Effect of Aflatoxin B1 on the Nervous System: A Systematic Review and Network Analysis Highlighting Alzheimer’s Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Search and Selection
2.3. Eligibility Criteria
2.4. A Compilation of Interconnected Genes Linked to AD
2.5. Evaluation of Essential Target Genes, Along with Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
3. Results
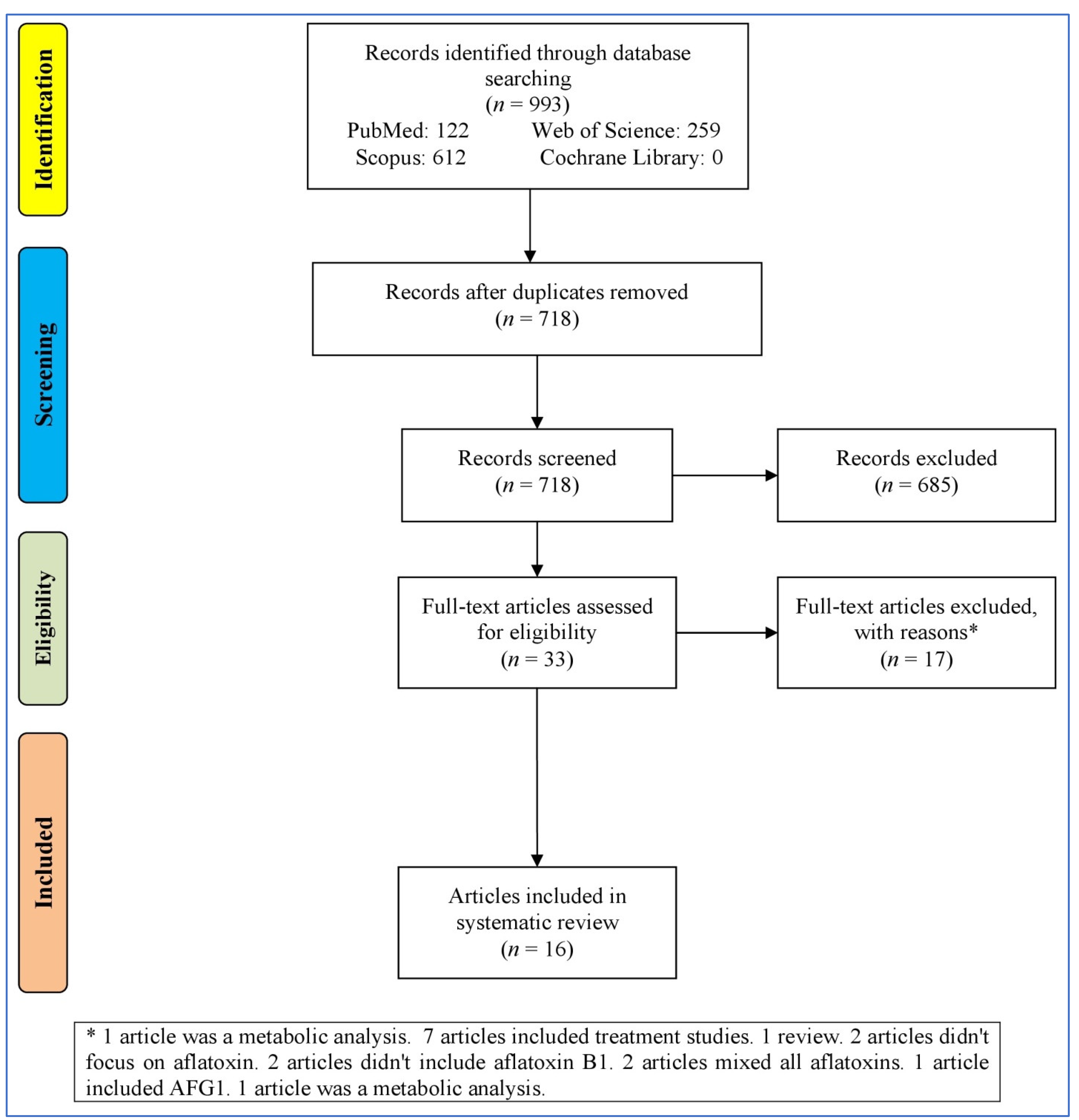
3.1. Study Selection Process
3.2. Characteristics of the Articles
3.3. Pathways
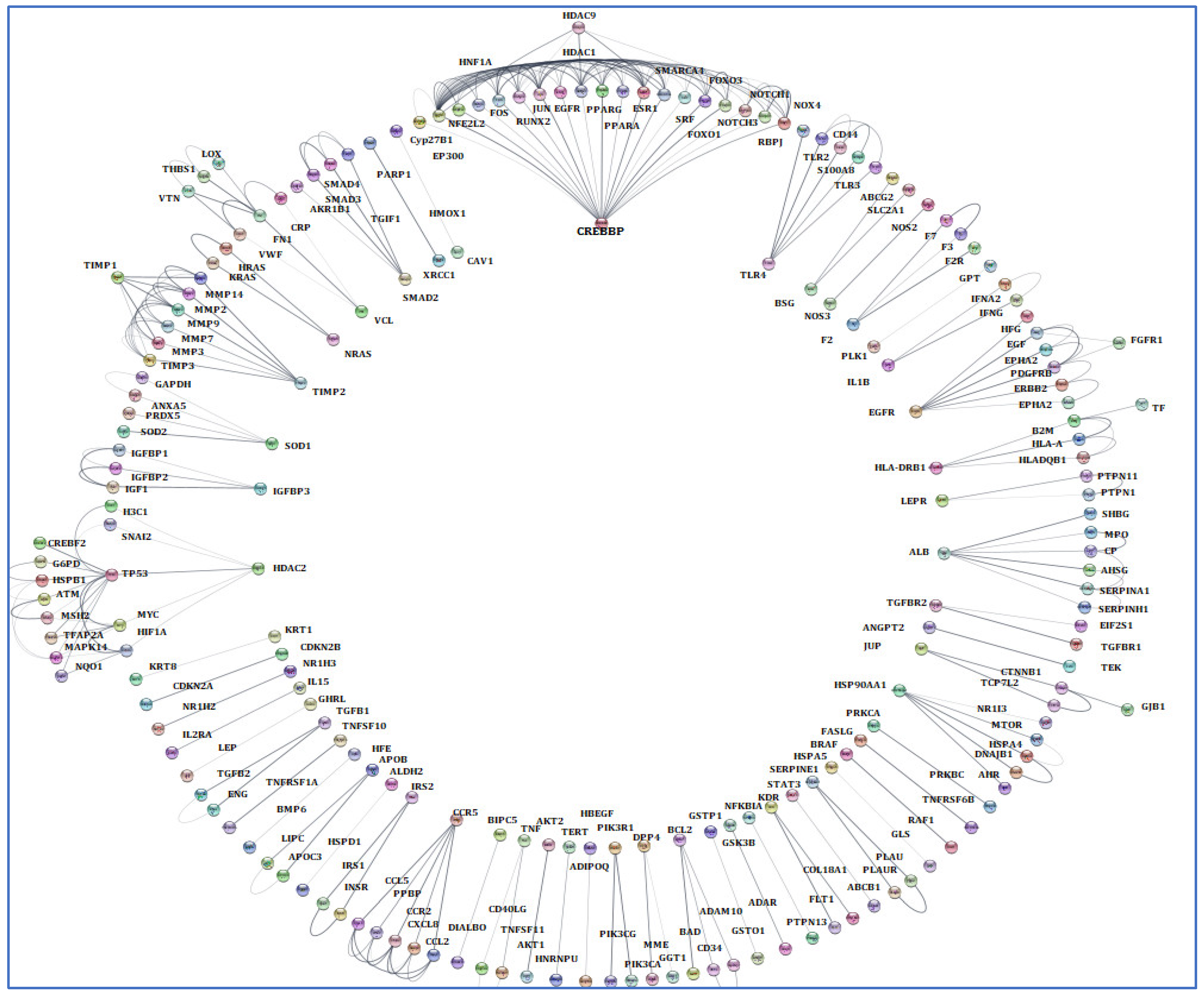
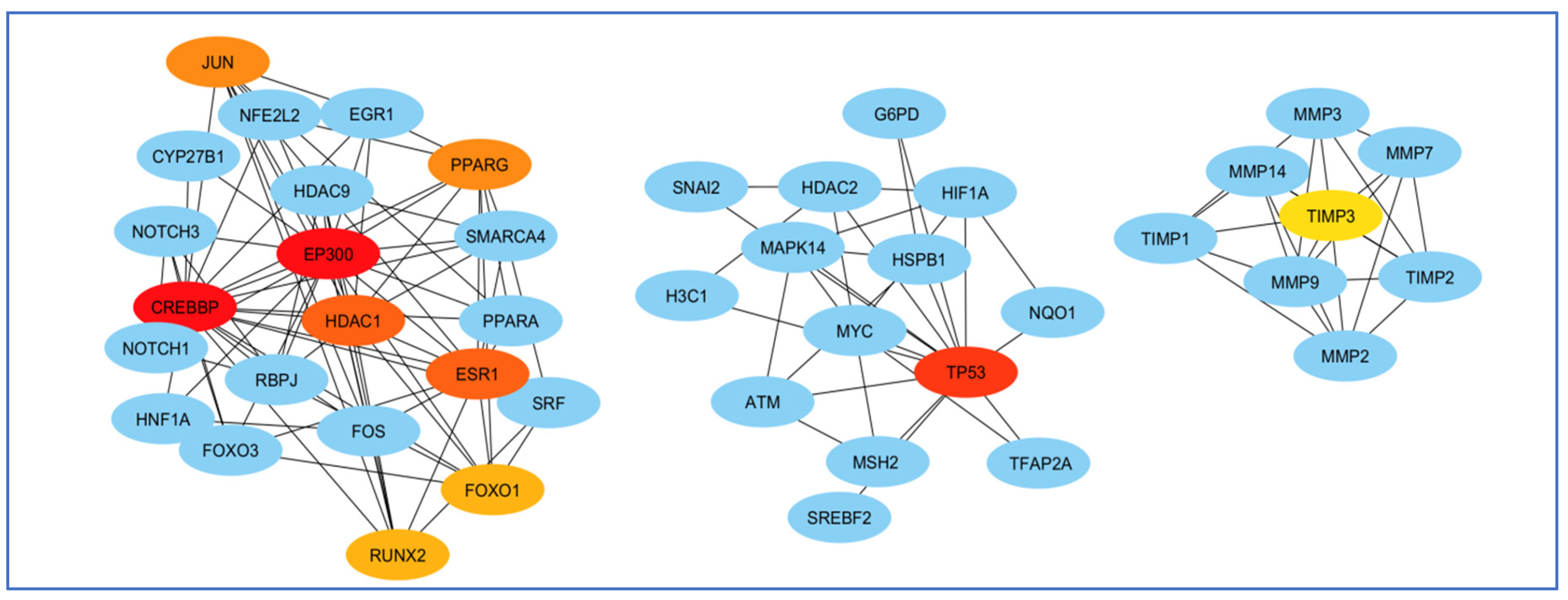
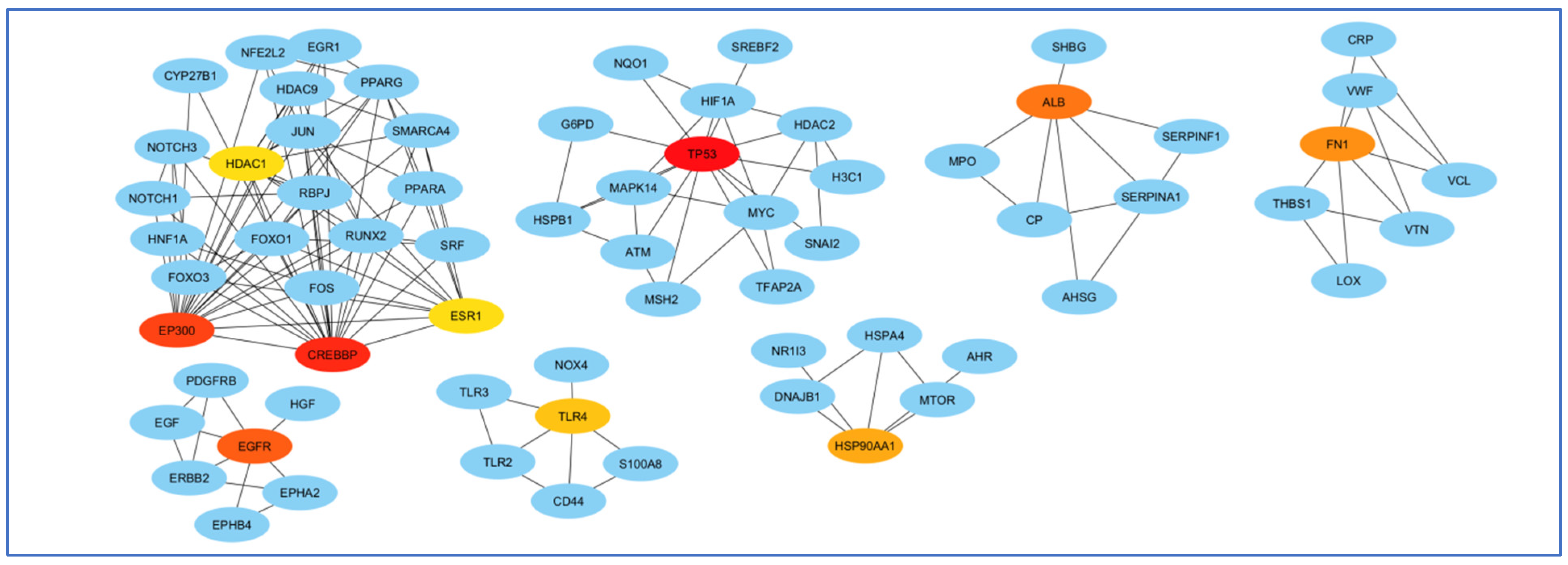
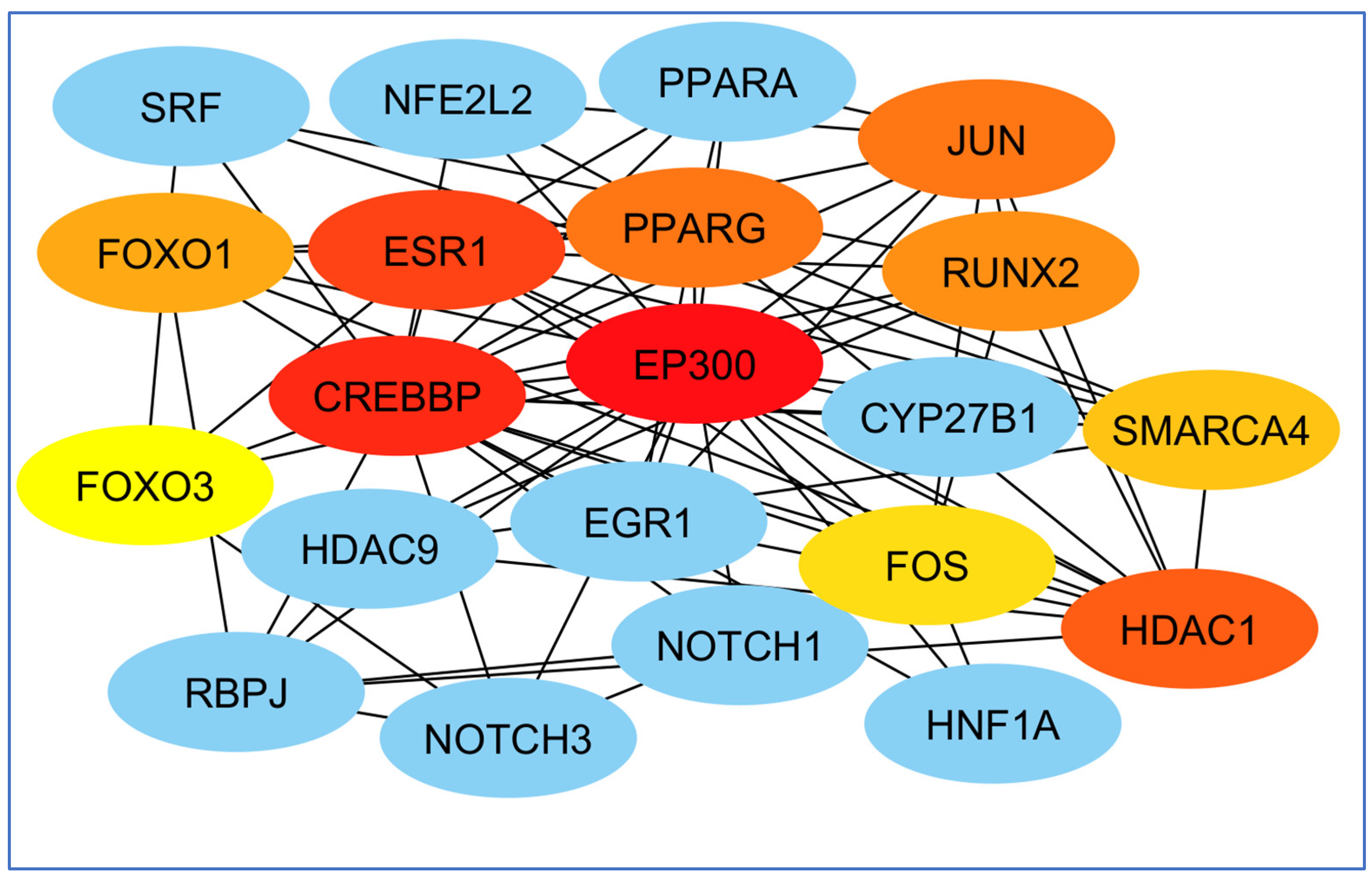
3.4. Screening and Analyzing the Genes Shared by “Alzheimer’s Disease”, “Inflammation”, “Angiopathy”, and “AFB1”, as Well as Performing Network Analysis
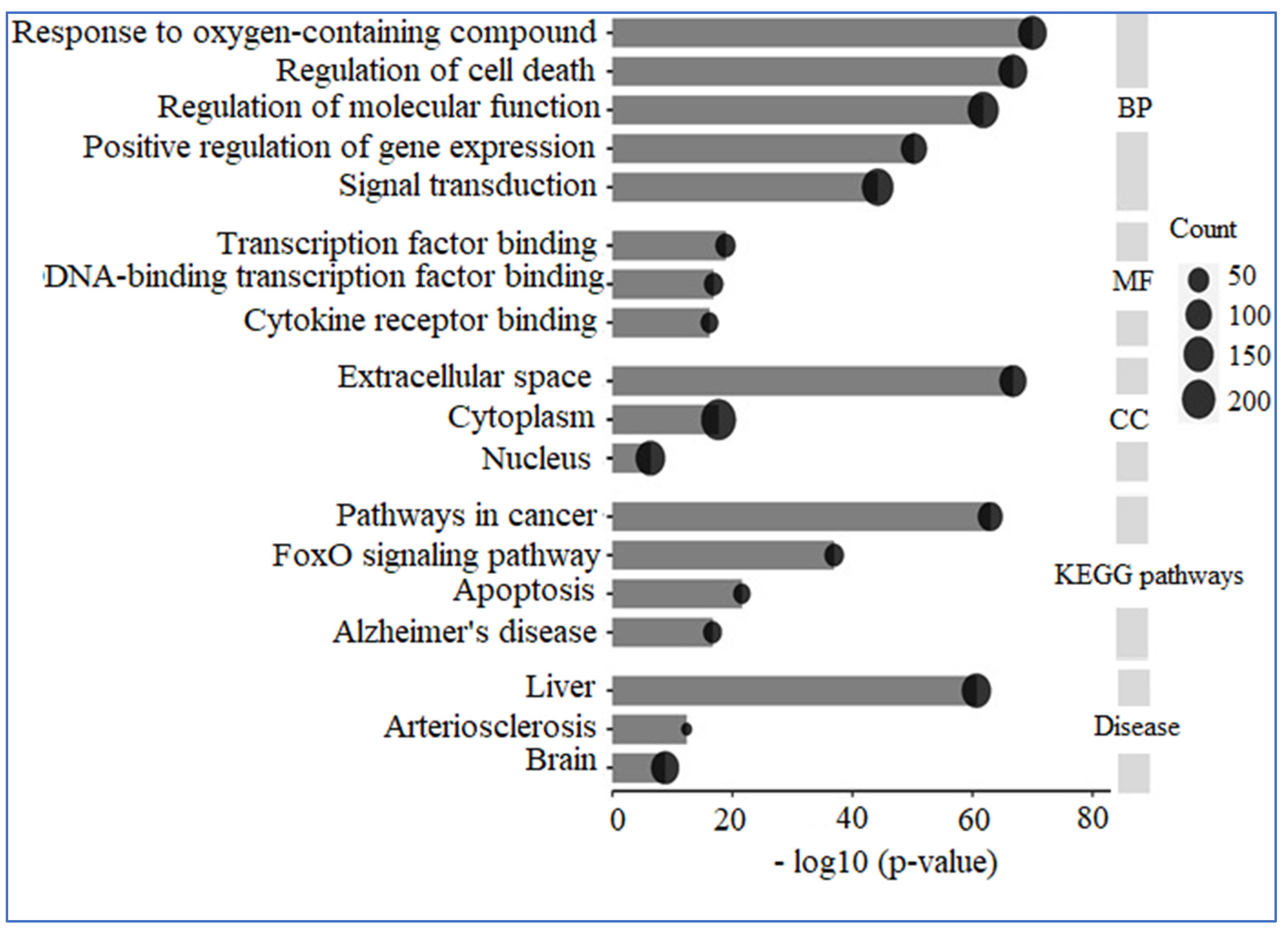
3.5. Enrichment Analysis
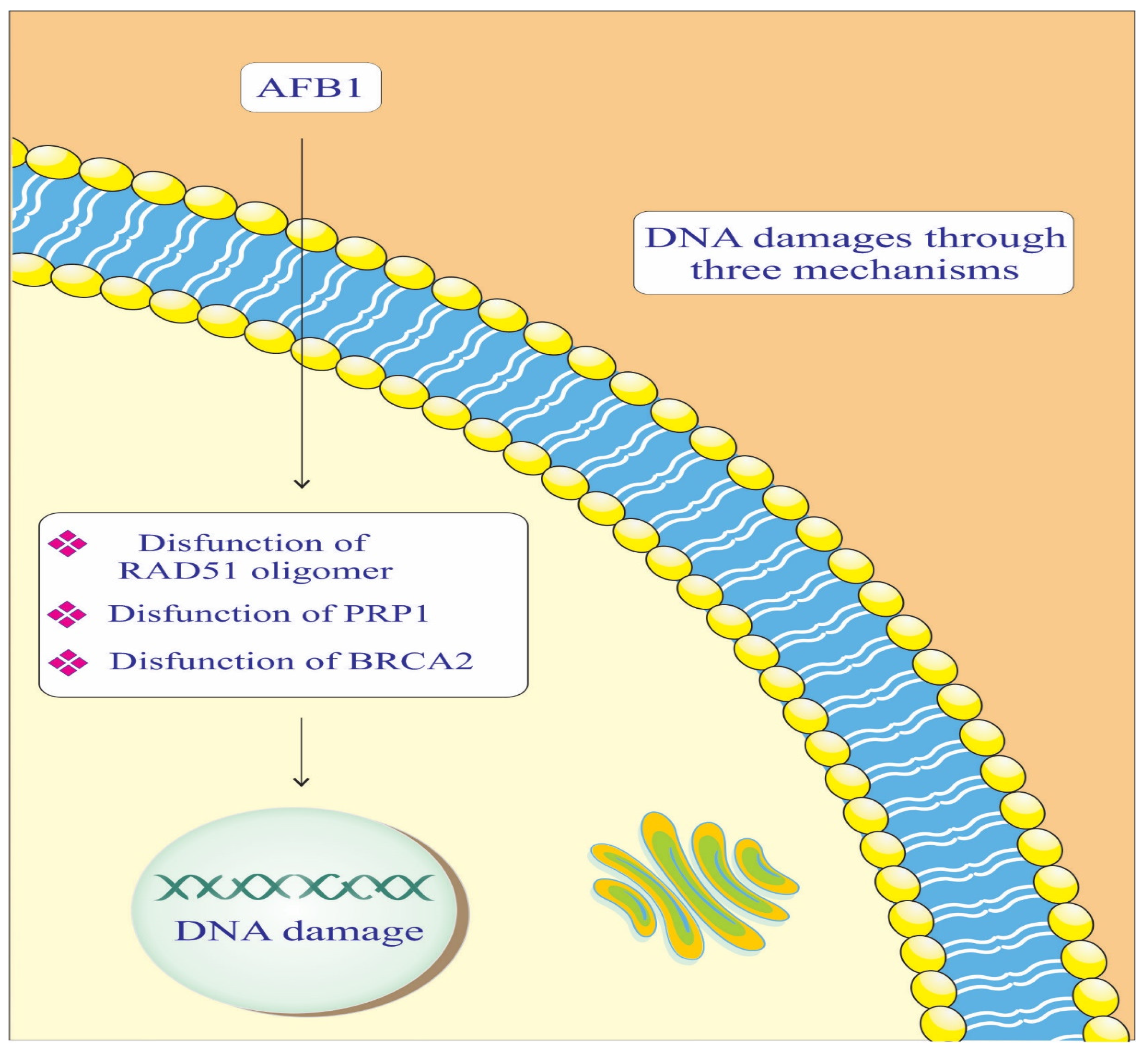
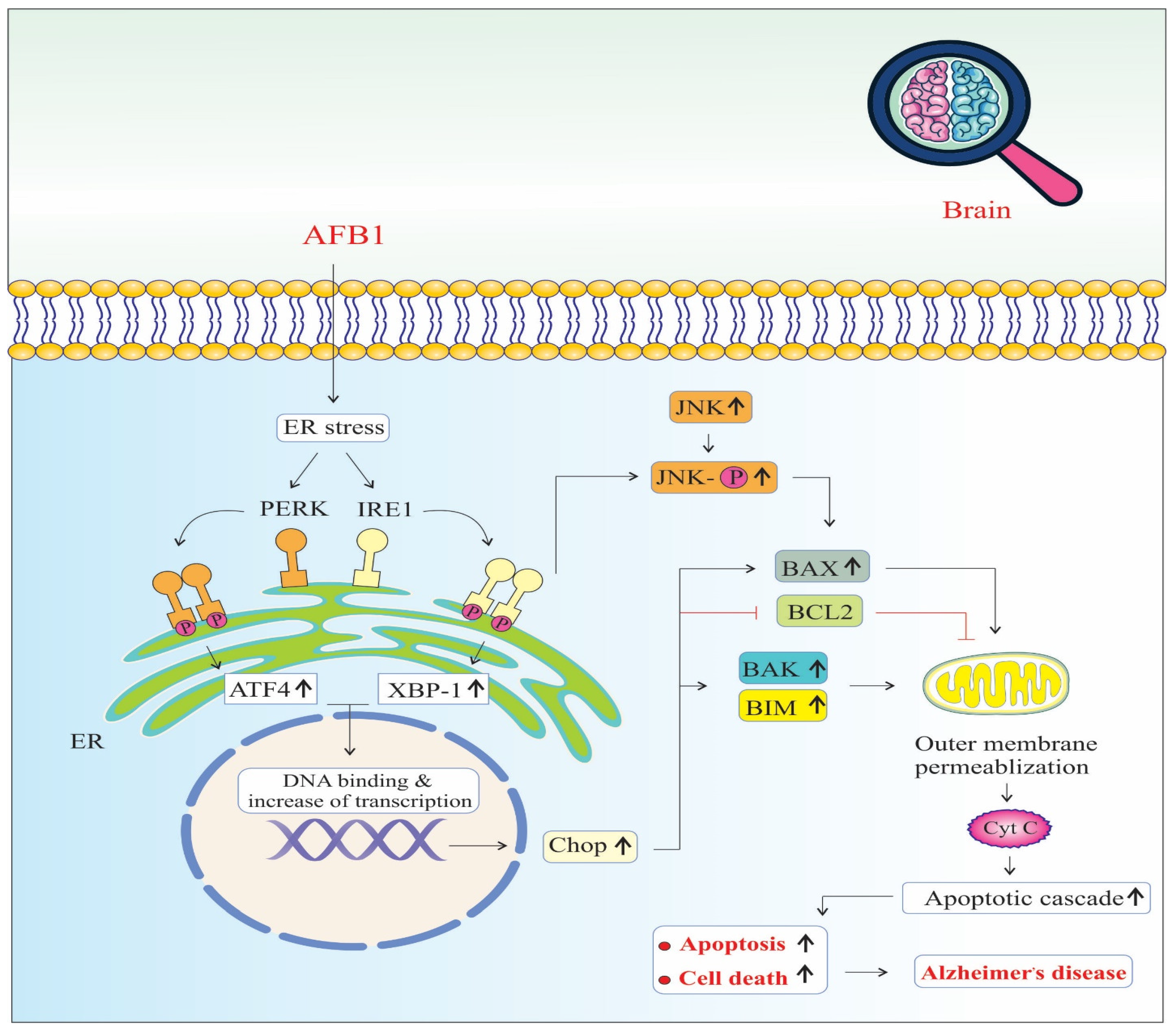
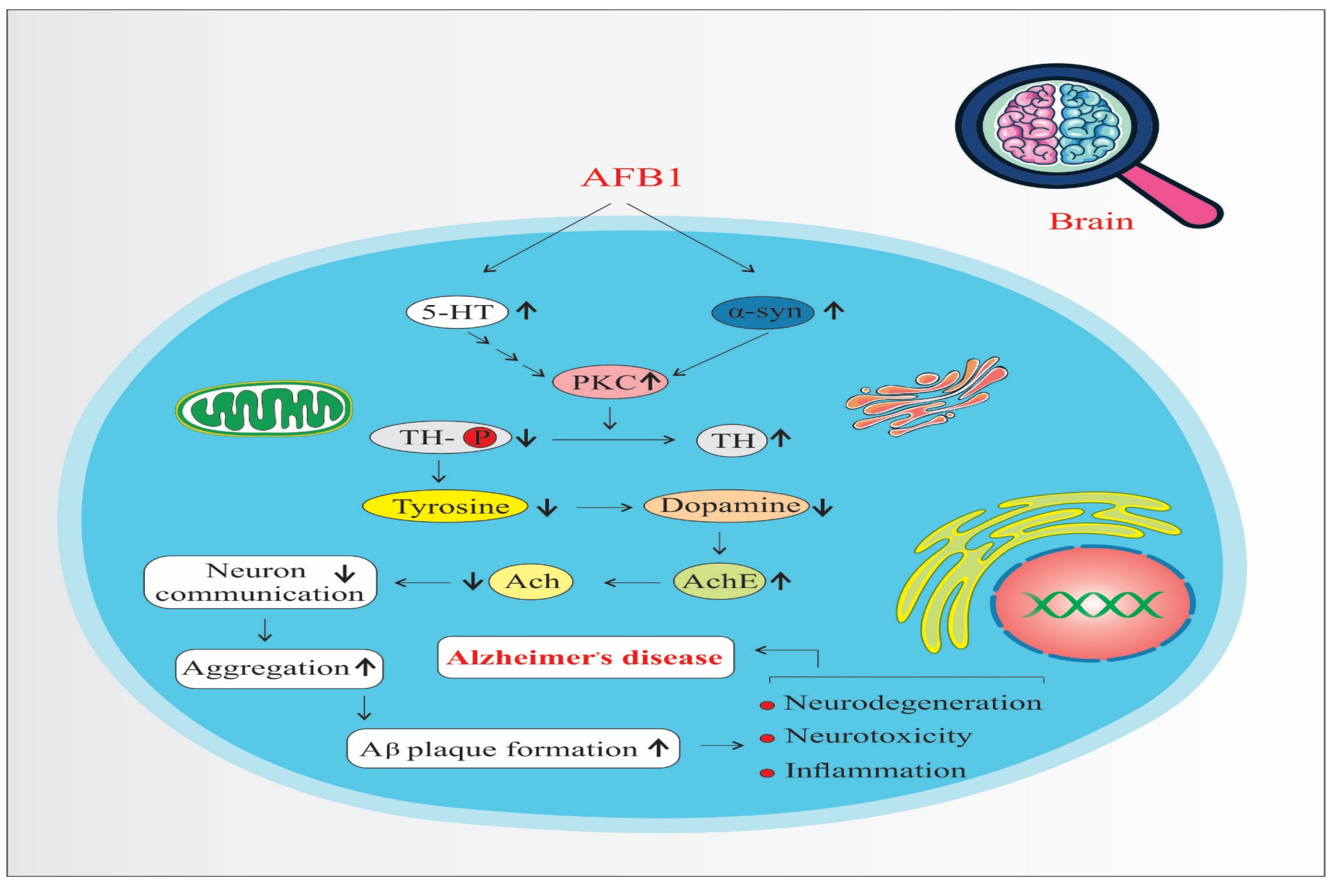
4. Discussion
5. Conclusions
5.1. Clinical Significance
5.2. Future Comment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Mehrabi, M.; Akbari, V.; Pashaei, S.; Khodarahmi, R. “Cyclophilin A” Enzymatic Effect on the Aggregation Behavior of 1N4R Tau Protein: An Overlooked Crucial Determinant that should be Re-considered in Alzheimer’s Disease Pathogenesis. Curr. Alzheimer Res. 2024, 21, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Akbari, V.; Bahramikia, S.; Jalalvand, A.R.; Mehrabi, M.; Ezati, M.; Khodarahmi, R. The induction of tau aggregation is restricted by sulfamethoxazole and provides new information regarding the use of the drug. J. Biomol. Struct. Dyn. 2024, 42, 12761–12775. [Google Scholar] [CrossRef]
- Akbari, V.; Mohammadi, S.; Mehrabi, M.; Ghobadi, S.; Farrokhi, A.; Khodarahmi, R. Investigation of the role of prolines 232/233 in RTPPK motif in tau protein aggregation: An in vitro study. Int. J. Biol. Macromol. 2022, 219, 1100–1111. [Google Scholar] [CrossRef]
- Fazelinejad, H.; Zahedi, E.; Nazarian, S.; Kaffash Siuki, Z.; Nasri, S.; Dadmehr, M.; Mehrabi, M.; Khodarahmi, R. Neuroprotective effect of Bis (Indolyl) phenylmethane in Alzheimer’s disease rat model through inhibition of hen Lysozyme amyloid fibril-induced neurotoxicity. J. Iran. Chem. Soc. 2023, 20, 551–562. [Google Scholar] [CrossRef]
- Mehrabi, M.; Bijari, N.; Akbari, V.; Ranjbar, S.; Karima, S.; Sankian, M.; Ojaghi, S.; Khodarahmi, R. Effective reduction of tau amyloid aggregates in the presence of cyclophilin from Platanus orientalis pollens; An alternative mechanism of action of the allergen. Curr. Protein Pept. Sci. 2023, 24, 518–532. [Google Scholar] [CrossRef]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s disease: Epidemiology and clinical progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Green, R.C.; Cupples, L.A.; Go, R.; Benke, K.S.; Edeki, T.; Griffith, P.A.; Williams, M.; Hipps, Y.; Graff-Radford, N.; Bachman, D.; et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002, 287, 329–336. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Masters, C.; Bateman, R.; Blennow, K. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2016, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 2022, 14, 853. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Scotter, M. A review of aflatoxin M1 in liquid milk. Iran. J. Health Saf. Environ. 2015, 2, 283–295. [Google Scholar]
- Womack, E.; Sparks, D.; Brown, A. Aflatoxin M1 in milk and milk products: A short review. World Mycotoxin J. 2016, 9, 305–315. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Li, H.; Gupta, S.D.; Hao, Z.; Wang, Z.; Velkov, T.; Shen, J. Molecular mechanisms of aflatoxin neurotoxicity and potential neuroprotective agents. Food Sci. Hum. Wellness 2024, 13, 2445–2455. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Neurotoxic mechanisms of mycotoxins: Focus on aflatoxin B1 and T-2 toxin. Environ. Pollut. 2024, 356, 124359. [Google Scholar] [CrossRef]
- Oyelami, O.; Maxwell, S.; Adelusola, K.; Aladekoma, T.; Oyelese, A. Aflatoxins in the lungs of children with kwashiorkor and children with miscellaneous diseases in Nigeria. J. Toxicol. Environ. Health 1997, 51, 623–628. [Google Scholar] [CrossRef]
- Qureshi, H.; Hamid, S.S.; Ali, S.S.; Anwar, J.; Siddiqui, A.A.; Khan, N.A. Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood-brain barrier. Med. Mycol. 2015, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Malvandi, A.M.; Alipour, M.; Hosseinkhani, S. Environmentally relevant level of aflatoxin B1 elicits toxic pro-inflammatory response in murine CNS-derived cells. Toxicol. Lett. 2017, 279, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Descovi, S.N.; Moreira, K.L.S.; da Rocha, M.I.U.; da Veiga, M.L.; da Silva, A.S.; Baldisserotto, B. Aflatoxin B1-contaminated diet disrupts the blood–brain barrier and affects fish behavior: Involvement of neurotransmitters in brain synaptosomes. Environ. Toxicol. Pharmacol. 2018, 60, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Guo, N.; Wang, C.; Zhao, M.; Yi, L.; Liu, C.; Kang, L.; Cao, L.; Lv, P.; Xing, L.; et al. Aflatoxin G1 induced TNF-α-Dependent lung inflammation to enhance DNA damage in alveolar epithelial cells. J. Cell. Physiol. 2019, 234, 9194–9206. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Sharma, N. Free radicals, antioxidants and disease. Biol. Med. 2014, 6, 1. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Stark, A.-A. Molecular and Biochemical Mechanisms of Action of Acute Toxicity and Carcinogenicity Induced by Aflatoxin B1, and of the Chemoprevention of Liver Cancer. In Microbial Food Contamination; CRC Press: Boca Raton, FL, USA, 2007; pp. 149–168. [Google Scholar]
- Stark, A.-A. Mechanisms of action of aflatoxin B1 at the biochemical and molecular levels. In Microbial Food Contamination; CRC Press: Boca Raton, FL, USA, 2001; pp. 81–94. [Google Scholar]
- Abou-Rawash, A.; Abdel-Megged, M. Effects of Enzyme Modulation on Aflatoxin Cytotoxicity and Carcinogenicity in Rats: Cyotgenetics and Histopathology. Egypt. J. Comp. Path. Clinic. Path. 2008, 21, 175–200. [Google Scholar]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Vasefi, M.; Ghaboolian-Zare, E.; Abedelwahab, H.; Osu, A. Environmental toxins and Alzheimer’s disease progression. Neurochem. Int. 2020, 141, 104852. [Google Scholar] [CrossRef] [PubMed]
- Ehsanifar, M.; Rajati, R.; Gholami, A.; Reiss, J.P. Mold and mycotoxin exposure and brain disorders. J. Integr. Neurosci. 2023, 22, 137. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, D.; Li, M.; Lao, G.; Zhou, Z.; Dinnyés, A.; Xu, W.; Sun, Q. A Novel Multicellular Placental Barrier Model to Investigate the Effect of Maternal Aflatoxin B(1) Exposure on Fetal-Side Neural Stem Cells. Toxins 2023, 15, 312. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Alwetaid, M.Y.; Bakheet, S.A.; Attia, S.M.; Ansari, M.A.; Nadeem, A.; Ahmad, S.F. Aflatoxin B(1) exposure deteriorates immune abnormalities in a BTBR T(+) Itpr3(tf)/J mouse model of autism by increasing inflammatory mediators’ production in CD19-expressing cells. J. Neuroimmunol. 2024, 391, 578365. [Google Scholar] [CrossRef]
- Sahoo, M.; Thakor, J.C.; Kumar, P.; Singh, R.; Kumar, P.; Singh, K.; Puvvala, B.; Kumar, A.; Gopinathan, A.; Palai, S.; et al. AFB1 induced free radicals cause encephalopathy in goat kids via intrinsic pathway of apoptosis: Pathological and immunohistochemical confirmation of non-hepatic neuroaflatoxicosis. Vet. Res. Commun. 2024, 48, 317–327. [Google Scholar] [CrossRef]
- Alwetaid, M.Y.; Almanaa, T.N.; Bakheet, S.A.; Ansari, M.A.; Nadeem, A.; Attia, S.M.; Ahmad, S.F. Aflatoxin B(1) exposure exacerbates chemokine receptor expression in the BTBR T(+) Itpr3(tf)/J Mouse Model, unveiling insights into autism spectrum disorder: A focus on brain and spleen. Reprod. Toxicol. 2024, 126, 108599. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Wagner, K.M.; Lee, R.D.; Hwang, S.H.; Morisseau, C.; Wulff, H.; Hammock, B.D. Aflatoxin B(1) Increases Soluble Epoxide Hydrolase in the Brain and Induces Neuroinflammation and Dopaminergic Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 9938. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef]
- Alwetaid, M.Y.; Almanaa, T.N.; Bakheet, S.A.; Ansari, M.A.; Nadeem, A.; Attia, S.M.; Hussein, M.H.; Ahmad, S.F. Aflatoxin B(1) Exposure Aggravates Neurobehavioral Deficits and Immune Dysfunctions of Th1, Th9, Th17, Th22, and T Regulatory Cell-Related Transcription Factor Signaling in the BTBR T(+)Itpr3(tf)/J Mouse Model of Autism. Brain Sci. 2023, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Bonsi, P.; Augusti-Tocco, G.; Palmery, M.; Giorgi, M. Aflatoxin B1 is an inhibitor of cyclic nucleotide phosphodiesterase activity. Gen. Pharmacol. 1999, 32, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Vahidi-Ferdowsi, P.; Mehrzad, J.; Malvandi, A.; Hosseinkhani, S. Bioluminescence-based detection of astrocytes apoptosis and ATP depletion induced by biologically relevant level aflatoxin B1. World Mycotoxin J. 2018, 11, 589–598. [Google Scholar] [CrossRef]
- Alsayyah, A.; ElMazoudy, R.; Al-Namshan, M.; Al-Jafary, M.; Alaqeel, N. Chronic neurodegeneration by aflatoxin B1 depends on alterations of brain enzyme activity and immunoexpression of astrocyte in male rats. Ecotoxicol. Environ. Saf. 2019, 182, 109407. [Google Scholar] [CrossRef]
- Ricordy, R.; Gensabella, G.; Cacci, E.; Augusti-Tocco, G. Impairment of cell cycle progression by aflatoxin B1 in human cell lines. Mutagenesis 2002, 17, 241–249. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, H.; Li, S.; Wang, J.; Liu, J.; Ren, H.; Gao, Y. Lactoferrin inhibits aflatoxin B1- and aflatoxin M1-induced cytotoxicity and DNA damage in Caco-2, HEK, Hep-G2, and SK-N-SH cells. Toxicon 2018, 150, 77–85. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Zeppenfeld, C.C.; Descovi, S.; Stefani, L.M.; Baldisserotto, B.; da Silva, A.S. Oxidative stress mediated the inhibition of cerebral creatine kinase activity in silver catfish fed with aflatoxin B(1)-contaminated diet. Fish. Physiol. Biochem. 2019, 45, 63–70. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Hesperetin protects hippocampal neurons from the neurotoxicity of Aflatoxin B1 in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115782. [Google Scholar] [CrossRef]
- Ahmed, N.; Singh, U.S. Effect of aflatoxin B1 on brain serotonin and catecholamines in chickens. Toxicol. Lett. 1984, 21, 365–367. [Google Scholar] [CrossRef]
- Richard, S.A.; Manaphraim, N.Y.; Kortei, N.K. The novel neurotoxic and neuroimmunotoxic capabilities of aflatoxin B1 on the nervous system: A review. Adv. Biosci. Clin. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Mahfuz, M.; Hossain, M.S.; Alam, M.A.; Gazi, M.A.; Fahim, S.M.; Nahar, B.; Ahmed, T. Chronic Aflatoxin Exposure and Cognitive and Language Development in Young Children of Bangladesh: A Longitudinal Study. Toxins 2022, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F.; Tannorella, P.; Stoccoro, A.; Chico, L.; Siciliano, G.; Bonuccelli, U.; Migliore, L. Methylation analysis of DNA repair genes in Alzheimer’s disease. Mech. Ageing Dev. 2017, 161, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S.; Li, X. DNA damage accumulation in aging brain and its links to Alzheimer’s disease progression. Genome Instab. Dis. 2022, 3, 172–178. [Google Scholar] [CrossRef]
- Genova, A.; Dix, O.; Thakur, M.; Dhillon, M.S. A review on the mutations of the BRCA 1 gene on neurocognitive disorders. Eur. J. Biomed. 2020, 7, 385–392. [Google Scholar]
- Zeng, J.; Libien, J.; Shaik, F.; Wolk, J.; Hernández, A.I. Nucleolar PARP-1 Expression Is Decreased in Alzheimer’s Disease: Consequences for Epigenetic Regulation of rDNA and Cognition. Neural Plast. 2016, 2016, 8987928. [Google Scholar] [CrossRef]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Gerakis, Y.; Hetz, C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018, 285, 995–1011. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009, 6, 41. [Google Scholar] [CrossRef]
- Hattori, N.; Kitagawa, K.; Higashida, T.; Yagyu, K.; Shimohama, S.; Wataya, T.; Perry, G.; Smith, M.A.; Inagaki, C. Cl−-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease brains. Neurosci. Lett. 1998, 254, 141–144. [Google Scholar] [CrossRef]
- Liguri, G.; Taddei, N.; Nassi, P.; Latorraca, S.; Nediani, C.; Sorbi, S. Changes in Na+, K+-ATPase, Ca2+–ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer’s disease. Neurosci. Lett. 1990, 112, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Jembrek, M.J.; Slade, N.; Hof, P.R.; Šimić, G. The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer’s disease. Prog. Neurobiol. 2018, 168, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Shimohama, S.; Kamoshima, W.; Matsuoka, Y.; Nomura, Y.; Taniguchi, T. Changes of p53 in the brains of patients with Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1997, 232, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.; Marques, S.C.; Ferreiro, E.; Simões, I.; Oliveira, C.R.; Pereira, C.M. Effect of α-synuclein on amyloid β-induced toxicity: Relevance to Lewy body variant of Alzheimer disease. Neurochem. Res. 2013, 38, 797–806. [Google Scholar] [CrossRef]
- Bridi, J.C.; Hirth, F. Mechanisms of α-synuclein induced synaptopathy in Parkinson’s disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
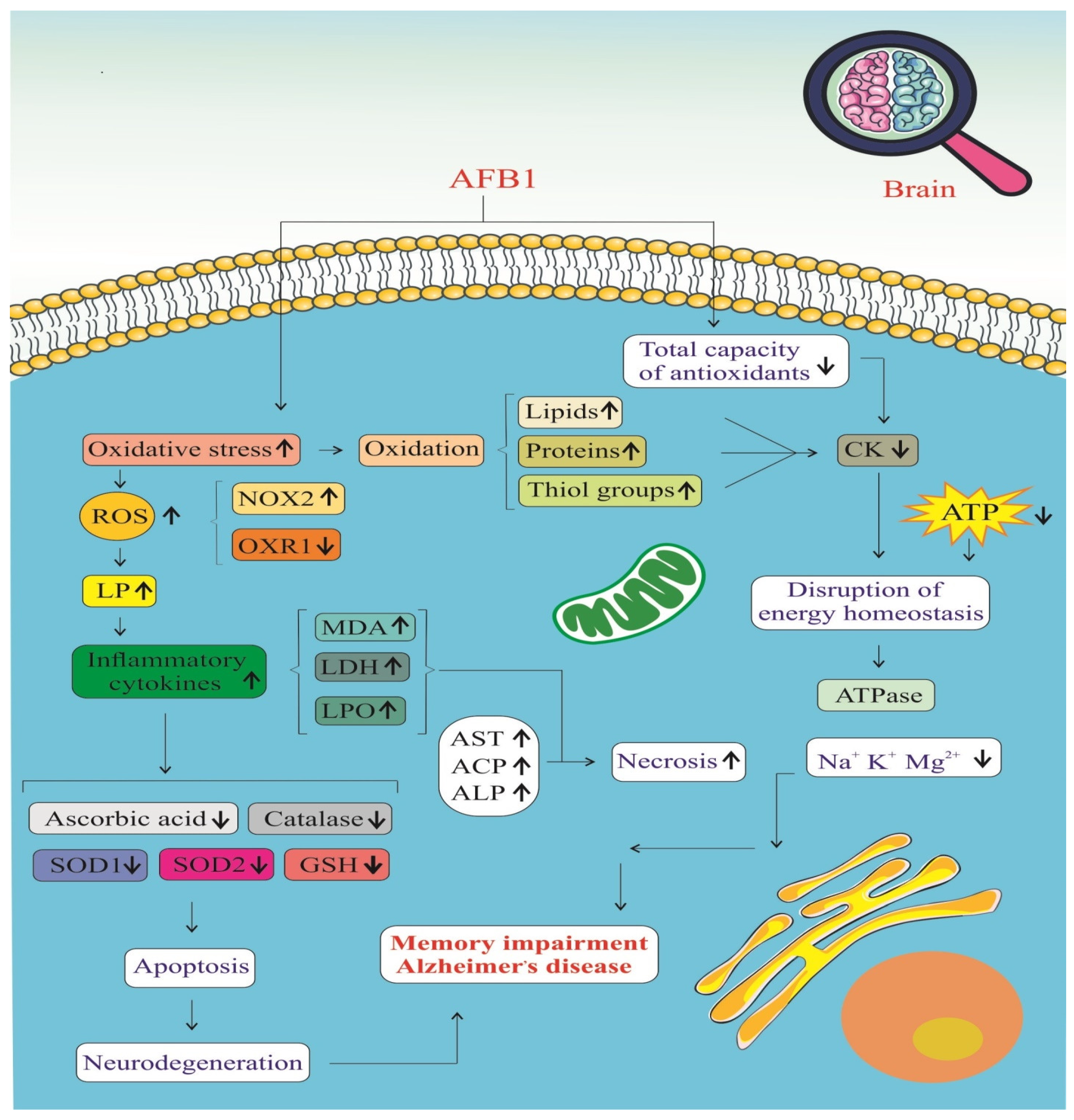
| The First Author, Publication Year | Country | Type of Cell | Cell Models | Main Results on AFB1-Exposed Brain Cells |
|---|---|---|---|---|
| Zhou, 2023 [37] | China | Neural stem cell | Human |
|
| Sahoo, 2024 [39] | India | Glia cells (oligodendrocytes, astrocytes, and ependymal cells) | Goat |
2. Reduced levels of antioxidant enzymes (Catalase, SOD, GSH). 3. Strong immunoreactivity of 8-OHdG in the brain, indicating high oxidative stress. 4. Increased immunosignaling of caspase-3 and caspase-9 in the brain, suggesting an association with the intrinsic pathway of apoptosis. |
| Almanaa, 2024 [38] | Saudi Arabia | NR | Mouse |
|
| Alwetaid, 2024 [40] | Saudi Arabia | NR | Mouse |
|
| Wang, 2023 [41] | USA | Microglial | Mouse |
|
| Baldissera, 2018 [23] | Brazil | Neuron | Fish |
|
| Alwetaid, 2023 [43] | Saudi Arabi | NR | Mouse |
|
| Huang, 2020 [42] | China | Neuroblastoma | Mouse |
|
| Bonsi, 1999 [44] | Italy | Neuroblastoma | Mouse |
|
| Vahidi-Ferdowsi, 2018 [45] | Iran | Astrocyte | Mouse |
|
| Alsayyah, 2019 [46] | Saudi Arabia | Astrocyte | Rat |
|
| Ahmed, 1984 [51] | India | NR | Chicken |
|
| Ricordy, 2002 [47] | Italy | Neuroblastoma (SK-N-SH) | Human |
|
| Zheng, 2018 [48] | China | Neuroblastoma (SK-N-SH) | Human |
|
| Souza, 2019 [49] | Brazil | NR | Silver catfish |
|
| Song, 2024 [50] | China | Neuron | Mouse |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjbar, S.; Mohammadi, P.; Pashaei, S.; Sadeghi, M.; Mehrabi, M.; Shabani, S.; Ebrahimi, A.; Brühl, A.B.; Khodarahmi, R.; Brand, S. Effect of Aflatoxin B1 on the Nervous System: A Systematic Review and Network Analysis Highlighting Alzheimer’s Disease. Biology 2025, 14, 436. https://doi.org/10.3390/biology14040436
Ranjbar S, Mohammadi P, Pashaei S, Sadeghi M, Mehrabi M, Shabani S, Ebrahimi A, Brühl AB, Khodarahmi R, Brand S. Effect of Aflatoxin B1 on the Nervous System: A Systematic Review and Network Analysis Highlighting Alzheimer’s Disease. Biology. 2025; 14(4):436. https://doi.org/10.3390/biology14040436
Chicago/Turabian StyleRanjbar, Samira, Pantea Mohammadi, Somayeh Pashaei, Masoud Sadeghi, Masomeh Mehrabi, Sasan Shabani, Ali Ebrahimi, Annette B. Brühl, Reza Khodarahmi, and Serge Brand. 2025. "Effect of Aflatoxin B1 on the Nervous System: A Systematic Review and Network Analysis Highlighting Alzheimer’s Disease" Biology 14, no. 4: 436. https://doi.org/10.3390/biology14040436
APA StyleRanjbar, S., Mohammadi, P., Pashaei, S., Sadeghi, M., Mehrabi, M., Shabani, S., Ebrahimi, A., Brühl, A. B., Khodarahmi, R., & Brand, S. (2025). Effect of Aflatoxin B1 on the Nervous System: A Systematic Review and Network Analysis Highlighting Alzheimer’s Disease. Biology, 14(4), 436. https://doi.org/10.3390/biology14040436










