Influence of Variation in Hind Leg Structure of Auchenorrhyncha on Their Jumping Performance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples for SEM
2.2. High-Speed Photography
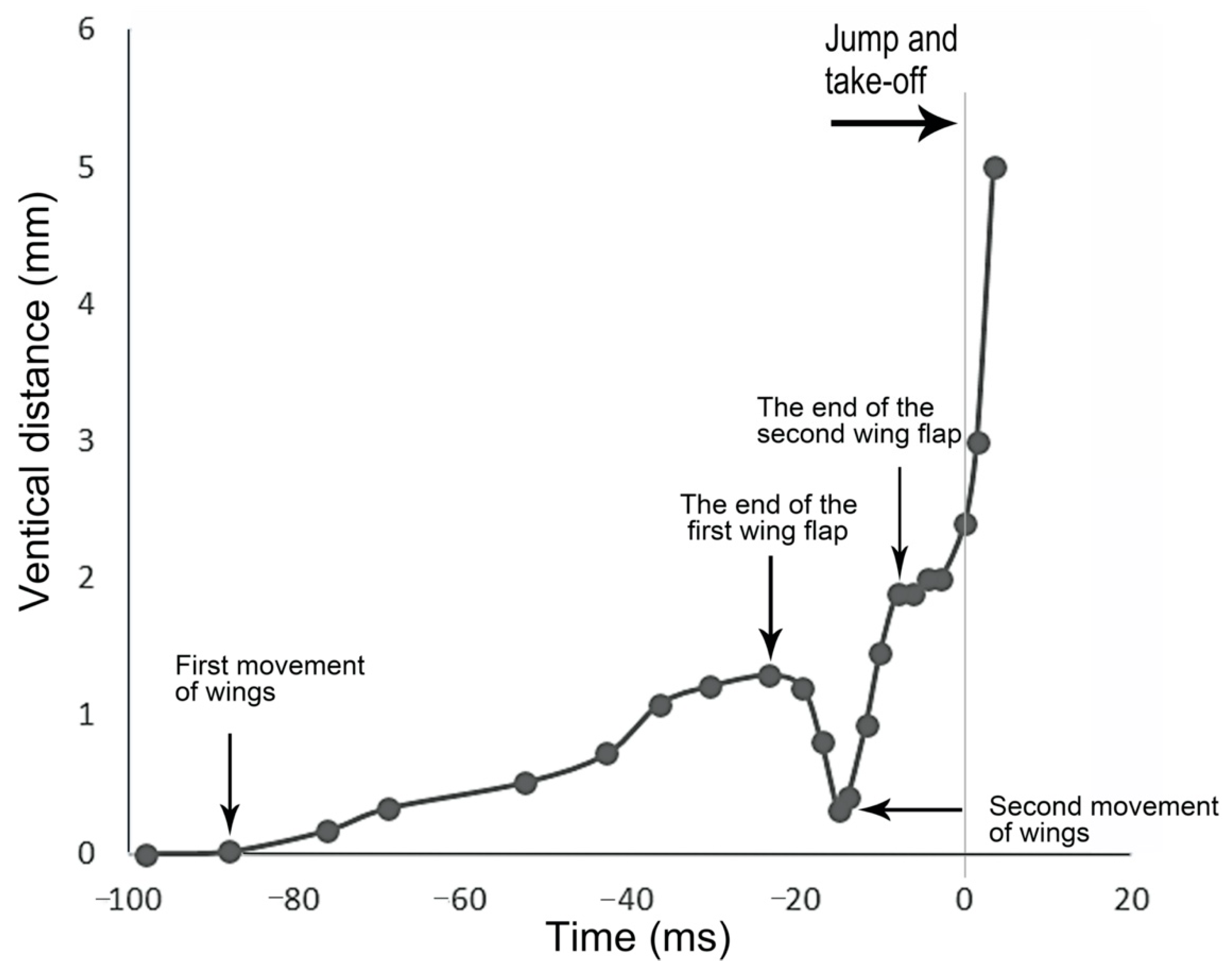
2.3. Image Processing and Measurement
3. Results
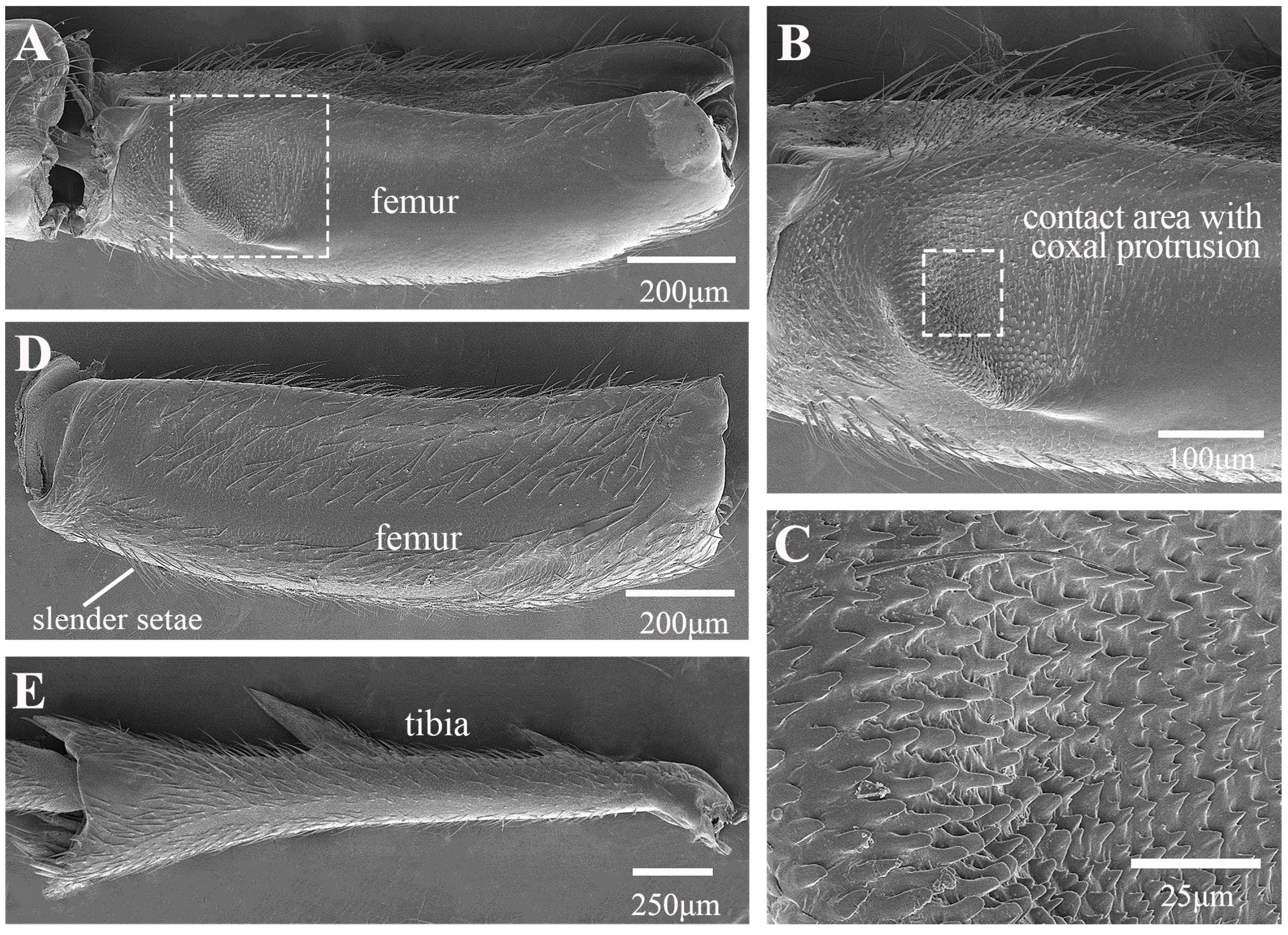
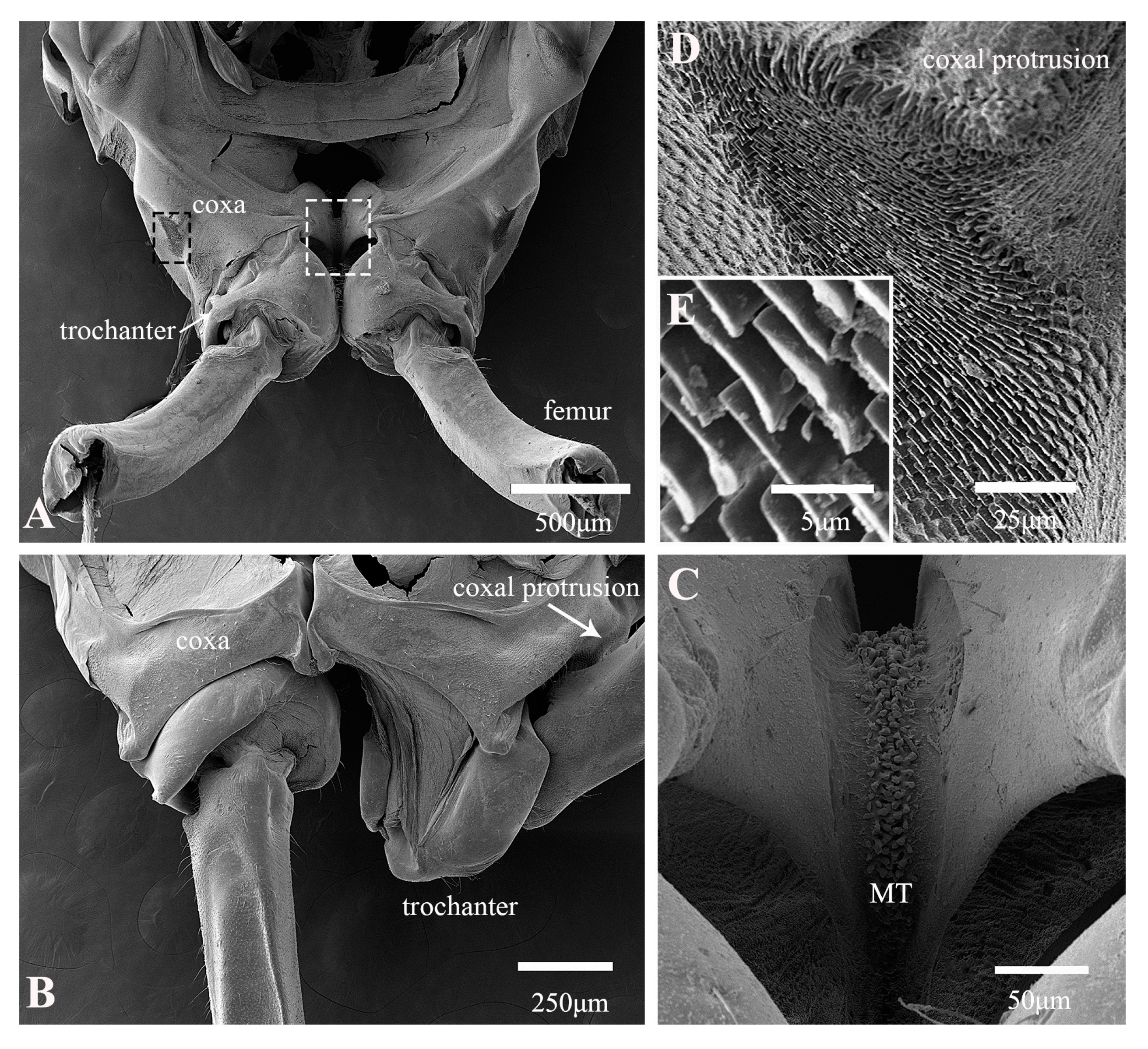
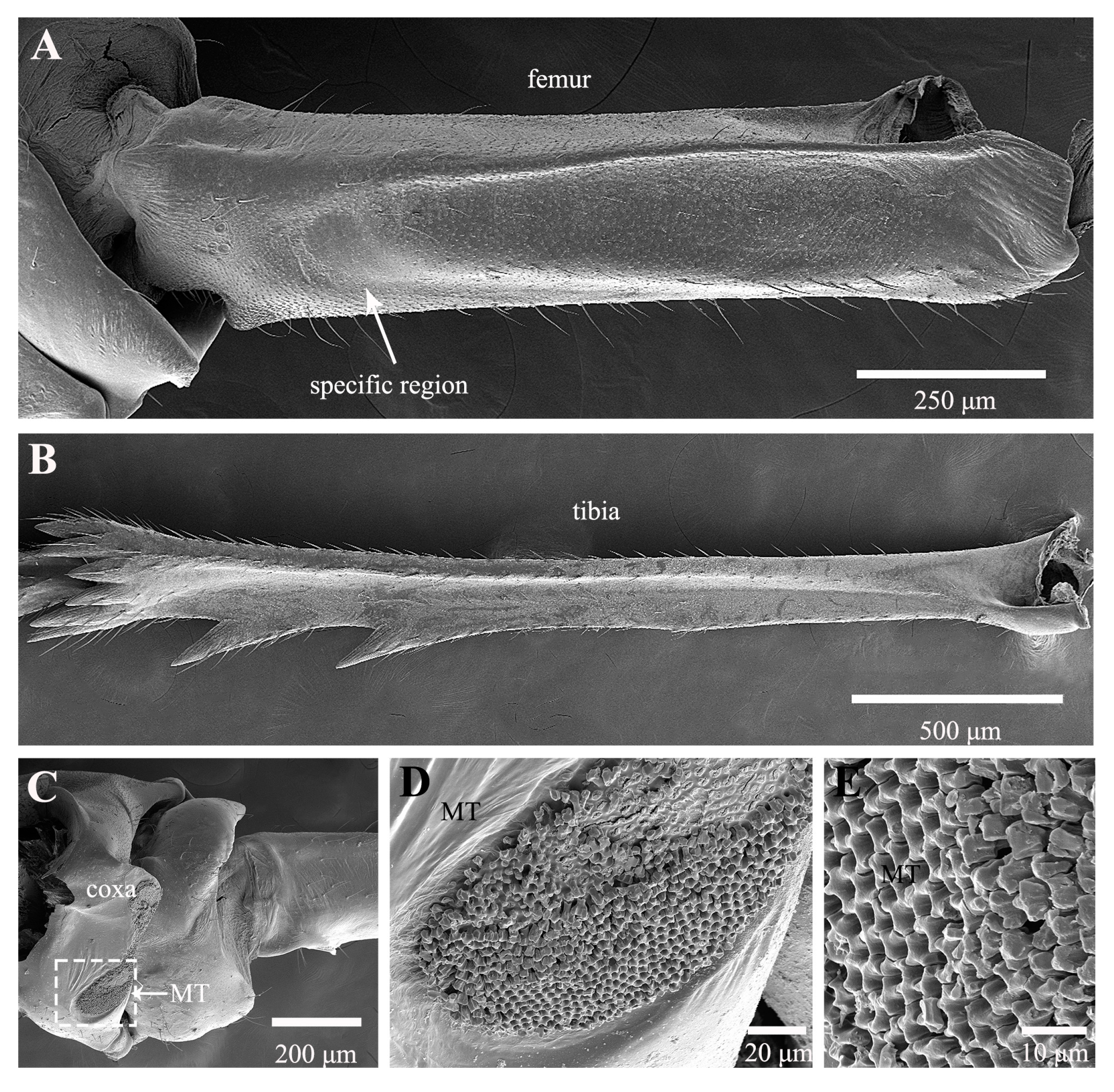
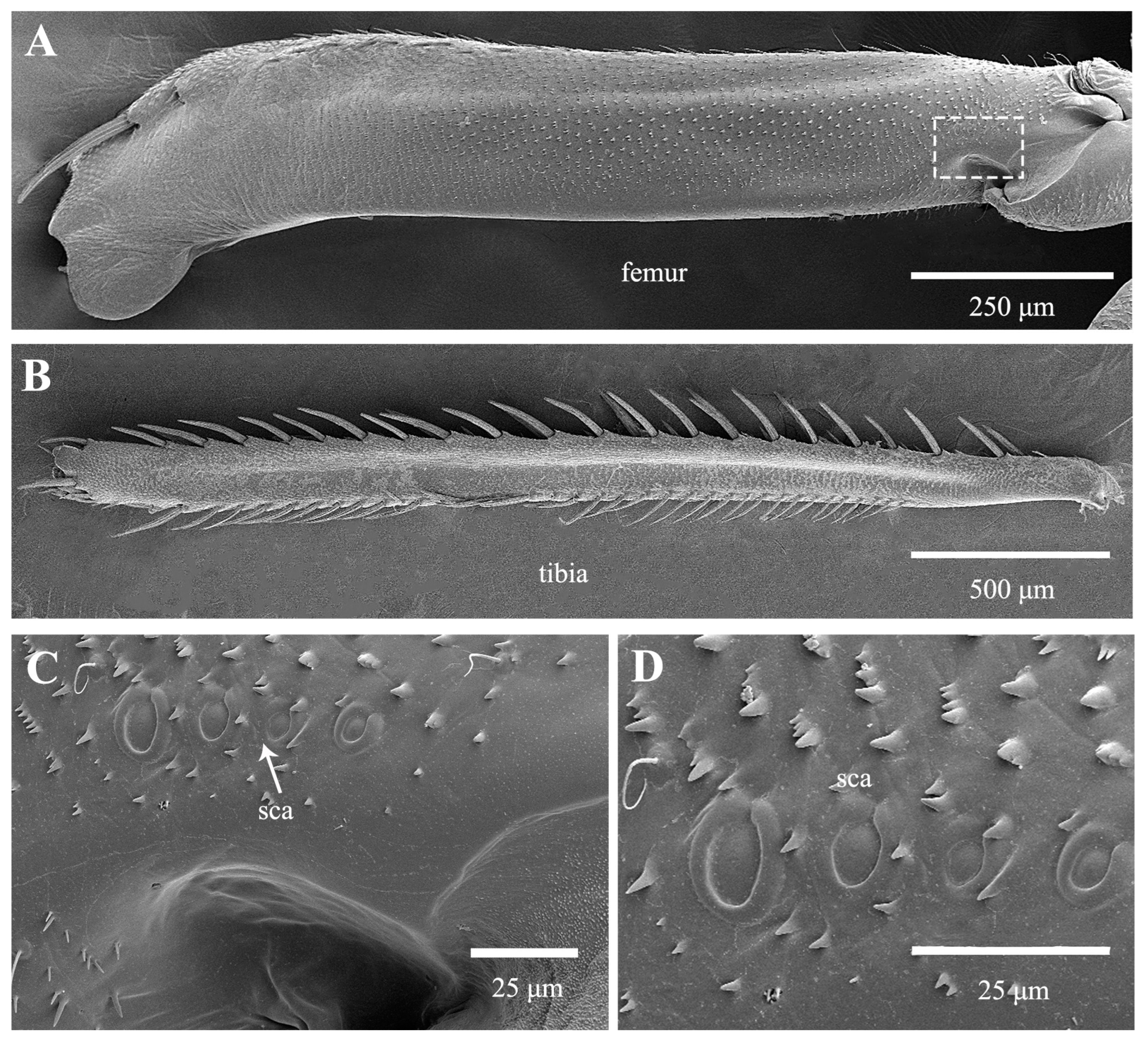
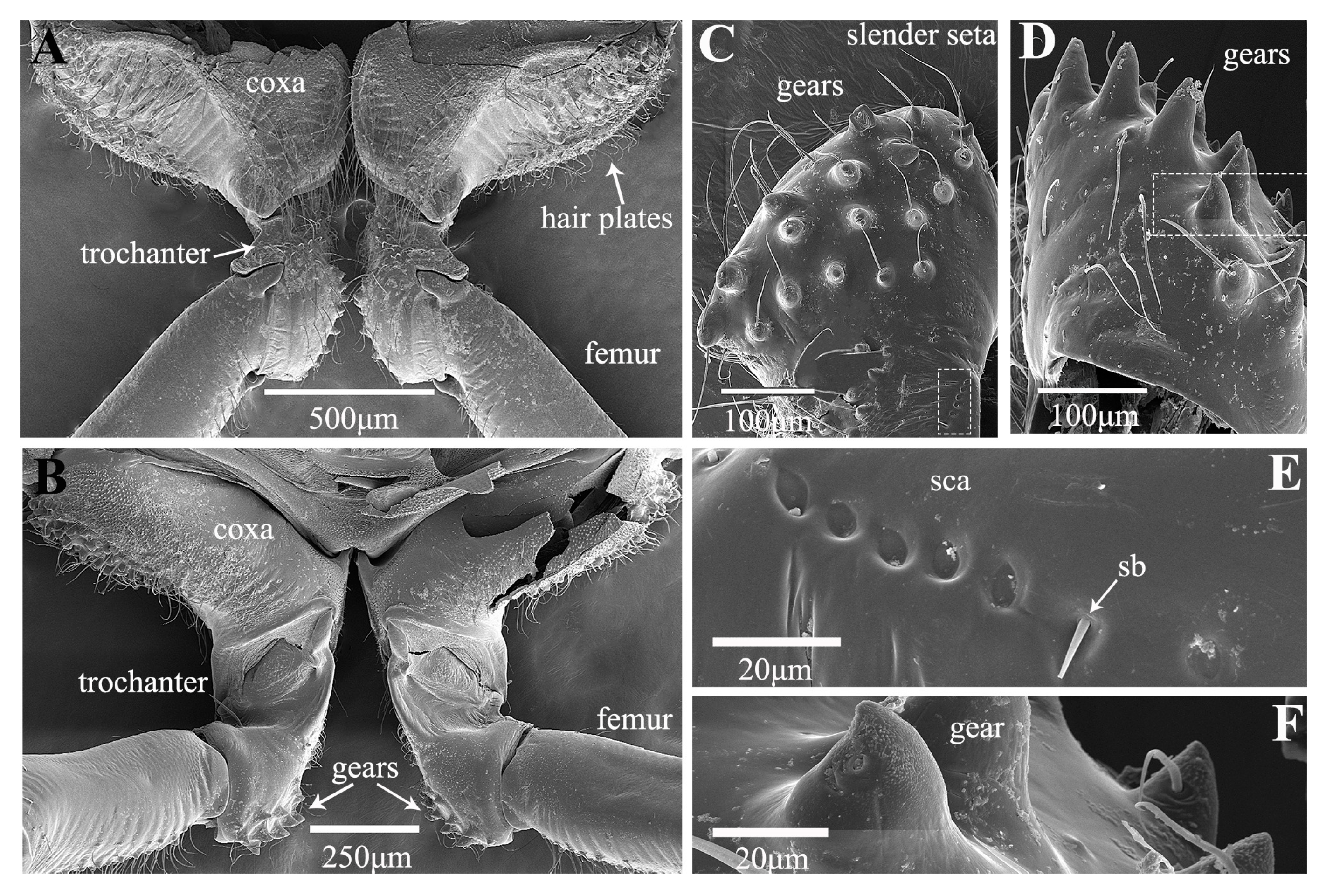
3.1. Morphological Structure
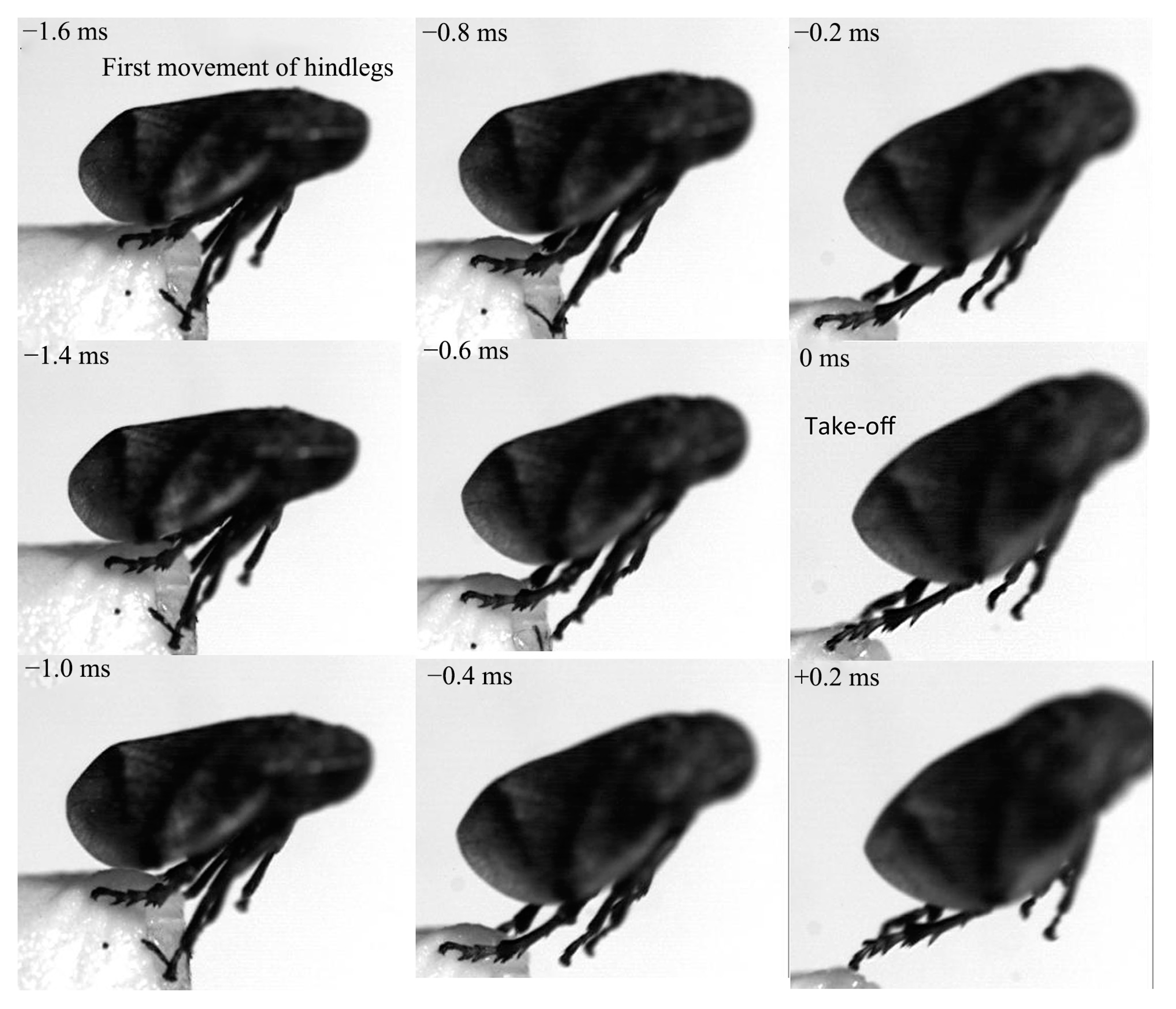
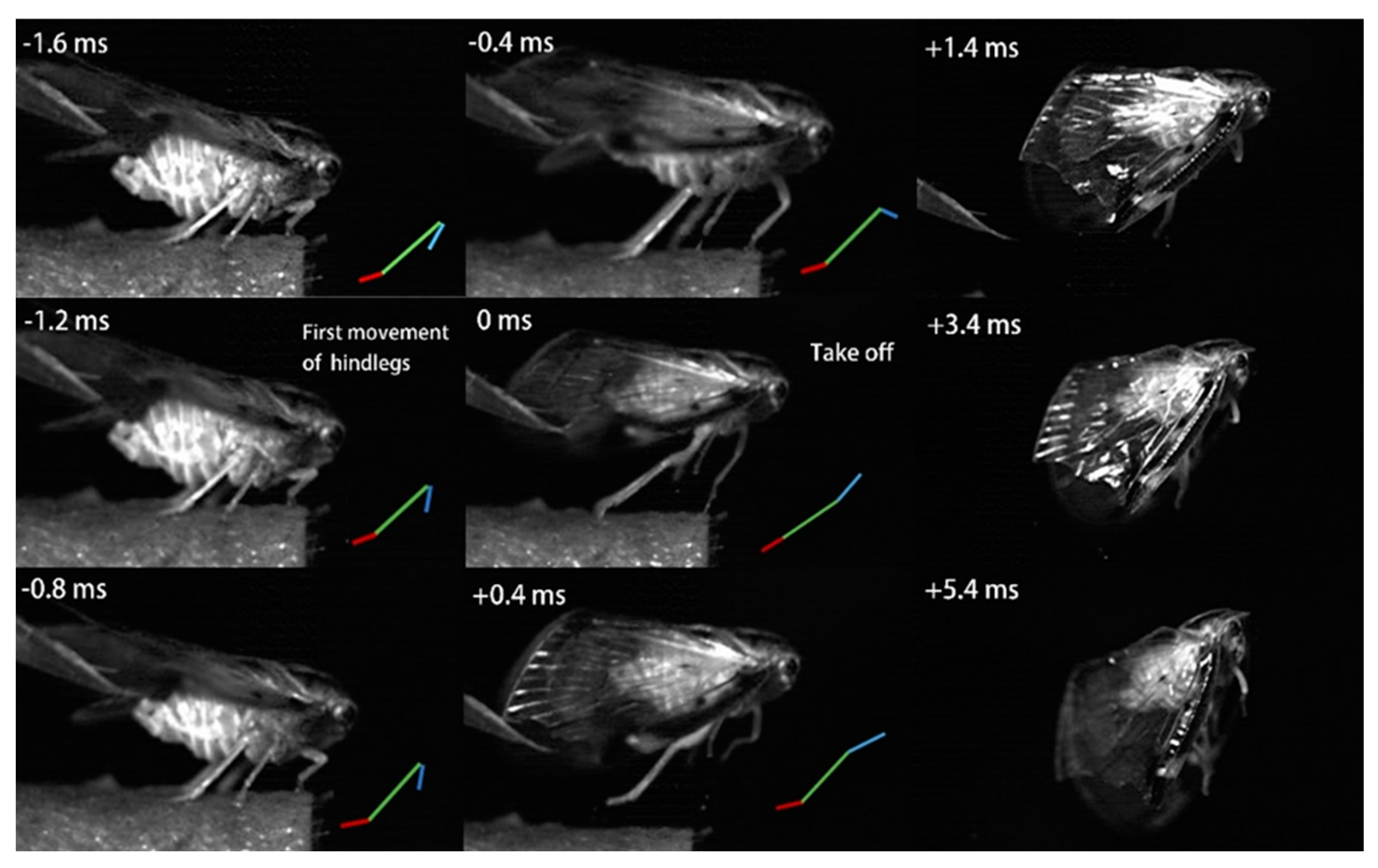
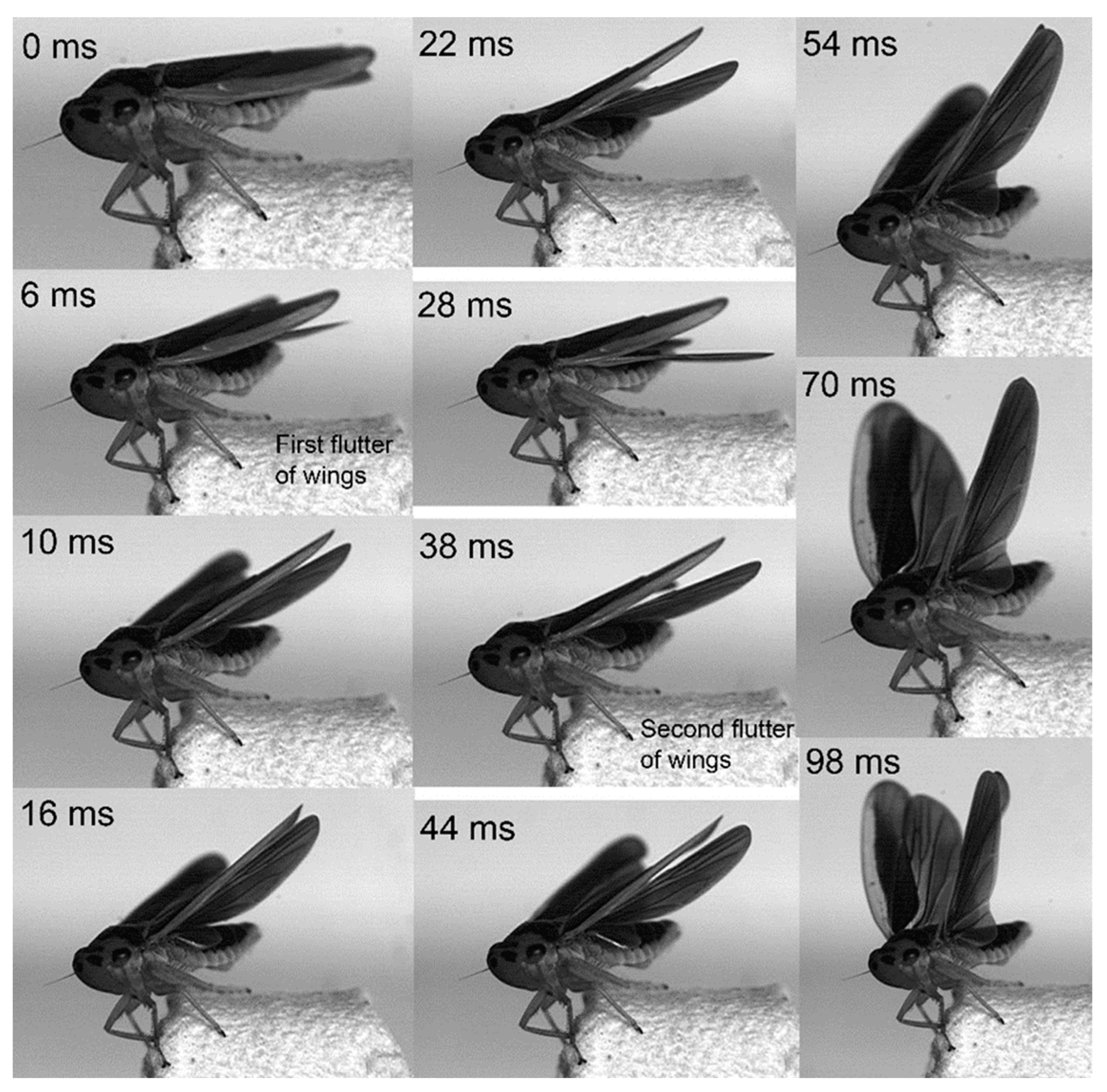
3.2. Kinematics of the Jump
3.3. Motion Physics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutton, G.P.; Burrows, M. The mechanics of elevation control in locust jumping. J. Comp. Physiol. A 2008, 194, 557–563. [Google Scholar] [CrossRef]
- Sutton, G.P.; Burrows, M. The mechanics of azimuth control in jumping by froghopper insects. J. Exp. Biol. 2010, 213, 1406–1416. [Google Scholar] [CrossRef]
- Burrows, M.; Picker, M.D. Jumping mechanisms and performance of pygmy mole crickets (Orthoptera, Tridactylidae). J. Exp. Biol. 2010, 213, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Bennet-Clark, H.C. The energetics of the jump of the locust Schistocerca gregaria. J. Exp. Biol. 1975, 63, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Ghosh, A.; Sutton, G.P.; Yeshwanth, H.M.; Rogers, S.M.; Sane, S.P. Jumping in lantern bugs (Hemiptera, Fulgoridae). J. Exp. Biol. 2021, 224, jeb243361. [Google Scholar] [CrossRef] [PubMed]
- Bennet-Clark, H.C.; Lucey, E.C.A. The jump of the flea: A study of the energetics and a model of the mechanism. J. Exp. Biol. 1967, 47, 59–76. [Google Scholar] [CrossRef]
- Burrows, M.; Morris, O. Jumping and kicking in bush crickets. J. Exp. Biol. 2003, 206, 1035–1049. [Google Scholar] [CrossRef]
- Burrows, M. Froghopper insects leap to new heights. Nature 2003, 424, 509. [Google Scholar] [CrossRef]
- Sutton, G.P.; Mendoza, E.; Azizi, E.; Longo, S.J.; Olberding, J.P.; Ilton, M.; Patek, S.N. Why do large animals never actuate their jumps with latchmediated springs? Because they can jump higher without them. Integr. Comp. Biol. 2019, 59, 1609–1618. [Google Scholar] [CrossRef]
- Nadein, K.; Betz, O. Jumping mechanisms and performance in beetles. I. Flea beetles (Coleoptera: Chrysomelidae: Alticini). J. Exp. Biol. 2016, 219, 2015–2027. [Google Scholar] [CrossRef]
- Ilton, M.; Bhamla, M.S.; Ma, X.; Cox, S.M.; Fitchett, L.L.; Kim, Y.; Koh, J.S.; Krishnamurthy, D.; Kuo, C.Y.; Temel, F.Z.; et al. The principles of cascading power limits in small, fast biological and engineered systems. Science 2018, 360, eaao1082. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.J.; Cox, S.M.; Azizi, E.; Ilton, M.; Olberding, J.P.; St Pierre, R.; Patek, S.N. Beyond power amplification: Latch-mediated spring actuation is an emerging framework for the study of diverse elastic systems. J. Exp. Biol. 2019, 222, jeb197889. [Google Scholar] [CrossRef] [PubMed]
- Askew, G.N.; Marsh, R.L. Muscle designed for maximum short-term power output: Quail flight muscle. J. Exp. Biol. 2002, 205, 2153–2160. [Google Scholar] [CrossRef]
- Josephson, R.K. Contraction dynamics and power output of skeletal muscle. Annu. Rev. Physiol. 1993, 55, 527–546. [Google Scholar] [CrossRef]
- Burrows, M. Jumping performance of planthoppers (Hemiptera, Issidae). J. Exp. Biol. 2009, 212, 2844–2855. [Google Scholar] [CrossRef]
- Burrows, M. Jumping strategies and performance in shore bugs (Hemiptera, Heteroptera, Saldidae). J. Exp. Biol. 2009, 212, 106–115. [Google Scholar] [CrossRef][Green Version]
- Burrows, M. Neural control and co-ordination of jumping in froghopper insects. J. Neurophysiol. 2007, 97, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Jumping mechanisms in flatid planthoppers (Hemiptera, Flatidae). J. Exp. Biol. 2014, 217, 2590–2600. [Google Scholar] [CrossRef]
- Burrows, M.; Bräunig, P. Actions of motor neurons and leg muscles in jumping by planthopper insects (Hemiptera, Issidae). J. Comp. Neurol. 2010, 518, 1349–1369. [Google Scholar] [CrossRef]
- Siwanowicz, I.; Burrows, M. Three dimensional reconstruction of energy stores for jumping in planthoppers and froghoppers from confocal laser scanning microscopy. eLife 2017, 6, e23824. [Google Scholar] [CrossRef]
- Burrows, M. Anatomy of the hind legs and actions of their muscles during jumping in leafhopper insects. J. Exp. Biol. 2007, 210, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- van de Kamp, T.; Vagovič, P.; Baumbach, T.; Riedel, A. A biological screw in a beetle’s leg. Science 2011, 333, 52. [Google Scholar] [CrossRef]
- Burrows, M.; Sutton, G. Interacting gears synchronize propulsive leg movements in a jumping insect. Science 2013, 341, 1254–1256. [Google Scholar] [CrossRef]
- Jung, G.P.; Cho, K.J. Froghopper-inspired direction-changing concept for miniature jumping robots. Bioinspir. Biomim. 2016, 11, 056015. [Google Scholar] [CrossRef]
- Burrows, M.; Shaw, S.R.; Sutton, G.P. Resilin and chitinous cuticle form a composite structure for energy storage in jumping by froghopper insects. BMC Biol. 2008, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Jumping mechanisms in dictyopharid planthoppers (Hemiptera, Dicytyopharidae). J. Exp. Biol. 2014, 217, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Jumping mechanisms of treehopper insects (Hemiptera, Auchenorrhyncha, Membracidae). J. Exp. Biol. 2013, 216, 788–799. [Google Scholar] [CrossRef]
- Burrows, M. Morphology and action of the hind leg joints controlling jumping in froghopper insects. J. Exp. Biol. 2006, 209, 4622–4637. [Google Scholar] [CrossRef]
- Gorb, S.N. The jumping mechanism of cicada Cercopis vulnerata (Auchenorrhyncha, Cercopidae): Skeleton–muscle organisation, frictional surfaces, and inverse-kinematic model of leg movements. Arthropod Struct. Dev. 2004, 33, 201–220. [Google Scholar] [CrossRef]
- Gorb, S.N. Attachment Devices of Insect Cuticle; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 1–305. [Google Scholar] [CrossRef]
- Burrows, M. Kinematics of jumping in leafhopper insects (Hemiptera, Auchenorrhyncha, Cicadellidae). J. Exp. Biol. 2007, 210, 3579–3589. [Google Scholar] [CrossRef]
- Alexander, R.M. Animal Mechanics; Blackwell Science: Oxford, UK, 1983; pp. 1–312. [Google Scholar]
- Bennet-Clark, H.C.; Alder, G.M. The effect of air resistance on the jumping performance of insects. J. Exp. Biol. 1979, 82, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S. Living in a physical world II. The bio-ballistics of small projectiles. J. Biosci. 2005, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]






| Body Mass (mg) | Body Length (mm) | Femur (mm) | Tibia (mm) | Hind Leg Length (% Body Length) | |
|---|---|---|---|---|---|
| Lepyronia coleoptrata (N = 17) | 18.6 ± 3.4 | 7.6 ± 0.3 | 1.3 ± 0.1 | 3.1 ± 0.1 | 84 |
| Euricania ocellus (N = 13) | 11.8 ± 0.3 | 6.1 ± 0.05 | 1.5 ± 0.1 | 2.2 ± 0.1 | 86 |
| Kolla sp. (N = 14) | 3.9 ± 0.4 | 6.7 ± 0.1 | 1.4 ± 0.05 | 2.4 ± 0.1 | 88 |
| Tricentrus sp. (N = 9) | 8.2 | 5.2 | 0.7 | 2.4 | 85 |
| Body Mass (mg) | Time to Take-Off (ms) | Take-Off Velocity | Take-Off Angle (deg) | Acceleration ) | g-Force (g) | Energy (μJ) | Power (mW) | Force (mN) | |
|---|---|---|---|---|---|---|---|---|---|
| Formula/symbol | m | v | f = v/t | g = f/9.81 | e = 0.5 m | P = e/t | F = mf | ||
| Lepyronia coleoptrata | 2 | ||||||||
| mean (N = 17, n = 3) | 18.6 ± 3.4 | 1.7 ± 0.1 | 37 ± 4 | 1118 | 114 | 33 | 19 | 21 | |
| best | 19.1 | 1.6 | 1250 | 127 | 38 | 23 | 24 | ||
| Euricania ocellus | 2.8 | ||||||||
| mean (N = 13, n = 7) | 11.8 ± 0.3 | 1.3 ± 0.1 | 48 ± 2 | 2077 | 212 | 43 | 33 | 25 | |
| best | 11.6 | 1.2 | 2333 | 238 | 45 | 38 | 27 | ||
| Kolla sp. | 3.9 | ||||||||
| mean (N = 14, n = 2) | 3.9 ± 0.4 | 4.2 ± 0.2 | 30 ± 6 | 857 | 87 | 25 | 6 | 3 | |
| best | 4.1 | 4 | 975 | 99. | 38 | 10 | 4 | ||
| Tricentrus sp. | |||||||||
| mean (N = 9, n = 1) | |||||||||
| best | 8.2 | 1.7 | 2.4 | 29 | 1412 | 144 | 24 | 14 | 12 |
| Species | Take-Off Velocity | Take-Off Angle (deg) | Body Length (mm) | Horizontal Distance (mm) | Horizontal Distance (Body Lengths) | Vertical Height (mm) | Vertical Height (Body Lengths) |
|---|---|---|---|---|---|---|---|
| Lepyronia coleoptrata | 2 | 37 | 7.6 | 392 | 52 | 74 | 10 |
| Euricania ocellus | 2.8 | 48 | 6.1 | 795 | 130 | 221 | 36 |
| Kolla sp. | 3.9 | 30 | 6.7 | 1343 | 200 | 737 | 110 |
| Tricentrus sp. | 2.4 | 29 | 5.2 | 498 | 96 | 93 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Dietrich, C.H.; Dai, W. Influence of Variation in Hind Leg Structure of Auchenorrhyncha on Their Jumping Performance. Biology 2025, 14, 418. https://doi.org/10.3390/biology14040418
Xu Y, Dietrich CH, Dai W. Influence of Variation in Hind Leg Structure of Auchenorrhyncha on Their Jumping Performance. Biology. 2025; 14(4):418. https://doi.org/10.3390/biology14040418
Chicago/Turabian StyleXu, Yifei, Christopher H. Dietrich, and Wu Dai. 2025. "Influence of Variation in Hind Leg Structure of Auchenorrhyncha on Their Jumping Performance" Biology 14, no. 4: 418. https://doi.org/10.3390/biology14040418
APA StyleXu, Y., Dietrich, C. H., & Dai, W. (2025). Influence of Variation in Hind Leg Structure of Auchenorrhyncha on Their Jumping Performance. Biology, 14(4), 418. https://doi.org/10.3390/biology14040418






