Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vector Construction
2.3. Pineapple Transformation
2.4. Tomato Transformation
2.5. Construction of Pineapple AcoPG3 Mutant Lines
2.6. Determination of Fruit Firmness
2.7. Polygalacturonase Activity Measurement
2.8. Extraction of Cell Wall
2.9. Cell Wall Component Analysis
2.10. Liquid Content in Intercellular Space
2.11. Cell Membrane Permeability Determination
3. Results
3.1. Increased Polygalacturonase Activity Is the Main Cause for the Softening of Pineapple Fruit
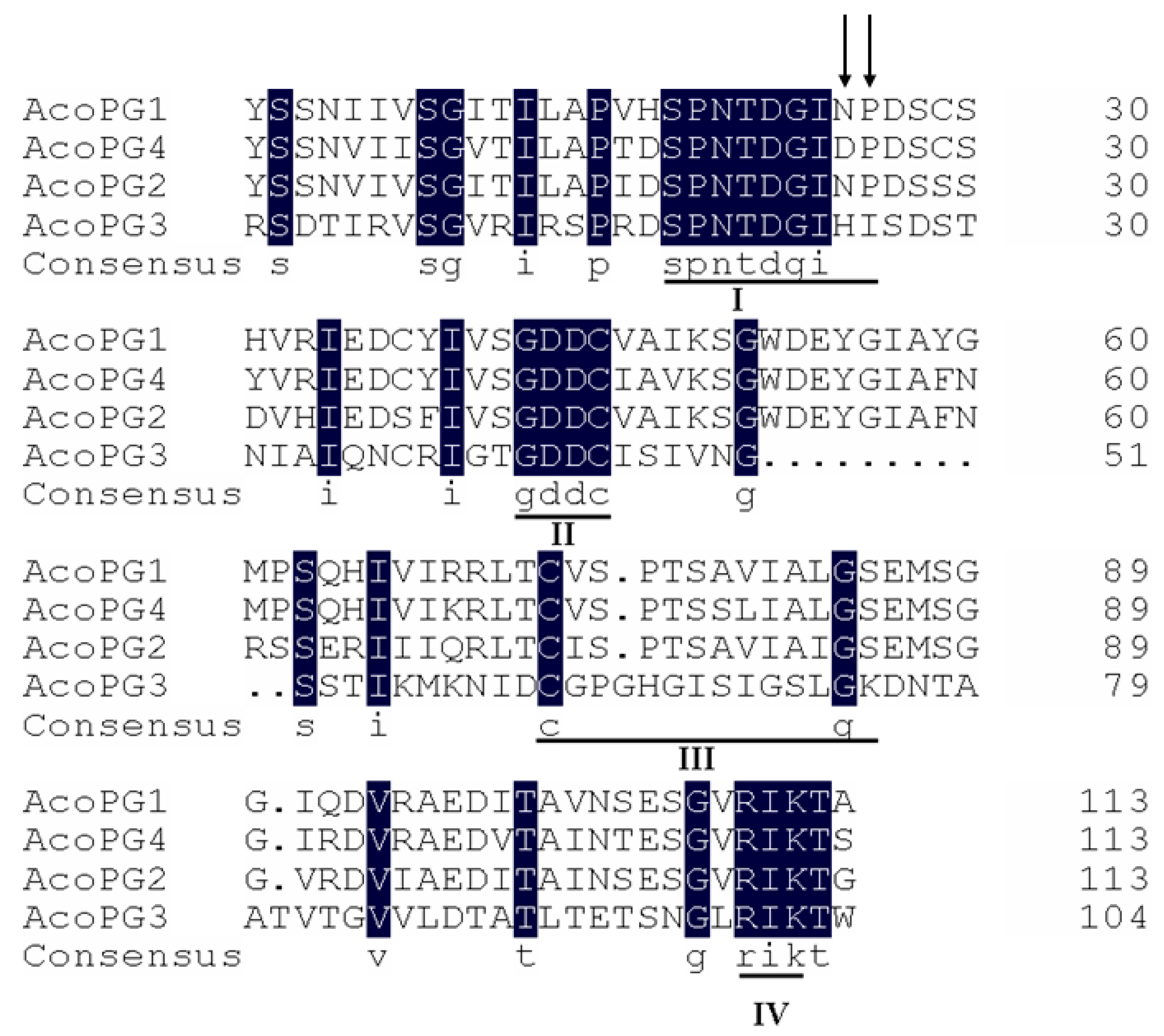
3.2. AcoPG3 Was the Main Gene Encoding Polygalacturonase in Pineapple Fruit
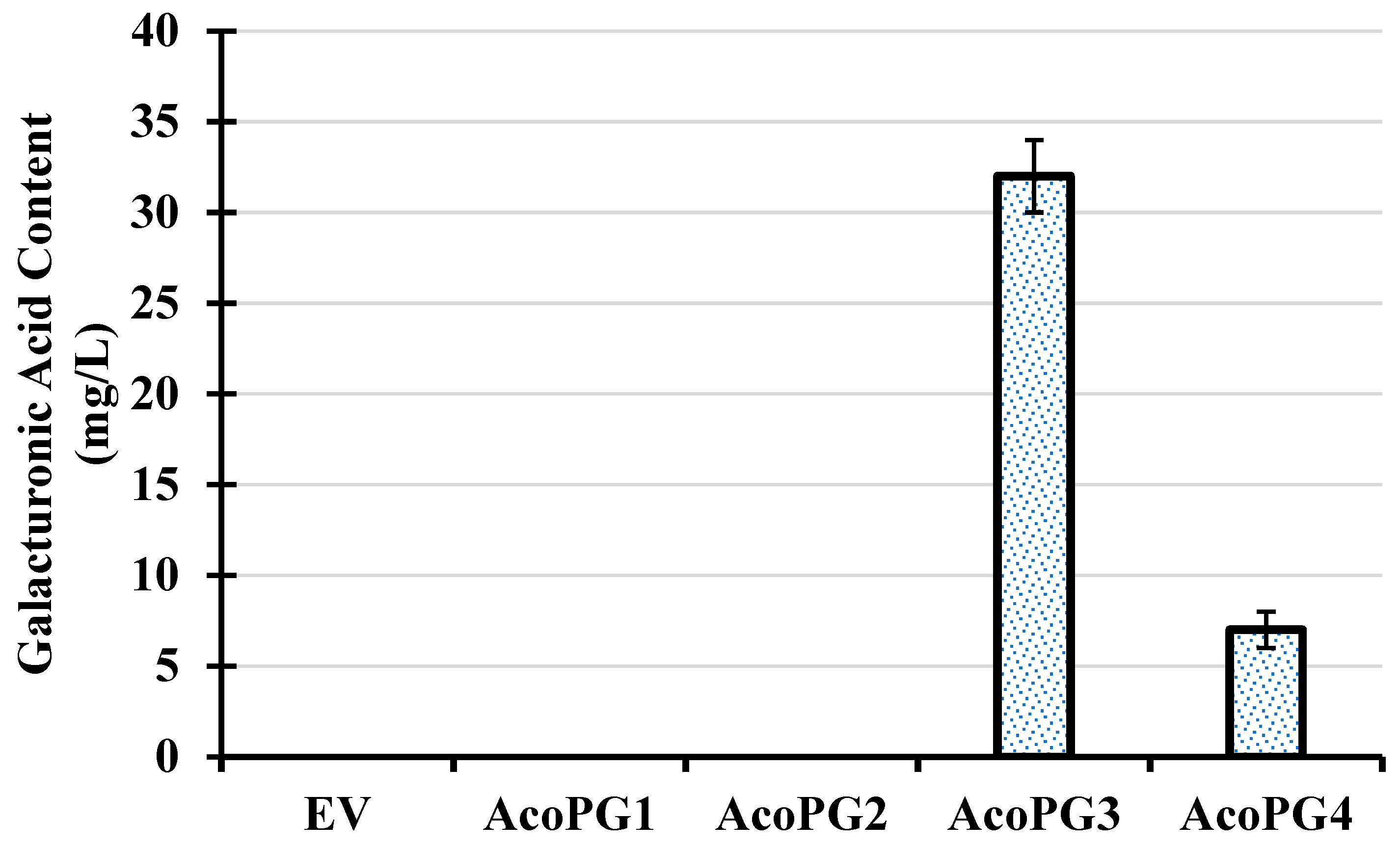
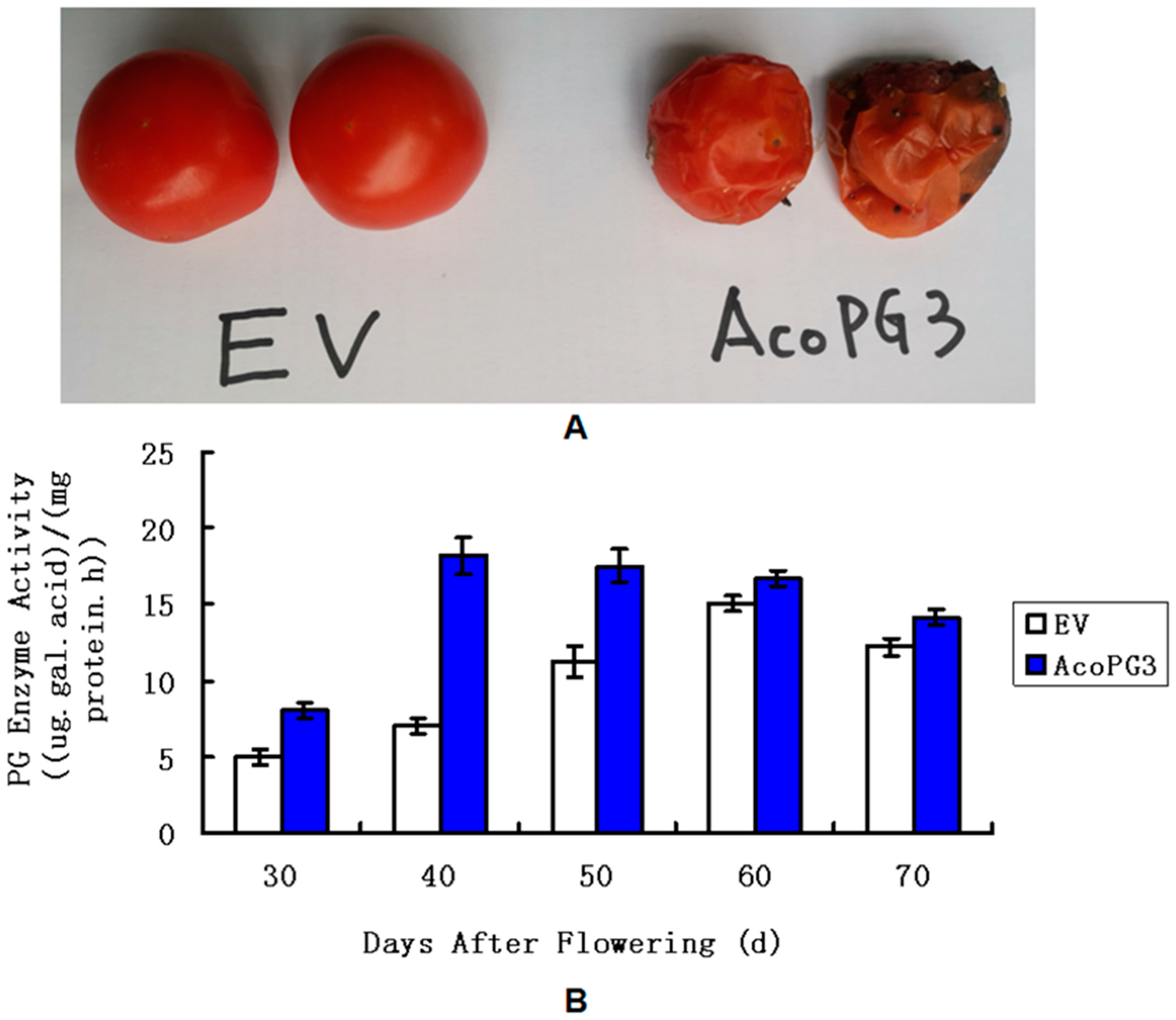
3.3. Tomato Fruits Transformed with AcoPG3 Began to Soften Earlier than Control
3.4. The Softening of Pineapple Fruit Was Regulated by the Expression of AcoPG3
3.5. AcoPG3 Was the Determinator for Polygalacturonase Activity in Pineapple Fruit
3.6. Component Contents in Cell Wall of Pineapple Pulp Were Modified by the Expression of AcoPG3
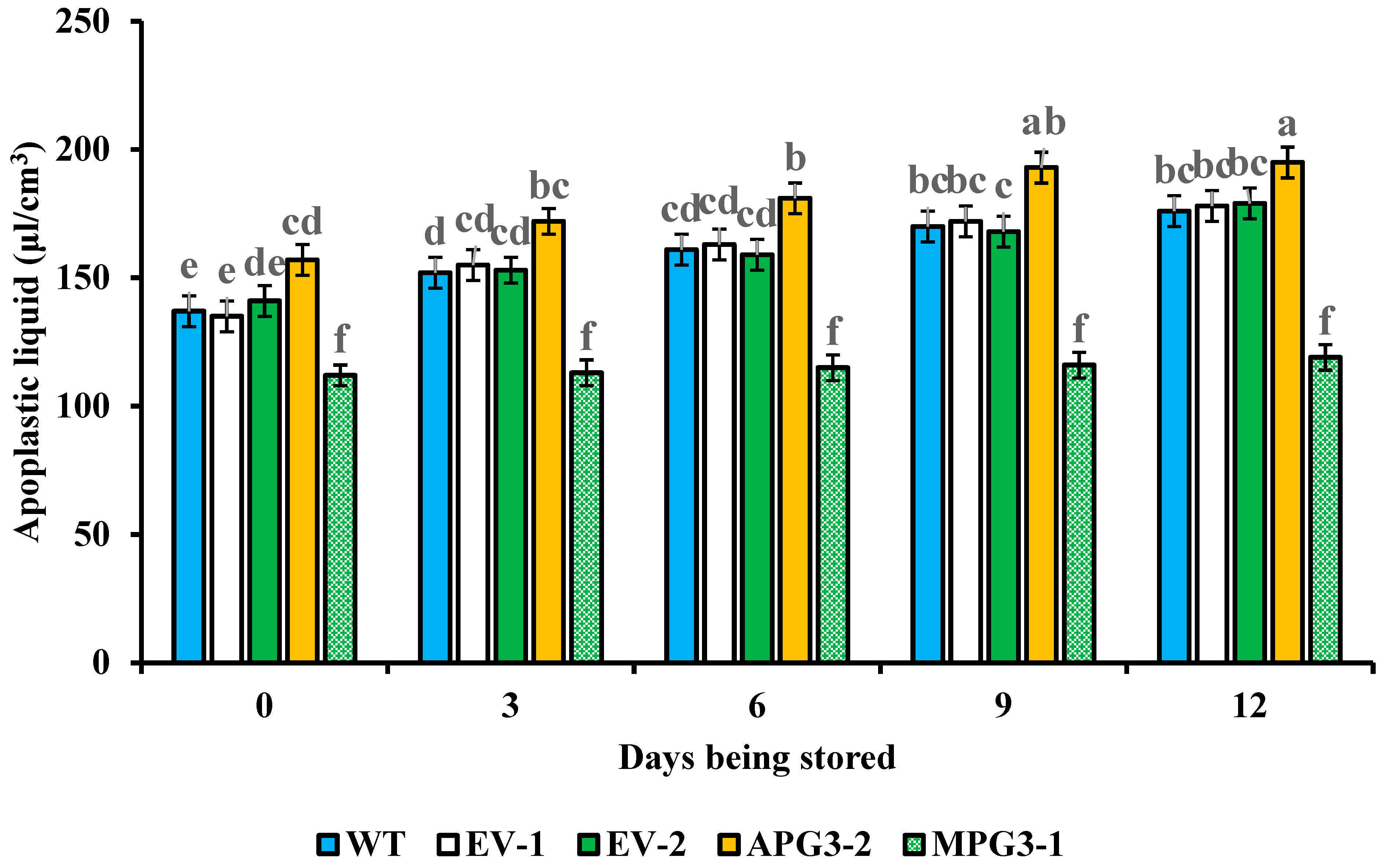
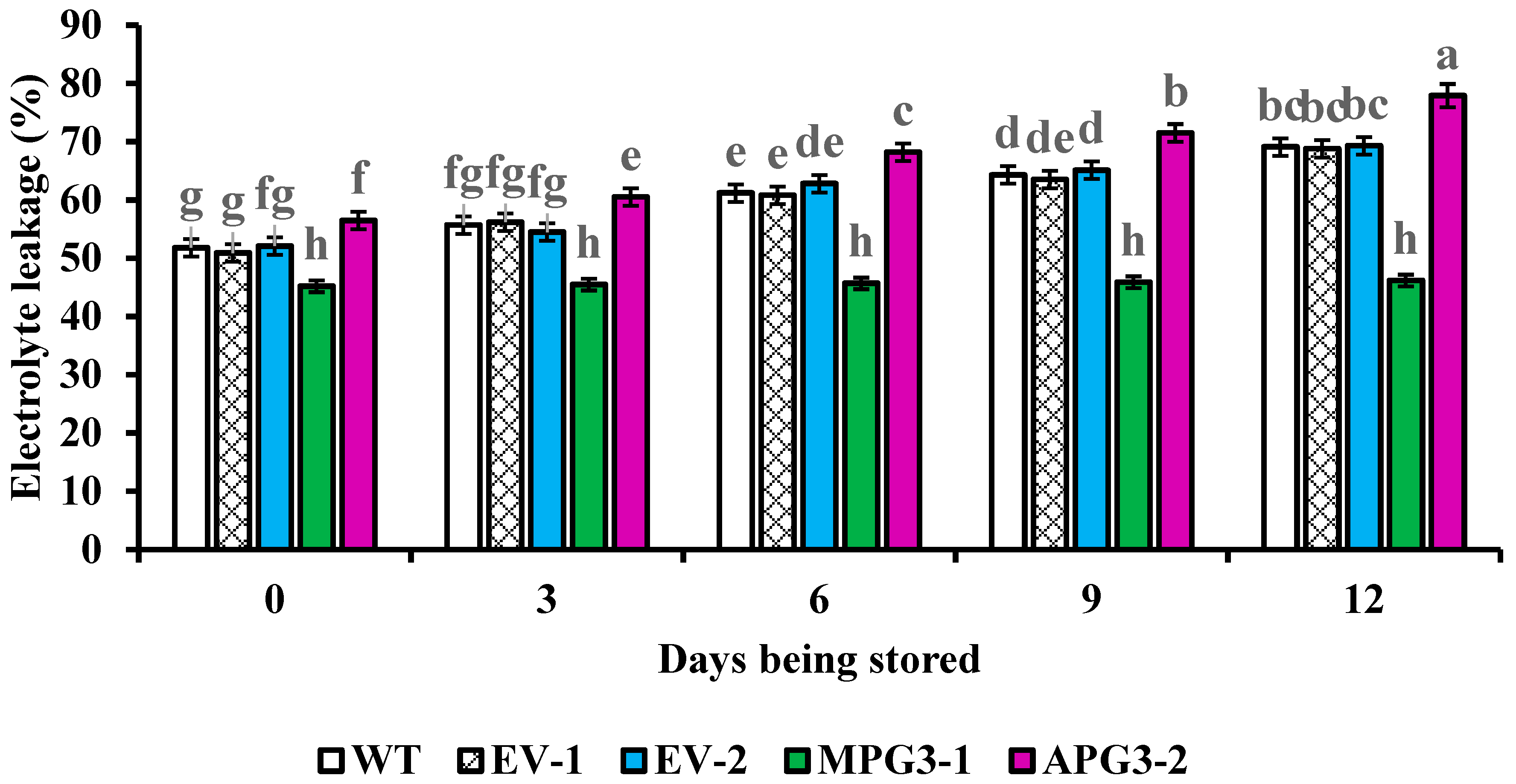
3.7. Liquid Contents in Apoplast and Electrolyte Leakage of Pineapple Pulp Were Regulated by AcoPG3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Liu, L.; Liang, D.; Zhang, M.; Jia, C.; Qi, M.; Liu, Y.; Shao, Z.; Meng, F.; Hu, S.; et al. SIBES1 promotes tomato fruit softening through transcriptional inhibition of PMEU1. iScience 2021, 24, 102926–102941. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Y.; Chen, Y.; Yu, N.; Liaqat, S.; Wu, W.; Chen, D.; Cheng, S.; Wei, X.; Cao, L.; et al. OsPG1 encodes a polygalacturonase that determines cell wall architecture and affects resistance to bacterial blight pathogen in rice. Rice 2021, 14, 36. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Zhao, T.; Han, F.; Zhang, Q.; Liu, X.; Jiang, C.; Zhong, C. Genome-wide identification and expression analysis of polygalacturonase gene family in kiwifruit (Actinidia chinensis) during fruit softening. Plants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, K.; Grierson, D. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Shi, Y.; Geng, X.; Xing, G. CRISRP/Cas9-mediated targeted mutagenesis of tomato polygalacturonase gene (SlPG) delays fruit softening. Front. Plant Sci. 2022, 13, 729128. [Google Scholar] [CrossRef]
- Santi, L.; Beys-da-Silva, W.O.; Berger, M.; Yates, J.R.; Brandelli, A.; Vainstein, M.H. Penicillium oxalicum secretomic analysis identify plant cell wall degrading enzymes important for fruit juice extraction. J. Food Sci. Technol. 2021, 58, 1764–1775. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Alba, K.; Sims, I.M.; Kontogiorgos, V. Structure and rheology of pectic polysaccharides from baobab fruit and leaves. Carbohydr. Polym. 2021, 273, 118540. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Ye, Y. Preliminary Study on the Mechanism of Ripening and Softening of Postharvest Pineapple fruit by Induced Degradation of Cell Wall Polysaccharides. Master’s Dissertation, Guangdong Ocean University, Zhanjiang, China, 2014; pp. 34–38. [Google Scholar]
- Wang, F.; Sun, X.; Liu, B.; Kong, F.; Pan, X.; Zhang, H. A polygalacturonase gene PG031 regulates seed coat permeability with a pleiotropic effect on seed weight in soybean. Theor. Appl. Genet. 2022, 135, 1603–1618. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, G.; Li, H.; Wang, Y.; Gao, H.; Jemrić, T.; Fu, D. Molecular and genetic events determining the softening of fleshy fruits: A comprehensive review. Int. J. Mol. Sci. 2022, 23, 12482. [Google Scholar] [CrossRef]
- Guo, S.; Wang, M.; Song, X.; Zhou, G.; Kong, Y. The evolving views of the simplest pectic polysaccharides: Homogalacturonan. Plant Cell Rep. 2022, 41, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Tatsumi, A.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Effects of polygalacturonase overexpression on pectin distribution in the elongation zones of roots under aluminium stress. AoB Plants 2022, 14, plac003. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G.; Schroder, R.; Hallett, I.C.; Cohen, D.; MacRae, E.A. Overexpression of polygalacturonase in transgenic apple trees to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002, 129, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nevado, J.; Moyano, E.; Medina-Escobar, N.; Caballero, J.L.; Muñoz-Blanco, J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria×ananassa cv. Chandler). J. Exp. Bot. 2001, 52, 1941–1945. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Aharoni, A.; Schaart, J.G.; Boone, M.J.; Krens, F.A. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol. Plant. 2010, 118, 571–578. [Google Scholar] [CrossRef]
- Villarreal, N.M.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol. Technol. 2008, 47, 141–150. [Google Scholar] [CrossRef]
- Quesada, M.A.; Blanco-Portales, R.; Pose, S.; Garcia-Gago, J.A.; Jimenez-Bermudez, S.; Muñoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Muñoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef]
- Pose, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef]
- Pose, S.; Kirby, A.R.; Paniagua, C.; Waldron, K.W.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. The nanostructural characterization of strawberry pectins in pectate lyase or polygalacturonase silenced fruits elucidates their role in softening. Carbohydr. Polym. 2015, 132, 134–145. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; García-Gago, J.A.; López-Casado, G.; Blanco-Portales, R.; Muñoz-Blanco, J.; Schückel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A.; et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, R.; Lin, Y.; Yang, Y.; Chen, Q.; Li, M.; Zhang, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Genome-wide analysis of polygalacturonase gene family reveals its role in strawberry softening. Plants 2024, 13, 1838. [Google Scholar] [CrossRef]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D.; et al. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 22. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Zhan, R.; Eissa, M.A.; Vafadar, F.; Ding, Z.; Wang, Y.; He, J.; Wei, Q.; Luan, A.; Chang, S. Propagation of pineapple (Ananas comosus L.) embryogenic cell suspension is regulated by LEAFY COTYLEDON1 gene AcoLEC1-1. Sci. Hortic. 2024, 332, 113173. [Google Scholar] [CrossRef]
- Wang, M.; Mao, Y.; Lu, Y.; Wang, Z.; Tao, X.; Zhu, J. Multiplex gene editing in rice with simplified CRISPR-Cpf1 and CRISPR-Cas9 systems. J. Integr. Plant Biol. 2018, 60, 626–631. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Lou, H.; Li, S.; Shi, Z.; Zou, Y.; Zhang, Y.; Huang, X.; Yang, D.; Yang, Y.; Li, Z.; Xu, C. Engineering source-sink relations by prime editing confers heat-stress resilience in tomato and rice. Cell 2025, 188, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Wang, X.Y.; Xu, Z.; Shi, P.; Gu, M.J.; Kang, T.Y.; Li, Q.; Xing, L.; Ma, J.; Zhang, D.; et al. PrupeFUL4 regulates ripening and softening of peach fruits through ethylene biosynthesis. Acta Physiol. Plant. 2022, 44, 23–36. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Fang, L.; Li, F.; Dong, Z.; Zhao, C. Identification and expression analysis of polygalacturonase family members during peach fruit softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef]
- Redgwell, R.; Melton, L. Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa): Solubilization of the pectic polymers. Plant Physiol. 1992, 98, 71–81. [Google Scholar] [CrossRef]
- Kračun, S.K.; Fangel, J.U.; Rydahl, M.G.; Pedersen, H.L.; Willats, W.G.T. Carbohydrate microarray technology applied to high-throughput mapping of plant cell wall glycans using comprehensive microarray polymer profiling (CoMPP). Methods Mol. Biol. 2017, 1503, 147–165. [Google Scholar]
- Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit ripening in Vitis vinifera: Apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 2008, 227, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lin, Q.Y.; Xie, L.H.; Wu, Z.J. The effects of three cucumber mosaic virus isolates on the defendant enzymes and cell membrane permeability in tobacco cells. Acta Phytopathol. Sin. 2001, 31, 43–49. [Google Scholar]
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: Evidence from solid-state nuclear magnetic resonance. Plant Physiol. 2015, 168, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y. Myricetin disturbs the cell wall integrity and increases the membrane permeability of Candida albicans. J. Microbiol. Biotechnol. 2022, 32, 37–45. [Google Scholar] [CrossRef]
- McManus, M.T.; Sharp, P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [Google Scholar] [CrossRef]



| Period | PG | PME | GAL | XET | CX |
|---|---|---|---|---|---|
| I | 11.5 ± 0.3 | 0.34 ± 0.03 | 0.12 ± 0.02 | 1.25 ± 0.03 | 1.29 ± 0.03 |
| II | 12.7 ± 0.4 | 0.36 ± 0.03 | 0.17 ± 0.03 | 1.42 ± 0.03 | 1.42 ± 0.04 |
| III | 14.9 ± 0.5 | 0.37 ± 0.04 | 0.22 ± 0.04 | 1.55 ± 0.04 | 1.02 ± 0.02 |
| IV | 14.2 ± 0.4 | 0.38 ± 0.04 | 0.21 ± 0.04 | 1.47 ± 0.03 | 1.42 ± 0.04 |
| V | 13.5 ± 0.3 | 0.36 ± 0.04 | 0.18 ± 0.02 | 1.18 ± 0.03 | 1.25 ± 0.03 |
| Period | WSP (mg/g) | Firmness (kg/cm2) |
|---|---|---|
| I | 1 ± 0.2 | 22 ± 0.5 |
| II | 4 ± 0.3 | 13 ± 0.4 |
| III | 17 ± 0.5 | 12 ± 0.4 |
| IV | 17.5 ± 0.5 | 9 ± 0.3 |
| V | 19 ± 0.6 | 7 ± 0.3 |
| DAH | WT | EV-1 | EV-2 | MPG3-1 | APG3-2 |
|---|---|---|---|---|---|
| 0 | 26 ± 2.0 ab | 25 ± 2.1 ab | 25.6 ± 2.3 ab | 29 ± 2.5 a | 20 ± 1.5 bc |
| 3 | 21 ± 1.1 bc | 22 ± 1.5 b | 21.7 ± 1.8 bc | 28 ± 2.3 ab | 10 ± 1.5 d |
| 6 | 13 ± 1.2 c | 12.6 ± 1.7 cd | 12.8 ± 1.5 cd | 28 ± 2.2 ab | 8 ± 1.4 de |
| 9 | 12 ± 1.5 cd | 12.3 ± 1.3 cd | 11.8 ± 1.3 cd | 28 ± 2.0 ab | 5 ± 1.6 f |
| 12 | 9 ± 1.0 de | 9.5 ± 1.2 de | 9.7 ± 1.0 de | 27.5 ± 2.0 ab | 2 ± 1.4 gh |
| 15 | 7 ± 0.7 ef | 8 ± 1.1 de | 7.5 ± 0.8 e | 27.3 ± 2.2 ab | 1 ± 0.5 h |
| 18 | 6 ± 0.7 ef | 7 ± 0.8 ef | 6.8 ± 0.8 ef | 27 ± 2.5 ab | 1 ± 0.4 h |
| 21 | 5 ± 0.9 fg | 6 ± 0.7 ef | 5.7 ± 0.7 ef | 26.8 ± 2.6 ab | 1 ± 0.4 h |
| 24 | 3.5 ± 0.6 fg | 4 ± 0.7 fg | 4.6 ± 0.6 fg | 26.3 ± 2.3 ab | 1 ± 0.4 h |
| 27 | 2 ± 0.5 gh | 2.7 ± 0.5 g | 3 ± 0.6 fg | 26 ± 2.0 ab | 1 ± 0.6 h |
| 30 | 0.7 ± 0.5 h | 1.1 ± 0.4 h | 1.6 ± 0.5 h | 25.5 ± 1.8 ab | 1 ± 0.5 h |
| DAH | WT | EV-1 | EV-2 | MPG3-1 | APG3-2 |
|---|---|---|---|---|---|
| 0 | 11.5 ± 0.5 de | 11.2 ± 0.5 e | 11.7 ± 0.5 e | 3.7 ± 0.3 j | 17.2 ± 0.6 a |
| 3 | 12.7 ± 0.5 cd | 12.5 ± 0.5 d | 12.9 ± 0.5 cd | 4.2 ± 0.3 ij | 16.8 ± 0.6 ab |
| 6 | 14.2 ± 0.6 bc | 14.1 ± 0.6 bc | 14.5 ± 0.6 bc | 4.5 ± 0.3 i | 16.5 ± 0.7 ab |
| 9 | 14.9 ± 0.7 bc | 15.1 ± 0.6 ab | 14.7 ± 0.6 bc | 5.1 ± 0.4 hi | 16.1 ± 0.6 ab |
| 12 | 13.5 ± 0.5 cd | 13.6 ± 0.5 c | 13.2 ± 0.5 cd | 5.3 ± 0.4 hi | 15.6 ± 0.6 ab |
| 15 | 13.2 ± 0.5 cd | 13.3 ± 0.5 cd | 13 ± 0.5 cd | 5.7 ± 0.4 h | 15 ± 0.6 b |
| 18 | 13 ± 0.5 cd | 13.2 ± 0.5 cd | 13.2 ± 0.5 cd | 6.2 ± 0.4 gh | 14.3 ± 0.6 bc |
| 21 | 12.5 ± 0.5 d | 12.7 ± 0.5 cd | 12.9 ± 0.5 cd | 6.6 ± 0.4 g | 13.1 ± 0.5 cd |
| 24 | 12 ± 0.5 de | 12.3 ± 0.4 de | 12.5 ± 0.4 d | 6.9 ± 0.4 fg | 12.8 ± 0.5 cd |
| 27 | 11.6 ± 0.4 de | 11.8 ± 0.4 de | 12 ± 0.4 de | 7.3 ± 0.4 fg | 12 ± 0.5 de |
| 30 | 11 ± 0.4 e | 11.5 ± 0.4 e | 11.7 ± 0.4 de | 7.6 ± 0.4 f | 11.6 ± 0.5 de |
| Fraction | Line | LM18 | LM19 | JIM7 | LM5 | RU1 | RU2 | LM15 | LM25 |
|---|---|---|---|---|---|---|---|---|---|
| Water | WT | 20 ± 3 | 5 ± 1 | 30 ± 3 | 0 | 0 | 0 | 0 | 0 |
| EV-1 | 19 ± 3 | 5 ± 1 | 31 ± 3 | 0 | 0 | 0 | 0 | 0 | |
| APG3-2 | 13 ± 2 | 2 ± 1 | 21 ± 2 | 0 | 0 | 0 | 0 | 0 | |
| EV-2 | 21 ± 3 | 5 ± 1 | 29 ± 3 | 0 | 0 | 0 | 0 | 0 | |
| MPG3-1 | 31 ± 4 | 8 ± 1 | 41 ± 4 | 0 | 0 | 0 | 0 | 0 | |
| Na2CO3 | WT | 21 ± 3 | 20 ± 3 | 0 | 10 ± 2 | 25 ± 3 | 11 ± 2 | 0 | 0 |
| EV-1 | 20 ± 3 | 20 ± 3 | 0 | 10 ± 2 | 24 ± 3 | 11 ± 2 | 0 | 0 | |
| APG3-2 | 12 ± 2 | 7 ± 2 | 0 | 3 ± 1 | 10 ± 2 | 5 ± 1 | 0 | 0 | |
| EV-2 | 22 ± 3 | 21 ± 3 | 0 | 10 ± 2 | 24 ± 3 | 12 ± 2 | 0 | 0 | |
| MPG3-1 | 32 ± 4 | 31 ± 4 | 0 | 16 ± 3 | 36 ± 4 | 19 ± 3 | 0 | 0 | |
| KOH | WT | 20 ± 3 | 20 ± 3 | 0 | 60 ± 4 | 40 ± 3 | 40 ± 3 | 40 ± 3 | 72 ± 6 |
| EV-1 | 21 ± 3 | 20 ± 3 | 0 | 59 ± 4 | 41 ± 3 | 40 ± 3 | 39 ± 3 | 71 ± 6 | |
| APG3-2 | 15 ± 2 | 16 ± 2 | 0 | 21 ± 2 | 22 ± 2 | 25 ± 2 | 40 ± 3 | 76 ± 6 | |
| EV-2 | 20 ± 3 | 21 ± 3 | 0 | 61 ± 4 | 42 ± 3 | 41 ± 3 | 43 ± 3 | 73 ± 5 | |
| MPG3-1 | 26 ± 4 | 27 ± 3 | 0 | 72 ± 4 | 65 ± 5 | 52 ± 4 | 41 ± 3 | 73 ± 6 | |
| Cadoxen | WT | 0 | 0 | 0 | 20 ± 3 | 0 | 0 | 30 ± 3 | 31 ± 3 |
| EV-1 | 0 | 0 | 0 | 21 ± 3 | 0 | 0 | 29 ± 3 | 32 ± 3 | |
| APG3-2 | 0 | 0 | 0 | 12 ± 2 | 0 | 0 | 30 ± 3 | 35 ± 4 | |
| EV-2 | 0 | 0 | 0 | 22 ± 3 | 0 | 0 | 31 ± 3 | 33 ± 4 | |
| MPG3-1 | 0 | 0 | 0 | 35 ± 4 | 0 | 0 | 31 ± 4 | 33 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Luan, A.; Wang, Y.; He, J.; Wei, Q.; Zhan, R.; Chang, S. Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit. Biology 2025, 14, 474. https://doi.org/10.3390/biology14050474
Shu H, Luan A, Wang Y, He J, Wei Q, Zhan R, Chang S. Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit. Biology. 2025; 14(5):474. https://doi.org/10.3390/biology14050474
Chicago/Turabian StyleShu, Haiyan, Aiping Luan, You Wang, Junhu He, Qing Wei, Rulin Zhan, and Shenghe Chang. 2025. "Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit" Biology 14, no. 5: 474. https://doi.org/10.3390/biology14050474
APA StyleShu, H., Luan, A., Wang, Y., He, J., Wei, Q., Zhan, R., & Chang, S. (2025). Mutation of the Polygalacturonase Gene AcoPG3 Deferred Softening of Pineapple Fruit. Biology, 14(5), 474. https://doi.org/10.3390/biology14050474






