Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
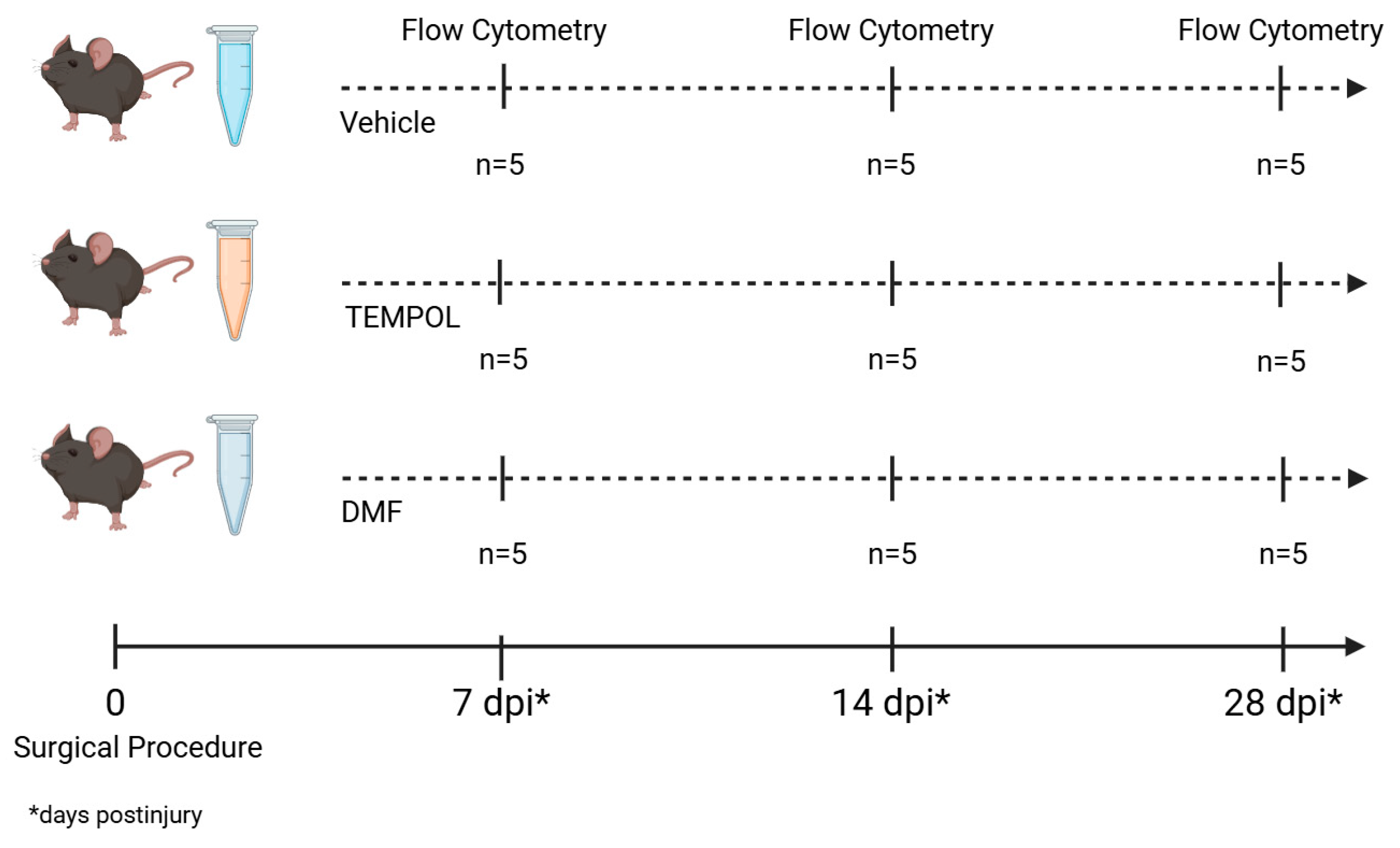
2.2. Surgical Procedure and Drug Treatment
2.3. Animals’ Euthanasia
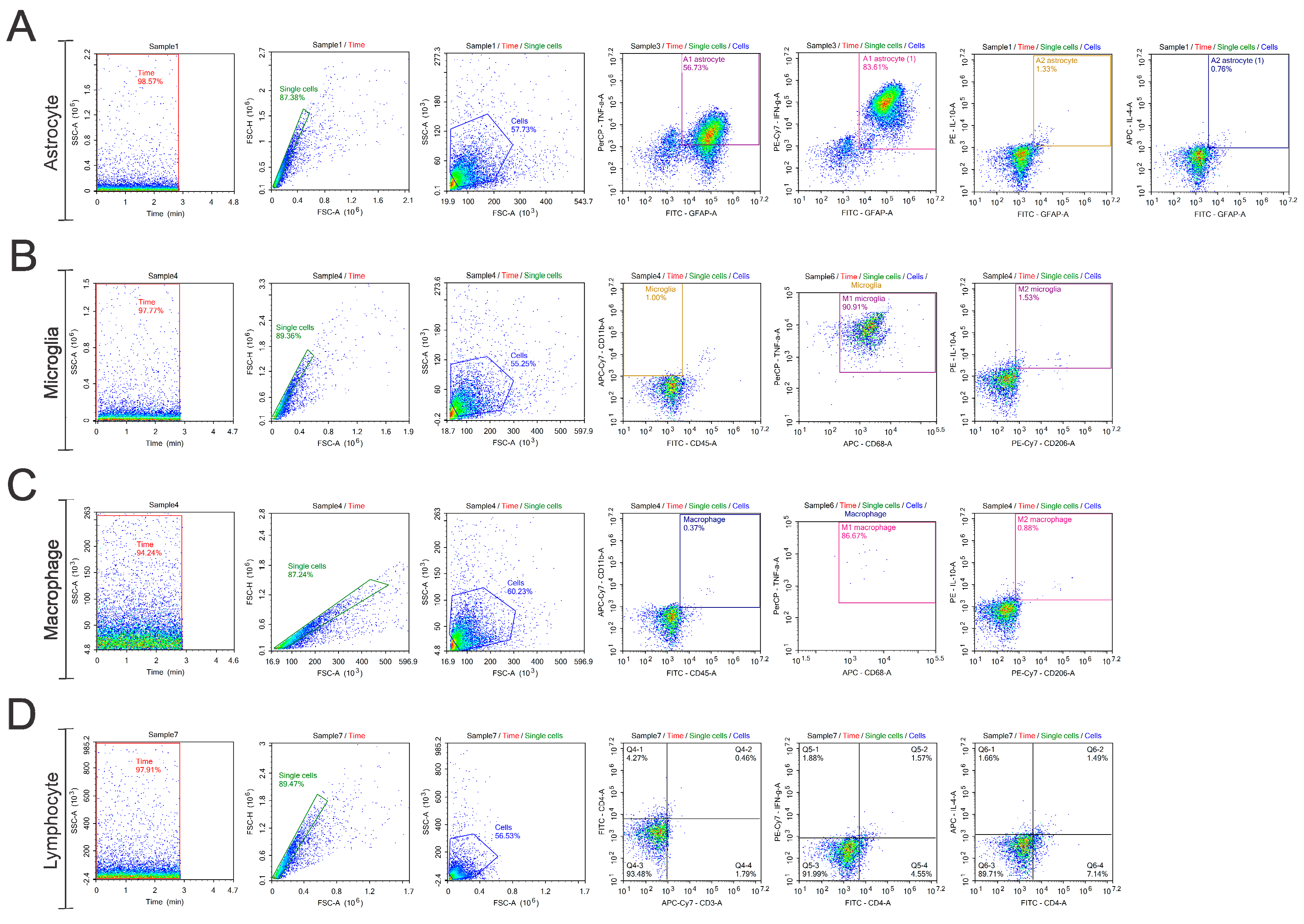
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
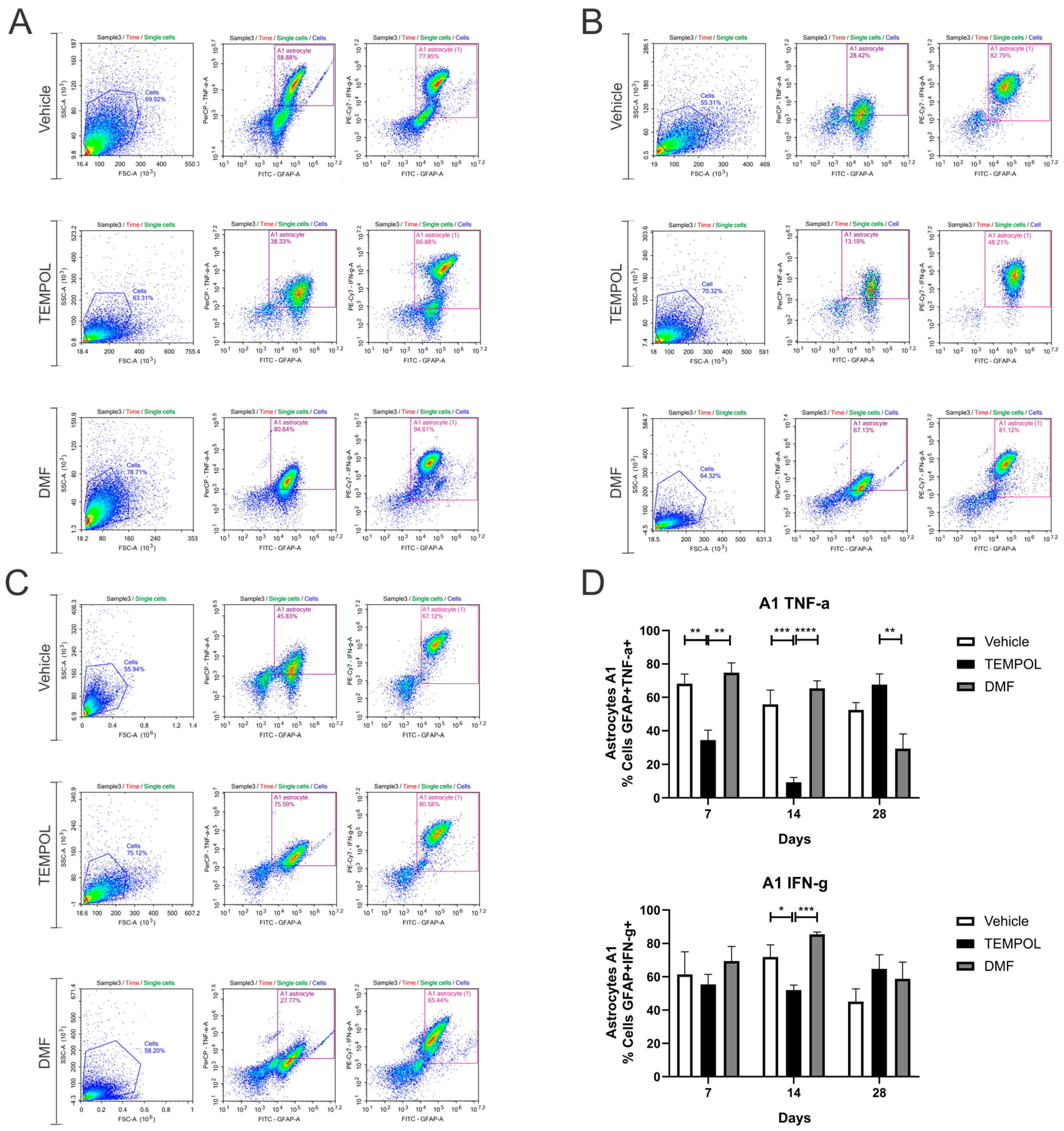
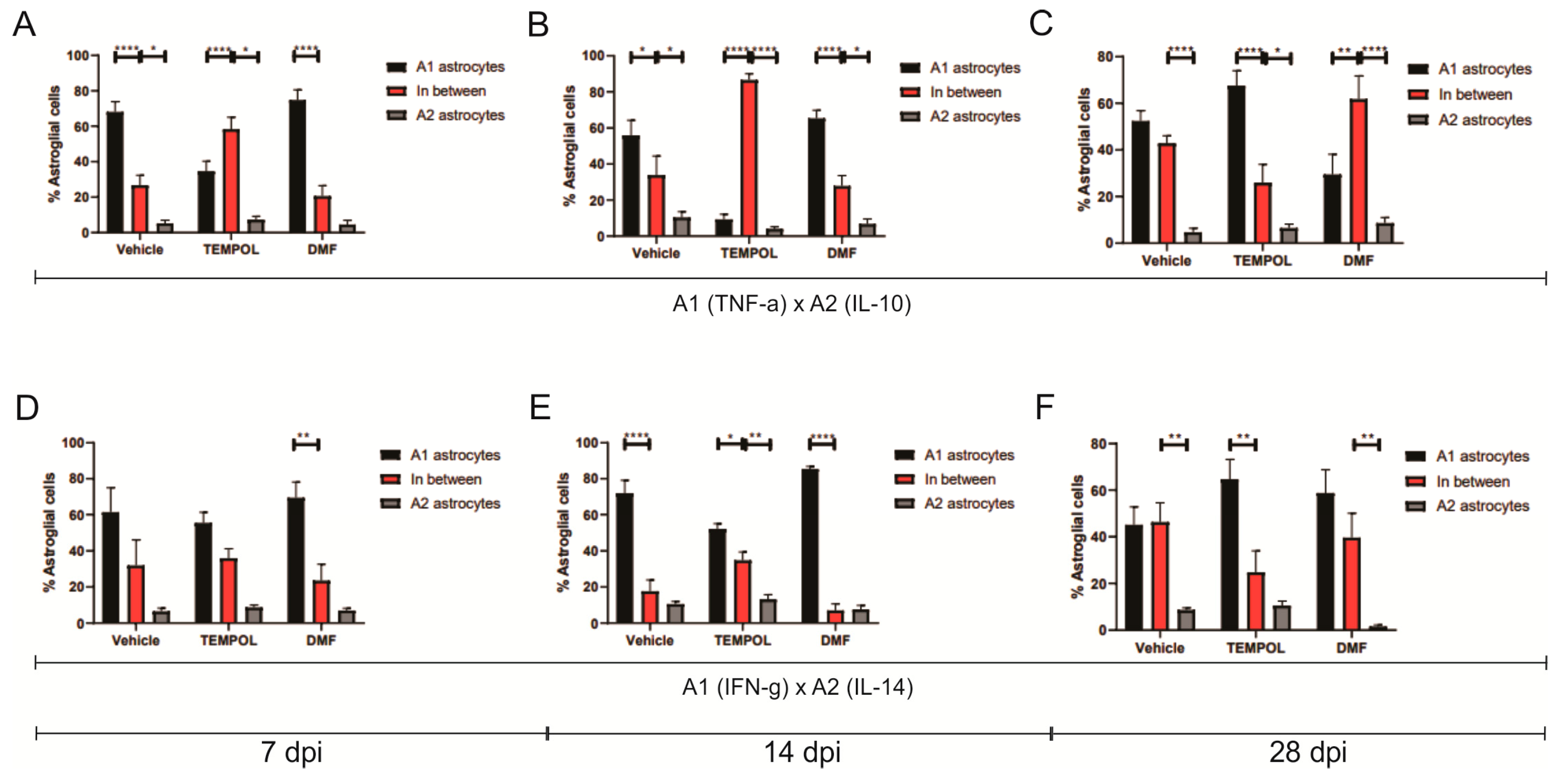
3.1. Astrocytes A1 and A2
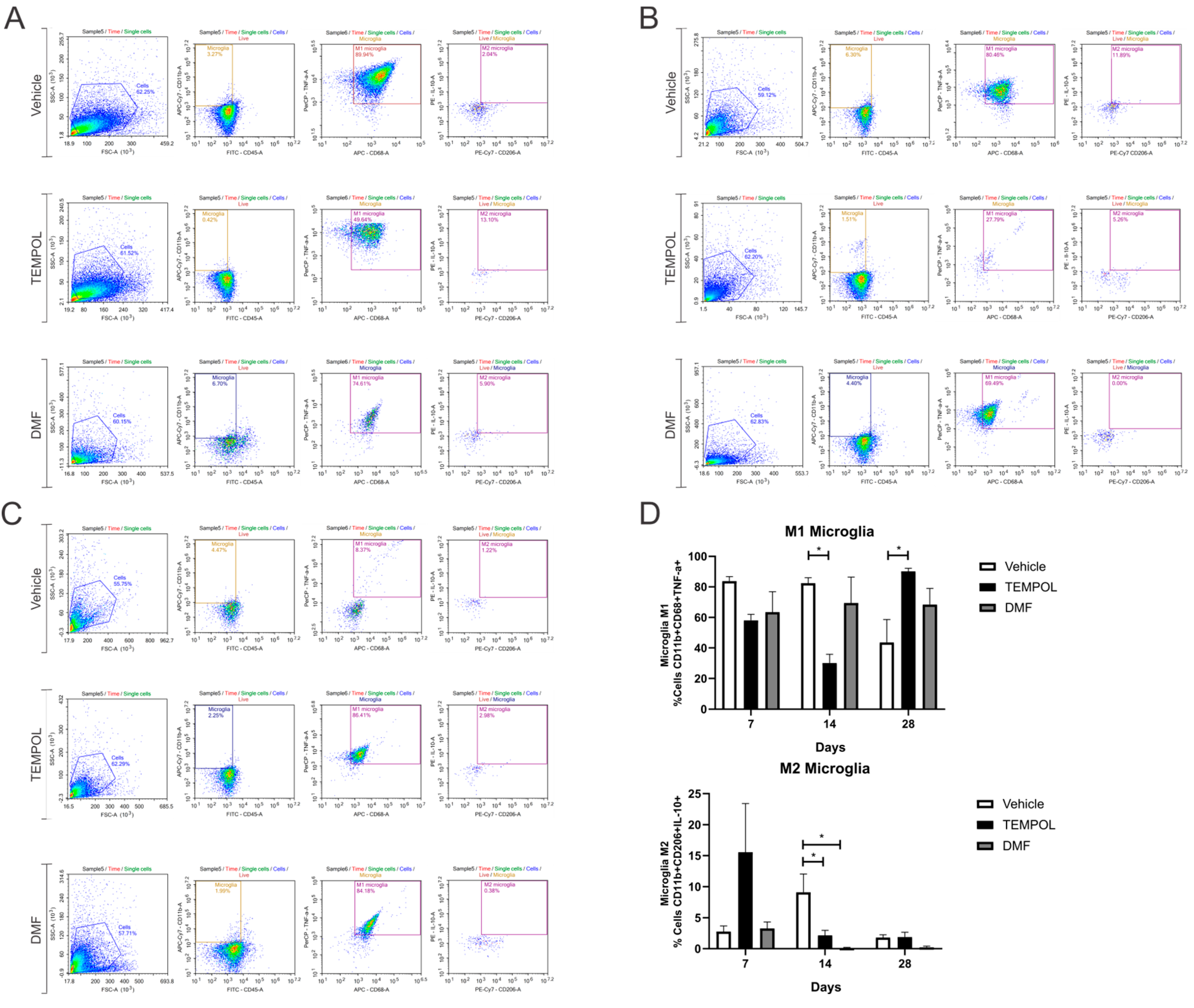
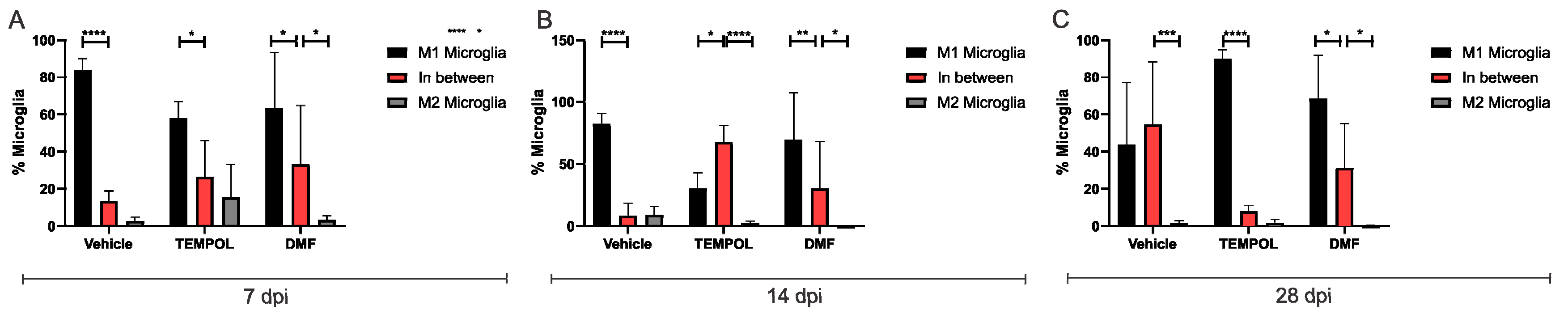
3.2. Microglia M1 and M2
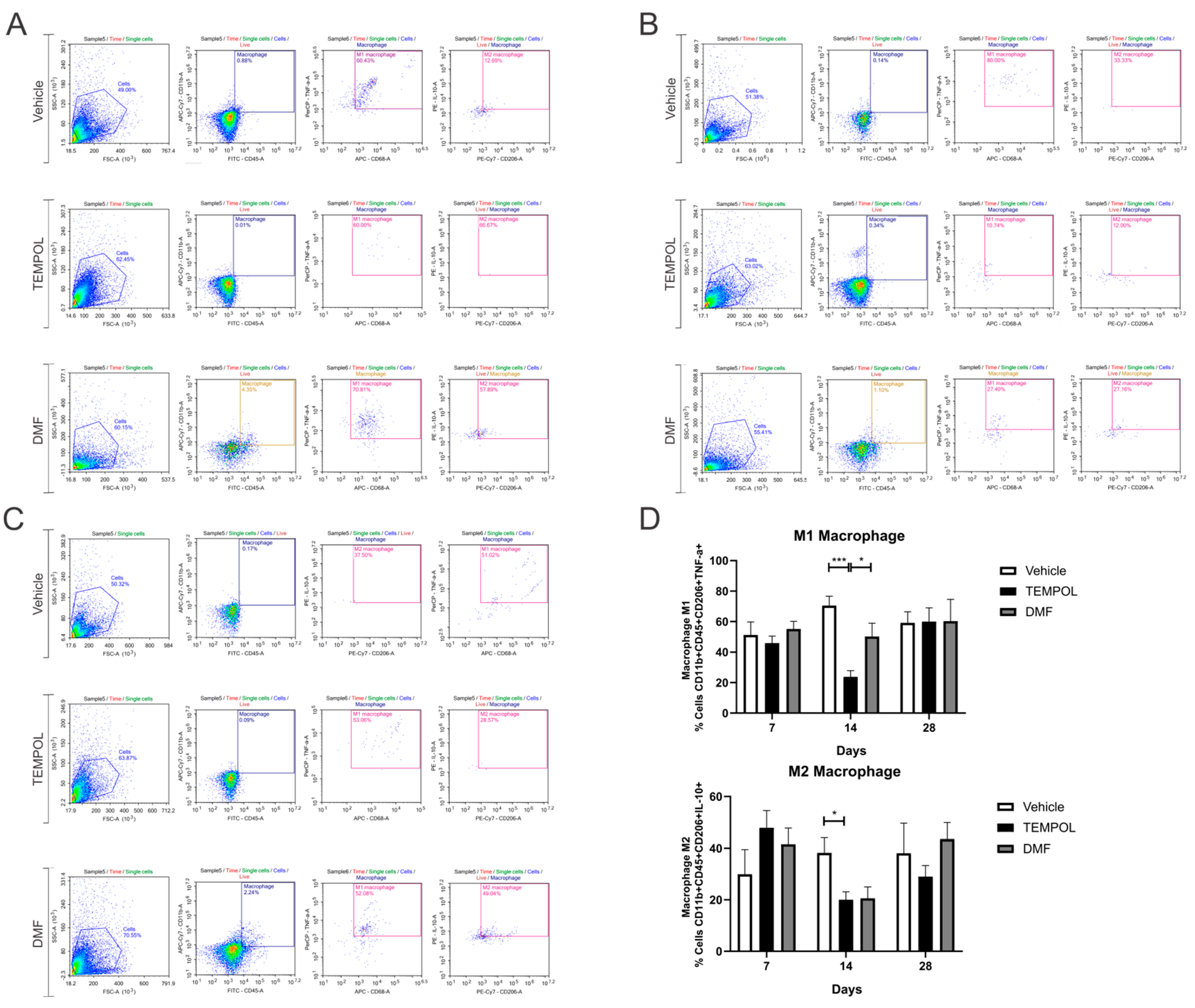
3.3. Macrophages M1 and M2
3.4. Lymphocytes Th1 and Th2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kempe, P.R.G.; Chiarotto, G.B.; Barraviera, B.; Ferreira, R.S., Jr.; de Oliveira, A.L.R. Neuroprotection and immunomodulation by dimethyl fumarate and a heterologous fibrin biopolymer after ventral root avulsion and reimplantation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190093. [Google Scholar] [CrossRef] [PubMed]
- Spejo, A.B.; Teles, C.B.; Zuccoli, G.D.S.; de Oliveira, A.L.R. Synapse preservation and decreased glial reactions following ventral root crush (VRC) and treatment with 4-hydroxy-tempo (TEMPOL). J. Neurosci. Res. 2019, 97, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.B.; Pacheco, M.B.; Aguilar, M.C.; Valenzuela, F.J.; Pérez, Z.J.J. Lesión de la médula espinal: Actualización bibliográfica: Fisiopatología y tratamiento inicial. Coluna/Columna 2012, 11, 73–76. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Botero, L.; Gómez, R.M.; Chaparro, O. Patogenia de la lesión medular y mecanismos de raparacíon inducidos por las células de la glía envolvente olfatoria. Rev. Neurol. 2024, 56, 521–531. [Google Scholar] [CrossRef]
- Mazzer, P.Y.; Barbieri, C.H.; Mazzer, N.; Fazan, V.P. Morphologic and morphometric evaluation of experimental acute crush injuries of the sciatic nerve of rats. J. Neurosci. Methods 2008, 173, 249–258. [Google Scholar] [CrossRef]
- Cartarozzi, L.P.; Perez, M.; Kirchhoff, F.; de Oliveira, A.L.R. Role of MHC-I Expression on Spinal Motoneuron Survival and Glial Reactions Following Ventral Root Crush in Mice. Cells 2019, 8, 483. [Google Scholar] [CrossRef]
- Aldskogius, H.; Kozlova, E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 1998, 55, 1–26. [Google Scholar] [CrossRef]
- David, S.; López-Vales, R.; Yong, V.W. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 109, pp. 485–502. [Google Scholar]
- Schnackenberg, C.G. Oxygen radicals in cardiovascular-renal disease. Curr. Opin. Pharmacol. 2002, 2, 121–125. [Google Scholar] [CrossRef]
- Bombeiro, A.L.; Pereira, B.T.N.; Bonfanti, A.P.; de Oliveira, A.L.R. Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res. Bull. 2020, 160, 24–32. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Samuni, A.; Krishna, M.C.; DeGraff, W.G.; Ahn, M.S.; Samuni, U.; Russo, A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 1990, 29, 2802–2807. [Google Scholar] [CrossRef]
- Amankwa, C.E.; Kodati, B.; Donkor, N.; Acharya, S. Therapeutic Potential of Antioxidants and Hybrid TEMPOL Derivatives in Ocular Neurodegenerative Diseases: A Glimpse into the Future. Biomedicines 2023, 11, 2959. [Google Scholar] [CrossRef]
- Chiarotto, G.B.; Cartarozzi, L.P.; Perez, M.; Biscola, N.P.; Spejo, A.B.; Gubert, F.; França, J.M.; Mendez-Otero, R.; de Oliveira, A.L.R. Tempol improves neuroinflammation and delays motor dysfunction in a mouse model (SOD1G93A) of ALS. J. Neuroinflamm. 2019, 16, 218. [Google Scholar] [CrossRef]
- Lipman, T.; Tabakman, R.; Lazarovici, P. Neuroprotective effects of the stable nitroxide compound Tempol on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in the Nerve Growth Factor-differentiated model of pheochromocytoma PC12 cells. Eur. J. Pharmacol. 2006, 549, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Gold, R. Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Curr. Neurol. Neurosci. Rep. 2013, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef]
- Werdenberg, D.; Joshi, R.; Wolffram, S.; Merkle, H.P.; Langguth, P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm. Drug Dispos. 2003, 24, 259–273. [Google Scholar] [CrossRef]
- Šilhavý, J.; Zídek, V.; Mlejnek, P.; Landa, V.; Šimáková, M.; Strnad, H.; Oliyarnyk, O.; Škop, V.; Kazdová, L.; Kurtz, T.; et al. Fumaric acid esters can block pro-inflammatory actions of human CRP and ameliorate metabolic disturbances in transgenic spontaneously hypertensive rats. PLoS ONE 2014, 9, e101906. [Google Scholar] [CrossRef]
- Wang, T.; Sobue, A.; Watanabe, S.; Komine, O.; Saido, T.C.; Saito, T.; Yamanaka, K. Dimethyl fumarate improves cognitive impairment and neuroinflammation in mice with Alzheimer’s disease. J. Neuroinflamm. 2024, 21, 55. [Google Scholar] [CrossRef]
- Coser, L.O.; Comelis, M.T.; Matoso, D.E.C.; Cartarozzi, L.P.; de Oliveira, A.L.R. Flow Cytometry Characterization and Analysis of Glial and Immune Cells from the Spinal Cord. Neuroglia 2024, 5, 129–144. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Wada, M.L.; Langone, F.; de Oliveira, A.L.R. Differential schwann cell migration in adult and old mice: An in vitro study. Brain Res. 2000, 881, 73–76. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2024, 15, 541–553. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Duarte, A.S.; Longhini, A.L.; Pradella, F.; Farias, A.S.; Luzo, A.C.; Oliveira, A.L.; Olalla Saad, S.T. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci. Rep. 2015, 5, 16167. [Google Scholar] [CrossRef] [PubMed]
- Rabchevsky, A.G.; Patel, S.P.; Springer, J.E. Pharmacological interventions for spinal cord injury: Where do we stand? How might we step forward? Pharmacol. Ther. 2011, 132, 15–29. [Google Scholar] [CrossRef]
- Neil, S.; Huh, J.; Baronas, V.; Li, X.; McFarland, H.F.; Cherukuri, M.; Mitchell, J.B.; Quandt, J.A. Oral administration of the nitroxide radical TEMPOL exhibits immunomodulatory and therapeutic properties in multiple sclerosis models. Brain Behav. Immun. 2017, 62, 332–343. [Google Scholar] [CrossRef]
- Kopf, M.; Bachmann, M.F.; Marsland, B.J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 2010, 9, 703–718. [Google Scholar] [CrossRef]
- Wu, J.; Lu, B.; Yang, R.; Chen, Y.; Chen, X.; Li, Y. EphB2 knockdown decreases the formation of astroglial-fibrotic scars to promote nerve regeneration after spinal cord injury in rats. CNS Neurosci. Ther. 2021, 27, 714–724. [Google Scholar] [CrossRef]
- Tomiyama, A.L.M.R.; Cartarozzi, L.P.; Coser, L.O.; Chiarotto, G.B.; de Oliveira, A.L.R. Neuroprotection by upregulation of the major histocompatibility complex class I (MHC I) in SOD1G93A mice. Front. Cell. Neurosci. 2023, 17, 1211486. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Aldskogius, H.; Liu, L.; Svensson, M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999, 58, 33–41. [Google Scholar] [CrossRef]
- Chitnis, T.; Khoury, S.J. 20. Immunologic neuromuscular disorders. J. Allergy Clin. Immunol. 2003, 111 (Suppl. 2), S659–S668. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Li, Y.; Irwin, N.; Fischer, D.; Harvey, A.R.; Benowitz, L.I. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003, 23, 2284–2293. [Google Scholar] [CrossRef]
- Tsuhako, M.H.; Augusto, O.; Linares, E.; Chadi, G.; Giorgio, S.; Pereira, C.A. Tempol ameliorates murine viral encephalomyelitis by preserving the blood-brain barrier, reducing viral load, and lessening inflammation. Free. Radic. Biol. Med. 2010, 48, 704–712. [Google Scholar] [CrossRef]
- Suneetha, A.; Raja, R.K. Role of dimethyl fumarate in oxidative stress of multiple sclerosis: A review. J. Chromatogr. B 2016, 1019, 15–20. [Google Scholar]
- Akino, N.; Wada-Hiraike, O.; Isono, W.; Terao, H.; Honjo, H.; Miyamoto, Y.; Tanikawa, M.; Sone, K.; Hirano, M.; Harada, M.; et al. Activation of Nrf2/Keap1 pathway by oral Dimethylfumarate administration alleviates oxidative stress and age-associated infertility might be delayed in the mouse ovary. Reprod. Biol. Endocrinol. RB&E 2019, 17, 23. [Google Scholar]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134 Pt 3, 678–692. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, B.; Shi, Z.; Wang, Y.; Yuan, Y.; Chen, X.; Wang, Y.; Hu, J.; Mao, L.; Gao, Y.; et al. LncRNA H19 knockdown promotes neuropathologic and functional recovery via the Nrf2/HO-1 axis after traumatic brain injury. CNS Neurosci. Ther. 2024, 30, e14870. [Google Scholar] [CrossRef]
- Carvalho, N.Z.M.; Chiarotto, G.B.; Bernardes, D.; Kempe, P.R.G.; de Oliveira, A.L.R. Neuroprotection by dimethyl fumarate following ventral root crush in C57BL/6J mice. Brain Res. Bull. 2020, 164, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Spejo, A.B.; Carvalho, J.L.; Goes, A.M.; de Oliveira, A.L.R. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: Synapse stability and axonal regeneration. Neuroscience 2013, 250, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Kempe, P.R.G.; de Castro, M.V.; Khuriyeh, V.C.; Barraviera, B.; Ferreira, R.S.; de Oliveira, A.L.R. Ultrastructural Evidence of Synapse Preservation and Axonal Regeneration Following Spinal Root Repair with Fibrin Biopolymer and Therapy with Dimethyl Fumarate. Polymers 2023, 15, 3171. [Google Scholar] [CrossRef]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflamm. 2011, 8, 109. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Bombeiro, A.L.; Santini, J.C.; Thomé, R.; Ferreira, E.L.R.; Nunes, S.L.O.; Moreira, B.M.; Bonet, I.J.M.; Sartori, C.R.; Verinaud, L.; Oliveira, A.L.R. Enhanced Immune Response in Immunodeficient Mice Improves Peripheral Nerve Regeneration Following Axotomy. Fontiers Cell. Neurosci. 2016, 10, 151. [Google Scholar] [CrossRef]
- Hosseini, A.; Ali, M.; Baradaran, B.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Anvari, E.; Jadidi-Niaragh, F. Dimethyl fumarate: Regulatory effects on the immune system in the treatment of multiple sclerosis. J. Cell. Physiol. 2018, 234, 9943–9955. [Google Scholar] [CrossRef]
- Moalem, G.; Xu, K.; Yu, L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004, 129, 767–777. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Wang, H. Imbalance of Th1 and Th2 Cytokines and Stem Cell Therapy in Pathological Pain. CNS Neurol. Disord. Drug Targets 2024, 23, 88–101. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Mao, G.; Dowling, C.A.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef] [PubMed]


| TUBE | FITC | PE | PerCP | PE-Cy7 | APC | APC-Cy7 |
|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | - |
| 2 | GFAP | IL-10 | IL-4 | |||
| 3 | GFAP | TNF-α | IFN-γ | |||
| 4 | - | - | - | - | - | - |
| 5 | CD45 | IL-10 | CD206 | CD11b | ||
| 6 | CD45 | TNF-α | CD68 | CD11b | ||
| 7 | - | - | - | - | - | - |
| 8 | CD4 | IFN-γ | IL-4 | CD3 |
| Analysis | Time | Comparation | Statistical Data | p Value | * |
|---|---|---|---|---|---|
| Flow Cytometry | |||||
| A1 Astrocyte (TNF-α+) | 7 dpi | VE vs. TE | 68.16 ± 5.75 vs. 34.52 ± 5.80 | 0.0044 | ** |
| 7 dpi | DMFE vs. TE | 74.79 ± 5.83 vs. 34.52 ± 5.80 | 0.011 | ** | |
| 14 dpi | VE vs. TE | 55.83 ± 8.45 vs. 9.33 ± 2.83 | 0.0003 | *** | |
| 14 dpi | DMFE vs. TE | 65.35 ± 4.53 vs. 9.33 ± 2.83 | <0.00001 | **** | |
| 28 dpi | TE vs. DMFE | 67.60 ± 6.42 vs. 29.49 ± 8.63 | 0.0051 | ** | |
| A1 Astrocyte (IFN-γ+) | 14 dpi | VE vs. TE | 71.84 ± 7.27 vs. 52.06 ± 3.03 | 0.0318 | * |
| 14 dpi | DMFE vs. TE | 85.43 ± 1.48 vs. 52.06 ± 3.03 | 0.0008 | *** | |
| A2 Astrocyte (IL-4) | 28 dpi | VE vs. DMFE | 8.65 ± 0.82 vs. 1.59 ± 0.63 | 0.0053 | ** |
| 28 dpi | TE vs. DMFE | 10.51 ± 1.90 vs. 1.59 ± 0.62 | 0.0008 | *** | |
| M1 Microglia | 14 dpi | VE vs. TE | 82.36 ± 3.65 vs. 30.17 ± 5.67 | 0.0129 | * |
| 28 dpi | VE vs. TE | 43.65 ± 14.99 vs. 90.12 ± 2.08 | 0.0284 | * | |
| M2 Microglia | 14 dpi | VE vs. TE | 9.12 ± 2.92 vs. 2.17 ± 0.81 | 0.0473 | * |
| 14 dpi | VE vs. DMFE | 9.12 ± 2.92 vs. 0.14 ± 0.09 | 0.0104 | * | |
| M1 Macrophage | 14 dpi | VE vs. TE | 70.45 ± 6.20 vs. 23.73 ± 4.10 | 0.001 | *** |
| 14 dpi | TE vs. DMFE | 23.73 ± 4.10 vs. 50.2 ± 8.79 | 0.0469 | * | |
| M2 Macrophage | 14 dpi | VE vs. TE | 38.21 ± 5.80 vs. 20.05 ± 3.10 | 0.0487 | * |
| Th1 Lymphocyte | 7 dpi | VE vs. TE. | 62.84 ± 9.72 vs. 31.04 ± 7.38 | 0.0372 | * |
| 14 dpi | VE vs. TE. | 48.05 ± 3.30 vs. 17.13 ± 5.61 | 0.0033 | ** | |
| 14 dpi | DMFE vs. TE | 64.50 ± 6.02 vs. 17.13 ± 5.61 | <0.00001 | **** | |
| 28 dpi | VE vs. DMFE | 47.58 ± 4.79 vs. 21.9 ± 3.83 | 0.0105 | * | |
| 28 dpi | TE vs. DMFE | 56.52 ± 6.14 vs. 21.90 ± 6.83 | 0.0011 | ** | |
| Th2 Lymphocyte | 14 | VE vs. TE | 10.46 ± 1.33 vs. 2.41 ± 0.71 | 0.0011 | ** |
| 14 | TE vs. DMFE | 2.41 ± 0.71 vs. 10.06 ± 1.34 | 0.0017 | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzani, M.F.V.; Coser, L.d.O.; Oliveira, A.L.R.d. Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis. Biology 2025, 14, 473. https://doi.org/10.3390/biology14050473
Balzani MFV, Coser LdO, Oliveira ALRd. Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis. Biology. 2025; 14(5):473. https://doi.org/10.3390/biology14050473
Chicago/Turabian StyleBalzani, Maria Fernanda Vannucci, Lilian de Oliveira Coser, and Alexandre Leite Rodrigues de Oliveira. 2025. "Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis" Biology 14, no. 5: 473. https://doi.org/10.3390/biology14050473
APA StyleBalzani, M. F. V., Coser, L. d. O., & Oliveira, A. L. R. d. (2025). Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis. Biology, 14(5), 473. https://doi.org/10.3390/biology14050473






