I.; Nacka-Aleksić, M.; Petrović, R.; Arsenović-Ranin, N.; Dimitrijević, M.; Leposavić, G. GM-CSF-Producing Th Cells in Rats Sensitive and Resistant to Experimental Autoimmune Encephalomyelitis. PLoS ONE 2016, 11, e0166498. [Google Scholar] [CrossRef] [PubMed]
- Stojić-Vukanić, Z.; Nacka-Aleksić, M.; Pilipović, I.; Vujnović, I.; Blagojević, V.; Kosec, D.; Dimitrijević, M.; Leposavić, G. Aging diminishes the resistance of AO rats to EAE: Putative role of enhanced generation of GM-CSF Expressing CD4+ T cells in aged rats. Immun. Ageing 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- BioRender. 2025. Available online: https://BioRender.com/x18w108 (accessed on 11 March 2025).






| DA | AO | |||
|---|---|---|---|---|
| Absolute | Per 100 g b.w. | Absolute | Per 100 g b.w. | |
| 3-month old animals | ||||
| Body mass (g) | 196.9 ± 20.1 | 245.7 ± 24.2 * | ||
| Naso-anal length (cm) | 21.2 ± 0.6 | 21.9 ± 0.9 | ||
| Food intake (g/day) | 17.3 ± 1.8 | 8.9 ± 1.7 | 22.6 ± 5.5 * | 9.7 ± 2.1 |
| Water intake (mL/day) | 23.8 ± 7.4 | 12.3 ± 4.7 | 33.1 ± 8.0 * | 14.4 ± 4.1 |
| Urine volume (mL/day) | 5.5 ± 2.1 | 2.8 ± 1.2 | 6.7 ± 1.1 | 3.0 ± 1.0 |
| Feces (g/day) | 9.2 ± 2.6 | 4.6 ± 1.4 | 10.1 ± 3.5 | 4.4 ± 1.4 |
| 6-month old animals | ||||
| Body mass (g) | 235.4 ± 7.2 | 371.5 ± 35.2 *** | ||
| Naso-anal length (cm) | 22.3 ± 0.4 | 24.4 ± 0.6 *** | ||
| Food intake (g/day) | 17.5 ± 3.7 | 7.5 ± 1.7 | 25.9 ± 4.5 *** | 7.3 ± 1.4 |
| Water intake (mL/day) | 21.1 ± 3.9 | 9.0 ± 1.8 | 31.5 ± 10.0 * | 8.9 ± 3.0 |
| Urine volume (mL/day) | 4.5 ± 2.4 | 1.9 ± 1.0 | 8.3 ± 4.1 * | 3.0 ± 1.0 |
| Feces (g/day) | 9.7 ± 3.0 | 4.1 ± 1.3 | 13.5 ± 4.1 | 3.8 ± 1.1 |
| DA | AO | |
|---|---|---|
| White blood cells (WBC) | ||
| Total WBC (×109/L) | 6.7 ± 1.6 | 4.2 ± 1.1 *** |
| Neutrophils (%) | 37.7 ± 6.6 | 44.3 ± 7.4 * |
| Lymphocytes (%) | 60.1 ± 6.6 | 52.4 ± 7.9 * |
| Monocytes (%) | 0.8 ± 0.2 | 1.0 ± 0.3 |
| Eosinophils (%) | 1.1 ± 0.3 | 1.8 ± 0.4 *** |
| Basophils (%) | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Red blood cells (RBC) | ||
| RBC number (×1012/L) | 8.1 ± 0.4 | 7.9 ± 0.5 * |
| Hemoglobin (g/L) | 136.7 ± 9.4 | 142.7 ± 8.0 * |
| Hematocrit (L/L) | 0.43 ± 0.03 | 0.43 ± 0.04 |
| MCV (fL) | 53.0 ± 1.0 | 56.3 ± 1.8 *** |
| MCH (pg) | 17.1 ± 0.8 | 18.3 ± 0.6 *** |
| MCHC (g/L) | 320.6 ± 7.7 | 326.4 ± 6.0 * |
| Neutrophil-to-lymphocyte ratio (NLR) | 0.61 ± 0.15 | 0.95 ± 0.28 *** |
| Systemic immune-inflammation index | 464.0 ± 149.8 | 737.0 ± 363.9 ** |
| DA | AO | |
|---|---|---|
| Alpha diversity | ||
| Observed species | 753.2 ± 165.9 | 807.5 ± 124.9 |
| Shannon index | 5.55 ± 1.36 | 5.87 ± 0.59 |
| Chao1 index | 847.9 ± 154.9 | 896.2 ± 170.1 |
| Relative abundance | ||
| Firmicutes | 0.645 ± 0.168 | 0.708 ± 0.078 |
| Bacteroidetes | 0.179 ± 0.141 | 0.092 ± 0.064 |
| Proteobacteria | 0.054 ± 0.078 | 0.033 ± 0.020 |
| Actinobacteria | 0.070 ± 0.064 | 0.024 ± 0.010 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucović, D.; Popov Aleksandrov, A.; Popović, D.; Malešević, A.; Subota, V.; Brdarić, E.; Soković Bajić, S.; Živković, M.; Kataranovski, M.; Mirkov, I.; et al. Differential Proneness to Obesity in Two Rat Strains with Diverse Immune Responses. Biology 2025, 14, 557. https://doi.org/10.3390/biology14050557
Tucović D, Popov Aleksandrov A, Popović D, Malešević A, Subota V, Brdarić E, Soković Bajić S, Živković M, Kataranovski M, Mirkov I, et al. Differential Proneness to Obesity in Two Rat Strains with Diverse Immune Responses. Biology. 2025; 14(5):557. https://doi.org/10.3390/biology14050557
Chicago/Turabian StyleTucović, Dina, Aleksandra Popov Aleksandrov, Dušanka Popović, Anastasija Malešević, Vesna Subota, Emilija Brdarić, Svetlana Soković Bajić, Milica Živković, Milena Kataranovski, Ivana Mirkov, and et al. 2025. "Differential Proneness to Obesity in Two Rat Strains with Diverse Immune Responses" Biology 14, no. 5: 557. https://doi.org/10.3390/biology14050557
APA StyleTucović, D., Popov Aleksandrov, A., Popović, D., Malešević, A., Subota, V., Brdarić, E., Soković Bajić, S., Živković, M., Kataranovski, M., Mirkov, I., Stanojević, S., & Kulaš, J. (2025). Differential Proneness to Obesity in Two Rat Strains with Diverse Immune Responses. Biology, 14(5), 557. https://doi.org/10.3390/biology14050557






