Microbiota-Driven Mechanisms in Multiple Sclerosis: Pathogenesis, Therapeutic Strategies, and Biomarker Potential
Simple Summary
Abstract
1. The Role of Gut Microbiota in the Pathogenesis of Multiple Sclerosis
1.1. Overview of the Gut–Brain Axis
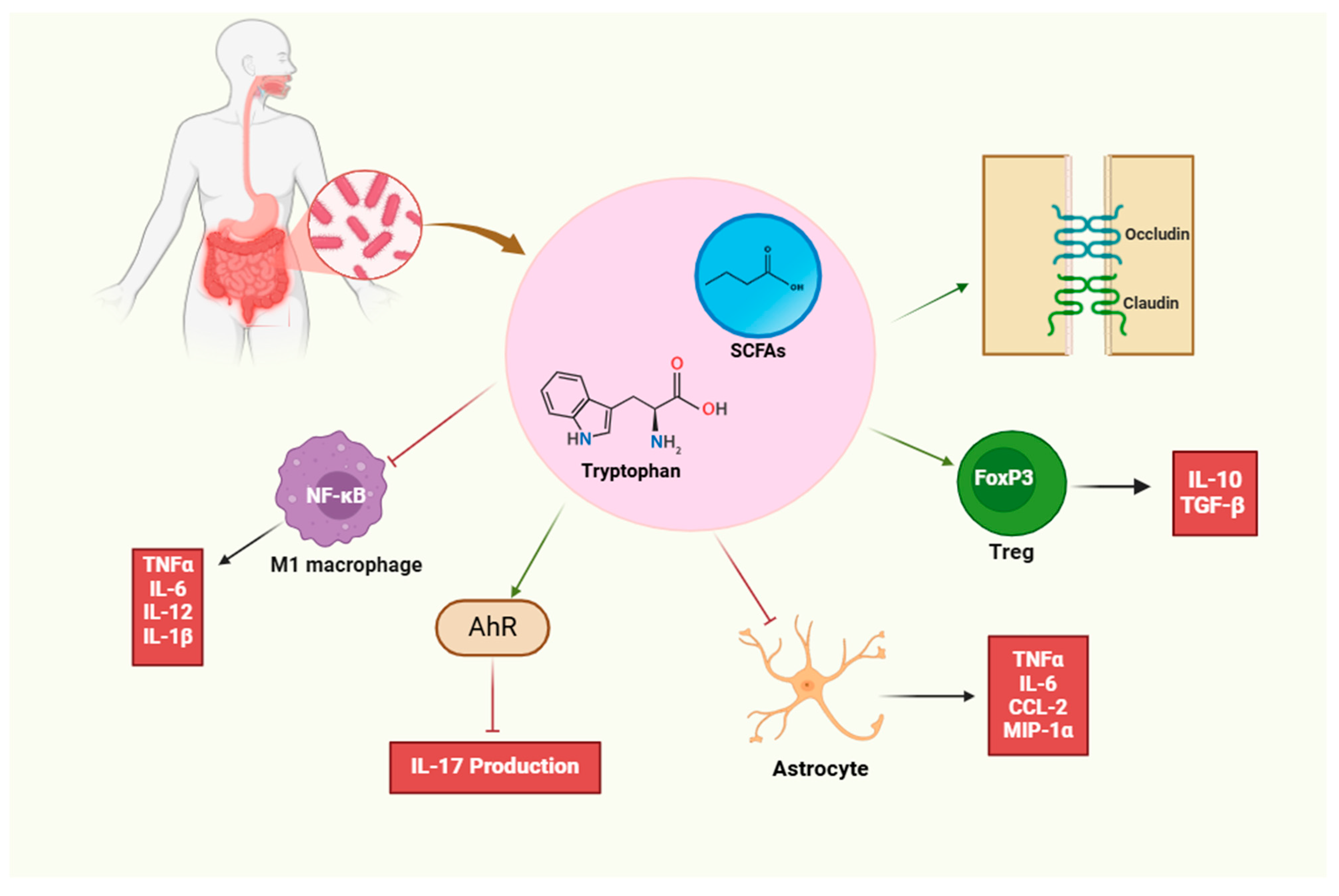
1.2. Immunomodulatory Effects of Microbiota
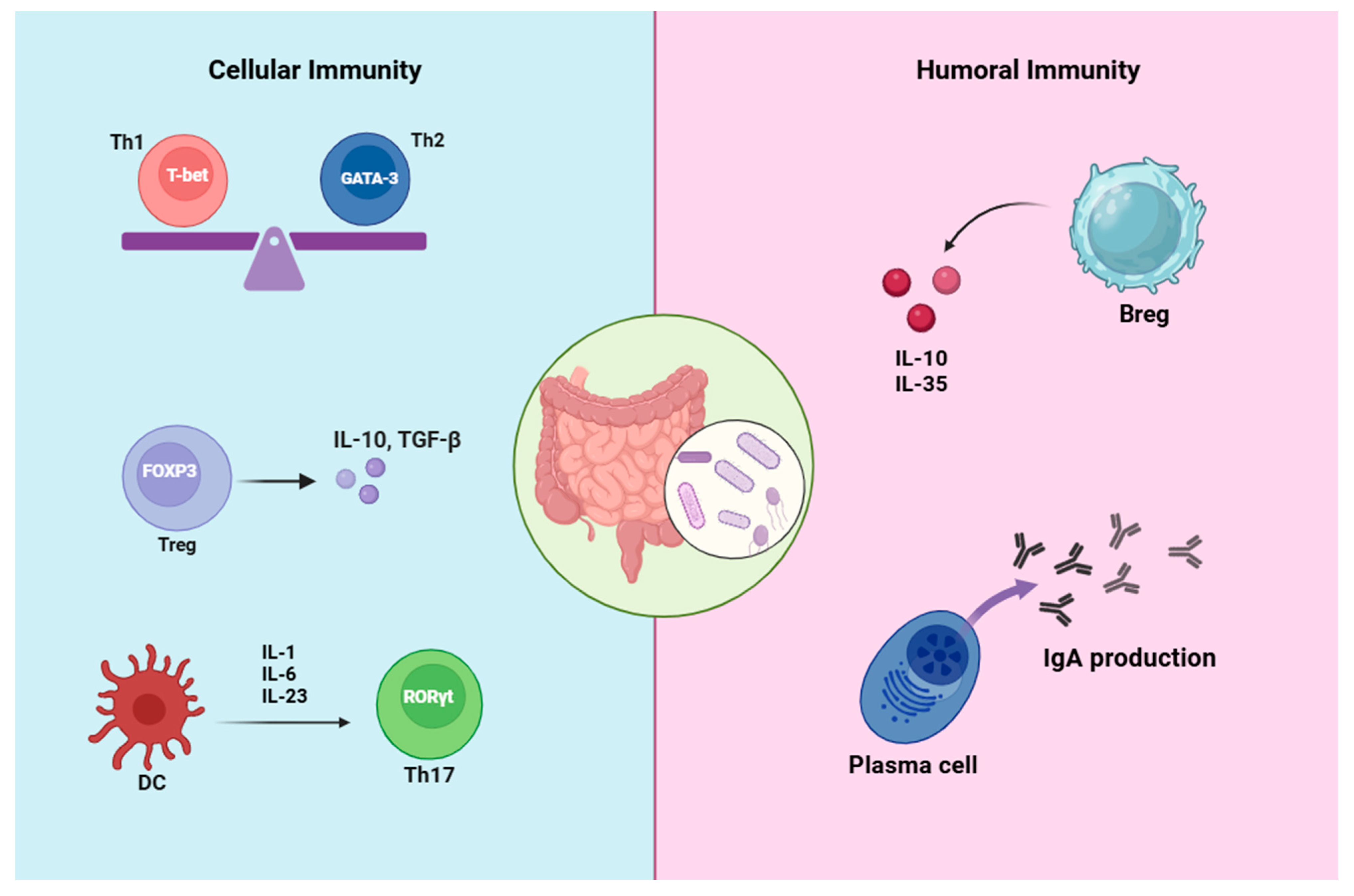
1.2.1. Immunomodulatory Effects on T Cells
1.2.2. Immunomodulatory Effects on B Cells
1.3. Key Microbial Players
2. Alterations in Gut Microbiota Composition in Multiple Sclerosis Patients
2.1. Microbiome Profiling
2.2. Disease Phenotypes and Microbiota
3. Therapeutic Potential of Modulating Gut Microbiota in Multiple Sclerosis
3.1. Probiotics and Prebiotics
3.2. Fecal Microbiota Transplantation (FMT)
3.3. Dietary Interventions
4. Microbiota-Driven Biomarkers for Multiple Sclerosis
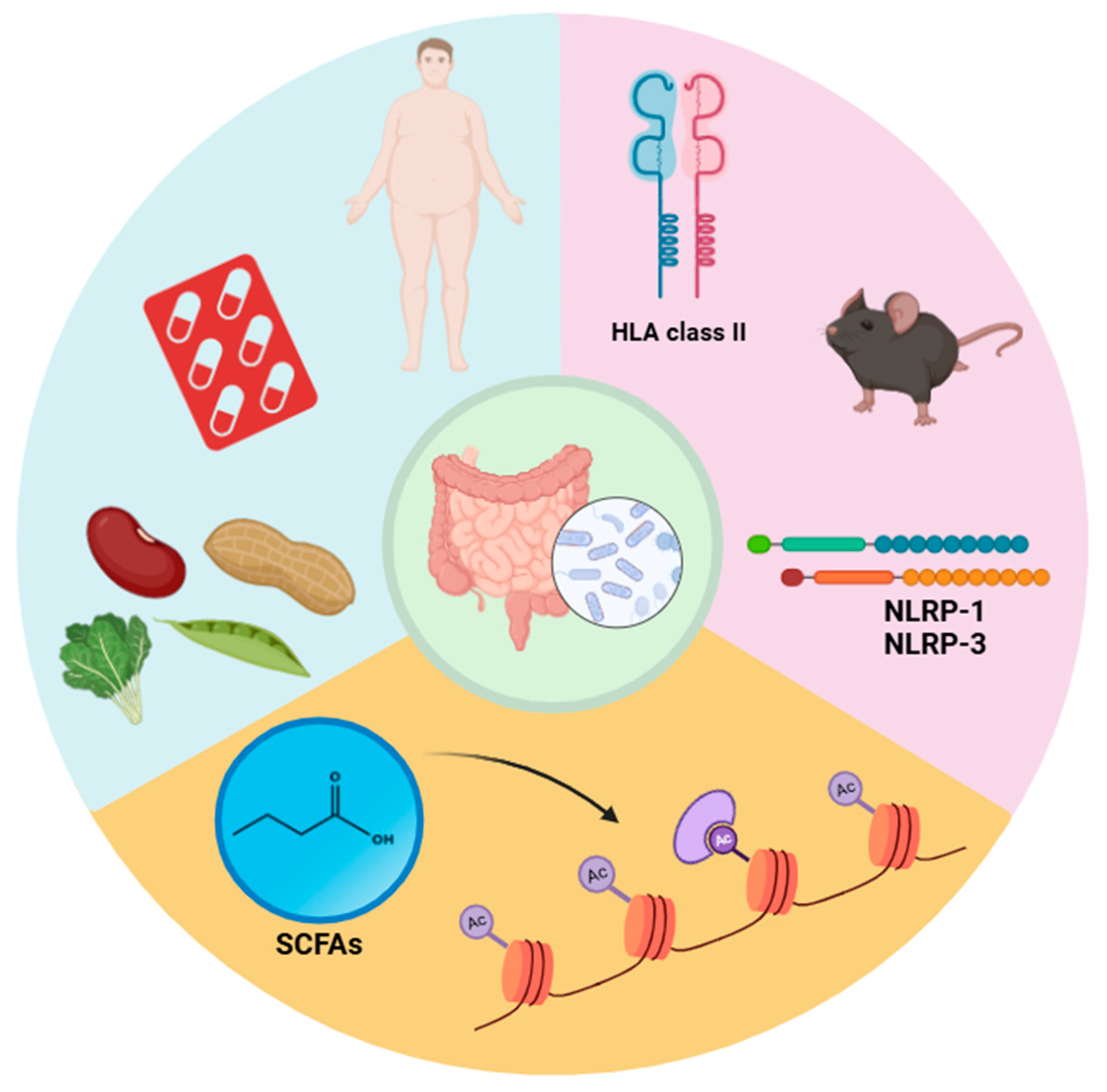
5. The Interplay Between Gut Microbiota, Genetics, and Environmental Factors in Multiple Sclerosis
5.1. Gene–Microbiota Interactions
5.2. Environmental Influences
5.3. Epigenetic Modifications
6. Future Directions and Practical Applications of Gut Microbiota Research in Multiple Sclerosis
Limitations of EAE Models in MS Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heravi, F.S. Gut microbiota and autoimmune diseases: Mechanisms, treatment, challenges, and future recommendations. Curr. Clin. Microbiol. Rep. 2024, 11, 18–33. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. Microbial metabolites in multiple sclerosis: Implications for pathogenesis and treatment. Front. Neurosci. 2022, 16, 885031. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Rob, M.; Yousef, M.; Lakshmanan, A.P.; Mahboob, A.; Terranegra, A.; Chaari, A. Microbial signatures and therapeutic strategies in neurodegenerative diseases. Biomed. Pharmacother. 2025, 184, 117905. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Layunta, E.; Buey, B.; Mesonero, J.E.; Latorre, E. Crosstalk Between Intestinal Serotonergic System and Pattern Recognition Receptors on the Microbiota–Gut–Brain Axis. Front. Endocrinol. 2021, 12, 748254. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [PubMed]
- Buey, B.; Layunta, E.; Latorre, E.; Mesonero, J.E. Potential role of milk bioactive peptides on the serotonergic system and the gut-brain axis. Int. Dairy J. 2023, 137, 105534. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. eBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut flora in multiple sclerosis: Implications for pathogenesis and treatment. Neural Regen. Res. 2024, 19, 1480–1488. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, C.-Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- A Shim, J.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178–1191. [Google Scholar] [CrossRef]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Z.; Wang, S.; Ma, D.; Zhu, M.; Feng, J. Role of gut microbiota in multiple sclerosis and potential therapeutic implications. Curr. Neuropharmacol. 2022, 20, 1413–1426. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Choden, T.; Cohen, N.A. The gut microbiome and the immune system. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.U. Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; Costa, P.D.S.S.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the human gut microbiota for immunomodulatory organisms. Cell 2017, 168, 928–943.e11. [Google Scholar] [CrossRef] [PubMed]
- Ahern, P.P.; Faith, J.J.; Gordon, J.I. Mining the human gut microbiota for effector strains that shape the immune system. Immunity 2014, 40, 815–823. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Qian, L.-Y.; Pang, J.; Lin, J.-Y.; Xu, Q.; Wang, L.-H.; Huang, D.-S.; Zou, H. The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget 2017, 8, 59915–59928. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Denning, T.L. Segmented filamentous bacteria-induced immune responses: A balancing act between host protection and autoimmunity. Immunology 2018, 154, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella induces the production of Th17 cells in the colon of mice. J. Immunol. Res. 2020, 2020, 9607328. [Google Scholar] [CrossRef]
- Brown, E.M.; Kenny, D.J.; Xavier, R.J. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, W.; Liang, Z.; Wang, J.; Zeng, Z.; Kołat, D.; Li, X.; Zhou, D.; Xu, X.; Zhao, L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 33. [Google Scholar] [CrossRef]
- Yu, B.; Wang, L.; Chu, Y. Gut microbiota shape B cell in health and disease settings. J. Leukoc. Biol. 2021, 110, 271–281. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.H. Regulation of humoral immunity by gut microbial products. Gut Microbes 2017, 8, 392–399. [Google Scholar] [CrossRef]
- Liu, Y.; Rhoads, J.M. Communication between B-Cells and Microbiota for the Maintenance of Intestinal Homeostasis. Antibodies 2013, 2, 535–553. [Google Scholar] [CrossRef]
- Xue, J.; Ajuwon, K.M.; Fang, R. Mechanistic insight into the gut microbiome and its interaction with host immunity and inflammation. Anim. Nutr. 2020, 6, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Talham, G.L.; Jiang, H.Q.; Bos, N.A.; Cebra, J.J. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 1999, 67, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Lundell, A.-C.; Björnsson, V.; Ljung, A.; Ceder, M.; Johansen, S.; Lindhagen, G.; Törnhage, C.-J.; Adlerberth, I.; Wold, A.E.; Rudin, A. Infant B cell memory differentiation and early gut bacterial colonization. J. Immunol. 2012, 188, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Ohno, H. IgA in human health and diseases: Potential regulator of commensal microbiota. Front. Immunol. 2022, 13, 1024330. [Google Scholar] [CrossRef]
- Rosser, E.C.; Oleinika, K.; Tonon, S.; Doyle, R.; Bosma, A.; A Carter, N.; A Harris, K.; A Jones, S.; Klein, N.; Mauri, C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 2014, 20, 1334–1339. [Google Scholar] [CrossRef]
- Mu, Q.; Edwards, M.R.; Swartwout, B.K.; Puig, X.C.; Mao, J.; Zhu, J.; Grieco, J.; Cecere, T.E.; Prakash, M.; Reilly, C.M.; et al. Gut Microbiota and Bacterial DNA Suppress Autoimmunity by Stimulating Regulatory B Cells in a Murine Model of Lupus. Front. Immunol. 2020, 11, 593353. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Tuganbaev, T.; Skelly, A.N.; Honda, K. T Cell Responses to the Microbiota. Annu. Rev. Immunol. 2022, 40, 559–587. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Alshehri, D.; Saadah, O.; Mosli, M.; Edris, S.; Alhindi, R.; Bahieldin, A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn. J. Basic Med. Sci. 2021, 21, 270–283. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef] [PubMed]
- Thoda, C.; Touraki, M. Immunomodulatory Properties of Probiotics and Their Derived Bioactive Compounds. Appl. Sci. 2023, 13, 4726. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Eixarch, H.; Montalban, X.; Espejo, C. Combined therapies to treat complex diseases: The role of the gut microbiota in multiple sclerosis. Autoimmun. Rev. 2018, 17, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.O.; Ochoa-Repáraz, J. The gut microbiome in multiple sclerosis: A potential therapeutic avenue. Med. Sci. 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Dunalska, A.; Saramak, K.; Szejko, N. The role of gut microbiome in the pathogenesis of multiple sclerosis and related disorders. Cells 2023, 12, 1760. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Frazzei, G.; van Vollenhoven, R.F.; de Jong, B.A.; Siegelaar, S.E.; van Schaardenburg, D. Preclinical autoimmune disease: A comparison of rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis and type 1 diabetes. Front. Immunol. 2022, 13, 899372. [Google Scholar] [CrossRef]
- Steimle, A.; Neumann, M.; Grant, E.T.; Willieme, S.; De Sciscio, A.; Parrish, A.; Ollert, M.; Miyauchi, E.; Soga, T.; Fukuda, S.; et al. Gut microbial factors predict disease severity in a mouse model of multiple sclerosis. Nat. Microbiol. 2024, 9, 2244–2261. [Google Scholar] [CrossRef]
- Altieri, C.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Gut-microbiota, and multiple sclerosis: Background, evidence, and perspectives. Nutrients 2023, 15, 942. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4615–4622. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut Microbiota in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Current Applications and Future Perspectives. Mediators Inflamm. 2018, 2018, 8168717. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Murra, A.C.; Zarei, K.; Sompallae, R.; Gibson-Corley, K.N.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K. Prevotella histicola, A Human Gut Commensal, Is as Potent as COPAXONE® in an Animal Model of Multiple Sclerosis. Front. Immunol. 2019, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Søndergaard, H.B.; et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef]
- Geuking, M.B.; Cahenzli, J.; Lawson, M.A.; Ng, D.C.; Slack, E.; Hapfelmeier, S.; McCoy, K.D.; Macpherson, A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011, 34, 794–806. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Pompura, S.L.; Hafler, D.A.; Dominguez-Villar, M. Fatty Acid Metabolism and T Cells in Multiple Sclerosis. Front. Immunol. 2022, 13, 869197. [Google Scholar] [CrossRef] [PubMed]
- Takewaki, D.; Yamamura, T. Gut microbiome research in multiple sclerosis. Neurosci. Res. 2021, 168, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mackay, C.R.; Gershwin, M.E. Immunomodulatory effects of microbiota-derived short-chain fatty acids in autoimmune liver diseases. J. Immunol. 2023, 210, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef]
- Yokote, H.; Miyake, S.; Croxford, J.L.; Oki, S.; Mizusawa, H.; Yamamura, T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 2008, 173, 1714–1723. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Al Rasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Pröbstel, A.-K.; Baranzini, S.E. The role of the gut microbiome in multiple sclerosis risk and progression: Towards characterization of the “MS microbiome”. Neurotherapeutics 2018, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; Aaen, G.; Belman, A.; et al. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J. Neurol. Sci. 2016, 363, 153–157. [Google Scholar] [CrossRef]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E. Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.-J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef]
- Ullah, H.; Tovchiga, O.; Daglia, M.; Khan, H. Modulating gut microbiota: An emerging approach in the prevention and treatment of multiple sclerosis. Curr. Neuropharmacol. 2021, 19, 1966. [Google Scholar]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef]
- Rovinaru, C.; Pasarin, D. Application of microencapsulated synbiotics in fruit-based beverages. Probiot. Antimicrob. Proteins 2020, 12, 764–773. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J.; Michel, C. How to manipulate the microbiota: Prebiotics. Microbiota Hum. Body Implic. Health Dis. 2016, 902, 119–142. [Google Scholar]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, FST_100005. [Google Scholar] [CrossRef]
- Macfarlane, G.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch-Stärke 2011, 7, 406–415. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Berer, K.; Martínez, I.; Walker, A.; Kunkel, B.; Schmitt-Kopplin, P.; Walter, J.; Krishnamoorthy, G. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci. Rep. 2018, 8, 10431. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Paknahad, Z.; Chitsaz, A. Dietary intake of energy and fiber in MS patients; an approach to prebiotics role. Nutr. Food Sci. 2019, 49, 1039–1050. [Google Scholar] [CrossRef]
- Samara, J.; Moossavi, S.; Alshaikh, B.; Ortega, V.A.; Pettersen, V.K.; Ferdous, T.; Hoops, S.L.; Soraisham, A.; Vayalumkal, J.; Dersch-Mills, D.; et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe 2022, 30, 696–711.e5. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Seghinsara, A.M.; Chollou, K.M.; Bahadori, A.; Abbaszadeh, S.; Taghdir, M.; Behniafar, H.; Riahi, S.M. The efficacy of probiotics in experimental autoimmune encephalomyelitis (an animal model for MS): A systematic review and meta-analysis. Lett. Appl. Microbiol. 2021, 73, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Kap, Y.S.; Bus-Spoor, C.; van Driel, N.; Dubbelaar, M.L.; Grit, C.; Kooistra, S.M.; Fagrouch, Z.C.; Verschoor, E.J.; Bauer, J.; Eggen, B.J.L.; et al. Targeted diet modification reduces multiple sclerosis–like disease in adult marmoset monkeys from an outbred colony. J. Immunol. 2018, 201, 3229–3243. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Kohl, H.M.; Castillo, A.R.; Ochoa-Repáraz, J. The microbiome as a therapeutic target for multiple sclerosis: Can genetically engineered probiotics treat the disease? Diseases 2020, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflamm. 2019, 16, 231. [Google Scholar] [CrossRef]
- Pushkala, K.; Gupta, P. Promising role of Faecal transplant therapy on Sclerosis. Clin. Trials Case Stud. 2024, 3, 1–4. [Google Scholar]
- Borody, T.; Leis, S.; Campbell, J.; Torres, M.; Nowak, A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Off. J. Am. Coll. Gastroenterol. 2011, 106, S352. [Google Scholar] [CrossRef]
- Makkawi, S.; Camara-Lemarroy, C.; Metz, L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e459. [Google Scholar] [CrossRef]
- Li, K.; Wei, S.; Hu, L.; Yin, X.; Mai, Y.; Jiang, C.; Peng, X.; Cao, X.; Huang, Z.; Zhou, H.; et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators Inflamm. 2020, 2020, 2058272. [Google Scholar] [CrossRef]
- Engen, P.A.; Zaferiou, A.; Rasmussen, H.; Naqib, A.; Green, S.J.; Fogg, L.F.; Forsyth, C.B.; Raeisi, S.; Hamaker, B.; Keshavarzian, A. Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis. Front. Neurol. 2020, 11, 978. [Google Scholar] [CrossRef]
- Al, K.F.; Craven, L.J.; Gibbons, S.; Parvathy, S.N.; Wing, A.C.; Graf, C.; A Parham, K.; Kerfoot, S.M.; Wilcox, H.; Burton, J.P.; et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173221086662. [Google Scholar] [CrossRef] [PubMed]
- Laeeq, T.; Vongsavath, T.; Tun, K.M.; Hong, A.S. The Potential Role of Fecal Microbiota Transplant in the Reversal or Stabilization of Multiple Sclerosis Symptoms: A Literature Review on Efficacy and Safety. Microorganisms 2023, 11, 2840. [Google Scholar] [CrossRef]
- Xu, D.; Ren, L.; Zhang, W.; Wu, S.; Yu, M.; He, X.; Wei, Z. Therapeutic effects and mechanisms of fecal microbiota transplantation on EAE partly through HPA axis-mediated neuroendocrine regulation. Heliyon 2024, 10, e33214. [Google Scholar] [CrossRef] [PubMed]
- Totsch, S.K.; Quinn, T.L.; Strath, L.J.; McMeekin, L.J.; Cowell, R.M.; Gower, B.A.; Sorge, R.E. The impact of the Standard American Diet in rats: Effects on behavior, physiology and recovery from inflammatory injury. Scand. J. Pain 2017, 17, 316–324. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Diet, gut microbiota, and vitamins D+ A in multiple sclerosis. Neurotherapeutics 2018, 15, 75–91. [Google Scholar] [CrossRef] [PubMed]
- E Parks, N.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 2020, CD004192. [Google Scholar]
- Sanchez, J.M.S.; DePaula-Silva, A.B.; Libbey, J.E.; Fujinami, R.S. Role of diet in regulating the gut microbiota and multiple sclerosis. Clin. Immunol. 2022, 235, 108379. [Google Scholar] [CrossRef]
- Valburg, C.; Sonti, A.; Stern, J.N.; Najjar, S.; Harel, A. Dietary factors in experimental autoimmune encephalomyelitis and multiple sclerosis: A comprehensive review. Mult. Scler. J. 2021, 27, 494–502. [Google Scholar] [CrossRef]
- Kujawa, D.; Laczmanski, L.; Budrewicz, S.; Pokryszko-Dragan, A.; Podbielska, M. Targeting gut microbiota: New therapeutic opportunities in multiple sclerosis. Gut Microbes 2023, 15, 2274126. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Eixarch, H.; Cornejo, T.; Costa, C.; Castillo, M.; Mestre, L.; Guaza, C.; Martínez-Cuesta, M.d.C.; Tanoue, T.; Honda, K.; et al. Selected clostridia strains from the human microbiota and their metabolite, butyrate, improve experimental autoimmune encephalomyelitis. Neurotherapeutics 2021, 18, 920–937. [Google Scholar] [CrossRef]
- Di Biase, A.; Salvati, S.; Di Benedetto, R.; Attorri, L.; Martinelli, A.; Malchiodi, F. Eicosapentaenoic acid pre-treatment reduces biochemical changes induced in total brain and myelin of weanling Wistar rats by cuprizone feeding. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, Ø.; Brunborg, L.A.; Thorsen, F.; Mørk, S.J.; Stangel, M.; Myhr, K.M.; Bø, L. Effects of dietary intervention on MRI activity, de-and remyelination in the cuprizone model for demyelination. Exp. Neurol. 2009, 215, 160–166. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-salt diet has a certain impact on protein digestion and gut microbiota: A sequencing and proteome combined study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Aden, L.A.; Barbaro, N.R.; Van Beusecum, J.P.; Xiao, L.; Simmons, A.J.; Warden, C.; Pasic, L.; Himmel, L.E.; Washington, M.K.; et al. High dietary salt–induced DC activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019, 4, 5. [Google Scholar] [CrossRef]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef]
- Farez, M.F.; Fiol, M.P.; I Gaitán, M.; Quintana, F.J.; Correale, J. Sodium intake is associated with increased disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 26–31. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Munger, K.L.; Hartung, H.; Freedman, M.S.; Montalbán, X.; Edan, G.; Wicklein, E.; Radue, E.; Kappos, L.; Pohl, C.; et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann. Neurol. 2017, 82, 20–29. [Google Scholar] [CrossRef]
- McDonald, J.; Graves, J.; Waldman, A.; Lotze, T.; Schreiner, T.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.-M.; et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 6, 87–92. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Graves, J.; Casper, T.C.; Lulu, S.; Waldman, A.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.-M.; et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1350–1353. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Bahr, L.S.; Bock, M.; Liebscher, D.; Bellmann-Strobl, J.; Franz, L.; Prüß, A.; Schumann, D.; Piper, S.K.; Kessler, C.S.; Steckhan, N.; et al. Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): Protocol of a randomized controlled study. Trials 2020, 21, 3. [Google Scholar] [CrossRef]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; LaRosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.L.; Healy, B.C.; Malik, M.T.; Carruthers, R.L.; Musallam, A.J.; Kivisakk, P.; Weiner, H.L.; Glanz, B.; Chitnis, T. Effect of vitamin D on MS activity by disease-modifying therapy class. Neuroimmunol. Neuroinflamm. 2015, 2, e167. [Google Scholar] [CrossRef]
- Devolder, L.; Pauwels, A.; Van Remoortel, A.; Falony, G.; Vieira-Silva, S.; Nagels, G.; De Keyser, J.; Raes, J.; D’hooghe, M.B. Gut microbiome composition is associated with long-term disability worsening in multiple sclerosis. Gut Microbes 2023, 15, 2180316. [Google Scholar] [CrossRef]
- Navarro-López, V.; Méndez-Miralles, M.Á.; Vela-Yebra, R.; Fríes-Ramos, A.; Sánchez-Pellicer, P.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Gómez-Gómez, H.; Chumillas-Lidón, S.; Picó-Monllor, J.A.; et al. Gut Microbiota as a Potential Predictive Biomarker in Relapsing-Remitting Multiple Sclerosis. Genes 2022, 13, 930. [Google Scholar] [CrossRef]
- Pellizoni, F.P.; Leite, A.Z.; Rodrigues, N.d.C.; Ubaiz, M.J.; Gonzaga, M.I.; Takaoka, N.N.C.; Mariano, V.S.; Omori, W.P.; Pinheiro, D.G.; Junior, E.M.; et al. Detection of Dysbiosis and Increased Intestinal Permeability in Brazilian Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Environ. Res. Public Health 2021, 18, 4621. [Google Scholar] [CrossRef]
- Torres-Chávez, M.E.; Torres-Carrillo, N.; Moreal-Lugo, A.; Garnés-Rancurello, S.; Murugesan, S.; Gutiérrez-Hurtado, I.A.; Beltrán-Ramírez, J.R.; Sandoval-Pinto, E. Association of intestinal dysbiosis with susceptibility to multiple sclerosis: Evidence from different population studies (Review). Biomed. Rep. 2023, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Forbes, J.D.; Zhu, F.; Bernstein, C.N.; Van Domselaar, G.; Graham, M.; Waubant, E.; Tremlett, H. The multiple sclerosis gut microbiota: A systematic review. Mult. Scler. Relat. Disord. 2020, 37, 101427. [Google Scholar] [CrossRef]
- Elsayed, N.S.; Valenzuela, R.K.; Kitchner, T.; Le, T.; Mayer, J.; Tang, Z.-Z.; Bayanagari, V.R.; Lu, Q.; Aston, P.; Anantharaman, K.; et al. Genetic risk score in multiple sclerosis is associated with unique gut microbiome. Sci. Rep. 2023, 13, 16269. [Google Scholar] [CrossRef]
- Toivanen, P.; Vaahtovuo, J.; Eerola, E. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect. Immun. 2001, 69, 2372–2377. [Google Scholar] [CrossRef]
- Kubinak, J.L.; Stephens, W.Z.; Soto, R.; Petersen, C.; Chiaro, T.; Gogokhia, L.; Bell, R.; Ajami, N.J.; Petrosino, J.F.; Morrison, L.; et al. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat. Commun. 2015, 6, 8642. [Google Scholar] [CrossRef]
- Shahi, S.K.; Ali, S.; Jaime, C.M.; Guseva, N.V.; Mangalam, A.K. HLA class II polymorphisms modulate gut microbiota and experimental autoimmune encephalomyelitis phenotype. ImmunoHorizons 2021, 5, 627–646. [Google Scholar] [CrossRef]
- Montgomery, T.L.; Künstner, A.; Kennedy, J.J.; Fang, Q.; Asarian, L.; Culp-Hill, R.; D’alessandro, A.; Teuscher, C.; Busch, H.; Krementsov, D.N. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc. Natl. Acad. Sci. USA 2020, 117, 27516–27527. [Google Scholar] [CrossRef] [PubMed]
- Digehsara, S.G.; Name, N.; Sartipnia, N.; Karim, E.; Taheri, S.; Ebrahimi, M.T.; Arasteh, J. Analysis of inflammasomes and CYP27B1 genes in cuprizone demyelinated C57BL/6 mice and evaluation of Th1 and Th2 patterns after oral administration of Lactobacillus casei strain T2 (IBRC-M10783). Microb. Pathog. 2021, 155, 104931. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Ghimire, S.; Lehman, P.; Mangalam, A.K. Obesity induced gut dysbiosis contributes to disease severity in an animal model of multiple sclerosis. Front. Immunol. 2022, 13, 966417. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.N.; Cady, N.M.; Shahi, S.K.; Peterson, S.R.; Gupta, A.; Gibson-Corley, K.N.; Mangalam, A.K. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci. Adv. 2021, 7, eabd4595. [Google Scholar] [CrossRef]
- Ghimire, S.; Cady, N.M.; Lehman, P.; Peterson, S.R.; Shahi, S.K.; Rashid, F.; Giri, S.; Mangalam, A.K. Dietary isoflavones alter gut microbiota and lipopolysaccharide biosynthesis to reduce inflammation. Gut Microbes 2022, 14, 2127446. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Liu, Y.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; Ma, X.; Chen, X. Gut microbiota interventions with clostridium butyricum and norfloxacin modulate immune response in experimental autoimmune encephalomyelitis mice. Front. Immunol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Turnea, M.; Blendea, C.D.; Rotariu, M.; Poștaru, M. Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review. Antioxidants 2024, 13, 1062. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J. Dietary modulation of intestinal microbiota: Future opportunities in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Microbiol. 2019, 10, 740. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clin. Nutr. ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef]
- Dora, D.; Bokhari, S.M.Z.; Aloss, K.; Takacs, P.; Desnoix, J.Z.; Szklenárik, G.; Hurley, P.D.; Lohinai, Z. Implication of the gut microbiome and microbial-derived metabolites in immune-related adverse events: Emergence of novel biomarkers for cancer immunotherapy. Int. J. Mol. Sci. 2023, 24, 2769. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Omran, H.S.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary polyphenols, microbiome, and multiple sclerosis: From molecular anti-inflammatory and neuroprotective mechanisms to clinical evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Lee, B.H.; Oh, D.-H. Challenges and perspective in integrated multi-omics in gut microbiota studies. Biomolecules 2021, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Fekete, E.E.; Figeys, D.; Zhang, X. Microbiota-directed biotherapeutics: Considerations for quality and functional assessment. Gut Microbes 2023, 15, 2186671. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e00338-20. [Google Scholar] [CrossRef] [PubMed]
- Soltanmohammadi, A.; Tavaf, M.; Zargarani, S.; Yazdanpanah, E.; Sadighi-Moghaddam, B.; Yousefi, B.; Sameni, H.; Haghmorad, D. Daphnetin alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating Th2 and regulatory T cells. Acta Neurobiol. Exp. 2022, 82, 273–283. [Google Scholar] [CrossRef]
- Haghmorad, D.; Khaleghian, A.; Eslami, M.; Sadeghnejad, A.; Tarahomi, M.; Yousefi, B. Bone marrow mesenchymal stem cells to ameliorate experimental autoimmune encephalomyelitis via modifying expression patterns of miRNAs. Mol. Biol. Rep. 2023, 50, 9971–9984. [Google Scholar] [CrossRef]
- Haghmorad, D.; Soltanmohammadi, A.; Tavaf, M.J.; Zargarani, S.; Yazdanpanah, E.; Shadab, A.; Yousefi, B. The protective role of interaction between vitamin D, sex hormones and calcium in multiple sclerosis. Int. J. Neurosci. 2024, 134, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Mace, J.W.; Calabresi, P.A. Animal models to investigate the effects of inflammation on remyelination in multiple sclerosis. Front. Mol. Neurosci. 2022, 15, 995477. [Google Scholar] [CrossRef] [PubMed]
- Keough, M.B.; Jensen, S.K.; Yong, V.W. Experimental demyelination and remyelination of murine spinal cord by focal injection of lysolecithin. J. Vis. Exp. 2015, 97, 52679. [Google Scholar]
- DePaula-Silva, A.B. The contribution of microglia and brain-infiltrating macrophages to the pathogenesis of neuroinflammatory and neurodegenerative diseases during TMEV infection of the central nervous system. Viruses 2024, 16, 119. [Google Scholar] [CrossRef]
| MS Subtype | Microbial Taxa | Dysbiosis Pattern | References |
|---|---|---|---|
| RRMS | Lachnospiraceae | ↑↓ | [140,143] |
| Ezakiella | ↑↓ | [140] | |
| Ruminococcaceae | ↑↓ | [140] | |
| Hungatella | ↑↓ | [140] | |
| Hungatella effluvia | ↑ | [66] | |
| Hungatella hathewayi | ↑ | [3] | |
| Roseburia | ↑↓ | [140,143] | |
| Clostridium leptum | ↑ | [66] | |
| Clostridium innocuum | ↑ | [66] | |
| Shuttleworthia | ↑↓ | [140] | |
| Bilophila wadsworthia | ↑ | [66] | |
| Prevotella | ↓ | [85,143,144] | |
| Streptococcus | ↑↓ | [85,145] | |
| Bacteroidaceae | ↓ | [82] | |
| Faecalibacterium | ↓ | [82,143] | |
| Faecalibacterium prausnitzii | ↓ | [3] | |
| Ruminococcus | ↑ | [82] | |
| Ruminococcus torques | ↑ | [66] | |
| Ruminococcus gnavus | ↑ | [66] | |
| Methanobrevibacter | ↑ | [79,146] | |
| Akkermansia | ↑ | [79,143] | |
| Akkermansia muciniphila | ↑ | [3,141] | |
| Bifidobacterium | ↓ | [141,143] | |
| Pseudomonas | ↑↓ | [60] | |
| Mycoplana | ↑↓ | [60] | |
| Haemophilus | ↑↓ | [60] | |
| Blautia | ↑↓ | [3,60,143] | |
| Blautia wexlerae | ↑ | [66] | |
| Blautia massiliensis | ↑ | [66] | |
| Dorea | ↑↓ | [60] | |
| Dysosmobacter welbionis | ↑ | [66] | |
| Flavonifractor plautii | ↑ | [66] | |
| Lawsonibacter phoceensis | ↑ | [66] | |
| Gordonibacter urolithinfaciens | ↑ | [66] | |
| Anaerobutyricum hallii | ↑ | [66] | |
| Pseudoflavonifractor capillosus | ↑ | [66] | |
| Anaerotruncus colihominis | ↑ | [66] | |
| Erysipelatoclostridium ramosum | ↑ | [66] | |
| Sellimonas intestinalis | ↑ | [66] | |
| Coprobacillus cateniformis | ↑ | [66] | |
| Ruthenibacterium lactatiformans | ↑ | [3] | |
| Eisenbergiella tayi | ↑ | [3] | |
| Bacteroides vulgatus | ↑ | [141] | |
| RR, Pediatric MS | Clostridium | ↑↓ | [84,140] |
| Bilophila | ↑↓ | [84,140] | |
| Bacteroides 2 enterotype | ↑ | [139] | |
| Pediatric MS | Escherichia | ↑ | [84] |
| Shigella | ↑ | [84] | |
| Eubacterium rectale | ↓ | [84] | |
| Corynebacterium | ↓ | [84] | |
| MS | Akkermansia genus | ↑ | [146] |
| Firmicutes | ↓ | [143] | |
| Roseburia | ↓ | [143] | |
| Coprococcus | ↓ | [143] | |
| Butyricicoccus | ↓ | [143] | |
| Lachnospira | ↓ | [143] | |
| Dorea | ↓ | [143] | |
| Bacteroidetes | ↑ | [143] | |
| Ruminocococcus | ↑ | [143] | |
| Acinetobacter | ↑ | [142] | |
| Parabacteroides distasonis | ↓ | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemati, M.H.; Yazdanpanah, E.; Kazemi, R.; Orooji, N.; Dadfar, S.; Oksenych, V.; Haghmorad, D. Microbiota-Driven Mechanisms in Multiple Sclerosis: Pathogenesis, Therapeutic Strategies, and Biomarker Potential. Biology 2025, 14, 435. https://doi.org/10.3390/biology14040435
Nemati MH, Yazdanpanah E, Kazemi R, Orooji N, Dadfar S, Oksenych V, Haghmorad D. Microbiota-Driven Mechanisms in Multiple Sclerosis: Pathogenesis, Therapeutic Strategies, and Biomarker Potential. Biology. 2025; 14(4):435. https://doi.org/10.3390/biology14040435
Chicago/Turabian StyleNemati, Mohammad Hosein, Esmaeil Yazdanpanah, Roya Kazemi, Niloufar Orooji, Sepehr Dadfar, Valentyn Oksenych, and Dariush Haghmorad. 2025. "Microbiota-Driven Mechanisms in Multiple Sclerosis: Pathogenesis, Therapeutic Strategies, and Biomarker Potential" Biology 14, no. 4: 435. https://doi.org/10.3390/biology14040435
APA StyleNemati, M. H., Yazdanpanah, E., Kazemi, R., Orooji, N., Dadfar, S., Oksenych, V., & Haghmorad, D. (2025). Microbiota-Driven Mechanisms in Multiple Sclerosis: Pathogenesis, Therapeutic Strategies, and Biomarker Potential. Biology, 14(4), 435. https://doi.org/10.3390/biology14040435






