Effects of Acidic Polysaccharide-Enriched Extracts from Holothuria tubulosa on Two- and Three-Dimensional Invasive Breast Cancer Cell Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Acidic Polysaccharides Enrichment
2.2.1. Hexuronic Acid Quantification
2.2.2. Carbohydrate Polyacrylamide Gel Electrophoresis (C-PAGE)
2.3. Cell Cultures
2.3.1. Cell Viability Assay
2.3.2. Wound Closure Assay
2.3.3. Collagen Type I Cell Adhesion Assay
2.3.4. Immunofluorescence Staining and Microscopy
2.4. RNA Isolation, cDNA Synthesis and Real-Time PCR
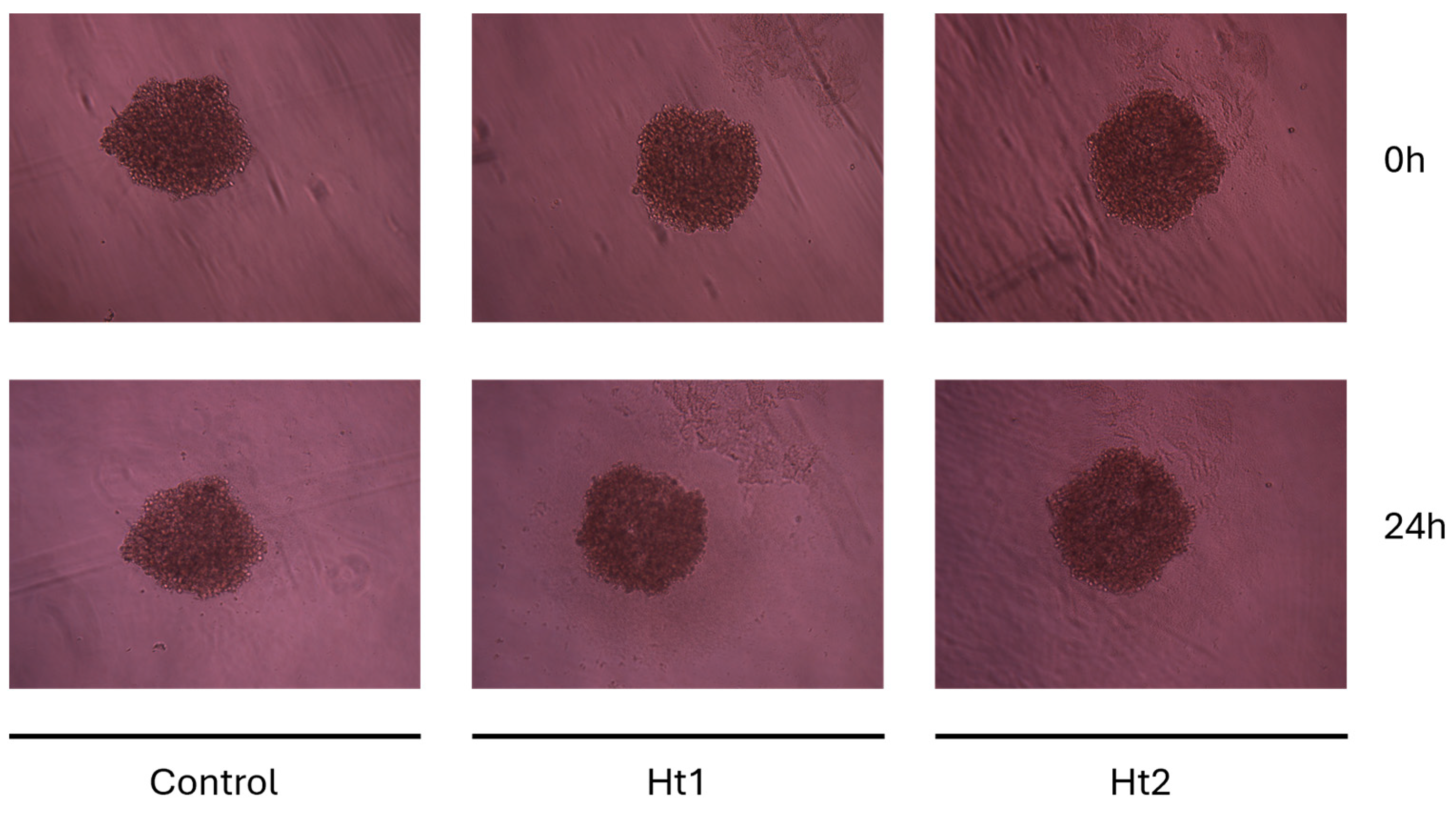
2.5. MDA-MB-231 Spheroid Formation and Treatment
2.6. Statistical Analysis
3. Results
3.1. Electrophoretic Profiles of Holothuria tubulosa-Derived APs
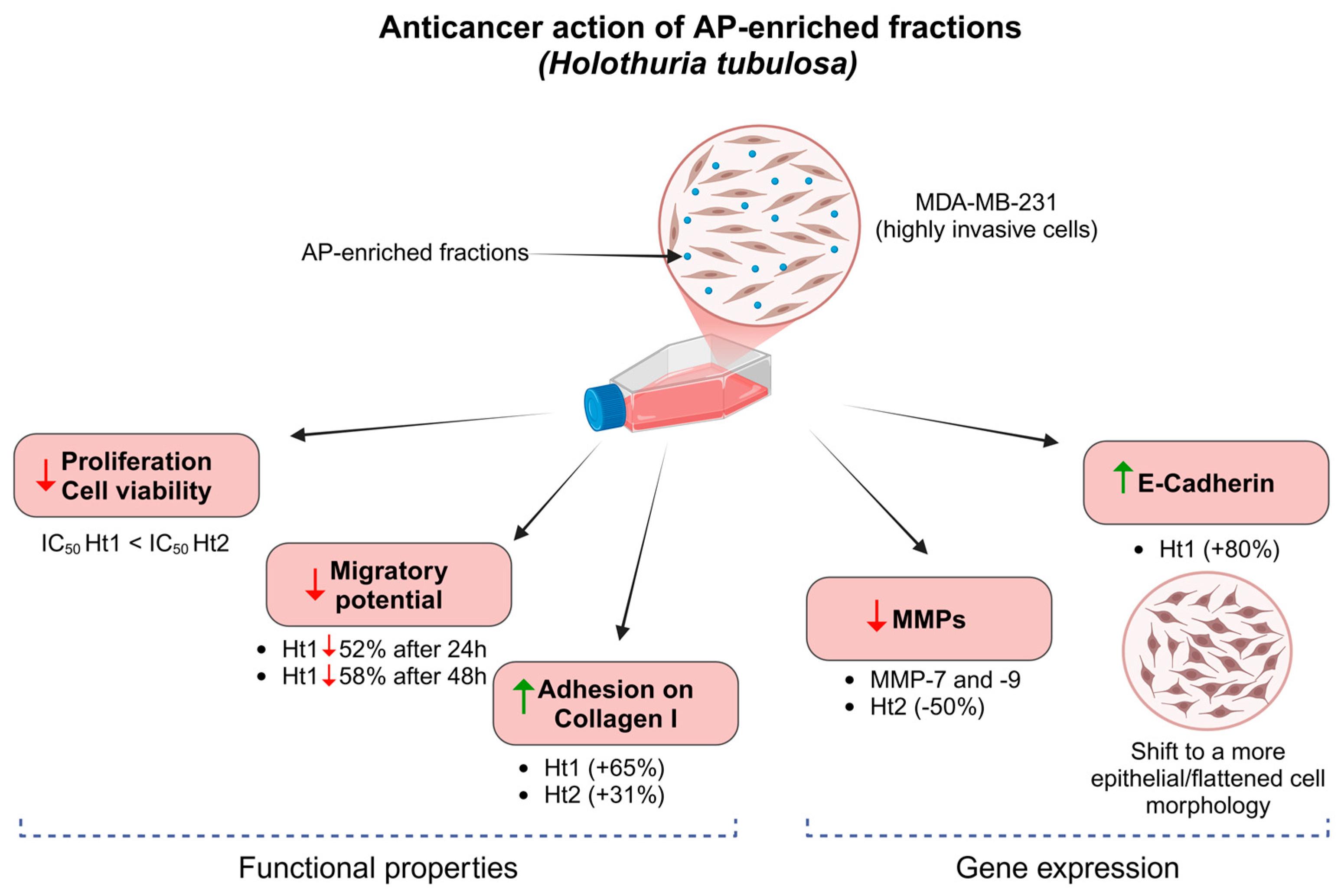
3.2. Biological Activities of AP Enriched Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | acidic polysaccharide |
| Ht | Holothuria tubulosa |
| Ht1 | polysaccharide-enriched fraction 1 eluted with 20 mM Tris–HCl buffer, pH 8.6, containing 0.5 M lithium chloride |
| Ht2 | polysaccharide-enriched fraction 2 eluted with 20 mM Tris–HCl buffer, pH 8.6, containing 1 M lithium chloride |
| EMT | epithelial–mesenchymal transition |
| ER | estrogen receptor |
| HER2 | human epidermal growth factor receptor 2 |
| PR | progesterone receptor |
| TNBC | triple-negative breast cancer |
| MDA-MB-231 | triple-negative breast cancer cell line |
| ECM | extracellular matrix |
| MMP | matrix metalloproteinases |
| FCS | fucosylated chondroitin sulfate |
| DDT | dehydrated and delipidated tissue |
| DEAE | diethylaminoethyl |
| UA | uronic acid |
| C-PAGE | carbohydrate polyacrylamide gel electrophoresis |
| WST-1 | water-soluble tetrazolium salt |
References
- Pangestuti, R.; Arifin, Z. Medicinal and Health Benefit Effects of Functional Sea Cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y. Pharmacological Potential of Sea Cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Wargasetia, T.L. Widodo Mechanisms of Cancer Cell Killing by Sea Cucumber-Derived Compounds. Investig. New Drugs 2017, 35, 820–826. [Google Scholar] [CrossRef]
- Felix, A.L.; Penno, S.M.; Bezerra, F.F.; Mourão, P.A.S. Fucosylated Chondroitin Sulfate, an Intriguing Polysaccharide from Sea Cucumber: Past, Present, and Future. Glycobiology 2025, 35, cwae098. [Google Scholar] [CrossRef]
- Nieddu, G.; Obino, G.; Ciampelli, C.; Brunetti, A.; Cubeddu, T.; Manconi, R.; Stocchino, G.A.; Deiana, G.A.; Formato, M.; Lepedda, A.J. Purification of an Acidic Polysaccharide with Anticoagulant Activity from the Marine Sponge Sarcotragus Spinosulus. Mar. Drugs 2024, 22, 139. [Google Scholar] [CrossRef]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast Cancer: Presentation, Investigation and Management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.U. Breast Cancer: A Review of Risk Factors and Diagnosis. Medicine 2024, 103, e36905. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, R.; Mazumder, A. Breast Cancer and Its Therapeutic Targets: A Comprehensive Review. Chem. Biol. Drug Des. 2024, 103, e14384. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Isert, L.; Mehta, A.; Loiudice, G.; Oliva, A.; Roidl, A.; Merkel, O.M. An In Vitro Approach to Model EMT in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7757. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Costa, R.M.; Helal-Neto, E.; Vieira, A.M.; Barcellos-de-Souza, P.; Morgado-Diaz, J.; Barja-Fidalgo, C. Extracellular Matrix Derived from High Metastatic Human Breast Cancer Triggers Epithelial-Mesenchymal Transition in Epithelial Breast Cancer Cells through Avβ3 Integrin. Int. J. Mol. Sci. 2020, 21, 2995. [Google Scholar] [CrossRef]
- Insua-Rodríguez, J.; Oskarsson, T. The Extracellular Matrix in Breast Cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Elfenbein, A.; Simons, M. Auxiliary and Autonomous Proteoglycan Signaling Networks. Methods Enzymol. 2010, 480, 3–31. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Kollet, O.; Das, A.; Karamanos, N.; Auf dem Keller, U.; Sagi, I. Redefining Metalloproteases Specificity through Network Proteolysis. Trends Mol. Med. 2024, 30, 147–163. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- BITTER, T.; MUIR, H.M. A Modified Uronic Acid Carbazole Reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Idini, M.; Wieringa, P.; Rocchiccioli, S.; Nieddu, G.; Ucciferri, N.; Formato, M.; Lepedda, A.; Moroni, L. Glycosaminoglycan Functionalization of Electrospun Scaffolds Enhances Schwann Cell Activity. Acta Biomater. 2019, 96, 188–202. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Alves, C.; Diederich, M. Marine Natural Products as Anticancer Agents. Mar. Drugs 2021, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Savci-Heijink, C.D.; Halfwerk, H.; Hooijer, G.K.J.; Koster, J.; Horlings, H.M.; Meijer, S.L.; van de Vijver, M.J. Epithelial-to-Mesenchymal Transition Status of Primary Breast Carcinomas and Its Correlation with Metastatic Behavior. Breast Cancer Res. Treat. 2019, 174, 649–659. [Google Scholar] [CrossRef]
- van Roy, F.; Berx, G. The Cell-Cell Adhesion Molecule E-Cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; Staes, K.; van Hengel, J.; Molemans, F.; Bussemakers, M.J.; van Bokhoven, A.; van Roy, F. Cloning and Characterization of the Human Invasion Suppressor Gene E-Cadherin (CDH1). Genomics 1995, 26, 281–289. [Google Scholar] [CrossRef]
- Mendonsa, A.M.; Na, T.-Y.; Gumbiner, B.M. E-Cadherin in Contact Inhibition and Cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix Metalloproteinases Participation in the Metastatic Process and Their Diagnostic and Therapeutic Applications in Cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Noë, V.; Fingleton, B.; Jacobs, K.; Crawford, H.C.; Vermeulen, S.; Steelant, W.; Bruyneel, E.; Matrisian, L.M.; Mareel, M. Release of an Invasion Promoter E-Cadherin Fragment by Matrilysin and Stromelysin-1. J. Cell Sci. 2001, 114, 111–118. [Google Scholar] [CrossRef]
- Joseph, C.; Alsaleem, M.; Orah, N.; Narasimha, P.L.; Miligy, I.M.; Kurozumi, S.; Ellis, I.O.; Mongan, N.P.; Green, A.R.; Rakha, E.A. Elevated MMP9 Expression in Breast Cancer Is a Predictor of Shorter Patient Survival. Breast Cancer Res. Treat. 2020, 182, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; Rodriguez de la Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Kefali, E.; Piperigkou, Z.; Riethmüller, C.; Greve, B.; Franchi, M.; Götte, M.; Karamanos, N.K. EGFR Is a Pivotal Player of the E2/ERβ—Mediated Functional Properties, Aggressiveness, and Stemness in Triple-Negative Breast Cancer Cells. FEBS J. 2022, 289, 1552–1574. [Google Scholar] [CrossRef] [PubMed]






| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACTβ | 5′-TCAAGATCATTGCTCCTCCTGAG-3′ | 5′-ACATCTGCTGGAAGGTGGACA-3′ |
| CDH1 (E-cadherin) | 5′-TACGCCTGGGACTCCACCTA-3′ | 5′-CCAGAAACGGAGGCCTGAT-3′ |
| MMP7 | 5′-GCTGGCTCATGCCTTTGC-3′ | 5′-TCCTCATCGAAGTGAGCATCTC-3′ |
| MMP9 | 5′-TTCCAGTACCGAGAGAAAGCCTAT-3′ | 5′-GGTCACGTAGCCCACTTGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciampelli, C.; Mangani, S.; Nieddu, G.; Formato, M.; Ioannou, P.; Kremmydas, S.; Karamanos, N.; Lepedda, A.J. Effects of Acidic Polysaccharide-Enriched Extracts from Holothuria tubulosa on Two- and Three-Dimensional Invasive Breast Cancer Cell Models. Biology 2025, 14, 334. https://doi.org/10.3390/biology14040334
Ciampelli C, Mangani S, Nieddu G, Formato M, Ioannou P, Kremmydas S, Karamanos N, Lepedda AJ. Effects of Acidic Polysaccharide-Enriched Extracts from Holothuria tubulosa on Two- and Three-Dimensional Invasive Breast Cancer Cell Models. Biology. 2025; 14(4):334. https://doi.org/10.3390/biology14040334
Chicago/Turabian StyleCiampelli, Cristina, Sylvia Mangani, Gabriele Nieddu, Marilena Formato, Paraskevi Ioannou, Spyros Kremmydas, Nikos Karamanos, and Antonio Junior Lepedda. 2025. "Effects of Acidic Polysaccharide-Enriched Extracts from Holothuria tubulosa on Two- and Three-Dimensional Invasive Breast Cancer Cell Models" Biology 14, no. 4: 334. https://doi.org/10.3390/biology14040334
APA StyleCiampelli, C., Mangani, S., Nieddu, G., Formato, M., Ioannou, P., Kremmydas, S., Karamanos, N., & Lepedda, A. J. (2025). Effects of Acidic Polysaccharide-Enriched Extracts from Holothuria tubulosa on Two- and Three-Dimensional Invasive Breast Cancer Cell Models. Biology, 14(4), 334. https://doi.org/10.3390/biology14040334














