Traces of a Primitive RNA Ring in Current Genomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Criteria
- (1)
- The AL must satisfy the principle “be as short as possible and contain at least one codon per synonymy class of the genetic code”;
- (2)
- The AL codon sequence obtained with overlap after 3 turns of its circular form (the theoretical ring) must begin with the start codon and end with the stop codon;
- (3)
- The AL must have a hairpin configuration in balance with its circular shape, and this hairpin must have a minimum head length (3 nt) and a maximum number (9) of codon pairs;
- (4)
- If multiple rings possess properties (1) to (3), they must have a single barycenter for classical inter-ring distances (circular Hamming, permutation, and editing distances), i.e., the AL ring.
2.2. AL-Codon-Counter, an Algorithm for Finding AL Traces in Current Genomes
3. Results and Discussion
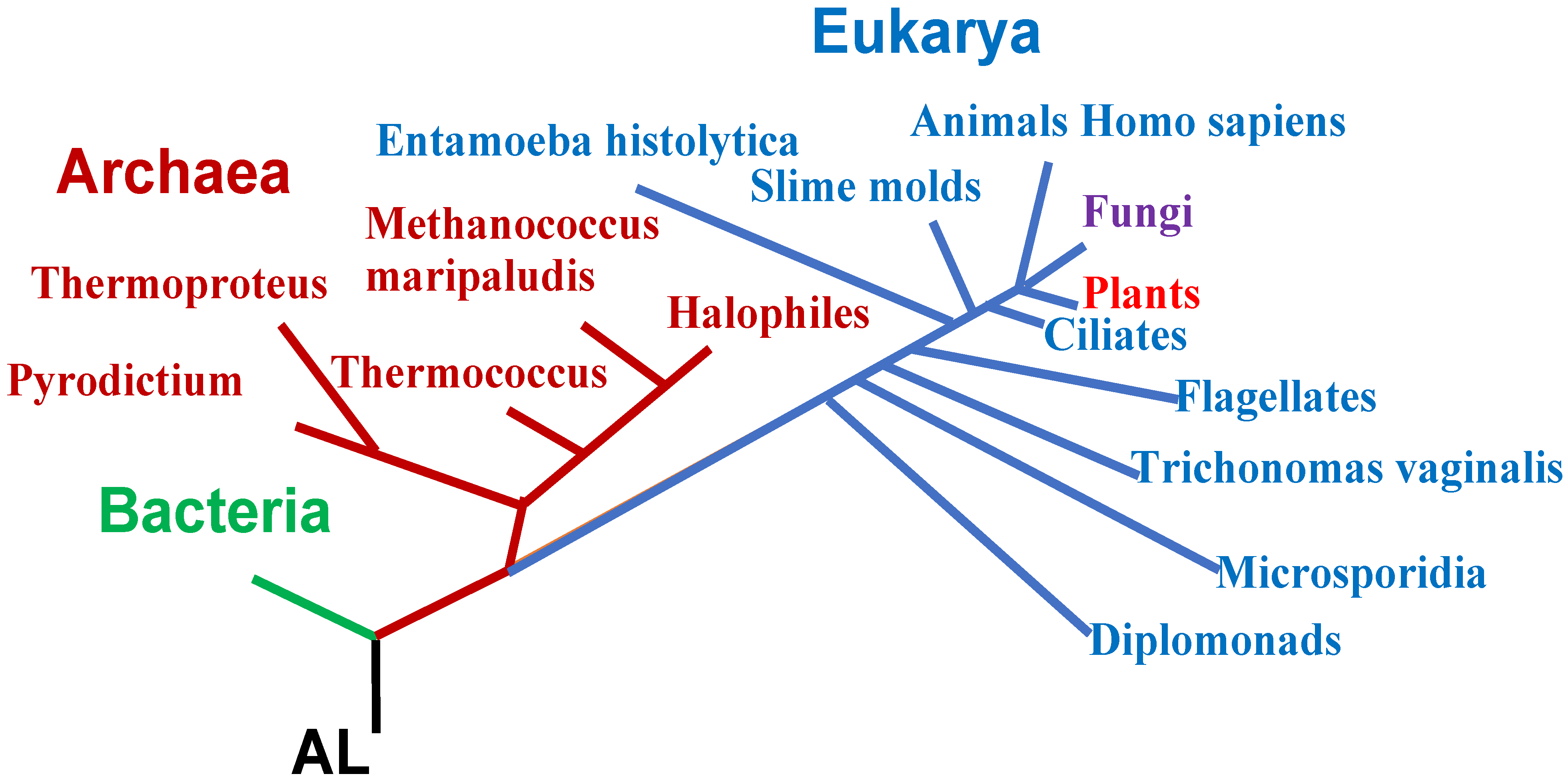
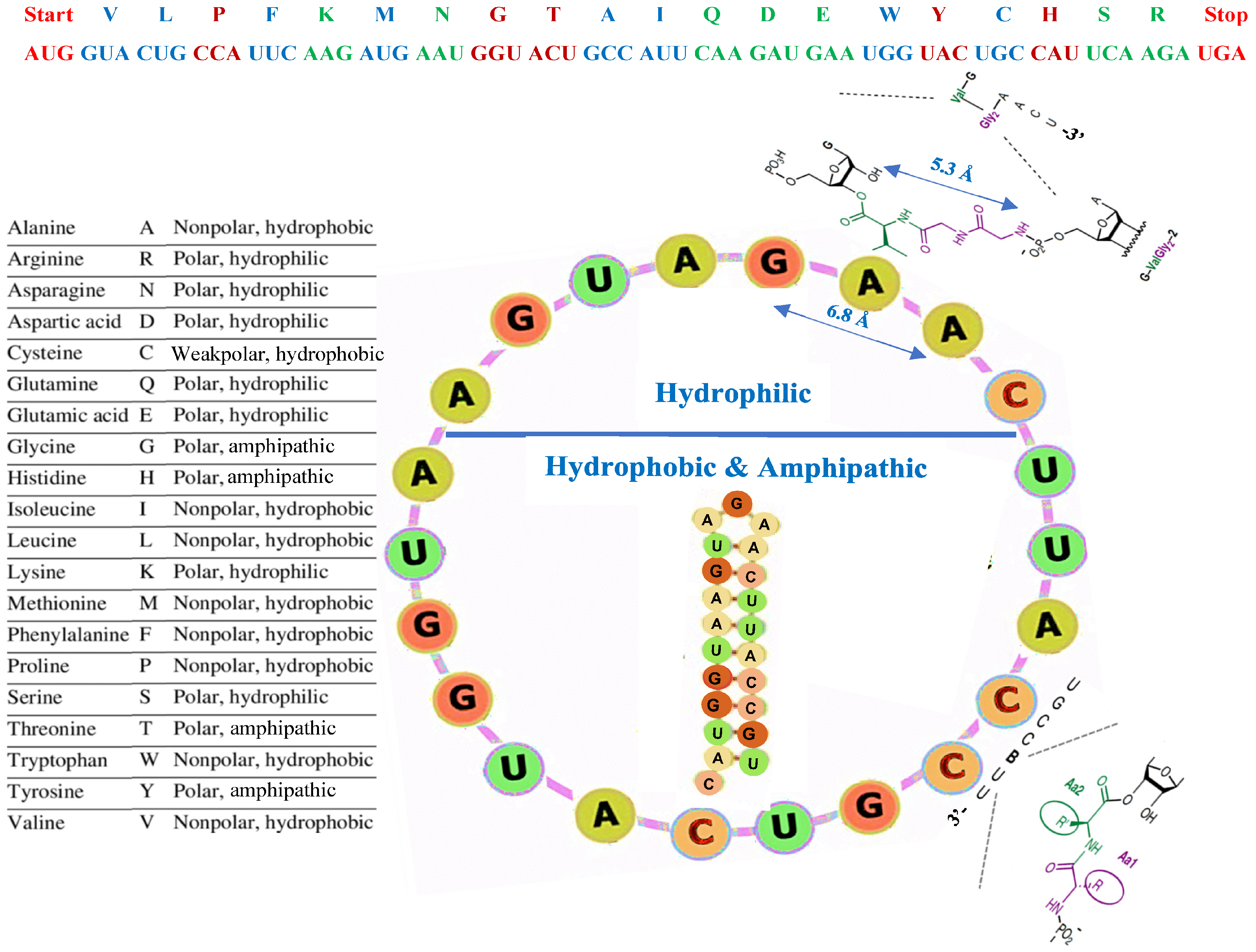
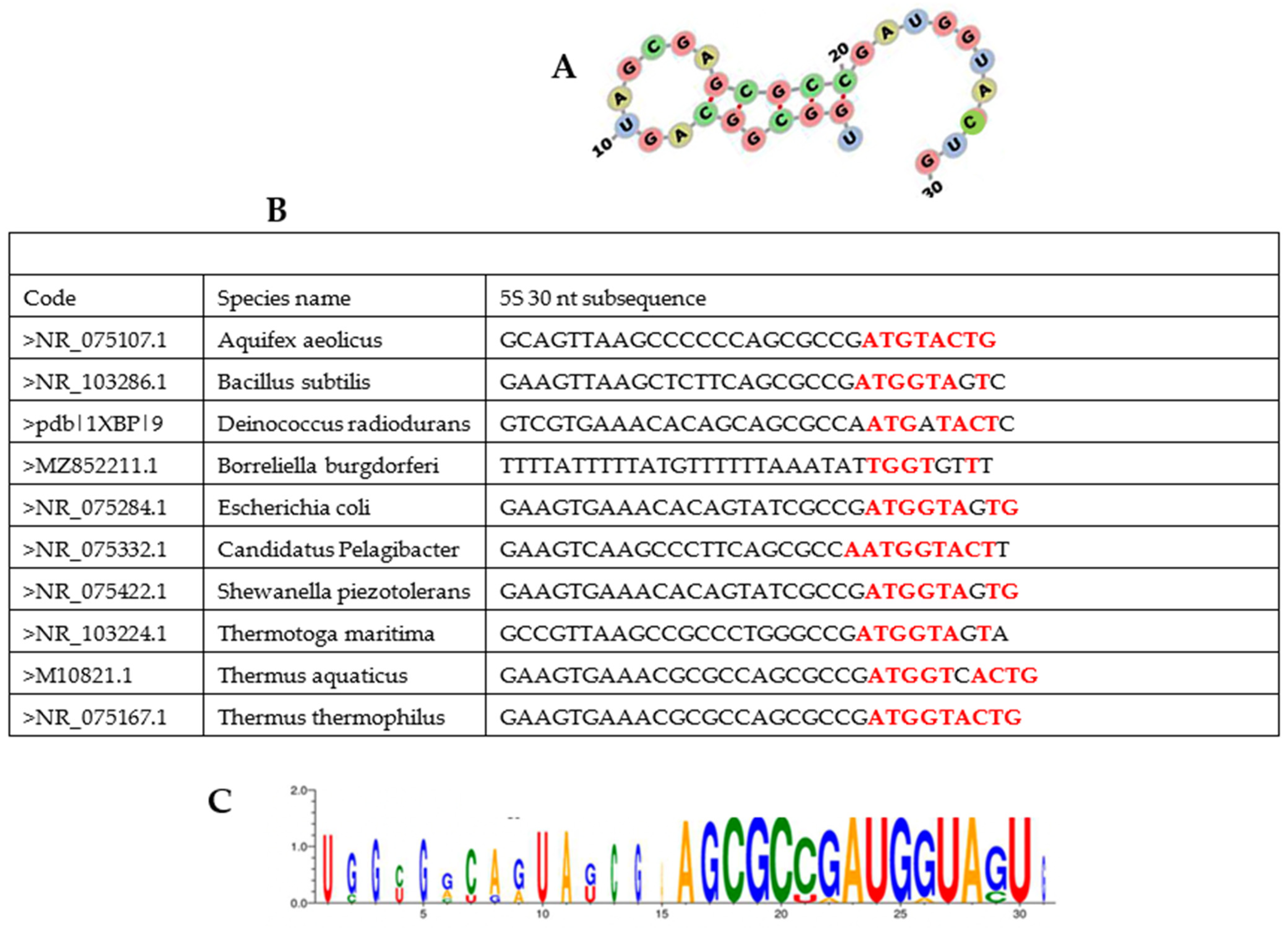
3.1. Presentation of AL
TGGTA TCAG T A
ACCAT AA CT T C
TGGTAAGTAT C
G
CATGGTAAGTA
3.2. Structural Properties of AL
3.3. Functional Properties of AL
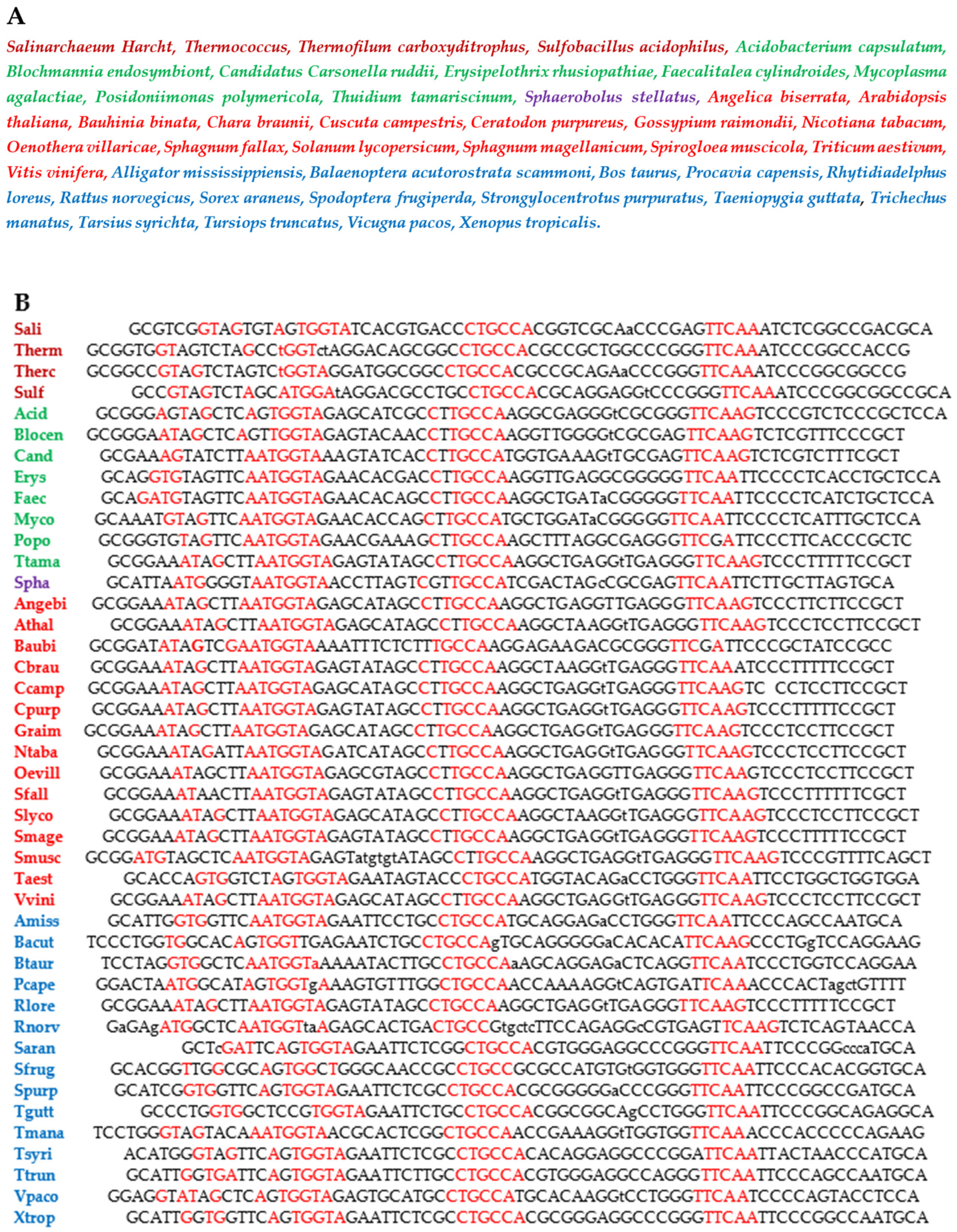
3.4. Searching for AL Motifs in Current Genomes
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic Synthesis of Polypeptides by Heterogeneous Polycondensation of Amino-acid Adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Paecht-Horowitz, M.; Katchalsky, A. Synthesis of amino acyl-adenylates under prebiotic conditions. J. Mol. Evol. 1973, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. Polymerisation en phase aqueuse d’acides aminés sur des argiles. Clay Miner. 1976, 11, 117–120. [Google Scholar] [CrossRef]
- Crick, F.H.C.; Brenner, S.; Klug, A.; Pieczenik, G. A speculation on the origin of protein synthesis. Orig. Life 1976, 7, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F. Evolution of protein synthesis from an RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003681. [Google Scholar] [CrossRef]
- Jash, B.; Tremmel, P.; Jovanovic, D.; Richert, C. Single nucleotide translation without ribosomes. Nat. Chem. 2021, 13, 751–757. [Google Scholar] [CrossRef]
- Ishida, T. Simulation of the emergence of cell-like morphologies with evolutionary potential based on virtual molecular interactions. Sci. Rep. 2024, 14, 2086. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The hypercycle: A principle of natural self-organization. Part C: The realistic hypercycle. Naturwissenschaften 1978, 65, 341–369. [Google Scholar] [CrossRef]
- Eigen, M.; Winkler-Oswatitsch, R. Transfer-RNA: The early adaptor. Naturwissenschaften 1981, 68, 217–228. [Google Scholar] [CrossRef]
- Bernal, D. The Physical Basis of Life; RoutIedge and Kegan Paul: London, UK, 1951. [Google Scholar]
- Miller, S.L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Parker, E.T.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Callahan, M.; Aubrey, A.; Lazcano, A.; Bada, J.L. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. USA 2011, 108, 5526–5531. [Google Scholar] [CrossRef] [PubMed]
- Oró, J.; Kimball, A.P. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Arch. Biochem. Biophys. 1961, 94, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [PubMed]
- Ponnamperuma, C.; Sagan, C.; Mariner, R. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature 1963, 199, 222–226. [Google Scholar] [CrossRef]
- Hobish, M.K.; Wickramasinghe, N.S.; Ponnamperuma, C. Direct interaction between amino acids and nucleotides as a possible physicochemical basis for the origin of the genetic code. Adv. Space Res. 1995, 15, 365–382. [Google Scholar] [CrossRef]
- Caetano-Anolles, G.; Kim, K.M. The Origin and Evolution of the Archaeal Domain; Hindawi Publishing Corporation: London, UK, 2014. [Google Scholar]
- Di Giulio, M. On the origin of protein synthesis: A speculative model based on hairpin RNA structures. J. Theor. Biol. 1994, 171, 303–308. [Google Scholar] [CrossRef]
- Woese, C.R. A New Biology for a New Century. Microbiol. Mol. Biol. Rev. 2004, 68, 173–186. [Google Scholar] [CrossRef]
- Shapiro, R. Small Molecule Interactions were Central to the Origin of Life. Q. Rev. Biol. 2006, 81, 105–126. [Google Scholar] [CrossRef]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others). Biol. Direct 2012, 7, 23. [Google Scholar] [CrossRef]
- Colín-García, M. Hydrothermal vents and prebiotic chemistry: A review. Bol. Soc. Geol. Mex. 2016, 68, 599–620. [Google Scholar] [CrossRef]
- Yarus, M. Life from an RNA World: The Ancestor Within; Harvard University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Yarus, M. Eighty routes to a ribonucleotide world; dispersion and stringency in the decisive selection. RNA 2018, 24, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. On an RNA-membrane protogenome. arXiv 2025, arXiv:2502.00647. [Google Scholar] [CrossRef]
- Lancet, D.; Zidovetzki, R.; Markovitch, O. Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 2018, 15, 20180159. [Google Scholar] [CrossRef] [PubMed]
- Raine, D.J.; Norris, V. Lipid domain boundaries as prebiotic catalysts of peptide bond formation. J. Theor. Biol. 2007, 246, 176–185. [Google Scholar] [CrossRef]
- Kahana, A.; Lancet, D. Protobiotic Systems Chemistry Analyzed by Molecular Dynamics. Life 2019, 9, 38. [Google Scholar] [CrossRef]
- Caforio, A.; Driessen, A.J.M. Archaeal phospho-lipids: Structural properties and biosynthesis. BBA-Mol. Cell Biol. Lipids 2016, 1862, 1325–1339. [Google Scholar] [CrossRef]
- Demongeot, J. Au Sujet de Quelques Modèles Stochastiques Appliqués à la Biologie. Ph.D. Thesis, Université Joseph Fourier, Grenoble, France, 1975. Available online: https://tel.archives-ouvertes.fr/tel-00286222 (accessed on 5 January 2025).
- Demongeot, J. Sur la possibilité de considérer le code génétique comme un code à enchaînement. Rev. Biomaths 1978, 62, 61–66. [Google Scholar]
- Demongeot, J.; Besson, J. Code génétique et codes à enchaînement I. C.R. Acad. Sc. III 1983, 296, 807–810. [Google Scholar]
- Demongeot, J.; Besson, J. Genetic code and cyclic codes II. C.R. Acad. Sc. III 1996, 319, 520–528. [Google Scholar]
- Weil, G.; Heus, K.; Faraut, T.; Demongeot, J. An archetypal basic code for the primitive genome. Theor. Comp. Sc. 2004, 322, 313–334. [Google Scholar] [CrossRef]
- Demongeot, J.; Elena, A.; Weil, G. Potential-Hamiltonian decomposition of cellular automata. Appl. Degeneracy Genet. Code Cycl. Codes III. C. R. Acad. Sc. Biol. 2006, 329, 953–962. [Google Scholar]
- Demongeot, J. Primitive genome and RNA relics. In Proceedings of the EMBC’ 07, Lyon, France, 22–26 August 2007; IEEE Proceedings: Piscataway, NJ, USA, 2007; pp. 6338–6342. [Google Scholar]
- Demongeot, J.; Moreira, A. A circular RNA at the origin of life. J. Theor. Biol. 2007, 249, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Drouet, E.; Moreira, A.; Rechoum, Y.; Sené, S. Micro-RNAs: Viral genome and robustness of the genes expression in host. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2009, 367, 4941–4965. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Glade, N.; Moreira, A.; Vial, L. RNA relics and origin of life. Int. J. Mol. Sci. 2009, 10, 3420–3441. [Google Scholar] [CrossRef]
- Demongeot, J.; Hazgui, H.; Bandiera, S.; Cohen, O.; Henrion-Caude, A. MitomiRs, ChloromiRs and general modelling of the microRNA inhibition. Acta Biotheor. 2013, 61, 367–383. [Google Scholar] [CrossRef]
- Demongeot, J. “Protoribosome” as new game of life. BioRxiv 2017. [Google Scholar] [CrossRef]
- Demongeot, J.; Hazgui, H. The Poitiers school of mathematical and theoretical biology: Besson-Gavaudan-Schützenberger’s conjectures on genetic code and RNA structures. Acta Biotheor. 2016, 64, 403–426. [Google Scholar] [CrossRef]
- Demongeot, J.; Norris, V. Emergence of a “Cyclosome” in a Primitive Network Capable of Building “Infinite” Proteins. Life 2019, 9, 51. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Theoretical minimal RNA rings recapitulate the order of the genetic code’s codon-amino acid assignments. J. Theor. Biol. 2019, 471, 108–116. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Spontaneous evolution of circular codes in theoretical minimal RNA rings. Gene 2019, 705, 95–102. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. More pieces of ancient than recent theoretical minimal proto-tRNA-like RNA rings in genes coding for tRNA synthetases. J. Mol. Evol. 2019, 87, 152–174. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Seligmann, H. Bias for 3′-dominant codon directional asymmetry in theoretical minimal RNA rings. J. Comput. Biol. 2019, 26, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Seligmann, H. Theoretical minimal RNA rings designed according to coding constraints mimick deamination gradients. Sci. Nat. 2019, 106, 44. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Seligmann, H. Pentamers with non-redundant frames: Bias for natural circular code codons. J. Mol. Evol. 2020, 88, 194–201. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. The primordial tRNA acceptor stem code from theoretical minimal RNA ring clusters. BMC Genet. 2020, 21, 7. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Accretion history of large ribosomal subunits deduced from theoretical minimal RNA rings is congruent with histories derived from phylogenetic and structural methods. Gene 2020, 738, 144436. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Deamination gradients within codons after 1<->2 position swap predict amino acid hydrophobicity and parallel β-sheet conformational preference. Biosystems 2020, 192, 104116. [Google Scholar]
- Demongeot, J.; Seligmann, H. Theoretical minimal RNA rings maximizing coding information overwhelmingly start with the universal initiation codon AUG. BioEssays 2020, 42, 1900201. [Google Scholar] [CrossRef]
- Demongeot, J.; Henrion-Caude, A. The old and the new on the prebiotic conditions of the origin of life. Biology 2020, 9, 88. [Google Scholar]
- Demongeot, J.; Seligmann, H. Theoretical minimal RNA rings mimick molecular evolution before tRNA-mediated translation: Codon-amino acid affinities increase from early to late RNA rings. Comptes Rendus Biol. 2020, 343, 111–122. [Google Scholar] [CrossRef]
- Demongeot, J.; Seligmann, H. Evolution of tRNA subelement accretion from small and large ribosomal RNAs. Biosystems 2022, 193, 104796. [Google Scholar] [CrossRef] [PubMed]
- Norris, V.; Demongeot, J. The Ring World hypothesis: The eversion of small, double-stranded polynucleotide circlets was at the origin of the double helix of DNA, the polymerisation of RNA and DNA, the triplet code, the twenty or so biological amino acids, and strand asymmetry. Int. J. Mol. Sci. 2022, 23, 12915. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Thellier, M. Primitive oligomeric RNAs at the origins of life on Earth. Int. J. Mol. Sci. 2023, 24, 2274. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Waku, J.; Cohen, O. Combinatorial and frequency properties of the ribosome ancestors. Math. Biosci. Eng. 2023, 21, 884–902. [Google Scholar] [CrossRef]
- Ben Khalfallah, H.; Jelassi, M.; Rissaoui, H.; Barchouchi, M.; Baraille, C.; Gardes, J.; Demongeot, J. Information Gradient among Nucleotide Sequences of Essential RNAs from an Evolutionary Perspective. Int. J. Mol. Sci. 2024, 25, 7521. [Google Scholar] [CrossRef]
- Ben Khalfallah, H.; Jelassi, M.; Rachdi, M.; Demongeot, J. The AL-Codon-Counter Program: An Advanced Tool for Pentamer Analysis in RNA Sequences and Evolutionary Insights. In Proceedings of the SAI Computing Conference 2025, London, UK, 19–20 June 2025; Lecture Notes in Networks & Systems. Springer Nature: New York, NY, USA, 2025. [Google Scholar]
- Staley, J.T. Domain Cell Theory supports the independent evolution of the Eukarya, Bacteria and Archaea and the Nuclear Compartment Commonality hypothesis. Open Biol. 2017, 7, 170041. [Google Scholar] [CrossRef]
- Li, S.; Yang, J. System analysis of synonymous codon usage biases in archaeal virus genomes. J. Theor. Biol. 2014, 355, 128–139. [Google Scholar] [CrossRef]
- Bahiri-Elitzur, S.; Tuller, T. Codon-based indices for modeling gene expression and transcript evolution. Comput. Struct. Biotechnol. J. 2021, 19, 2646–2663. [Google Scholar] [CrossRef]
- GtRNAdb. Available online: http://gtrnadb.ucsc.edu/ (accessed on 22 February 2025).
- NCBI Nucleotide. Available online: https://www.ncbi.nlm.nih.gov/nucleotide (accessed on 22 February 2025).
- MiRBase. Available online: http://www.mirbase.org/ (accessed on 22 February 2025).
- Georg, R.C.; Stefani, R.M.; Gomes, S.L. Environmental stresses inhibit splicing in the aquatic fungus Blastocladiella emersonii. BMC Microbiol. 2009, 9, 231. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Carmel, L.; Csuros, M.; Koonin, E.V. Origin and evolution of spliceosomal introns. Biol. Direct. 2012, 7, 11. [Google Scholar] [CrossRef]
- Brochier-Armanet, C.; Forterre, P.; Gribaldo, S. Phylogeny and evolution of the Archaea: One hundred genomes later. Curr. Opin. Microbiol. 2011, 14, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Forterre, P. The Common Ancestor of Archaea and Eukarya Was Not an Archaeon. Archaea 2013, 2013, 372396. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Yadav, S. The Origin of Prebiotic Information System in the Peptide/RNA World: A Simulation Model of the Evolution of Translation and the Genetic Code. Life 2019, 9, 25. [Google Scholar] [CrossRef]
- Slonimski, P.P. Periodic oscillations of the genomic nucleotide sequences disclose major differences in the way of constructing homologous proteins from different procaryotic species. Comptes. Rendus. Biol. 2007, 330, 13–32. [Google Scholar] [CrossRef]
- Yarus, M. The meaning of a minuscule ribozyme. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Schimmel, P. Oligonucleotide-directed peptide synthesis in a ribosome- and ribozyme-free system. Proc. Natl. Acad. Sci. USA 2001, 98, 1393–1397. [Google Scholar] [CrossRef]
- Tamura, K.; Schimmel, P. Peptide synthesis with a template-like RNA guide and aminoacyl phosphate adaptors. Proc. Natl. Acad. Sci. USA 2003, 100, 8666–8669. [Google Scholar] [CrossRef]
- Tamura, K.; Schimmel, P. Chiral-selective aminoacylation of an RNA minihelix. Science 2004, 305, 1253. [Google Scholar] [CrossRef]
- Tamura, K.; Schimmel, P. Chiral-selective aminoacylation of an RNA minihelix: Mechanistic features and chiral suppression. Proc. Natl. Acad. Sci. USA 2006, 103, 13750–13752. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.K.; Lee, S.; Seo, J.H.; Choi, J.W.; Park, J.; Min, S.; Yoon, S.; Cho, S.; Kim, H.H. Prediction of the sequence-specific cleavage activity of Cas9 variants. Nat. Biotechnol. 2020, 38, 1328–1336. [Google Scholar] [CrossRef]
- Miller, S.M.; Wang, T.; Randolph, P.B.; Arbab, M.; Shen, M.W.; Huang, T.P.; Matuszek, Z.; Newby, G.A.; Rees, H.A.; Liu, D.R. Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat. Biotechnol. 2020, 38, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Brinkmann, H.; Pradella, S. Diversity and evolution of repABC type plasmids in Rhodobacterales. Environ. Microbiol. 2009, 11, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Pérez, M.; José, M.V. A Proposal of the Ur-RNAome. Genes 2023, 14, 2158. [Google Scholar] [CrossRef]
- Trifonov, E.N. Consensus temporal order of amino acids and evolution of the triplet code. Gene 2000, 261, 139–151. [Google Scholar] [CrossRef]
- Trifonov, E.N.; Bettecken, T. Sequence fossils, triplet expansion, and reconstruction of earliest codons. Gene 1997, 205, 1–6. [Google Scholar] [CrossRef]
- Sobolevsky, Y.; Guimarães, R.C.; Trifonov, E.N. Towards functional repertoire of the earliest proteins. J. Biomol. Struct. Dyn. 2013, 31, 1293–1300. [Google Scholar] [CrossRef]
- Fontecilla-Camps, J. Geochemical Continuity and Catalyst/Cofactor Replacement in the Emergence and Evolution of Life. Angew. Chem. 2018, 130, 08438. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Catalan, P.; Cuesta, J.A.; Manrubia, S. On the networked architecture of genotype spaces and its critical effects on molecular evolution. Open Biol. 2018, 8, 180069. [Google Scholar] [CrossRef]
- Seligmann, H.; Raoult, D. Stem-Loop RNA Hairpins in Giant Viruses: Invading rRNA-Like Repeats and a Template Free RNA. Front. Microbiol. 2018, 9, 101. [Google Scholar] [CrossRef]
- Muller, H.J. The gene as the basis of life. In Proceedings of the International Congress of Plant Sciences, Ithaca, NY, USA, 16–23 August 1926; Duggar, B.M., Ed.; Menasha: Banta, WI, USA, 1929; pp. 897–921. [Google Scholar]
- Eigen, M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef]
- Eigen, M.; Lindemann, B.; Winkler-Oswatitsch, R.; Clarke, C.H. Pattern Analysis of 5S rRNA. Proc. Natl. Acad. Sci. USA 1985, 82, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, P.; Stewart, J. Autopoiesis and cognition. Artif. Life 2004, 10, 327–345. [Google Scholar] [CrossRef]
- Ono, N.; Ikegami, T. Self-maintenance and self-reproduction in an abstract cell model. J. Theor. Biol. 2000, 206, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Ikegami, T. Artificial chemistry: Computational studies on the emergence of self-reproducing units. In Proceedings of the 6th European Conference on Artificial Life (ECAL’01), Prague, Czech Republic, 10–14 September 2001; Kelemen, J., Sosik, S., Eds.; Springer: Berlin, Germany, 2001; pp. 186–195. [Google Scholar]
- Noble, D. Genes are not the blueprint for life. Nature 2019, 626, 254–255. [Google Scholar] [CrossRef]
- Noble, D.; Joyner, M. The physiology of evolution. J. Physiol. 2024, 602, 2361–2365. [Google Scholar] [CrossRef]
- Dufton, M.J. Genetic code synonym quotas and amino acid complexity: Cutting the cost of proteins? J. Theor. Biol. 1997, 187, 165–173. [Google Scholar] [CrossRef]
- Davis, B.K. Evolution of the genetic code. Prog. Biophys. Mol. Biol. 1999, 72, 157–243. [Google Scholar] [CrossRef]
- Wong, J.T.F. Coevolution theory of the genetic code at age thirty. Bioessays 2005, 27, 416–425. [Google Scholar] [CrossRef]
- Wong, J.T.F.; Ng, S.K.; Mat, W.K.; Hu, T.; Xue, H. Coevolution theory of the genetic code at age forty: Pathway to translation and synthetic life. Life 2016, 6, 12. [Google Scholar] [CrossRef]
- Takeuchi, N.; Kaneko, K. The origin of the central dogma through conflicting multilevel selection. Proc. R. Soc. B 2019, 286, 20191359. [Google Scholar] [CrossRef]
- Fried, S.D.; Fujishima, K.; Makarov, M.; Cherepashuk, I.; Hlouchova, K. Peptides before and during the nucleotide world: An origins story emphasizing cooperation between proteins and nucleic acids. J. R. Soc. Interface 2022, 19, 20210641. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2023, 41, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Cognat, V.; Duchêne, A.M.; Maréchal-Drouard, L. A global picture of tRNA genes in plant genomes. Plant J. 2011, 66, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Fonville, N.C.; Velmurugan, K.R.; Tae, H.; Vaksman, Z.; McIver, L.J.; Garner, H.R. Genomic leftovers: Identifying novel microsatellites, over-represented motifs and functional elements in the human genome. Sci. Rep. 2016, 6, 27722. [Google Scholar] [CrossRef]
- Fujishima, K.; Sugahara, J.; Tomita, M.; Kanai, A. Sequence Evidence in the Archaeal Genomes that tRNAs Emerged Through the Combination of Ancestral Genes as 59 and 39 tRNA Halves. PLoS ONE 2008, 3, e1622. [Google Scholar] [CrossRef]
- Levy, N.; Schabanel, N. ENSnano: A 3D Modeling Software for DNA Nanostructures. DNA 2021, 5, 1–5. [Google Scholar]
- Spang, A.; Caceres, E.F.; Ettema, T.J.G. Genomic exploration of the diversity, ecology, and evolution of the archaeal domain of life. Science 2017, 357, eaaf3883. [Google Scholar] [CrossRef]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the origin of eukaryotes. Nature 2017, 15, 711–723. [Google Scholar] [CrossRef]
- Legendre, M.; Fabre, E.; Poirot, O.; Jeudy, S.; Lartigue, A.; Alempic, J.M.; Beucher, L.; Philippe, N.; Bertaux, L.; Christo-Foroux, E.; et al. Diversity and evolution of the emerging Pandoraviridae family. Nat. Commun. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Case, R.J.; Boucher, Y.; Dahllöf, I.; Holmström, C.; Doolittle, W.F.; Kjelleberg, S. Use of 16S rRNA and rpoB Genes as Molecular Markers for Microbial Ecology Studies. Appl. Environ. Microbiol. 2010, 73, 278–288. [Google Scholar] [CrossRef]
- Bartnik, E.; Borsuk, P. A glycine tRNA gene from lupine mitochondria. Nucleic Acids Res. 1986, 14, 2407. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, K.; Fütterer, J.; Potrykus, I. Horizontal Gene Transfer from a Transgenic Potato Line to a Bacterial Pathogen (Erwinia chrysanthemi) Occurs—If at All—At an Extremely Low Frequency. Biotechnology 1995, 13, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.A.; Seitzer, P.M.; Tritt, A.; Larsen, D.; Krusor, M.; Yao, A.I.; Wu, D.; Madern, D.; Eisen, J.A.; Darling, A.E.; et al. Phylogenetically Driven Sequencing of Extremely Halophilic Archaea Reveals Strategies for Static and Dynamic Osmo-response. PLoS Genet. 2014, 10, e1004784. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.; Silva, L.; Silva, L.S.; Khalil, J.Y.B.; Rodrigues, R.; Arantes, T.; Assis, F.; Boratto, P.; Andrade, M.; Kroon, E.G.; et al. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat. Commun. 2018, 9, 749. [Google Scholar] [CrossRef]
- Buzayan, J.M.; Hampel, A.; Bruening, G. Nucleotide sequence and newly formed phosphodiester bond of spontaneously ligated satellite tobacco ringspot virus RNA. Nucleic Acids Res. 1986, 14, 9729–9743. [Google Scholar] [CrossRef]
- Salter, J.; Krucinska, J.; Alam, S.; Grum-Tokars, V.; Wedekind, J.E. Water in the Active Site of an All-RNA Hairpin Ribozyme and Effects of Gua8 Base Variants on the Geometry of Phosphoryl Transfer. Biochemistry 2006, 45, 686–700. [Google Scholar] [CrossRef]
- Pérez-Ruiz, M.; Barroso-delJesus, A.; Berzal-Herranz, A. Specificity of the Hairpin Ribozyme. J. Biol. Chem. 1999, 274, 29376–29380. [Google Scholar] [CrossRef]
- Müller, U.F. Design and Experimental Evolution of trans-Splicing Group I Intron Ribozymes. Molecules 2017, 22, 75. [Google Scholar] [CrossRef]
- Paul, N.; Joyce, G.F. A self-replicating ligase ribozyme. Proc. Natl. Acad. Sci. USA 2002, 99, 12733–12740. [Google Scholar] [CrossRef]
- Perreault, J.; Weinberg, Z.; Roth, A.; Popescu, O.; Chartrand, P.; Ferbeyre, G.; Breaker, R.R. Identification of Hammerhead Ribozymes in All Domains of Life Reveals Novel Structural Variations. PLoS Comput. Biol. 2011, 7, e1002031. [Google Scholar] [CrossRef]
- Hammann, C.; Luptak, A.; Perreault, J.; De La Peña, M. The ubiquitous hammerhead ribozyme. RNA 2012, 18, 871–885. [Google Scholar] [CrossRef]
- Harris, K.A.; Lünse, C.E.; Li, S.; Brewer, K.I.; Breaker, R.R. Biochemical analysis of hatchet self-cleaving ribozymes. RNA 2015, 21, 1852–1858. [Google Scholar] [CrossRef]
- Agmon, I.C. Could a Proto-Ribosome Emerge Spontaneously in the PrebioticWorld? Molecules 2016, 21, 1701. [Google Scholar] [CrossRef]
- Arquès, D.G.; Michel, C.J. A complementary circular code in the protein coding genes. J. Theor. Biol. 1996, 182, 45–58. [Google Scholar] [CrossRef]
- Dila, G.; Ripp, R.; Mayer, C.; Poch, O.; Michel, C.J.; Thompson, J.D. Circular code motifs in the ribosome: A missing link in the evolution of translation? RNA 2019, 25, 1714–1730. [Google Scholar] [CrossRef]
- Kim, Y.; Opron, K.; Burton, Z.F. A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life 2019, 9, 37. [Google Scholar] [CrossRef]
- Kunnev, D.; Gospodinov, A. Possible Emergence of Sequence Specific RNA Aminoacylation via Peptide Intermediary to Initiate Darwinian Evolution and Code Through Origin of Life. Life 2018, 8, 44. [Google Scholar] [CrossRef]
- Seligmann, H. Protein Sequences Recapitulate Genetic Code Evolution. Comput. Struct. Biotechnol. J. 2018, 16, 177–189. [Google Scholar] [CrossRef]
- Zaia, D.A.; Zaia, C.T.; De Santana, H. Which amino acids should be used in prebiotic chemistry studies? Orig. Life Evol. Biosph. 2008, 38, 469–488. [Google Scholar] [CrossRef]
- Robinson, R. Jump-starting a cellular world: Investigating the origin of life, from soup to networks. PLoS Biol. 2005, 3, e396. [Google Scholar] [CrossRef]
- Beringer, M.; Rodnina, M.V. Importance of tRNA interactions with 23S rRNA for peptide bond formation on the ribosome: Studies with substrate analogs. Biol. Chem. 2007, 388, 687–691. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the genetic code: The universal enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef]
- Rodin, A.S.; Szathmáry, E.; Rodin, S.N. On origin of genetic code and tRNA before translation. Biol. Direct. 2011, 6, 14. [Google Scholar] [CrossRef]
- Koonin, E.V. Frozen Accident Pushing 50: Stereochemistry, Expansion, and Chance in the Evolution of the Genetic Code. Life 2017, 7, 22. [Google Scholar] [CrossRef]
- Gonzalez, D.L.; Giannerini, S.; Rosa, R. On the origin of degeneracy in the genetic code. Interface Focus 2019, 9, 20190038. [Google Scholar] [CrossRef]
- Chang, L.H.; Seitz, O. RNA-templated chemical synthesis of proapoptotic L- and d-peptides. Bioorganic Med. Chem. 2022, 66, 116786. [Google Scholar] [CrossRef]
- Zhao, F.; Frandsen, M.; Capodaglio, S.; Sleiman, H.F. DNA-Mediated Peptide Assembly into Protein Mimics. J. Am. Chem. Soc. 2024, 146, 1946–1956. [Google Scholar] [CrossRef]
- Radakovic, A.; Wright, T.H.; Lelyveld, V.S.; Szostak, J.W. A Potential Role for Aminoacylation in Primordial RNA Copying Chemistry. Biochemistry 2021, 60, 477–488. [Google Scholar] [CrossRef]
- Węgrzyn, E.; Mejdrová, I.; Müller, F.M.; Nainytė, M.; Escobar, L.; Carell, T. RNA-Templated Peptide Bond Formation Promotes L-Homochirality. Angew. Chem. Int. Ed. Engl. 2024, 63, e202319235. [Google Scholar] [CrossRef]
- Węgrzyn, E.; Mejdrová, I.; Carell, T. Gradual evolution of a homo-l-peptide world on homo-d-configured RNA and DNA. Chem. Sci. 2024, 15, 14171–14176. [Google Scholar] [CrossRef]
- Kauffman, S.A.; Lehman, N. Mixed anhydrides at the intersection between peptide and RNA autocatalytic sets: Evolution of biological coding. Interface Focus 2023, 13, 20230009. [Google Scholar] [CrossRef]
- Su, M.; Schmitt, C.; Liu, Z.; Roberts, S.J.; Liu, K.C.; Röder, K.; Jäschke, A.; Wales, D.J.; Sutherland, J.D. Triplet-Encoded Prebiotic RNA Aminoacylation. J. Am. Chem. Soc. 2023, 145, 15971–15980. [Google Scholar] [CrossRef]
- Wickramasinghe, N.S.; Staves, M.P.; Lacey, J.C., Jr. Stereoselective, nonenzymatic, intramolecular transfer of amino acids. Biochemistry 1991, 30, 2768–2772. [Google Scholar] [CrossRef]
- Gless, B.H.; Jones, E.; Labão-Almeida, C.; Tang, C.; Gottscheber, N.; Couto, R.; Bernardes, G.J.L. Conditional Activation of Protein Therapeutics by Templated Removal of Peptide Nucleic Acid Masking Groups. Angew. Chem. Int. Ed. Engl. 2025, 64, e202502268. [Google Scholar]
- Räuchle, M.; Leveau, G.; Richert, C. Synthesis of Peptido RNAs from Unprotected Peptides and Oligoribonucleotides via Coupling in Aqueous Solution. Eur. J. Org. Chem. 2020, 2020, 6966–6975. [Google Scholar] [CrossRef]
- Altrichter, Y.; Bou-Dib, P.; Kuznia, C.; Seitz, O. Towards a templated reaction that translates RNA in cells into a proaptotic peptide-PNA conjugate. J. Pept. Sci. 2023, 29, e3477. [Google Scholar] [CrossRef]
- Di Pisa, M.; Hauser, A.; Seitz, O. Maximizing Output in RNA-Programmed Peptidyl-Transfer Reactions. ChemBioChem 2017, 18, 872–879. [Google Scholar] [CrossRef]
- Middel, S.; Panse, C.H.; Nawratil, S.; Diederichsen, U. Native Chemical Ligation Directed by Photocleavable Peptide Nucleic Acid (PNA) Templates. ChemBioChem 2017, 18, 2328–2332. [Google Scholar] [CrossRef]
- Vázquez, O.; Seitz, O. Templated native chemical ligation: Peptide chemistry beyond protein synthesis. J. Pept. Sci. 2014, 20, 78–86. [Google Scholar] [CrossRef]
- Saha, P.; Panda, D.; Dash, J. Nucleic acids as templates and catalysts in chemical reactions: Target-guided dynamic combinatorial chemistry and in situ click chemistry and DNA/RNA induced enantioselective reactions. Chem. Soc. Rev. 2023, 52, 4248–4291. [Google Scholar] [CrossRef]
- Escobar, L. Covalent Linkages Used in Prebiotic Chemistry for RNA-Templated Amino Acid Transfer and Peptide Synthesis. ChemSystemsChem 2024, 6, e202400030. [Google Scholar] [CrossRef]
- Guo, X.; Su, M. The Origin of Translation: Bridging the Nucleotides and Peptides. Int. J. Mol. Sci. 2022, 24, 197. [Google Scholar] [CrossRef]
- Kuila, S.; Nanda, J. Cysteine-Based Dynamic Self-Assembly and Their Importance in the Origins of Life. ChemSystemsChem 2024, 6, e202400022. [Google Scholar] [CrossRef]
- Ianeselli, A.; Salditt, A.; Mast, C.; Dieter Braun, D. Physical non-equilibria for prebiotic nucleic acid chemistry. Nat. Rev. Phys. 2023, 5, 185–195. [Google Scholar] [CrossRef]
- Bandela, A.K.; Wagner, N.; Sadihov, H.; Morales-Reina, S.; Chotera-Ouda, A.; Basu, K.; Cohen-Luria, R.; de la Escosura, A.; Ashkenasy, G. Primitive selection of the fittest emerging through functional synergy in nucleopeptide networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2015285118. [Google Scholar] [CrossRef]
- Sadihov-Hanoch, H.; Bandela, A.K.; Chotera-Ouda, A.; Ben David, O.; Cohen-Luria, R.; Lynn, D.G.; Ashkenasy, G. Dynamic exchange controls the assembly structure of nucleic-acid-peptide chimeras. Soft Matter 2023, 19, 3940–3945. [Google Scholar] [CrossRef]
- Chotera, A.; Sadihov, H.; Cohen-Luria, R.; Monnard, P.A.; Ashkenasy, G. Functional Assemblies Emerging in Complex Mixtures of Peptides and Nucleic Acid-Peptide Chimeras. Chemistry 2018, 24, 10128–10135. [Google Scholar] [CrossRef]
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.Y.; Pichler, A.; Carell, T. A prebiotically plausible scenario of an RNA-peptide world. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef]
- Singer, J.N.; Müller, F.M.; Węgrzyn, E.; Hölzl, C.; Hurmiz, H.; Liu, C.; Escobar, L.; Carell, T. Loading of Amino Acids onto RNA in a Putative RNA-Peptide World. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302360. [Google Scholar] [CrossRef]
- Nainytė, M.; Müller, F.; Ganazzoli, G.; Chan, C.Y.; Crisp, A.; Globisch, D.; Carell, T. Amino Acid Modified RNA Bases as Building Blocks of an Early Earth RNA-Peptide World. Chemistry 2020, 26, 14856–14860. [Google Scholar] [CrossRef]
- Prosdocimi, F.; de Farias, S.T.; José, M.V. Prebiotic chemical refugia: Multifaceted scenario for the formation of biomolecules in primitive Earth. Theory Biosci. 2022, 141, 339–347. [Google Scholar] [CrossRef]
- Turk, R.M.; Chumachenko, N.V.; Yarus, M. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl Acad. Sci. USA 2010, 107, 4585–4589. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Bai, J.; Qiao, H. Prebiotic Peptide Synthesis: How Did Longest Peptide Appear? J. Mol. Evol. 2025, 93, 193–211. [Google Scholar] [CrossRef]
- Sutherland, J.D. The origin of life—Out of the blue. Angew. Chem. Int. Ed. Engl. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Jash, B.; Richert, C. Templates direct the sequence-specific anchoring of the C-terminus of peptido RNAs. Chem. Sci. 2020, 11, 3487–3494. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Wills, P.R. The Roots of Genetic Coding in Aminoacyl-tRNA Synthetase Duality. Annu. Rev. Biochem. 2021, 90, 349–373. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Wang, M.; Caetano-Anollés, D. Structural phylogenomics retrodicts the origin of the genetic code and uncovers the evolutionary impact of protein flexibility. PLoS ONE 2013, 8, e72225. [Google Scholar] [CrossRef]
- Harish, A.; Caetano-Anollés, G. Ribosomal history reveals origins of modern protein synthesis. PLoS ONE 2012, 7, e32776. [Google Scholar] [CrossRef]
- Mughal, F.; Caetano-Anollés, G. Evolution of intrinsic disorder in the structural domains of viral and cellular proteomes. Sci. Rep. 2025, 15, 2878. [Google Scholar] [CrossRef]


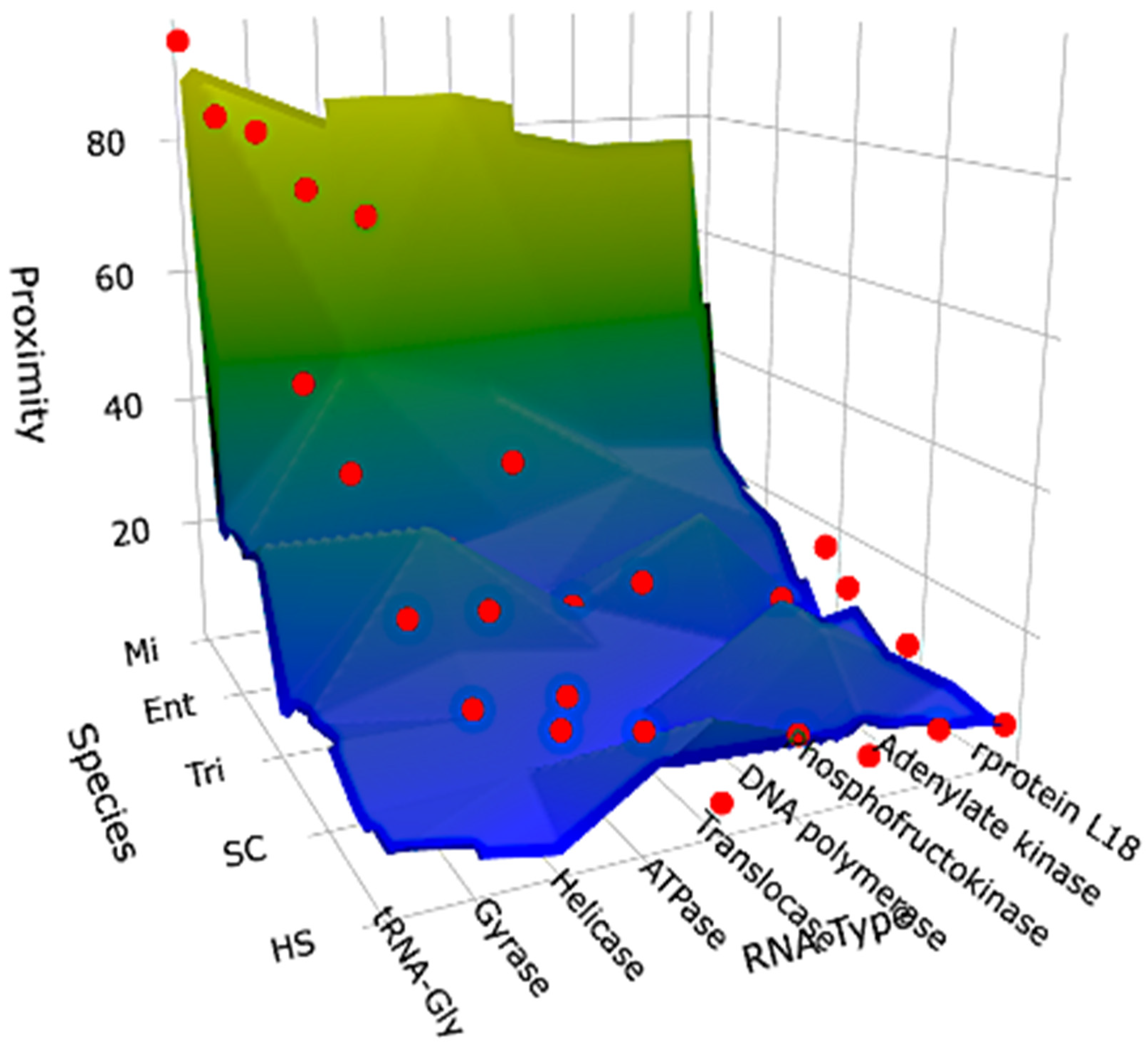
| Molecule | Species | no | N | ne | (se) | PPAL = | Mean PPAL | PPAL Doublet | Mean PPAL Doublet |
|---|---|---|---|---|---|---|---|---|---|
| rprotein L18 | HS | 14 | 639 | 5.7 | (2.4) | 7 | 10.1 | 4 | 8 |
| SC | 19 | 536 | 4.7 | (2.2) | 13.1 | 7.4 | |||
| Ent | 9 | 536 | 4.7 | (2.2) | 4 | 5 | |||
| Tri | 25 | 562 | 5 | (2.2) | 18 | 7.1 | |||
| Mm | 8 | 227 | 2 | (1.4) | 8.4 | 16.2 | |||
| mRNA FtsH | HS | 36 | 1918 | 16.9 | (4.1) | 9.3 | 11.4 | 1.3 | 13 |
| SC | 53 | 2968 | 26.1 | (5.1) | 10.5 | 4.4 | |||
| Ent | 21 | 944 | 8.3 | (2.9) | 8.8 | 27.1 | |||
| Tri | 58 | 1598 | 14.1 | (3.8) | 23.4 | 23.4 | |||
| Mm | 23 | 1457 | 12.8 | (3.6) | 5.7 | 9 | |||
| mRNA PFK | HS | 43 | 3036 | 26.7 | (5.2) | 6.3 | 10.6 | 8.6 | 13.5 |
| SC | 79 | 2960 | 26 | (5.1) | 20.8 | 26.8 | |||
| Ent | 21 | 1413 | 12.5 | (3.5) | 4.8 | 2 | |||
| Tri | 25 | 1286 | 11.3 | (3.4) | 8.1 | 9.5 | |||
| Mm | 35 | 1385 | 12.2 | (3.5) | 13 | 20.7 | |||
| mRNA Gly-tRNA ligase | HS | 34 | 2230 | 19.6 | (4.4) | 6.5 | 10.8 | 6.1 | 19.5 |
| SC | 42 | 1856 | 16.3 | (4) | 12.7 | 20.1 | |||
| Ent | 44 | 1880 | 16.5 | (4.1) | 13.6 | 36.6 | |||
| Tri | 39 | 1946 | 17.1 | (4.1) | 10.6 | 8.7 | |||
| Mm | 36 | 1721 | 15.2 | (3.9) | 10.8 | 26 | |||
| mRNA DNA polymerase | HS | 75 | 3959 | 34.8 | (5.9) | 5.5 | 13.5 | 8.6 | 18.9 |
| SC | 30 | 1316 | 11.6 | (3.4) | 14.5 | 16 | |||
| Ent | 72 | 3194 | 28.1 | (5.3) | 10 | 11.7 | |||
| Tri | 29 | 1040 | 9.2 | (3) | 13.2 | 8.2 | |||
| Mm | 45 | 2351 | 20.7 | (4.6) | 10.7 | 18.2 | |||
| mRNA ATPase | HS | 78 | 3414 | 30 | (5.5) | 17.5 | 15.7 | 19.1 | 21.6 |
| SC | 42 | 1850 | 16.3 | (4) | 12.8 | 14.3 | |||
| Ent | 52 | 1832 | 16.1 | (4) | 17.9 | 30.1 | |||
| Tri | 35 | 1366 | 12 | (3.5) | 13.2 | 10.4 | |||
| Mm | 98 | 2978 | 26.2 | (5.1) | 28 | 39.4 | |||
| mRNA Translocase | HS | 15 | 1027 | 9.1 | (3) | 4 | 17.9 | 4 | 19.1 |
| SC | 133 | 4856 | 42.7 | (6.5) | 27.6 | 23 | |||
| Ent | 106 | 3002 | 26.4 | (5.1) | 31 | 38.1 | |||
| Tri | 33 | 1066 | 9.4 | (3) | 15.4 | 14.8 | |||
| Mm | 20 | 1325 | 11.7 | (3.4) | 4.9 | 15.3 | |||
| mRNA Helicase | HS | 73 | 2716 | 23.9 | (4.9) | 20.1 | 20.5 | 16.4 | 19.7 |
| SC | 110 | 4541 | 39.9 | (6.3) | 22.2 | 16.6 | |||
| Ent | 63 | 2573 | 22.6 | (4.8) | 17 | 30.9 | |||
| Tri | 46 | 1256 | 11.1 | (3.3) | 21 | 11.3 | |||
| Mm | 56 | 2236 | 19.6 | (4.4) | 22.2 | 23.2 | |||
| mRNA Gyrase | HS | 138 | 5691 | 50 | (7.1) | 24.9 | 32.5 | 26.2 | 29.9 |
| SC | 122 | 4283 | 37.7 | (6.1) | 27.4 | 34.6 | |||
| Ent | 176 | 4046 | 35.6 | (6) | 47 | 55.7 | |||
| Tri | 162 | 4376 | 38.5 | (6.2) | 39.8 | 33.6 | |||
| Mm | 66 | 1094 | 9.7 | (3.1) | 23.2 | 24 | |||
| tRNA-Gly | HS | 18 | 22 | 0.19 | (0.44) | 81 | 84.6 | ||
| SC | 17 | 22 | 0.19 | (0.44) | 76.4 | ||||
| Ent | 19 | 22 | 0.19 | (0.44) | 85.5 | ||||
| Tri | 19 | 22 | 0.19 | (0.44) | 85.5 | ||||
| Mm | 21 | 22 | 0.19 | (0.44) | 94.6 | ||||
| AL | 22 | 22 | 0.19 | (0.44) | 100 | 100 |
| Doublet | Homo Sapiens | Saccharomyces | Entamoeba | Trichomonas | Methanococcus | Total |
|---|---|---|---|---|---|---|
| ATT ATT | 3 | 8 | 20 | 0 | 5 | 36 |
| ATT CAA | 2 | 5 | 14 | 4 | 11 | 36 |
| ATT GAT | 3 | 11 | 12 | 0 | 8 | 34 |
| ATT GAA | 16 | 17 | 25 | 3 | 27 | 88 |
| ATT CCA | 4 | 2 | 2 | 6 | 7 | 21 |
| ATT ACT | 1 | 10 | 4 | 3 | 3 | 21 |
| ATT AGA | 3 | 5 | 4 | 3 | 1 | 16 |
| ATT TAC | 0 | 0 | 2 | 0 | 3 | 5 |
| CAA ATT | 6 | 4 | 9 | 0 | 1 | 20 |
| CAA CAA | 2 | 5 | 7 | 4 | 0 | 18 |
| CAA GAT | 7 | 6 | 4 | 5 | 0 | 22 |
| CAA GAA | 11 | 12 | 11 | 6 | 6 | 46 |
| CAA CCA | 0 | 0 | 4 | 0 | 0 | 4 |
| CAA AGA | 6 | 4 | 9 | 0 | 4 | 23 |
| CAA TAC | 2 | 6 | 1 | 0 | 0 | 9 |
| CAA ACT | 7 | 0 | 5 | 0 | 0 | 12 |
| GAT ATT | 9 | 14 | 21 | 0 | 9 | 53 |
| GAT GAT | 19 | 14 | 26 | 15 | 4 | 78 |
| GAT GAA | 22 | 23 | 35 | 16 | 26 | 122 |
| GAT CCA | 5 | 3 | 4 | 3 | 0 | 15 |
| GAT AGA | 5 | 2 | 9 | 1 | 4 | 21 |
| GAT ACT | 1 | 6 | 6 | 0 | 7 | 20 |
| GAT CAA | 1 | 2 | 6 | 0 | 1 | 10 |
| GAT TAC | 2 | 0 | 0 | 5 | 5 | 12 |
| GAA ATT | 9 | 6 | 27 | 2 | 7 | 51 |
| GAA CAA | 6 | 11 | 12 | 2 | 7 | 38 |
| GAA GAT | 20 | 21 | 33 | 14 | 14 | 102 |
| GAA GAA | 20 | 33 | 43 | 16 | 37 | 149 |
| GAA CCA | 3 | 9 | 11 | 6 | 2 | 31 |
| GAA ACT | 8 | 10 | 8 | 1 | 6 | 33 |
| GAA AGA | 8 | 10 | 7 | 3 | 7 | 35 |
| GAA TAC | 0 | 10 | 0 | 4 | 4 | 18 |
| CCA ATT | 2 | 3 | 7 | 4 | 6 | 22 |
| CCA GAA | 8 | 6 | 7 | 3 | 4 | 28 |
| CCA AGA | 8 | 0 | 3 | 4 | 1 | 16 |
| CCA GAT | 8 | 0 | 3 | 0 | 0 | 11 |
| CCA ACT | 6 | 0 | 6 | 0 | 0 | 12 |
| ACT CAA | 0 | 3 | 9 | 4 | 1 | 17 |
| ACT GAT | 4 | 0 | 7 | 0 | 4 | 15 |
| ACT GAA | 8 | 0 | 5 | 3 | 6 | 22 |
| ACT ATT | 2 | 6 | 9 | 0 | 2 | 19 |
| ACT CCA | 7 | 9 | 4 | 0 | 1 | 21 |
| ACT TAC | 1 | 0 | 0 | 0 | 2 | 3 |
| ACT ACT | 0 | 7 | 4 | 2 | 13 | |
| AGA ATT | 3 | 5 | 7 | 1 | 5 | 21 |
| AGA GAT | 6 | 12 | 11 | 0 | 5 | 34 |
| AGA GAA | 13 | 11 | 12 | 9 | 20 | 65 |
| AGA CAA | 4 | 9 | 2 | 11 | 0 | 26 |
| AGA AGA | 7 | 6 | 7 | 0 | 4 | 24 |
| AGA CCA | 0 | 0 | 2 | 0 | 0 | 2 |
| AGA TAC | 1 | 0 | 0 | 0 | 4 | 5 |
| TAC ATT | 2 | 3 | 0 | 4 | 1 | 0 |
| TAC GAT | 1 | 5 | 1 | 0 | 6 | 13 |
| TAC GAA | 1 | 0 | 1 | 5 | 10 | 17 |
| TAC TAC | 3 | 0 | 0 | 3 | 2 | 8 |
| TAC AGA | 0 | 0 | 0 | 3 | 2 | 5 |
| TAC CCA | 3 | 0 | 1 | 1 | 5 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demongeot, J. Traces of a Primitive RNA Ring in Current Genomes. Biology 2025, 14, 538. https://doi.org/10.3390/biology14050538
Demongeot J. Traces of a Primitive RNA Ring in Current Genomes. Biology. 2025; 14(5):538. https://doi.org/10.3390/biology14050538
Chicago/Turabian StyleDemongeot, Jacques. 2025. "Traces of a Primitive RNA Ring in Current Genomes" Biology 14, no. 5: 538. https://doi.org/10.3390/biology14050538
APA StyleDemongeot, J. (2025). Traces of a Primitive RNA Ring in Current Genomes. Biology, 14(5), 538. https://doi.org/10.3390/biology14050538







