Inhibition of Thioredoxin Reductase Activity and Oxidation of Cellular Thiols by Antimicrobial Agent, 2-Bromo-2-nitro-1,3-propanediol, Causes Oxidative Stress and Cell Death in Cultured Noncancer and Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Materials
2.2. Detection of Free Formaldehyde in Cell Culture Medium Containing BP
2.3. Inhibition of TR Activity
2.4. Reactivation of BP-Inhibited TR, by DTT
2.5. Quantification of Total Thiols in BP-Treated and Untreated Cells
2.6. Detection of Intracellular ROS Concentration Using Fluorescence Dye DCFH-DA
2.7. Cell Viability by MTT Assay
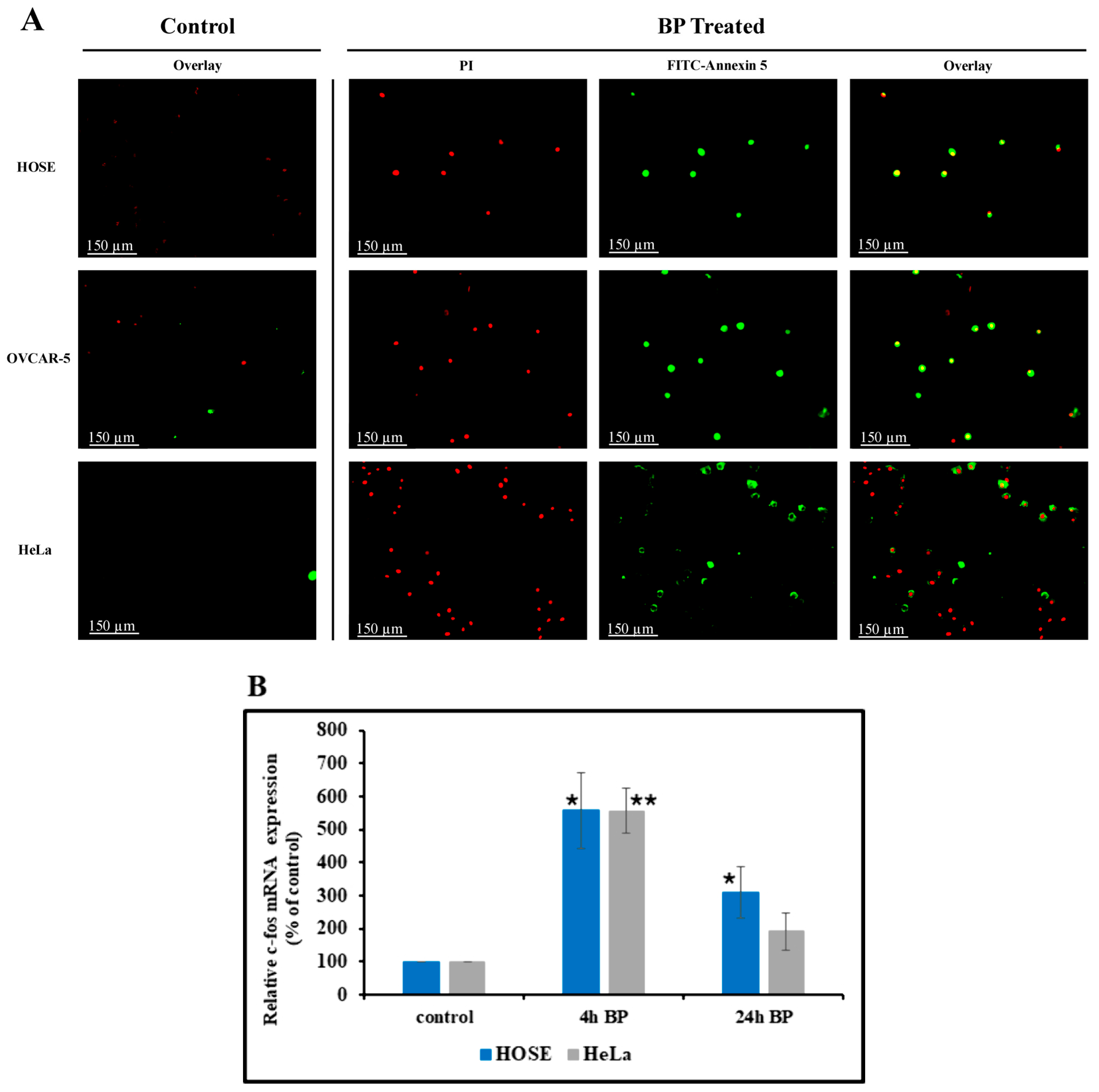
2.8. Detection of Apoptosis by Hoechst 33342 Staining Method and Annexin V and Propidium Iodide (PI) Double Staining Method
2.9. Detection of c-fos mRNA by Droplet Digital PCR (ddPCR)
2.10. Detection of ATP Concentration in Treated and Untreated HeLa Cells
2.11. TrxR Activity
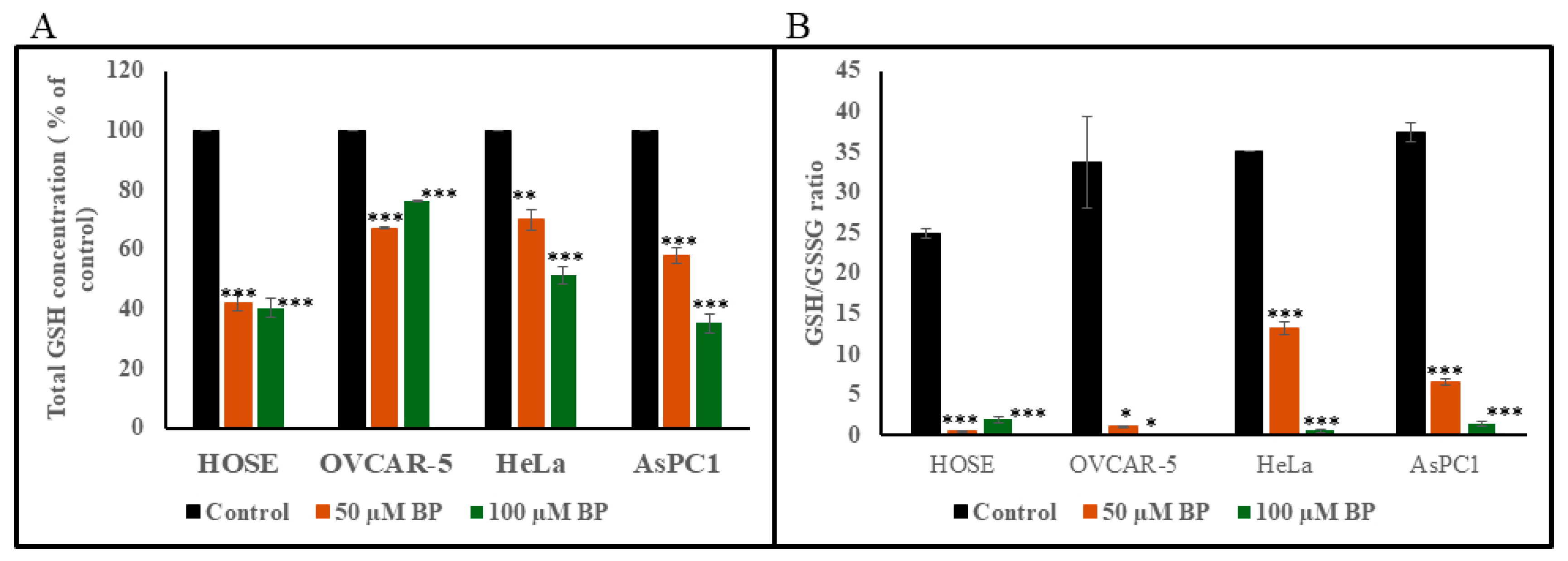
2.12. Detection of Cellular Total Glutathione (GSH) and GSH/GSSG) Ratio
2.13. Statistical Analysis
3. Results
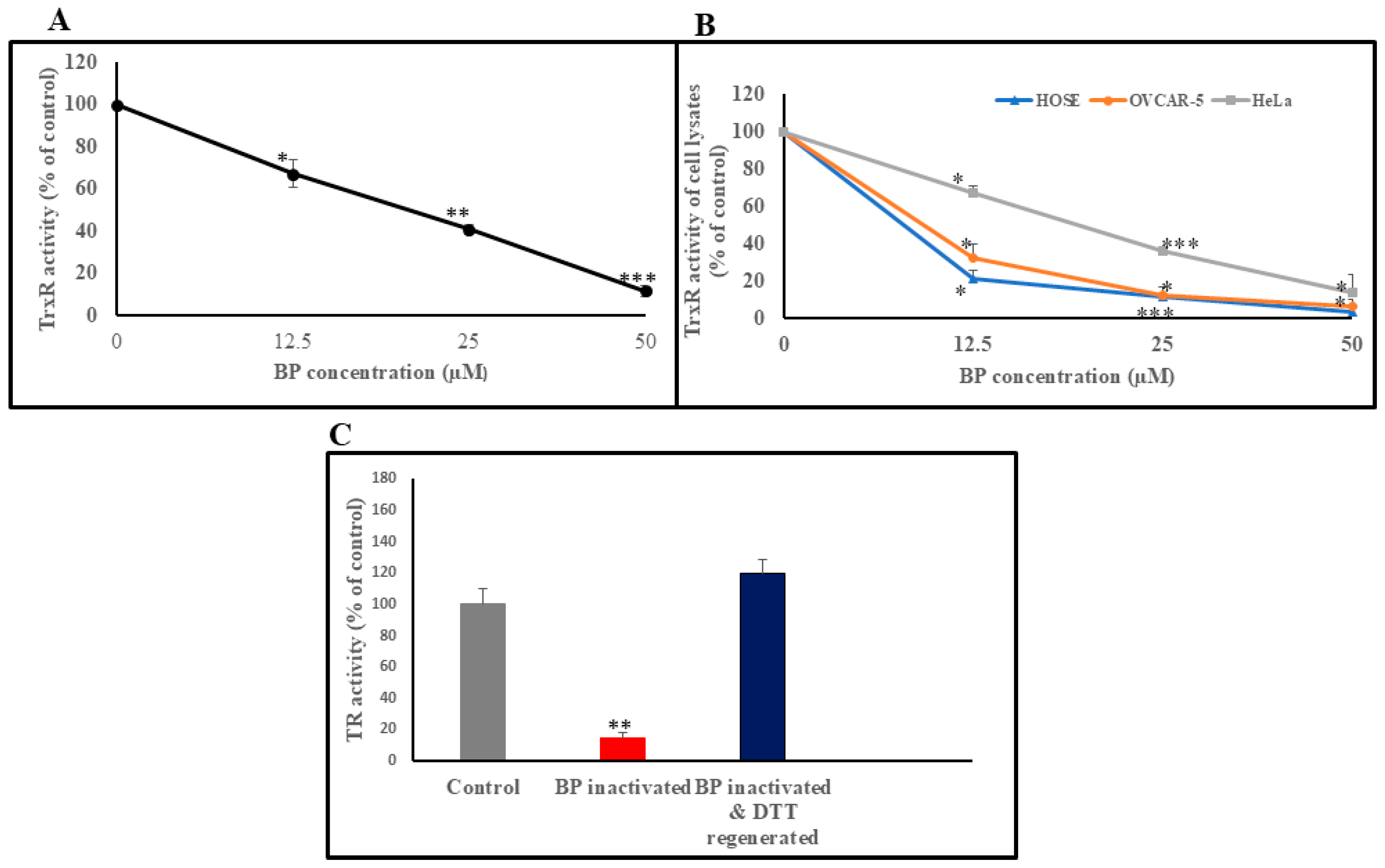
3.1. BP Reversibly Inhibits TrxR Enzyme Activity in In Vitro and in Cultured Cells
3.2. Reactivation of BP-Inhibited TrxR, by DTT
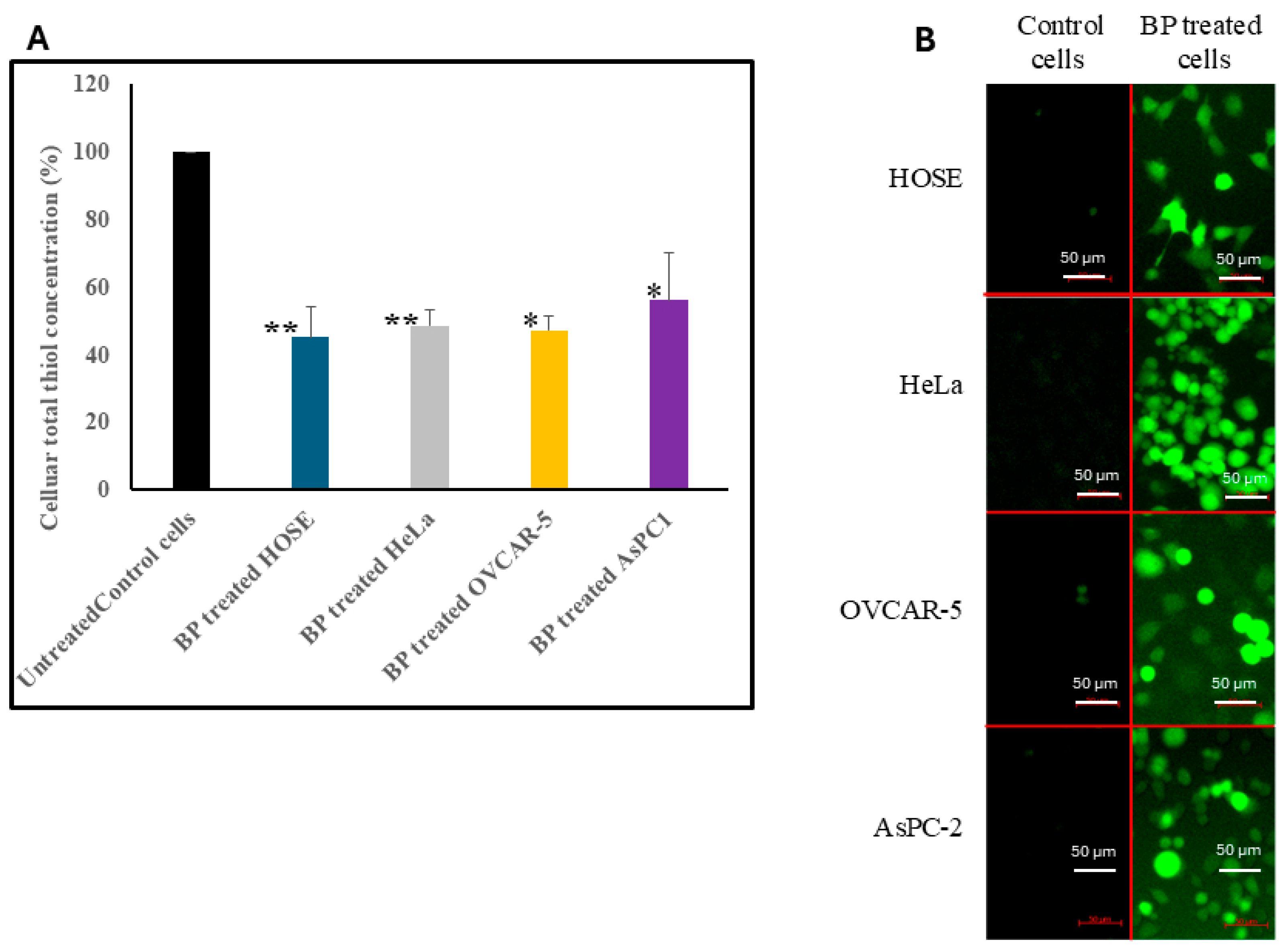
3.3. Effect of BP on Total Thiol Concentration in Cells
3.4. Effect of BP on Intracellular ROS Concentrations
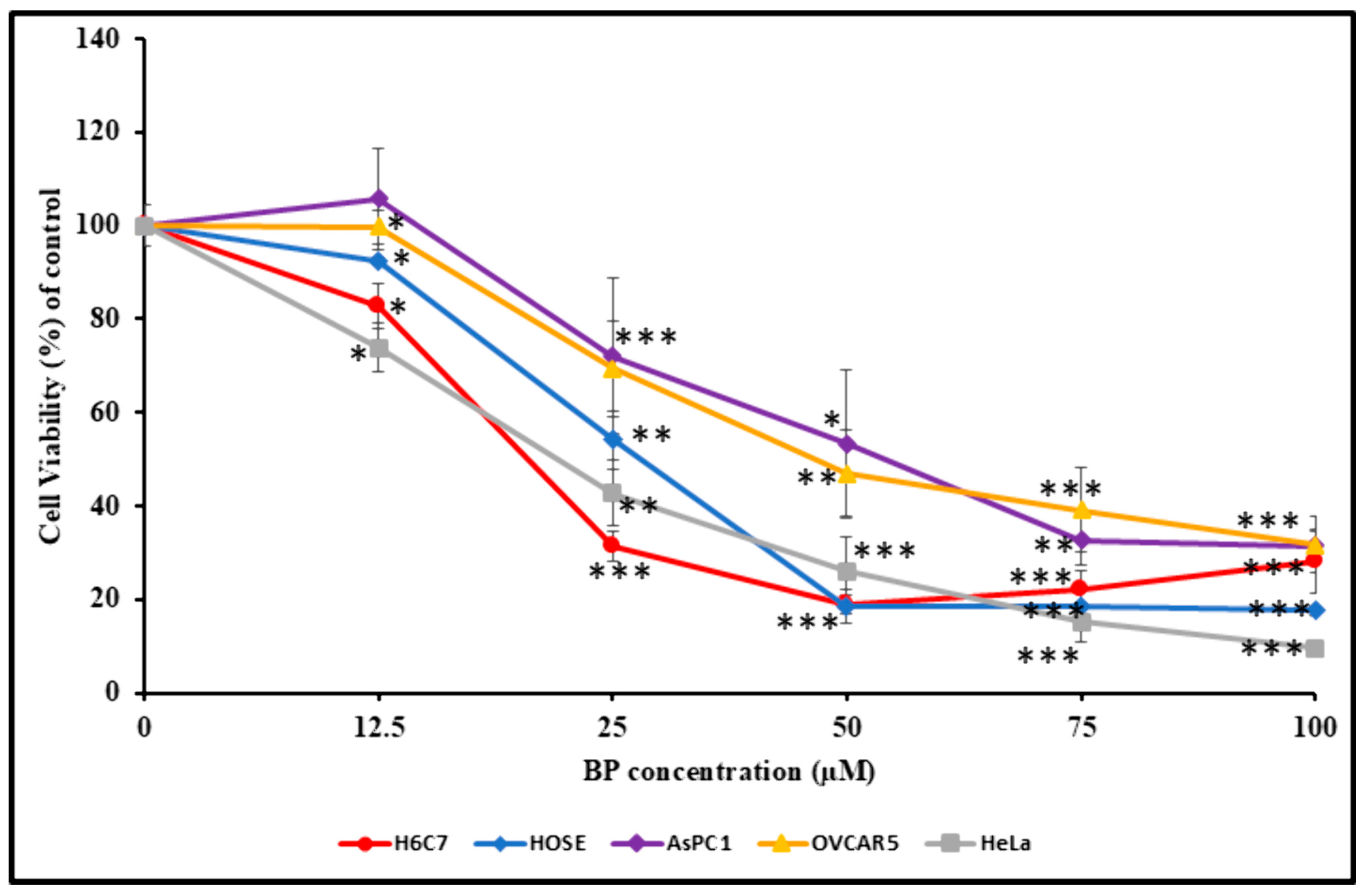
3.5. Effect of BP on Cell Viability
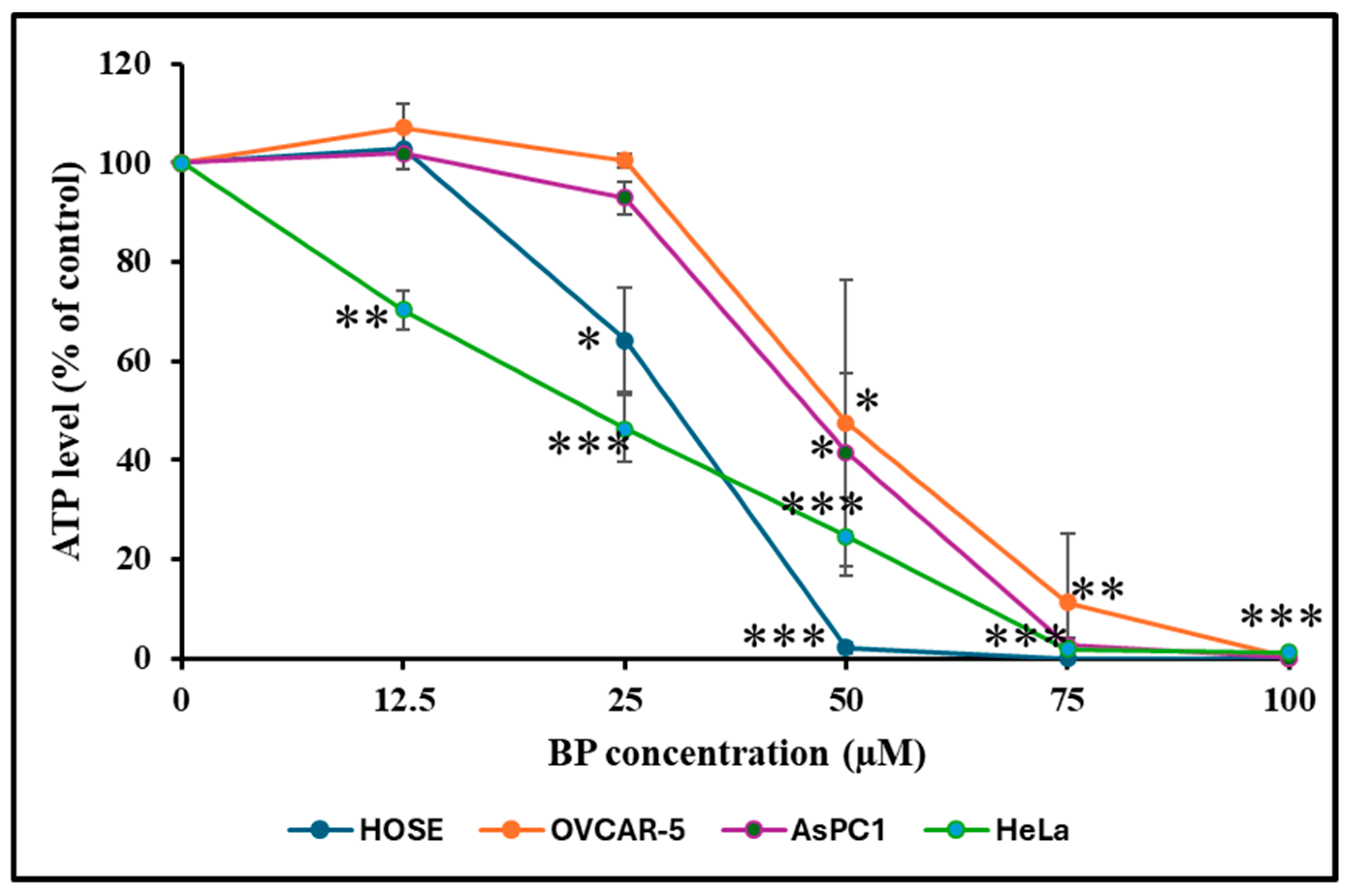
3.6. Effect of BP on Cellular ATP Level
3.7. BP-Induced Apoptosis
3.8. Effect of BP on Cellular c-fos mRNA Level
3.9. Detection of Total GSH and GSH/GSSG Ratio
3.10. Detection of Free Formaldehyde in Cell Culture Medium Containing BP
4. Discussion
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TrxS | Thioredoxin system |
| Trx | Thioredoxin |
| TrxR | Thioredoxin reductase |
| BP | Bronopol |
References
- Lunn, C.A.; Pak, P.; Van Savage, J.; Pigiet, V. The catalytic active site of thioredoxin: Conformation and homology with bovine pancreatic trypsin inhibitor. Biochim. Biophys. Acta 1986, 871, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Johansson, L.; Bauer, H.; Arscott, L.D.; Rauch, S.; Ballou, D.P.; Williams, C.H., Jr.; Schirmer, R.H.; Arnér, E.S. Active sites of thioredoxin reductases: Why selenoproteins? Proc. Natl. Acad. Sci. USA 2003, 100, 12618–12623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilbert, H.F. Molecular and cellular aspects of thioldisulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 1990, 63, 69–172. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Enzymatic reduction-oxidation of protein disulfides by thioredoxin. Methods Enzymol. 1984, 107, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.J.; Aslund, F.; Beckwith, J. Disulfide bond formation in the Escherichia coli cytoplasm: An invivo role reversal for the thioredoxins. EMBO J. 1998, 17, 5543–5550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmgren, A. Thioredoxin and Glutaredoxin Systems. J. Biol. Chem. 1989, 264, 13963–13966. [Google Scholar] [CrossRef] [PubMed]
- Brot, N.; Weissbach, H. Biochemistry of methionine sulfoxide residues in proteins. Biofactors 1991, 3, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.; Klein, M.; Erdbrügger, W.; Dröge, W.; Schulze-Osthoff, K. Distinct effects of thioredoxin and antioxidants onthe activation of transcription factors NF-kappa B and AP-1. Proc. Natl. Acad. Sci. USA 1994, 91, 1672–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirota, K.; Murata, M.; Sachi, Y.; Nakamura, H.; Takeuchi, J.; Mori, K.; Yodoi, J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999, 274, 27891–27897. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Nanri, H.; Yoshitake, S.; Nagata-Kuno, K.; Minakami, S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur. J. Biochem. 1992, 209, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-J.; Geng, W.-S.; Wang, Z.-Q.; Chen, L.; Zeng, X.-S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers 2010, 2, 209–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Kahlos, K.; Näpänkangas, U.; Kaarteenaho-Wiik, R.; Säily, M.; Koistinen, P.; Pääakkö, P.; Holmgren, A.; Kinnula, V.L. Widespread expression of thioredoxin and thioredoxin reductase in non-small cell lung carcinoma. Clin. Cancer Res. 2001, 7, 1750–1757. [Google Scholar] [PubMed]
- Lincoln, D.T.; Al-Yatama, F.; Mohammed, F.M.; Al-Banaw, A.G.; Al-Bader, M.; Burge, M.; Sinowatz, F.; Singal, P.K. Thioredoxin and thi-oredoxin reductase expression in thyroid cancer depends on tumor aggressiveness. Anticancer Res. 2010, 30, 767–775. [Google Scholar] [PubMed]
- Nguyen, P.; Awwad, R.T.; Smart, D.D.; Spitz, D.R.; Gius, D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006, 236, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, M.; Fan, R.; Zhao, S.; Liu, W. Targeting the Thioredoxin System as a Strategy for Cancer Therapy. J. Med. Chem. 2019, 62, 7309–7321. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Liang, Z.; Ma, X.; Liu, L.; Wen, Z.; Tolbatov, I.; Marrone, A.; Liu, W. NHC-gold(I)-alkyne complexes induced hepatocellular carcinoma cell death through bioorthogonal activation by palladium complex in living system. Chin. Chem. Lett. 2023, 34, 108413. [Google Scholar] [CrossRef]
- Croshaw, B.; Groves, M.J.; Lessel, B. Some properties of Bronopol, a new antimicrobial agent active against Pseudomonas aeruginosa. J. Pharm. Pharmacol. 1964, 16, 127T–130T. [Google Scholar] [CrossRef] [PubMed]
- Storrs, F.J.; Bell, D.E. Allergic contact dermatitis to 2-bromo-2-nitropropane-1,3-diol in a hydrophilic ointment. J. Am. Acad. Dermatol. 1983, 8, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.A.; Waigh, R.D.; Gilbert, P. Antibacterial action of 2-bromo-2-nitropropane-1,3-diol (bronopol). Antimicrob. Agents Chemother. 1988, 32, 1693–1698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stretton, R.J.; Manson, T.W. Some aspects of the mode of action of the antibacterial compound Bronopol (2-bromo-2-nitropropan-1,3-diol). J. Appl. Bacteriol. 1973, 36, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Duan, D.; Yao, J.; Gao, K.; Fang, J. Inhibition of thioredoxin reductase by alantolactone prompts oxidative stress-mediated apoptosis of HeLa cells. Biochem. Pharmacol. 2016, 102, 34–44, Erratum in Biochem. Pharmacol. 2024, 225, 116317. https://doi.org/10.1016/j.bcp.2024.116317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Pecha, J.; Hoshino, I.; Ankrapp, D.; Xiao, H. TIP30 mutant derived from hepatocellular carcinoma specimens promotes growth of HepG2 cells through up-regulation of N-cadherin. Cancer Res. 2007, 67, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, 1101. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Doroudi, R.; Hägg, U.; Johansson, A.; Selin-Sjögren, L.; Jern, S. Differential immediate-early gene responses to shear stress and intraluminal pressure in intact human conduit vessels. FEBS Lett. 2000, 477, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tanvetthayanont, P.; Yata, T.; Boonnil, J.; Temisak, S.; Ponglowhapan, S. Validation of droplet digital PCR for cytokeratin 19 mRNA detection in canine peripheral blood and mammary gland. Sci. Rep. 2022, 12, 13623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, W.; Zhou, Z.; Zhong, Y.; Sun, Y.; Wang, Y.; Zhu, Z.; Jiao, W.; Bai, M.; Sun, J.; Lu, J.; et al. Plasma activity of Thioredoxin Reductase as a Novel Biomarker in Gastric Cancer. Sci. Rep. 2019, 9, 19084, Erratum in Sci. Rep. 2020, 10, 17254. https://doi.org/10.1038/s41598-020-70071-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, C.; Zhang, L.; Sun, R.; Liu, J.; Yin, H.; Li, X.; Zheng, X.; Zeng, H. Role of thioredoxin reductase 1 in dysplastic transformation of human breast epithelial cells triggered by chronic oxidative stress. Sci. Rep. 2016, 6, 36860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preston, G.A.; Srinivasan, D.; Barrett, J.C. Apoptotic response to growth factor deprivation involves cooperative interactions between c-Fos and p300. Cell Death Differ. 2000, 7, 215–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cunnea, P.; Fernandes, A.P.; Capitanio, A.; Eken, S.; Spyrou, G.; Björnstedt, M. Increased expression of specific thioredoxin family proteins; a pilot immunohistochemical study on human hepatocellular carcinoma. Int. J. Immunopathol. Pharmacol. 2007, 20, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Effects of Mammalian Thioredoxin Reductase Inhibitors. Handb. Exp. Pharmacol. 2021, 264, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.D.; McCammon, J.A. Computer-aided drug design: Reversible covalent inhibitors. J. Mol. Graph. Model. 2011, 29, 670–676. [Google Scholar]
- Smeyne, R.J.; Vendrell, M.; Hayward, M.; Baker, S.J.; Miao, G.G.; Schilling, K.; Robertson, L.M.; Curran, T.; Morgan, J.I. Continuous c-fos expression precedes programmed cell death in vivo. Nature 1993, 363, 166–169, Erratum in Nature 1993, 365, 279. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Steinbach, J.P.; Marti, A.; Munz, K.; Wang, Z.Q.; Wagner, E.F.; Aguzzi, A.; Remé, C.E. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat. Med. 1997, 3, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Rich, K.A.; Zhan, Y.; Blanks, J.C. Aberrant expression of c-Fos accompanies photoreceptor cell death in therd mouse. J. Neurobiol. 1997, 32, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Kushnareva, Y.; Newmeyer, D.D. Bioenergetics and cell death. Ann. N. Y. Acad. Sci. 2010, 1201, 50–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leist, M.; Single, B.; Castoldi, A.F.; Kühnle, S.; Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997, 185, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, M.J.; Kim, I.Y.; Lee, D.M.; Park, Y.J.; Cho, M.-Y.; Jin, H.J.; Choi, K.S. Dual inhibition of thioredoxin reductase and proteasome is required for auranofin-induced paraptosis in breast cancer cells. Cell Death Dis. 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.; Masutani, H.; Oka, S.-I.; Tanaka, T.; Yamaguchi-Iwai, Y.; Nakamura, H.; Yodoi, J. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J. Biol. Chem. 2006, 281, 7384–7391. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Morton, S.U.; Fernhoff, N.B.; Marletta, M.A. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11609–11614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyihák, E.; Bocsi, J.; Timár, F.; Rácz, G.; Szende, B. Formaldehyde promotes and inhibits the proliferation of cultured tumorand endothelial cells. Cell Prolif. 2001, 34, 135–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Krzyzanowski, G.; Chandel, D.S.; Tom, W.A.; Fernando, N.; Olou, A.; Fernando, M.R. Inhibition of Thioredoxin Reductase Activity and Oxidation of Cellular Thiols by Antimicrobial Agent, 2-Bromo-2-nitro-1,3-propanediol, Causes Oxidative Stress and Cell Death in Cultured Noncancer and Cancer Cells. Biology 2025, 14, 509. https://doi.org/10.3390/biology14050509
Jiang C, Krzyzanowski G, Chandel DS, Tom WA, Fernando N, Olou A, Fernando MR. Inhibition of Thioredoxin Reductase Activity and Oxidation of Cellular Thiols by Antimicrobial Agent, 2-Bromo-2-nitro-1,3-propanediol, Causes Oxidative Stress and Cell Death in Cultured Noncancer and Cancer Cells. Biology. 2025; 14(5):509. https://doi.org/10.3390/biology14050509
Chicago/Turabian StyleJiang, Chao, Gary Krzyzanowski, Dinesh S. Chandel, Wesley A. Tom, Nirmalee Fernando, Appolinaire Olou, and M. Rohan Fernando. 2025. "Inhibition of Thioredoxin Reductase Activity and Oxidation of Cellular Thiols by Antimicrobial Agent, 2-Bromo-2-nitro-1,3-propanediol, Causes Oxidative Stress and Cell Death in Cultured Noncancer and Cancer Cells" Biology 14, no. 5: 509. https://doi.org/10.3390/biology14050509
APA StyleJiang, C., Krzyzanowski, G., Chandel, D. S., Tom, W. A., Fernando, N., Olou, A., & Fernando, M. R. (2025). Inhibition of Thioredoxin Reductase Activity and Oxidation of Cellular Thiols by Antimicrobial Agent, 2-Bromo-2-nitro-1,3-propanediol, Causes Oxidative Stress and Cell Death in Cultured Noncancer and Cancer Cells. Biology, 14(5), 509. https://doi.org/10.3390/biology14050509




