Status and Best Management Practices of Potato Early Dying Disease in New Brunswick, Canada
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
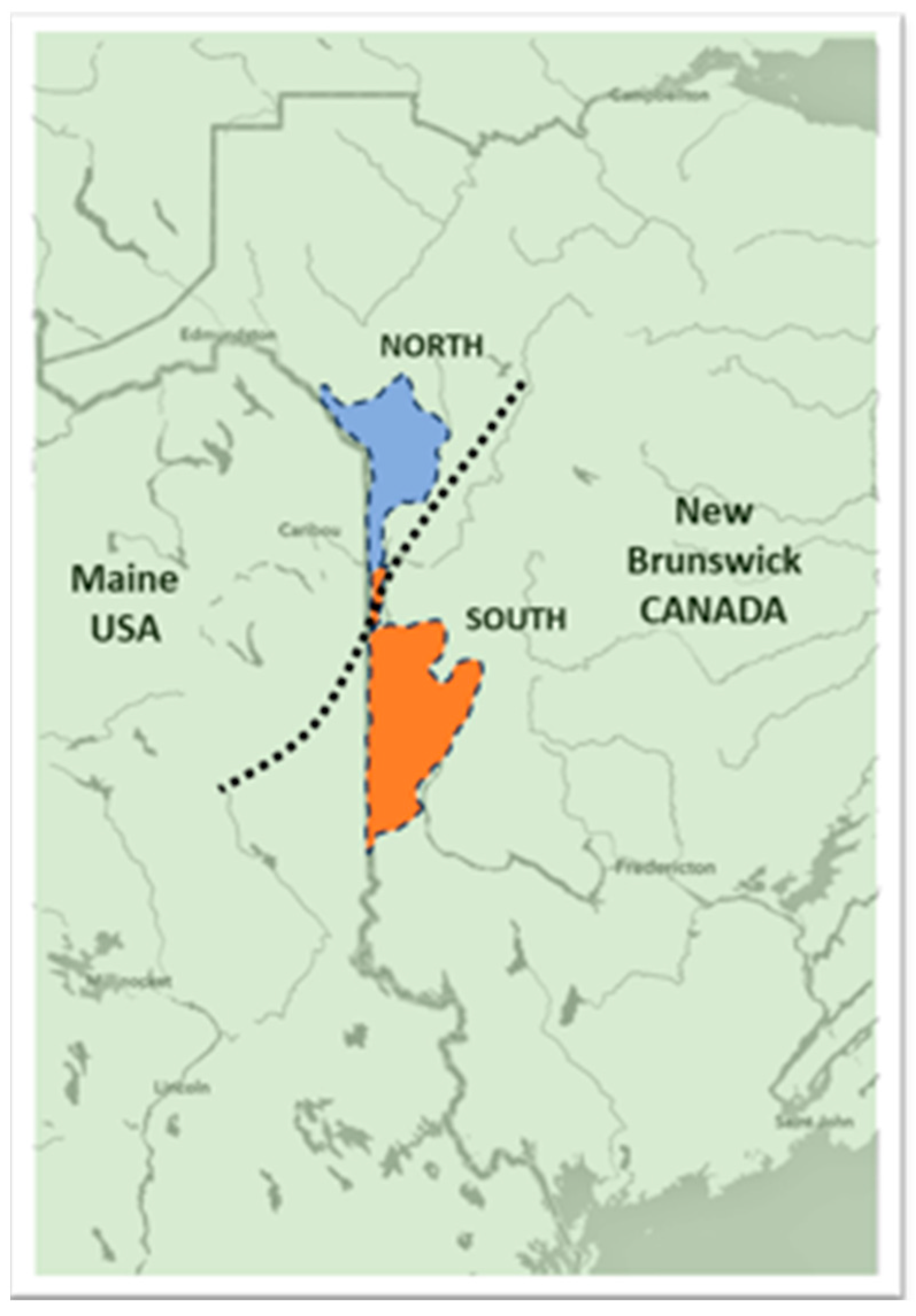
2.1. Soil Survey for Verticillium Species and Nematodes in NB Potato Fields
2.2. Nematode Extraction and Quantification
2.3. Plate Counts
2.3.1. Suspension Solution
2.3.2. Sorenson’s NP-10 Semi-Selective Medium
2.3.3. Plating Soil
2.3.4. Colony Identification and Count
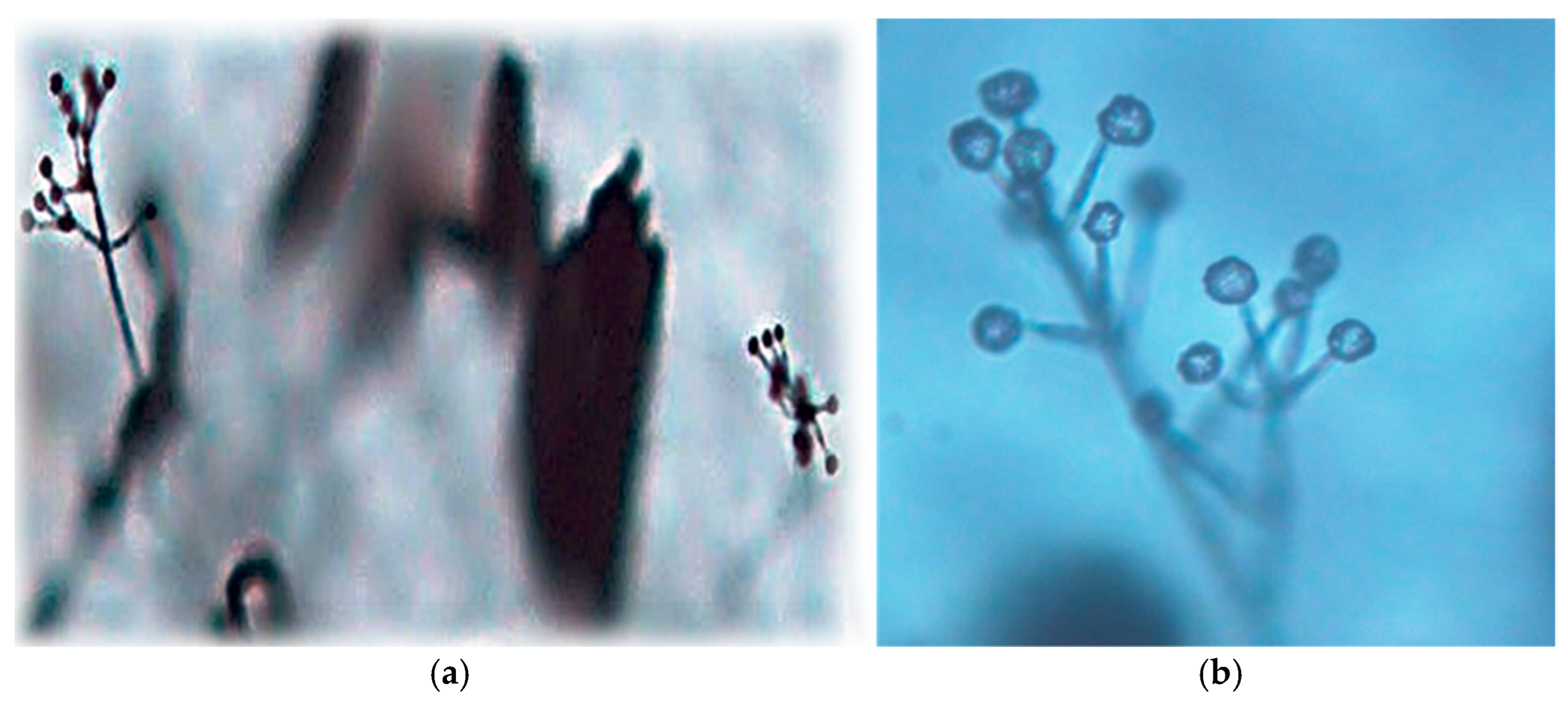
2.4. Pathogen and Nematode Identification
2.5. Verticillium Species Identification and Quantification Using RT-qPCR
2.6. Evaluation of Chemical Fumigation (Chloropicrin)
2.7. Evaluation of Various Disease Control Products Against Nematodes and Verticillium spp.
- Control: No fungicide or nematicide applied.
- Aprovia: A fungicide applied in-furrow at the recommended rate for control of Verticillium wilt (0.625 L/ha; 100 L water/ha).
- Velum: A nematicide applied in-furrow at the recommended rate for control of pathogenic nematodes (4.5 mL/100m row; 100 L water/ha).
- Velum + Aprovia (Treatments #2 + #3) combined.
- Mustgrow: An “oriental mustard seed meal” that is surface applied and incorporated in the soil (1680.5 kg/ha).
- Senator PSPT: A “seed piece treatment” registered for the control of Verticillium wilt (500 g/100 kg of cut seed).
- Vapam: Soil fumigant for control of pathogenic nematodes applied in-furrow a minimum of 5 days prior to planting (487.5 L/ha).
- Ammonium-lignosulfonate: Applied as a soil amendment 14 days prior to planting, mixed with water applied directly to the soil (6 T/ha).
- Nimitz: Fast-acting contact nematicide for controlling root-knot and root lesion nematodes in fruiting vegetables. Applied in-furrow 7 days before planting at a depth of 15–20 cm (6 L/ha; 200 L water/ha).
2.8. Field Visual Disease Assessment of Potato Early Dying (PED)
3. Results
3.1. Plant and Soil Survey for Verticillium Species and Nematodes in NB Potato Fields
3.2. Effect of Fumigation with Chloropicrin on Root Lesion Nematode and Verticillium dahliae Population Density in Soil of New Brunswick Fields in the Fall of 2017
3.3. Evaluation of Various Disease Control Products Against Nematodes and Verticillium (Field Plot Trial)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powelson, M.L.; Rowe, R.C. Biology and management of early dying of potatoes. Annu. Rev. Phytopathol. 1993, 31, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, T.; Lafond-Lapalme, J.; Gonzalez, C.E.Q.; Jordan, K.S.; Yevtushenko, D.; Barrett, R.; Mimee, B. A Genome Resource for 192 Verticillium dahliae Isolates Infecting Potatoes Across Canada. PhytoFrontiers 2023, 3, 753–775. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Talboys, P.W. Verticillium dahliae. [Descriptions of Pathogenic Fungi and Bacteria]; Commonwealth Mycological Institute: Kew, UK, 1970; Volume 26, p. Sheet-256. [Google Scholar]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K.V. Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef] [PubMed]
- Li, K. Determining Effects of Management Practices on Potato Early Dying and Soil Microbiome and Assessing Risk of Fungicide Resistance in Verticillium dahliae. Master’s Thesis, The University of Maine, Orono, ME, USA, 2021. Available online: https://digitalcommons.library.umaine.edu/etd/3403 (accessed on 30 March 2025).
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Schneider, A.T. Verticillium wilt of potato: A model of key factors related to disease severity and tuber yield in Southeastern Idaho. Am. J. Potato Res. 2001, 78, 291–300. [Google Scholar] [CrossRef]
- Rowe, R.C.; Powelson, M.L. Potato early dying: Management challenges in a changing production environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C. Management Strategies for Potato Early Dying Complex in Southern Alberta. Master’s Thesis, University of Lethbridge, Lethbridge, AB, Canada, 2020. [Google Scholar]
- Robb, J.; Moukhamedov, R.; Hu, X.; Platt, H.; Nazar, R. Putative subgroups of Verticillium albo-atrum distinguishable by PCR-based assays. Physiol. Mol. Plant Pathol. 1993, 43, 423–436. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Platt, H.W.B.; Maxwell, P. Comparison of polymerase chain reaction-based methods with plating on media to detect and identify Verticillium wilt pathogens of potato. Can. J. Plant Pathol. 1999, 21, 125–131. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Platt, H.W.B. Quantifying Verticillium dahliae in soils collected from potato fields using a competitive PCR assay. Am. J. Potato Res. 2002, 79, 107–117. [Google Scholar] [CrossRef]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1955, 45, 180–181. [Google Scholar]
- Ettema, C.H.; Wardle, D.A. Spatial soil ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Harris, D.C.; Yang, J.R. The relationships between the amount of Verticillium dahliae in soil and the incidence of strawberry wilt as a basis for disease risk prediction. Plant Pathol. J. 1996, 45, 106–114. [Google Scholar] [CrossRef]
- Xiao, C.L.; Subbarao, K.V. Relationships between Verticillium dahliae inoculum density and wilt incidence, severity, and growth of cauliflower. Phytopathology 1998, 88, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, C.A.; Simons, S.A.; Heide, G.A. Inoculum density and spatial patterns of Rhizoctonia solani in field plots of Solanum tuberosum: Effects of cropping frequency. Plant Pathol. J. 1996, 45, 232–244. [Google Scholar] [CrossRef]
- Botseas, D.D.; Rowe, R.C. Development of potato early dying in response to infection by two pathotypes of Verticillium dahliae and co-infection by Pratylenchus penetrans. Phytopathology 1994, 84, 275–282. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C. Comments on the feature “Potato early dying: Management challenges in a changing production environment”. Plant Dis. 2004, 88, 1168–1171. [Google Scholar] [CrossRef]
- Palmisano, A.; Parrado, L.; Quintanilla, M. Exploring the synergistic relationship between Pratylenchus penetrans and Verticillium dahliae in potato cropping systems: Recent developments and research gaps. Front. Hortic. 2024, 3, 1401147. [Google Scholar] [CrossRef]
- Otieno, H.M. Impacts and management strategies of common potato (Solanum tuberosum L.) pests and diseases in East Africa. Front. Sci. 2019, 9, 33–40. [Google Scholar]
- Baribeau, B. Verticillium wilt and seed potato certification. Am. J. Potato Res. 1952, 29, 157–159. [Google Scholar] [CrossRef]
- IsaacC, I.; Harrison, J.A.C. The symptoms and causal agents of early-dying disease (Verticillium wilt) of potatoes. Ann. Appl. Biol. 1968, 61, 231–244. [Google Scholar] [CrossRef]
- Rowe, R.C.; Davis, J.R.; Powelson, M.L.; Rouse, D.I. Potato early dying: Causal agents and management strategies. Plant Dis. 1987, 71, 482–489. [Google Scholar] [CrossRef]
- EL Hadrami, A.; Wally, O.; Adam, L.R.; Daayf, F. PCR-based determination of colonization patterns during potato tuber infection by single and multiple pathogens. Eur. J. Plant Pathol. 2007, 117, 201–218. [Google Scholar] [CrossRef]
- Powelson, M.L.; Rowe, R.C. Managing diseases caused by seedborne and soilborne fungi and fungus-like pathogens. In Potato Health Management, 2nd ed.; Johnson, D.A., Ed.; American Phytopathological Society (APS) Press: St. Paul, MN, USA, 2008; pp. 183–195. [Google Scholar]
- Stevenson, W.R.; Loria, R.; Franc, G.D.; Weingartner, D.P. Compendium of Potato Diseases, 2nd ed.; Stevenson, W.R., Ed.; American Phytopathological Society: St. Paul, MN, USA, 2001. [Google Scholar]
- Chen, D.; Barrett, R.; Mimee, B.; Arseneault, T.; Comeau, L.P.; Nahar, K.; Jimenez, S.L.; Zebarth, B.J. Prevalence of Verticillium spp. and Pratylenchus spp. in Commercial Potato Fields in Atlantic Canada. Am. J. Potato Res. 2024, 101, 291–305. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Griffin, T.S.; Olanya, O.M.; He, Z.; Halloran, J.M. Cumulative and residual effects of different potato cropping system management strategies on soilborne diseases and soil microbial communities over time. Plant Pathol. J. 2016, 66, 437–449. [Google Scholar] [CrossRef]
- Goicoechea, N. To what extent are soil amendments useful to control Verticillium wilt? Pest Manag. Sci. 2009, 65, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Lazarovits, G. Managing soilborne diseases of potatoes using ecologically based approaches. Am. J. Potato Res. 2010, 87, 401–411. [Google Scholar] [CrossRef]
- Lazarovits, G.; Subbarao, K. Challenges in controlling Verticillium wilt by the use of nonchemical methods. In Recent Developments in Management of Plant Diseases; Springer: Dordrecht, The Netherlands, 2010; pp. 247–264. [Google Scholar]
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Nolte, P.; Sorensen, L.H.; Schneider, A.T. Ecological relationships of Verticillium wilt suppression of potato by green manures. Am. J. Potato Res. 2010, 87, 315–326. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soilborne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Forge, T.A.; Larney, F.J.; Kawchuk, L.M.; Pearson, D.C.; Koch, C.; Blackshaw, R.E. Crop rotation effects on Pratylenchus neglectus populations in the root zone of irrigated potatoes in southern Alberta. Can. J. Plant Pathol. 2015, 37, 363–368. [Google Scholar] [CrossRef]
- Ochiai, N.; Crowe, F.J.; Dick, R.P.; Powelson, M.L. Effects of green manure type and amendment rate on Verticillium wilt severity and yield of Russet Burbank potato. Plant Dis. 2007, 91, 400–406. [Google Scholar] [CrossRef]
- Kelling, K.A.; Rouse, D.I.; Speth, E. Fumigation and Fertilizer Nitrogen Source Effects on Potato Yield, Quality, and Early Dying. Am. J. Potato Res. 2017, 94, 481–489. [Google Scholar] [CrossRef]
- Smolinska, U.; Horbowicz, M. Fungicidal activity of volatiles from selected cruciferous plants against resting propagules of soil-borne fungal pathogens. J. Phytopathol. 1999, 147, 119–124. [Google Scholar] [CrossRef]
- Montasser, S.A.; Korayem, A.M.; Youssef, M.M.A.; Mohamed, M.M.M. Vertical distribution of the root lesion nematode., Pratylenchus zeae infesting sugarcane in relation to soil type and growing season. Sci. Agric. 2015, 10, 95–97. [Google Scholar]
- Townshend, J.L. A modification and evaluation of the apparatus for the Oostenbrink direct cottonwool filter extraction method. Nematologica 1963, 9, 106–110. [Google Scholar] [CrossRef]
- Butterfield, E.J.; DeVay, J.E. Reassessment of soil assays for Verticillium dahliae. Phytopathology 1977, 67, 1073–1078. [Google Scholar] [CrossRef]
- Kabir, Z.; Bhat, R.; Subbarao, K.V. Comparison of media for recovery of Verticillium dahliae from soil. Plant Dis. 2004, 88, 49–55. [Google Scholar] [CrossRef]
- Goud, J.C.; Termorshuizen, A.J. Quality of methods to quantify microsclerotia of Verticillium dahliae in soil. Eur. J. Plant Pathol. 2003, 109, 523–534. [Google Scholar] [CrossRef]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 2nd ed.; Bruns, T., Lee, S.J.W.T., Taylor, J., Eds.; Academica Press Inc.: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Borza, T.; Beaton, B.; Govindarajan, A.; Gao, X.; Liu, Y.; Ganga, Z.; Wang-Pruski, G. Incidence and abundance of Verticillium dahliae in soil from various agricultural fields in Prince Edward Island, Canada. Eur. J. Plant Pathol. 2018, 151, 825–830. [Google Scholar] [CrossRef]
- Debode, J.; Van Poucke, K.; Franca, S.C.; Maes, M.; Hofte, M.; Heungens, K. Detection of multiple Verticillium species in soil using density flotation and real-time polymerase chain reaction. Plant Dis. 2011, 95, 1571–1580. [Google Scholar] [CrossRef]
- LaMondia, J.A. Management of lesion nematodes and potato early dying with rotation crops. J. Nematol. 2006, 38, 442–448. [Google Scholar]
- Rowe, R.C.; Riedel, R.M.; Martin, M.J. Synergistic Interactions Between Verticillium dahliae and Pratylenchus penetrans in Potato Early Dying Disease. Phytopathology 1985, 45, 412–418. [Google Scholar] [CrossRef]
- Johnson, D.A.; Dung, J.K.S. Verticillium wilt of potato—The pathogen, disease, and management. Can. J. Plant Pathol. 2010, 32, 58–67. [Google Scholar] [CrossRef]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato soil-borne diseases. A review. Agron. Sustain. Dev. 2012, 32, 93–132. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Collins, H.P.; Alva, A.; Boydston, R.A.; Cochran, R.L.; Hamm, P.B.; McGuire, A.; Riga, E. Soil microbial, fungal, and nematode responses to soil fumigation and cover crops under potato production. Biol. Fertil. Soils 2006, 42, 247–257. [Google Scholar] [CrossRef]
- Mahran, A.; Conn, K.L.; Tenuta, M.; Lazarovits, G.; Daayf, F. Effectiveness of liquid hog manure and acidification to kill Pratylenchus spp. in soil. J. Nematol. 2008, 40, 266–275. [Google Scholar]
- Borza, T.; Govindarajan, A.; Stephen, J.; Best, K.; Pruski, K.; Wang-Pruski, G. Verticillium dahliae and Verticillium nonalfalfae occurrence and abundance in several agricultural fields from Nova Scotia, Canada, assessed by real-time quantitative PCR. Eur. J. Plant Pathol. 2019, 154, 1171–1177. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Moreau, G.; Dixon, T.; Fillmore, S.; Smith, A.; Hann, S.; Comeau, L.P. Soil properties and topographic features influence within-field variation in potato tuber yield in New Brunswick, Canada. Soil Sci. Soc. Am. J. 2022, 86, 134–145. [Google Scholar] [CrossRef]
- Wang-Pruski, G.; Borza, T.; Govindarajan, A.; Gao, X.; Beaton, B.; Best, K.; Ganga, Z.; Pruski, K. Maritimes regional meeting, 2015/Réunion régionale des Maritimes, 2015. Detection and quantification of Verticillium dahliae in soil of potato and strawberry fields and its distribution in PEI and Nova Scotia. Can. J. Plant Pathol. 2016, 38, 141–147. [Google Scholar]
- Tsror, L.; Shlevin, E.; Peretz-Alon, I. Efficacy of metam sodium for controlling Verticillium dahliae prior to potato production in sandy soils. Am. J. Potato Res. 2005, 82, 419–423. [Google Scholar] [CrossRef]
- Molina, O.I.; Tenuta, M.; El Hadrami, A.; Buckley, K.; Cavers, C.; Daayf, F. Potato early dying and yield responses to compost, green manures, seed meal and chemical treatments. Am. J. Potato Res. 2014, 91, 414–428. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Westermann, D.T.; Hafez, S.L.; Everson, D.O.; Sorensen, L.H.; Schneider, A.T. Effects of green manures on Verticillium wilt of potato. Phytopathology 1996, 86, 444–453. [Google Scholar] [CrossRef]
- Jansky, S.H. Identification of Verticillium wilt resistance in US potato breeding programs. Am. J. Potato Res. 2009, 86, 504–512. [Google Scholar] [CrossRef]
- Couture, J.J.; Singh, A.; Charkowski, A.O.; Groves, R.L.; Gray, S.M.; Bethke, P.C.; Townsend, P.A. Integrating spectroscopy with potato disease management. Plant Dis. 2018, 102, 2233–2240. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M.; Matthiessen, J.N. Assessing the biofumigation potential of crucifers. Acta Hortic. 1998, 459, 105–112. [Google Scholar] [CrossRef]
- Bélair, G.; Dauphinais, N.; Fournier, Y.; Dangi, O.P.; Clément, M.F. Effect of forage and grain pearl millet on Pratylenchus penetrans and potato yields in Quebec. J. Nematol. 2005, 37, 78. [Google Scholar]
- Chen, D.; Zebarth, B.J.; Goyer, C.; Comeau, L.P.; Nahar, K.; Dixon, T. Effect of Biofumigation on population densities of Pratylenchus spp. and Verticillium spp. and potato yield in Eastern Canada. Am. J. Potato Res. 2022, 99, 229–242. [Google Scholar] [CrossRef]
- Ngala, B.M.; Haydock, P.P.J.; Woods, S.; Back, M.A. Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest Manag. Sci. 2015, 71, 759–769. [Google Scholar] [CrossRef]
- Watts, W.D.J.; Grove, I.G.; Tomalin, G.R.; Back, M.A. Field screening of biofumigant species for the reduction of potato cyst nematodes (Globodera sp.). Asp. Appl. Biol. 2014, 126, 1–7. [Google Scholar]
- Larkin, R.P.; Griffin, T.S. Control of soilborne diseases of potato using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- MacGuidwin, A.E.; Knuteson, D.L.; Connell, T.; Bland, W.L.; Bartelt, K.D. Manipulating inoculum densities of Verticillium dahliae and Pratylenchus penetrans with green manure amendments and solarization influence potato yield. Phytopathology 2012, 102, 519–527. [Google Scholar] [CrossRef] [PubMed]





| Field # | Sample ID | Parish | Nematode #/kg of Soil | Verticillium Content | |||||
|---|---|---|---|---|---|---|---|---|---|
| Root-Lesion | Pin | Root-Knot | Stunt | Spiral | # V. d * Cells/g | V. w ** CFU/g | |||
| 1 | VN2017-001 | Drummond | 5320 | 5920 | 0 | 0 | 0 | 3234.6 | 8 |
| 2 | VN2017-002 | Drummond | 8720 | 0 | 20 | 0 | 0 | 13,991.1 | 20 |
| 3 | VN2017-003 | Drummond | 5720 | 0 | 0 | 0 | 0 | 1866.7 | 6 |
| 4 | VN2017-004 | Drummond | 5000 | 0 | 0 | 0 | 0 | 11,489.8 | 30 |
| 5 | VN2017-005 | Drummond | 3640 | 80 | 0 | 40 | 0 | 3382.2 | 2 |
| 6 | VN2017-006 | Drummond | 6360 | 0 | 0 | 0 | 0 | 261.4 | 16 |
| 7 | VN2017-007 | Drummond | 4800 | 40 | 0 | 0 | 0 | 1267.5 | 36 |
| 8 | VN2017-008 | St-André | 1820 | 20 | 0 | 0 | 0 | 19,688.5 | 34 |
| 9 | VN2017-009 | St-André | 5080 | 240 | 0 | 0 | 0 | 2480.9 | 14 |
| 10 | VN2017-010 | St-André | 1160 | 0 | 0 | 0 | 0 | 4254.9 | 14 |
| 11 | VN2017-011 | St-André | 4620 | 0 | 0 | 0 | 0 | 1876.1 | 12 |
| 12 | VN2017-012 | Grand Falls | 3820 | 20 | 0 | 0 | 0 | 2287.7 | 10 |
| 13 | VN2017-013 | Grand Falls | 9400 | 0 | 0 | 0 | 0 | 4126.0 | 14 |
| 14 | VN2017-014 | New Denmark | 2120 | 0 | 0 | 0 | 0 | 814.7 | 10 |
| 15 | VN2017-016 | New Denmark | 1080 | 140 | 0 | 0 | 0 | 1364.4 | 12 |
| 16 | VN2017-017 | New Denmark | 5320 | 260 | 0 | 0 | 20 | 0.0 | 2 |
| 17 | VN2017-018 | Four Falls | 4400 | 160 | 0 | 0 | 100 | 3489.6 | 10 |
| 18 | VN2017-019 | Four Falls | 1480 | 4320 | 0 | 0 | 180 | 4359.2 | 2 |
| 19 | VN2017-020 | St-André | 580 | 0 | 0 | 0 | 300 | 423.1 | 24 |
| 20 | VN2017-021 | St-André | 5460 | 120 | 560 | 0 | 660 | 1294.3 | 0 |
| 21 | VN2017-022 | Grand Falls | 3580 | 0 | 0 | 0 | 0 | 27,471.4 | 14 |
| 22 | VN2017-023 | Grand Falls | 5960 | 40 | 0 | 0 | 0 | 3704.2 | 8 |
| 23 | VN2017-024 | Grand Falls | 7320 | 0 | 0 | 0 | 0 | 6025.8 | 16 |
| 24 | VN2017-025 | St-André | 680 | 0 | 120 | 0 | 400 | 18,147.0 | 16 |
| 25 | VN2017-026 | St-André | 13,640 | 0 | 0 | 0 | 0 | 4304.3 | 6 |
| 26 | VN2017-027 | Greenfield | 1100 | 100 | 0 | 0 | 0 | 16,287.5 | 0 |
| 27 | VN2017-028 | Greenfield | 10,680 | 0 | 0 | 0 | 0 | 2557.6 | 2 |
| 28 | VN2017-029 | Jacksonville | 2680 | 360 | 0 | 0 | 0 | 16,487.5 | 0 |
| 29 | VN2017-030 | Jacksonville | 2160 | 280 | 0 | 0 | 40 | 943.7 | 2 |
| 30 | VN2017-032 | St-Thomas | 4260 | 0 | 0 | 0 | 0 | 2583.9 | 0 |
| 31 | VN2017-033 | St-Thomas | 6600 | 740 | 20 | 0 | 180 | 2529.7 | 16 |
| 32 | VN2017-034 | Simonds | 2180 | 20 | 0 | 0 | 3291.6 | 6 | |
| 33 | VN2017-036 | Knoxford | 1080 | 60 | 0 | 0 | 20 | 4971.1 | 0 |
| 34 | VN2017-038 | Jacksonville | 1540 | 0 | 660 | 0 | 0 | 13,623.5 | 8 |
| 35 | VN2017-039 | Jacksonville | 8520 | 0 | 0 | 0 | 0 | 4589.5 | 4 |
| 36 | VN2017-041 | Killoween | 3680 | 320 | 1320 | 0 | 0 | 3193.4 | 0 |
| 37 | VN2017-042 | Bloomfield | 10,760 | 0 | 0 | 0 | 0 | 443.4 | 0 |
| 38 | VN2017-043 | Bloomfield | 14,240 | 0 | 0 | 0 | 0 | 1839.5 | 14 |
| 39 | VN2017-044 | Hartland | 1900 | 40 | 0 | 0 | 0 | 21,503.0 | 66 |
| 40 | VN2017-045 | Hartland | 8400 | 2600 | 0 | 0 | 0 | 1350.7 | 2 |
| 41 | VN2017-046 | Connell | 1580 | 0 | 0 | 0 | 0 | 10,287.6 | 24 |
| 42 | VN2017-047 | Connell | 2380 | 0 | 0 | 0 | 0 | 1390.5 | 10 |
| 43 | VN2017-048 | Richmond Corner | 2740 | 20 | 0 | 0 | 100 | 3027.5 | 6 |
| 44 | VN2017-049 | Richmond Corner | 1400 | 0 | 0 | 0 | 0 | 2797.5 | 12 |
| 45 | VN2017-050 | Grand Falls | 9840 | 0 | 0 | 0 | 0 | 3793.2 | 0 |
| 46 | VN2017-051 | Knoxford | 1400 | 20 | 1080 | 0 | 680 | 0.0 | 0 |
| 47 | VN2017-052 | Wicklow | 1080 | 60 | 0 | 0 | 20 | 4971.1 | 0 |
| 48 | VN2017-053 | Wakefield | 560 | 0 | 0 | 0 | 0 | 0.0 | 2 |
| 49 | VN2017-054 | Wakefield | 1480 | 0 | 0 | 0 | 0 | 4838.0 | 0 |
| 50 | VN2017-055 | Wakefield | 1120 | 0 | 0 | 0 | 40 | 5282.7 | 4 |
| 51 | VN2017-056 | Wakefield | 3360 | 60 | 0 | 0 | 0 | 1703.2 | 10 |
| 52 | VN2017-057 | Wakefield | 2300 | 120 | 0 | 0 | 0 | 3259.8 | 0 |
| 53 | VN2017-058 | Wakefield | 4520 | 0 | 0 | 0 | 0 | 5427.2 | 18 |
| 54 | VN2017-059 | Wicklow | 820 | 0 | 0 | 0 | 0 | 7894.1 | 0 |
| 55 | VN2017-060 | Wicklow | 820 | 0 | 0 | 0 | 0 | 7894.1 | 0 |
| 56 | VN2017-061 | Wakefield | 1580 | 0 | 0 | 0 | 20 | 7600.1 | 6 |
| 57 | VN2017-062 | Wicklow | 1180 | 0 | 0 | 0 | 80 | 3734.0 | 0 |
| 58 | VN2017-063 | Wicklow | 1780 | 0 | 0 | 0 | 20 | 1690.8 | 6 |
| 59 | VN2017-064 | Simonds | 900 | 0 | 0 | 0 | 0 | 13,737.5 | 18 |
| 60 | VN2017-065 | Aberdeen | 1480 | 0 | 0 | 0 | 20 | 8368.3 | 4 |
| 61 | VN2017-066 | Wakefield | 3840 | 60 | 0 | 0 | 0 | 6075.3 | 18 |
| 62 | VN2017-067 | Aberdeen | 1100 | 20 | 0 | 0 | 0 | 24,055.6 | 34 |
| 63 | VN2017-068 | Simonds | 3840 | 0 | 0 | 0 | 40 | 6159.4 | 20 |
| 64 | VN2017-069 | Simonds | 9080 | 0 | 0 | 0 | 20 | 2178.1 | 26 |
| 65 | VN2017-070 | Wilmot | 6160 | 0 | 0 | 0 | 0 | 1848.8 | 2 |
| 66 | VN2017-071 | Wilmot | 620 | 0 | 0 | 0 | 0 | 2438.0 | 0 |
| 67 | VN2017-072 | Wilmot | 5580 | 0 | 0 | 0 | 0 | 7705.0 | 24 |
| 68 | VN2017-073 | Wakefield | 2500 | 0 | 0 | 0 | 60 | 1564.4 | 14 |
| 69 | VN2017-074 | Wilmot | 6720 | 0 | 0 | 0 | 0 | 303.3 | 4 |
| 70 | VN2017-075 | Wilmot | 3960 | 0 | 0 | 100 | 0 | 1788.3 | 10 |
| 71 | VN2017-076 | Wakefield | 8000 | 0 | 0 | 0 | 0 | 1042.2 | 20 |
| Parish | No. of Samples | No. of Soil Samples Containing Nematodes | ||||
|---|---|---|---|---|---|---|
| Root Lesion | Pin | Root-Knot | Stunt | Spiral | ||
| Drummond | 12 | 12 | 3 | 1 | 1 | 0 |
| Grand Falls | 8 | 8 | 4 | 0 | 0 | 2 |
| St-André | 8 | 8 | 3 | 2 | 0 | 3 |
| Brighton | 2 | 2 | 2 | 0 | 0 | 0 |
| Kent | 1 | 1 | 1 | 1 | 0 | 0 |
| Richmond | 2 | 2 | 1 | 0 | 0 | 1 |
| Simonds | 9 | 9 | 3 | 1 | 0 | 4 |
| Wakefield | 11 | 11 | 3 | 0 | 0 | 3 |
| Wicklow | 9 | 9 | 4 | 1 | 0 | 5 |
| Wilmot | 9 | 9 | 1 | 1 | 1 | 0 |
| Sample ID | Nematode (#/kg Soil) | Verticillium Content/g Soil | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root Lesion | Pin | Root-Knot | Spiral | # V. d *a Cells/g | V. w ** (CFU/g) | ||||||||||
| *** BF | **** AF | % Control | BF | AF | BF | AF | BF | AF | BF | AF | % Control | BF | AF | % Control | |
| Field 1 | 2500 | 140 | 94.4 | 0 | 0 | 0 | 0 | 0 | 0 | 10,837 ± 4504 | 962.8 ± 324 | 91.1 | 8.0 | 0 | 100.0 |
| Field 2 | 4120 | 40 | 99.0 | 0 | 0 | 0 | 0 | 0 | 0 | 10,398.2 ± 2302 | 1968.4 ± 1053 | 81.1 | 10.0 | 1 | 90.0 |
| Field 3 | 11,080 | 7300 | 34.1 | 280 | 20 | 0 | 0 | 600 | 0 | 807.6 ± 387 | 501.5 ± 130 | 37.9 | 4.0 | 2 | 50.0 |
| Field 4 | 8520 | 1160 | 86.4 | 80 | 0 | 0 | 0 | 200 | 0 | 797.4 ± 115 | 156.3 ± 93 | 80.4 | 6.0 | 1 | 83.3 |
| Field 5 | 3120 | 140 | 95.5 | 0 | 0 | 0 | 0 | 0 | 0 | 830.3 ± 382 | 38.1 ± 18 | 95.4 | 8.0 | 2 | 75.0 |
| Field 6 | 2040 | 200 | 90.2 | 80 | 0 | 0 | 0 | 20 | 0 | 1736.4 ± 209 | 220.1 ± 125 | 87.3 | 6.0 | 0 | 100.0 |
| Field 7 | 780 | 280 | 64.1 | 380 | 80 | 0 | 0 | 20 | 0 | 4291.3 ± 740 | 536.8 ± 251 | 87.5 | 6.0 | 0 | 100.0 |
| Field 8 | 3660 | 140 | 96.2 | 80 | 0 | 20 | 0 | 0 | 0 | 14,412.8 ± 7682 | 1567.3 ± 550 | 89.1 | 14.0 | 0 | 100.0 |
| Treatment | Root Lesion Nematode (#/kg Soil) * | % Increase (+)/Decrease (−) of Root Lesion Nematodes ** | |
|---|---|---|---|
| Spring Samples | Fall Samples | ||
| Control | 190 | 365 | +92.11 a |
| Aprovia | 265 | 290 | +9.43 d |
| Velum | 450 | 150 | −66.67 h |
| Velum + Aprovia | 605 | 360 | −40.50 g |
| Mustgrow | 360 | 480 | +33.33 c |
| Senator PSPT | 585 | 675 | +15.38 d |
| Vapam | 440 | 330 | −25.00 f |
| Ammonium-lignosulfonate | 765 | 515 | −32.68 fg |
| Nimitz | 505 | 510 | +0.99 e |
| Treatment | Verticillium dahliae CFU/g Soil * | % Increase (+)/Decrease (−) of CFU/g Soil ** | |
|---|---|---|---|
| Spring Samples | Fall Samples | ||
| Control | 4.5 | 20.0 | +344.4 a |
| Aprovia | 7.5 | 2.0 | −73.3 c |
| Velum | 46.0 | 27.5 | −40.0 c |
| Velum + Aprovia | 15.5 | 13.0 | −16.1 c |
| Mustgrow | 25.5 | 14.5 | −43.1 c |
| Senator PSPT | 19.5 | 12.0 | −38.5 c |
| Vapam | 11.5 | 9.5 | −17.4 c |
| Ammonium-lignosulfonate | 7.50 | 3.5 | −53.3 c |
| Nimitz | 4.50 | 13.3 | +195.6 b |
| Treatment | Verticillium dahliae Content Cell#/g Soil * | % Increase (+)/Decrease (−) Cell#/g Soil ** | |
|---|---|---|---|
| Spring Samples | Fall Samples | ||
| Control | 820.3 | 3840.1 | +78.64 a |
| Aprovia | 6825.6 | 3914.8 | −74.35 b |
| Velum | 2301.4 | 1517.5 | −51.66 b |
| Velum + Aprovia | 9787.4 | 3368.6 | −190.55 d |
| Mustgrow | 2281.1 | 2088.6 | −9.22 b |
| Senator PSPT | 3102.3 | 2831.0 | −9.58 b |
| Vapam | 2272.5 | 997.0 | −127.93 c |
| Ammonium-lignosulfonate | 4917.3 | 1313.9 | −274.24 e |
| Nimitz | 1151.5 | 3477.7 | +66.89 a |
| Treatment | Marketable Yield (T/ha) * | % Increase Relative to Untreated Control |
|---|---|---|
| Control | 13.62 c | 0.00 |
| Aprovia | 22.89 ab | 67.99 |
| Velum | 25.09 ab | 84.12 |
| Velum + Aprovia | 19.95 abc | 46.41 |
| Mustgrow | 25.06 ab | 83.91 |
| Senator PSPT | 22.74 ab | 66.89 |
| Vapam | 17.36 bc | 27.38 |
| Ammonium-lignosulfonate | 26.66 a | 95.67 |
| Nimitz | 26.94 a | 97.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mughrabi, K.I.; Poirier, R.; Khabbaz, S.E. Status and Best Management Practices of Potato Early Dying Disease in New Brunswick, Canada. Biology 2025, 14, 514. https://doi.org/10.3390/biology14050514
Al-Mughrabi KI, Poirier R, Khabbaz SE. Status and Best Management Practices of Potato Early Dying Disease in New Brunswick, Canada. Biology. 2025; 14(5):514. https://doi.org/10.3390/biology14050514
Chicago/Turabian StyleAl-Mughrabi, Khalil I., Rene Poirier, and Salah Eddin Khabbaz. 2025. "Status and Best Management Practices of Potato Early Dying Disease in New Brunswick, Canada" Biology 14, no. 5: 514. https://doi.org/10.3390/biology14050514
APA StyleAl-Mughrabi, K. I., Poirier, R., & Khabbaz, S. E. (2025). Status and Best Management Practices of Potato Early Dying Disease in New Brunswick, Canada. Biology, 14(5), 514. https://doi.org/10.3390/biology14050514






