Human Milk Microbiome from Polish Women Giving Birth via Vaginal Delivery—Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Materials
2.3. Methods
2.3.1. Assessment of the Presence of Bacteria (Culture-Based Method)
2.3.2. Species Identification
2.3.3. Statistical Analysis
3. Results
4. Discussion
4.1. Core Microbiota of Human Milk
4.2. Potential Pathogens and Contamination Risks
4.3. Geographical and Maternal Influences
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, P.; Al Mohannadi, N.; Murugesan, S.; Almarzooqi, F.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T.; Terranegra, A.; McGready, R.; et al. Unveiling the dynamics of the breast milk microbiome: Impact of lactation stage and gestational age. J. Transl. Med. 2023, 21, 784. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Yang, N.; Muhlhausler, B.S.; Leghi, G.E.; Netting, M.J.; Elmes, M.J.; Langley-Evans, S.C. Acute changes to breast milk composition following consumption of high-fat and high-sugar meals. Matern. Child Nutr. 2021, 17, e13168, Erratum in: Matern. Child Nutr. 2022, 18, e13292. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Langa, S.; Reviriego, C.; Jimínez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’callaghan, T.F.; O’shea, C.-A.; Dempsey, E.M.; O’toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [CrossRef]
- Hunt, K.M.; Preuss, J.; Nissan, C.; Davlin, C.A.; Williams, J.E.; Shafii, B.; Richardson, A.D.; McGuire, M.K.; Bode, L.; McGuire, M.A. Human Milk Oligosaccharides Promote the Growth of Staphylococci. Appl. Environ. Microbiol. 2012, 78, 4763–4770. [Google Scholar] [CrossRef]
- Beghetti, I.; Biagi, E.; Martini, S.; Brigidi, P.; Corvaglia, L.; Aceti, A. Human Milk’s Hidden Gift: Implications of the Milk Microbiome for Preterm Infants’ Health. Nutrients 2019, 11, 2944. [Google Scholar] [CrossRef]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leyva, L.L.; Brereton, N.J.; Koski, K.G. Emerging frontiers in human milk microbiome research and suggested primers for 16S rRNA gene analysis. Comput. Struct. Biotechnol. J. 2020, 19, 121–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moubareck, C.A. Human Milk Microbiota and Oligosaccharides: A Glimpse into Benefits, Diversity, and Correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Pannaraj, P.S.; Rautava, S.; Rodríguez, J.M. The Microbiota of the Human Mammary Ecosystem. Front. Cell. Infect. Microbiol. 2020, 10, 586667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stinson, L.F.; Sindi, A.S.M.; Cheema, A.S.; Lai, C.T.; Mühlhäusler, B.S.; E Wlodek, M.; Payne, M.S.; Geddes, D.T. The human milk microbiome: Who, what, when, where, why, and how? Nutr. Rev. 2021, 79, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Aceti, A.; Quercia, S.; Beghetti, I.; Rampelli, S.; Turroni, S.; Soverini, M.; Zambrini, A.V.; Faldella, G.; Candela, M.; et al. Microbial Community Dynamics in Mother’s Milk and Infant’s Mouth and Gut in Moderately Preterm Infants. Front. Microbiol. 2018, 9, 2512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiménez, E.; Delgado, S.; Maldonado, A.; Arroyo, R.; Albújar, M.; García, N.; Jariod, M.; Fernández, L.; Gómez, A.; Rodríguez, J.M. Staphylococcus epidermidis: A differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol. 2008, 8, 143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boix-Amorós, A.; Martinez-Costa, C.; Querol, A.; Collado, M.C.; Mira, A. Multiple Approaches Detect the Presence of Fungi in Human Breastmilk Samples from Healthy Mothers. Sci. Rep. 2017, 7, 13016, Erratum in: Sci. Rep. 2018, 8, 16829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boix-Amorós, A.; Puente-Sánchez, F.; du Toit, E.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Salminen, S.; Isolauri, E.; Tamames, J.; Mira, A.; et al. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019, 85, e02994-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moossavi, S.; Atakora, F.; Miliku, K.; Sepehri, S.; Robertson, B.; Duan, Q.L.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Integrated Analysis of Human Milk Microbiota with Oligosaccharides and Fatty Acids in the CHILD Cohort. Front. Nutr. 2019, 6, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J. Hum. Lact. 2015, 31, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Royo, M.; Lerma, J.C.; Cortés-Macías, E.; Collado, M.C. Human milk microbiome: From actual knowledge to future perspective. Semin. Perinatol. 2021, 45, 151450. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, T.L.; Hosid, S.; Ioshikhes, I.; Altosaar, I. Human milk metagenome: A functional capacity analysis. BMC Microbiol. 2013, 25, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Douglas, C.A.; Ivey, K.L.; Papanicolas, L.E.; Best, K.P.; Muhlhausler, B.S.; Rogers, G.B. DNA extraction approaches substantially influence the assessment of the human breast milk microbiome. Sci. Rep. 2020, 10, 123. [Google Scholar] [CrossRef]
- Lee, S.; Heo, S.; Park, M.-K.; Sung, M.-H.; Jeong, D.-W. Bacterial Community of Breast Milk in Breastfeeding Women Using Culture-Dependent and Culture-Independent Approaches. J. Microbiol. Biotechnol. 2024, 34, 2005–2011. [Google Scholar] [CrossRef]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr. Clin. Pract. 2017, 32, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.R.; Avershina, E.; Storrø, O.; Johnsen, R.; Rudi, K.; Øien, T. Breastfeeding-associated microbiota in human milk following supplementation with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12. J. Dairy Sci. 2018, 101, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Brereton, N.J.B.; Li, C.; Leyva, L.L.; Solomons, N.W.; Agellon, L.B.; Scott, M.E.; Koski, K.G. Distinct Changes Occur in the Human Breast Milk Microbiome Between Early and Established Lactation in Breastfeeding Guatemalan Mothers. Front. Microbiol. 2021, 12, 557180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adamczyk, I.; Kaliszczak, K.; Skowron, K.; Grudlewska-Buda, K.; Twarużek, M.; Sinkiewicz-Darol, E. Microbiological status of donor human milk—A single center study from Poland. Food Microbiol. 2024, 122, 104528. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, M.P.; Saris, P.E. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Soeorg, H.; Metsvaht, T.; Eelmäe, I.; Merila, M.; Treumuth, S.; Huik, K.; Jürna-Ellam, M.; Ilmoja, M.-L.; Lutsar, I. The role of breast milk in the colonization of neonatal gut and skin with coagulase-negative staphylococci. Pediatr. Res. 2017, 82, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-W.; Tseng, S.-Y.; Huang, M.-S. Antibiotic Susceptibility of Commensal Bacteria from Human Milk. Curr. Microbiol. 2016, 72, 113–119. [Google Scholar] [CrossRef]
- Miura, K.; Tanaka, M.; Date, M.; Ito, M.; Mizuno, N.; Mizuno, K. Comparison of bacterial profiles in human milk from mothers of term and preterm infants. Int. Breastfeed. J. 2023, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, H.; Ni, Y.; Peng, Y. Contrasting Diversity and Composition of Human Colostrum Microbiota in a Maternal Cohort with Different Ethnic Origins but Shared Physical Geography (Island Scale). Front. Microbiol. 2022, 13, 934232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González, R.; Mandomando, I.; Fumadó, V.; Sacoor, C.; Macete, E.; Alonso, P.L.; Menendez, C. Breast Milk and Gut Microbiota in African Mothers and Infants from an Area of High HIV Prevalence. PLoS ONE 2013, 8, e80299, Erratum in: PLoS ONE 2014, 9, e92930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; et al. What’s normal? Microbiomes in human milk and infant feces are related to each other but vary geographically: The INSPIRE study. Front. Nutr. 2019, 6, 45. [Google Scholar]
- Könönen, E. Development of oral bacterial flora in young children. Ann. Med. 2000, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.S.; Cheng, C.C.; Tseng, S.Y.; Lin, Y.L.; Lo, H.M.; Chen, P.W. Most commensally bacterial strains in human milk of healthy mothers display multiple antibiotic resistance. Microbiologyopen. 2019, 8, e00618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Wu, G.; Chen, H.; Li, H.; Li, S.; Zhang, C.; Pang, X.; Wang, L.; Zhao, L.; Shen, J. Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene. Front. Microbiol. 2019, 10, 2910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franzosa, E.A.; Morgan, X.C.; Segata, N.; Waldron, L.; Reyes, J.; Earl, A.M.; Giannoukos, G.; Boylan, M.R.; Ciulla, D.; Gevers, D.; et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. USA 2014, 111, E2329–E2338. [Google Scholar] [CrossRef]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Oral microbiota maturation during the first 7 years of life in relation to allergy development. Allergy 2018, 73, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- van den Bogert, B.; Erkus, O.; Boekhorst, J.; de Goffau, M.; Smid, E.J.; Zoetendal, E.G.; Kleerebezem, M. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol. Ecol. 2013, 85, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Songjinda, P.; Nakayama, J.; Kuroki, Y.; Tanaka, S.; Fukuda, S.; Kiyohara, C.; Yamamoto, T.; Izuchi, K.; Shirakawa, T.; Sonomoto, K. Molecular Monitoring of the Developmental Bacterial Community in the Gastrointestinal Tract of Japanese Infants. Biosci. Biotechnol. Biochem. 2005, 69, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, N.; Wang, C.; Zhao, Y.; Cao, J.; Li, X.; Zhang, Z.; Li, Y.; Yang, X.; Wang, X.; et al. Gut Microbiota and Immune Modulatory Properties of Human Breast Milk Streptococcus salivarius and S. parasanguinis Strains. Front. Nutr. 2022, 9, 798403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damaceno, Q.S.; Souza, J.P.; Nicoli, J.R.; Paula, R.L.; Assis, G.B.; Figueiredo, H.C.; Azevedo, V.; Martins, F.S. Evaluation of Potential Probiotics Isolated from Human Milk and Colostrum. Probiotics Antimicrob. Proteins 2017, 9, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Moine, D.; Delley, M.; Combremont, S.; Rezzonico, E.; Descombes, P.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Microbiota in Breast Milk of Chinese Lactating Mothers. PLoS ONE 2016, 11, e0160856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urbaniak, C.; McMillan, A.; Angelini, M.; Gloor, G.B.; Sumarah, M.; Burton, J.P.; Reid, G. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome 2014, 2, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct Patterns in Human Milk Microbiota and Fatty Acid Profiles Across Specific Geographic Locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drago, L.; Toscano, M.; De Grandi, R.; Grossi, E.; Padovani, E.M.; Peroni, D.G. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. ISME J. 2017, 11, 875–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blum, W.E.H.; Zechmeister-Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef]
- Celik, C.; Lee, S.T.T.; Tanoto, F.R.; Veleba, M.; Kline, K.; Thibault, G. Decoding the complexity of delayed wound healing following Enterococcus faecalis infection. eLife 2024, 13, RP95113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Anjum, J.; Zaidi, A.; Barrett, K.; Tariq, M. A potentially probiotic strain of Enterococcus faecalis from human milk that is avirulent, antibiotic sensitive, and nonbreaching of the gut barrier. Arch. Microbiol. 2022, 204, 158. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Larsson, M.W.; Lind, M.V.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bahl, M.I.; Licht, T.R. Intestinal Enterococcus abundance correlates inversely with excessive weight gain and increased plasma leptin in breastfed infants. FEMS Microbiol. Ecol. 2020, 96, fiaa066, Erratum in: FEMS Microbiol. Ecol. 2020, 96, fiaa150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyons, K.E.; Shea, C.-A.O.; Grimaud, G.; Ryan, C.A.; Dempsey, E.; Kelly, A.L.; Ross, R.P.; Stanton, C. The human milk microbiome aligns with lactation stage and not birth mode. Sci. Rep. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Leyva, L.L.; Gonzalez, E.; Li, C.; Ajeeb, T.; Solomons, N.W.; Agellon, L.B.; E Scott, M.; Koski, K.G. Human Milk Microbiota in an Indigenous Population Is Associated with Maternal Factors, Stage of Lactation, and Breastfeeding Practices. Curr. Dev. Nutr. 2021, 5, nzab013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ojo-Okunola, A.; Claassen-Weitz, S.; Mwaikono, K.S.; Gardner-Lubbe, S.; Stein, D.J.; Zar, H.J.; Nicol, M.P.; du Toit, E. Influence of Socio-Economic and Psychosocial Profiles on the Human Breast Milk Bacteriome of South African Women. Nutrients 2019, 11, 1390. [Google Scholar] [CrossRef]
- Gunyakti, A.; Asan-Ozusaglam, M. Lactobacillus gasseri from human milk with probiotic potential and some technological properties. LWT 2019, 109, 261–269. [Google Scholar]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of Infectious Mastitis during Lactation: Antibiotics versus Oral Administration of Lactobacilli Isolated from Breast Milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Łubiech, K.; Twarużek, M. Lactobacillus Bacteria in Breast Milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, Y.; Li, R.; Chen, X. Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front. Cell. Infect. Microbiol. 2023, 13, 1153894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neu, J.; Rushing, J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and bifidobacteria in human breast milk: Influence of antibiotherapy and other host and clinical factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanco-Rojo, R.; Maldonado, J.; Schaubeck, M.; Özen, M.; López-Huertas, E.; Olivares, M. Beneficial Effects of Limosilactobacillus fermentum CECT 5716 Administration to Infants Delivered by Cesarean Section. Front. Pediatr. 2022, 10, 906924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.J.; Adisetiyo, H.; Woodward, C.; Pannaraj, P.S.; Tobin, N.H.; et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS ONE 2020, 15, e0219633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bo, X.; Chen, J.; Mu, J.; Dong, X.; Ren, Z.; Liu, J.; Wang, S. Quercetin promotes the secretion of musk by regulating the hormone level and microbial structure of forest musk deer. Integr. Zool. 2024, 19, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Pekmez, C.T.; Larsson, M.W.; Lind, M.V.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Dragsted, L.O.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiology 2015, 161, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Orden-Martínez, B.; Martínez-Ruiz, R.; Millán-Pérez, R. Qué estamos aprendiendo de Staphylococcus saprophyticus? Enferm. Infecc. Microbiol. Clín 2008, 495–499. (In Spanish) [Google Scholar] [CrossRef]
- Amir, L.H.; Cullinane, M.; Garland, S.M.; Tabrizi, S.N.; Donath, S.M.; Bennett, C.M.; Cooklin, A.R.; Fisher, J.R.; Payne, M.S. The role of micro-organisms (Staphylococcus aureus and Candida albicans) in the pathogenesis of breast pain and infection in lactating women: Study protocol. BMC Pregnancy Childbirth 2011, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.-M. Fermentation of Propionibacterium acnes, a Commensal Bacterium in the Human Skin Microbiome, as Skin Probiotics against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbott, I.; Cerqueira, G.M.; Bhuiyan, S.; Peleg, A.Y. Carbapenem Resistance in Acinetobacter baumannii: Laboratory Challenges, Mechanistic Insights and Therapeutic Strategies. Expert Rev. Anti-Infect. Ther. 2013, 11, 395–409. [Google Scholar] [PubMed]
- Spicer, S.K.; Moore, R.E.; Lu, J.; Guevara, M.A.; Marshall, D.R.; Manning, S.D.; Damo, S.M.; Townsend, S.D.; Gaddy, J.A. Antibiofilm Activity of Human Milk Oligosaccharides against Multidrug Resistant and Susceptible Isolates of Acinetobacter baumannii. ACS Infect. Dis. 2021, 7, 3254–3263. [Google Scholar] [CrossRef]
- Ackerman, D.L.; Craft, K.M.; Doster, R.S.; Weitkamp, J.-H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infect. Dis. 2018, 4, 315–324. [Google Scholar] [CrossRef]
- Jovanovic, J.; Ornelis, V.F.M.; Madder, A.; Rajkovic, A. Bacillus cereus food intoxication and toxicoinfection. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3719–3761. [Google Scholar] [CrossRef] [PubMed]
- Decousser, J.-W.; Ramarao, N.; Duport, C.; Dorval, M.; Bourgeois-Nicolaos, N.; Guinebretière, M.-H.; Razafimahefa, H.; Doucet-Populaire, F. Bacillus cereus and severe intestinal infections in preterm neonates: Putative role of pooled breast milk. Am. J. Infect. Control 2013, 41, 918–921. [Google Scholar] [CrossRef]
- Cormontagne, D.; Rigourd, V.; Vidic, J.; Rizzotto, F.; Bille, E.; Ramarao, N. Bacillus cereus Induces Severe Infections in Preterm Neonates: Implication at the Hospital and Human Milk Bank Level. Toxins 2021, 13, 123. [Google Scholar] [CrossRef]
- Lewin, A.; Quach, C.; Rigourd, V.; Picaud, J.-C.; Perreault, T.; Frange, P.; Domingo, M.-C.; Lalancette, C.; Delage, G.; Germain, M. Bacillus cereus infection in neonates and the absence of evidence for the role of banked human milk: Case reports and literature review. Infect. Control Hosp. Epidemiol. 2019, 40, 787–793. [Google Scholar] [CrossRef]
- Mule, P.; Patil, N.; Gaikwad, S. Corynebacterium tuberculostearicum a potential pathogen in breast abscess—A case report. IP Int. J. Med. Microbiol. Trop. Dis. 2018, 4, 196541734. [Google Scholar] [CrossRef]
- Wang, K.; Xia, X.; Sun, L.; Wang, H.; Li, Q.; Yang, Z.; Ren, J. Microbial Diversity and Correlation between Breast Milk and the Infant Gut. Foods 2023, 12, 1740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pawlik, D.; Chmielarczyk, A.; Lorenc, K.; Michalski, P.; Lauterbach, J.; Radziszewska, R.; Wójkowska-Mach, J. Klebsiella pneumoniae in Breast Milk-A Cause of Sepsis in Neonate. Arch. Med. 2017, 9, 6. [Google Scholar] [CrossRef]
- Lozano, G.L.; Bravo, J.I.; Handelsman, J. Draft Genome Sequence of Pseudomonas koreensis CI12, a Bacillus cereus “Hitchhiker” from the Soybean Rhizosphere. Genome Announc. 2017, 5, e00570-17. [Google Scholar] [CrossRef]
- Kaur, M.; Jangra, M.; Singh, H.; Tambat, R.; Singh, N.; Jachak, S.M.; Mishra, S.; Sharma, C.; Nandanwar, H.; Pinnaka, A.K. Pseudomonas koreensis Recovered from Raw Yak Milk Synthesizes a β-Carboline Derivative with Antimicrobial Properties. Front. Microbiol. 2019, 10, 1728. [Google Scholar] [CrossRef]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human Milk Oligosaccharides Protect Against Enteropathogenic Escherichia coli Attachment In Vitro and EPEC Colonization in Suckling Mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melas, D.S.; Papageorgiou, D.K.; Mantis, A.I. Enumeration and Confirmation of Aeromonas hydrophila, Aeromonas caviae, and Aeromonas sobria Isolated from Raw Milk and Other Milk Products in Northern Greece. J. Food Prot. 1999, 62, 463–466. [Google Scholar] [CrossRef]
- Namdari, H.; Bottone, E.J. Microbiologic and clinical evidence supporting the role of Aeromonas caviae as a pediatric enteric pathogen. J. Clin. Microbiol. 1990, 28, 837–840. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mirlohi, M.; Poursina, F.; Madani, G.; Khoshhali, M.; Bahreini, N.; Safaei, H.G. The influence of impact delivery mode, lactation time, infant gender, maternal age and rural or urban life on total number of Lactobacillus in breast milk Isfahan–Iran. Adv. Biomed. Res. 2015, 4, 141. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.E.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Mantziari, A.; Rautava, S. Factors influencing the microbial composition of human milk. Semin. Perinatol. 2021, 45, 151507. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gritz, E.C.; Bhandari, V. The Human Neonatal Gut Microbiome: A Brief Review. Front. Pediatr. 2015, 3, 17, Erratum in: Front. Pediatr. 2015, 3, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beharry, K.D.; Latkowska, M.; Valencia, A.M.; Allana, A.; Soto, J.; Cai, C.L.; Golombek, S.; Hand, I.; Aranda, J.V. Factors Influencing Neonatal Gut Microbiome and Health with a Focus on Necrotizing Enterocolitis. Microorganisms 2023, 11, 2528. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LeMay-Nedjelski, L.; Butcher, J.; Ley, S.H.; Asbury, M.R.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W.; Zinman, B.; et al. Examining the relationship between maternal body size, gestational glucose tolerance status, mode of delivery and ethnicity on human milk microbiota at three months post-partum. BMC Microbiol. 2020, 20, 219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
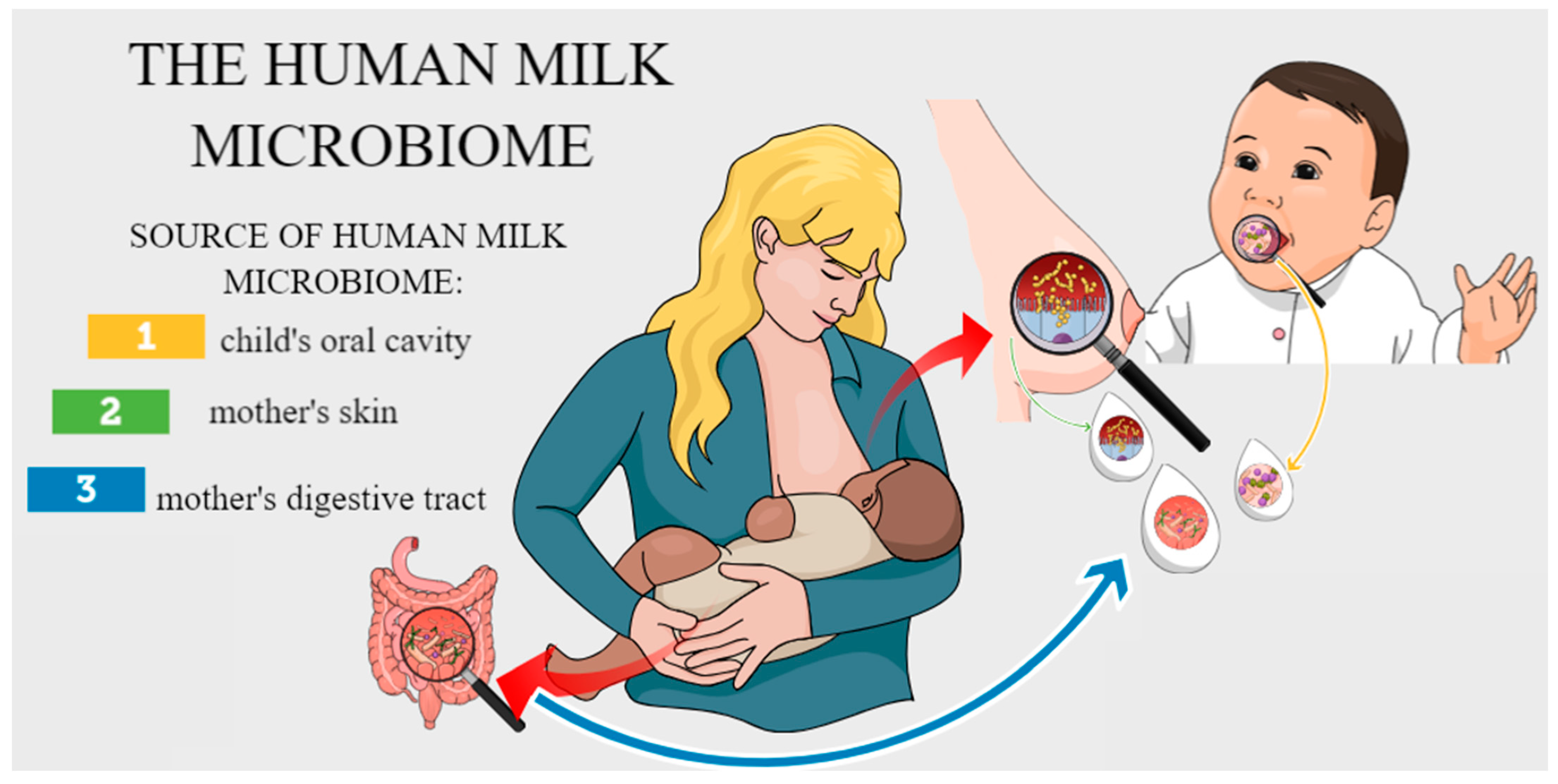

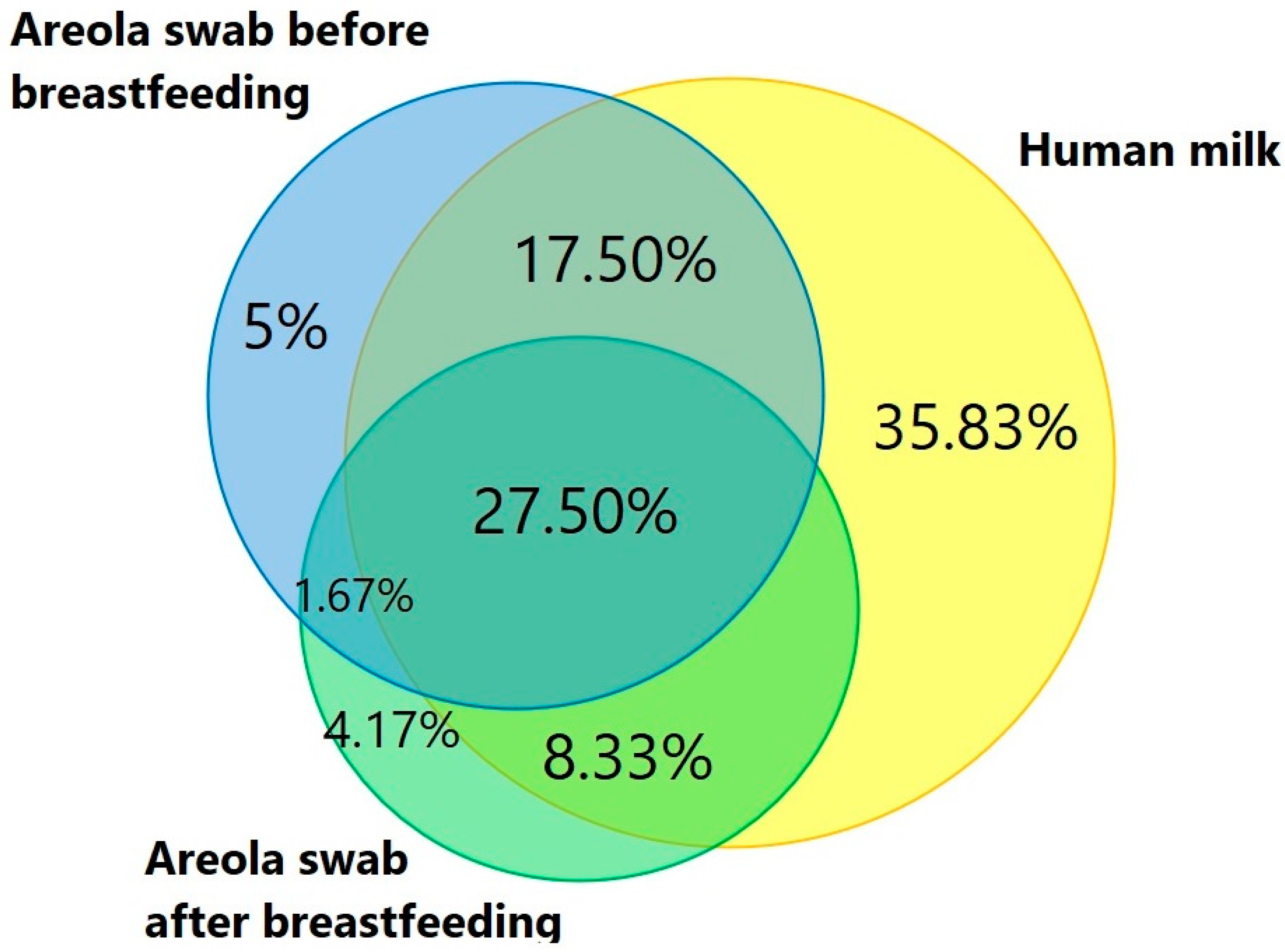
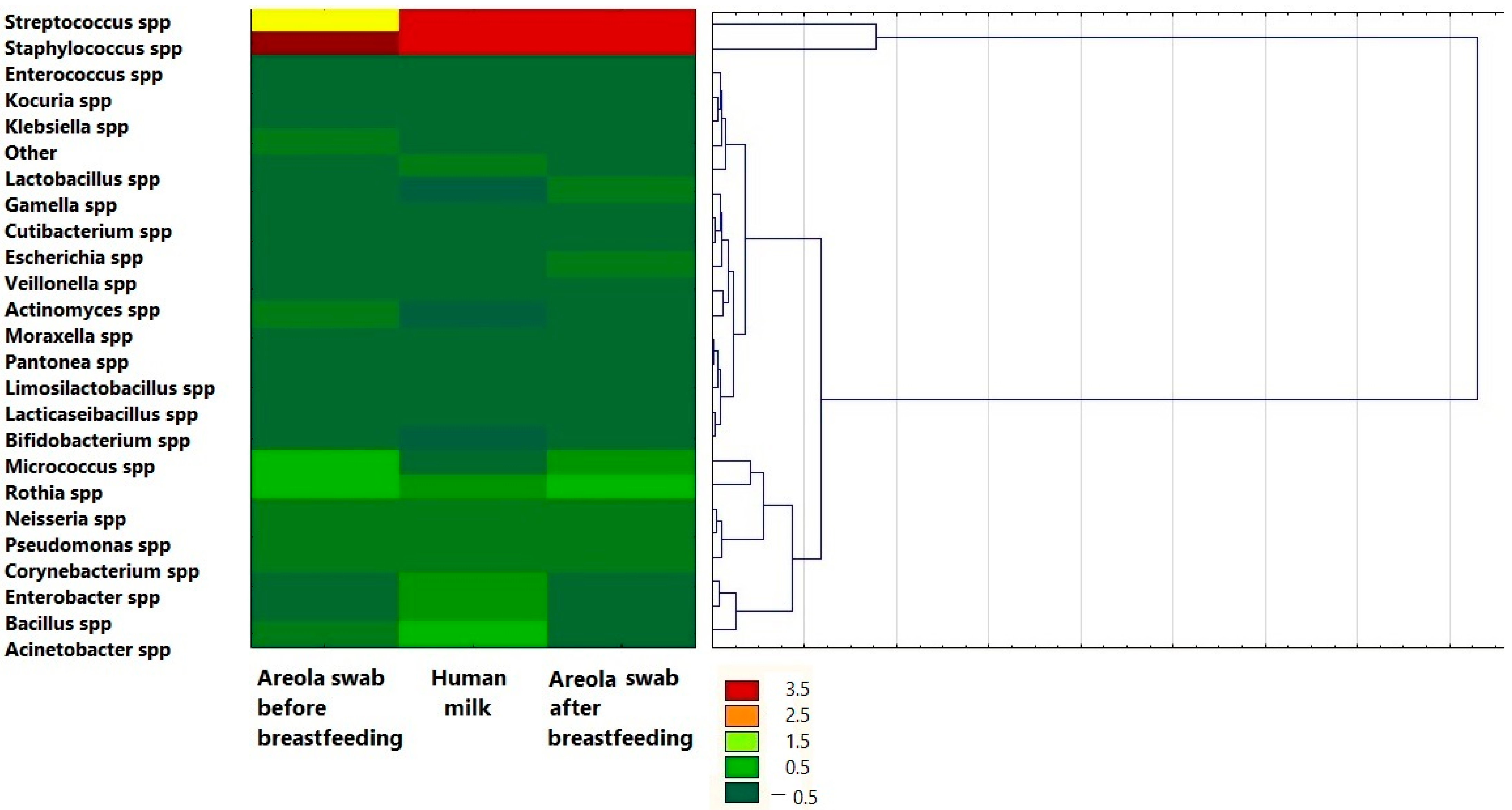
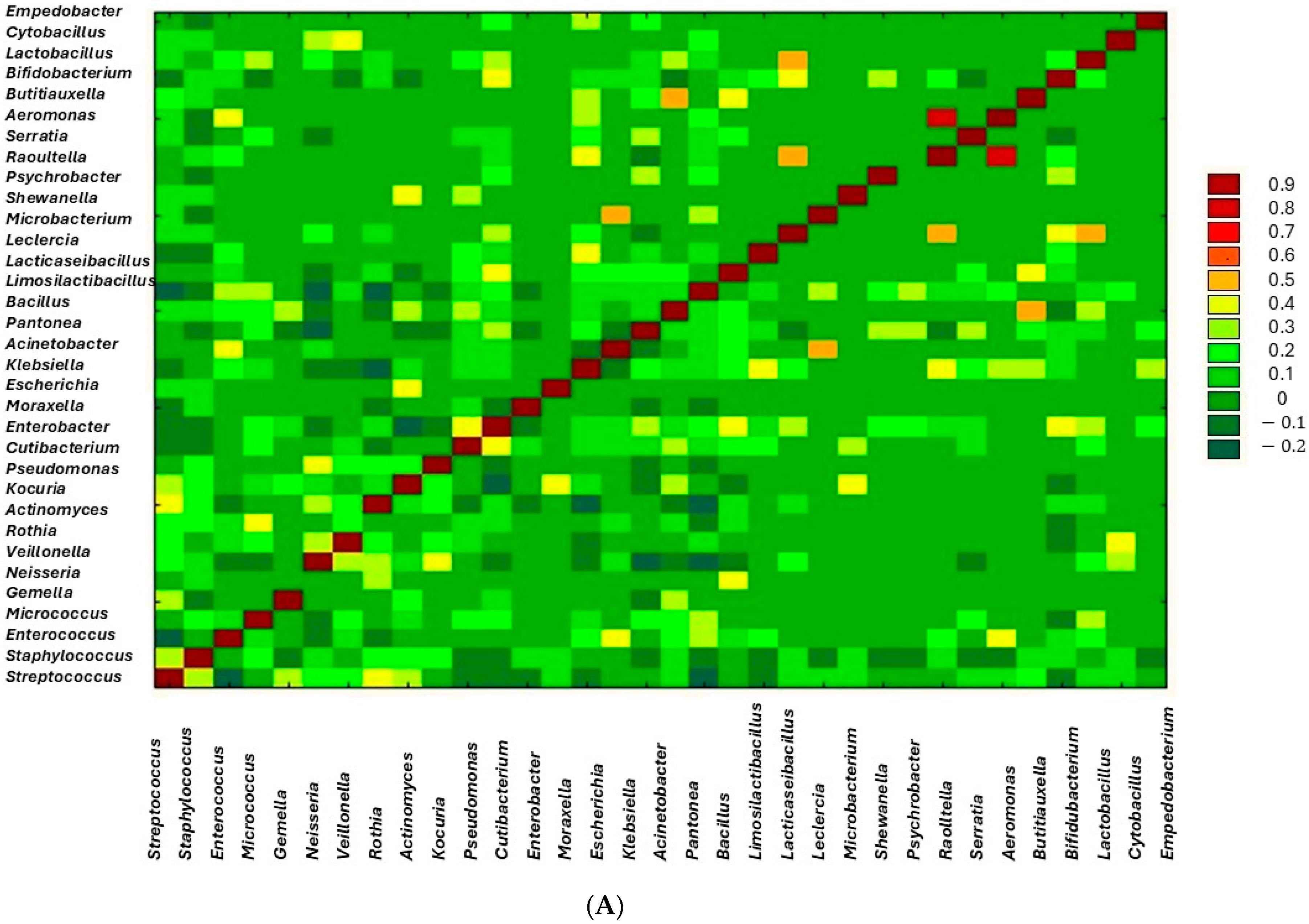
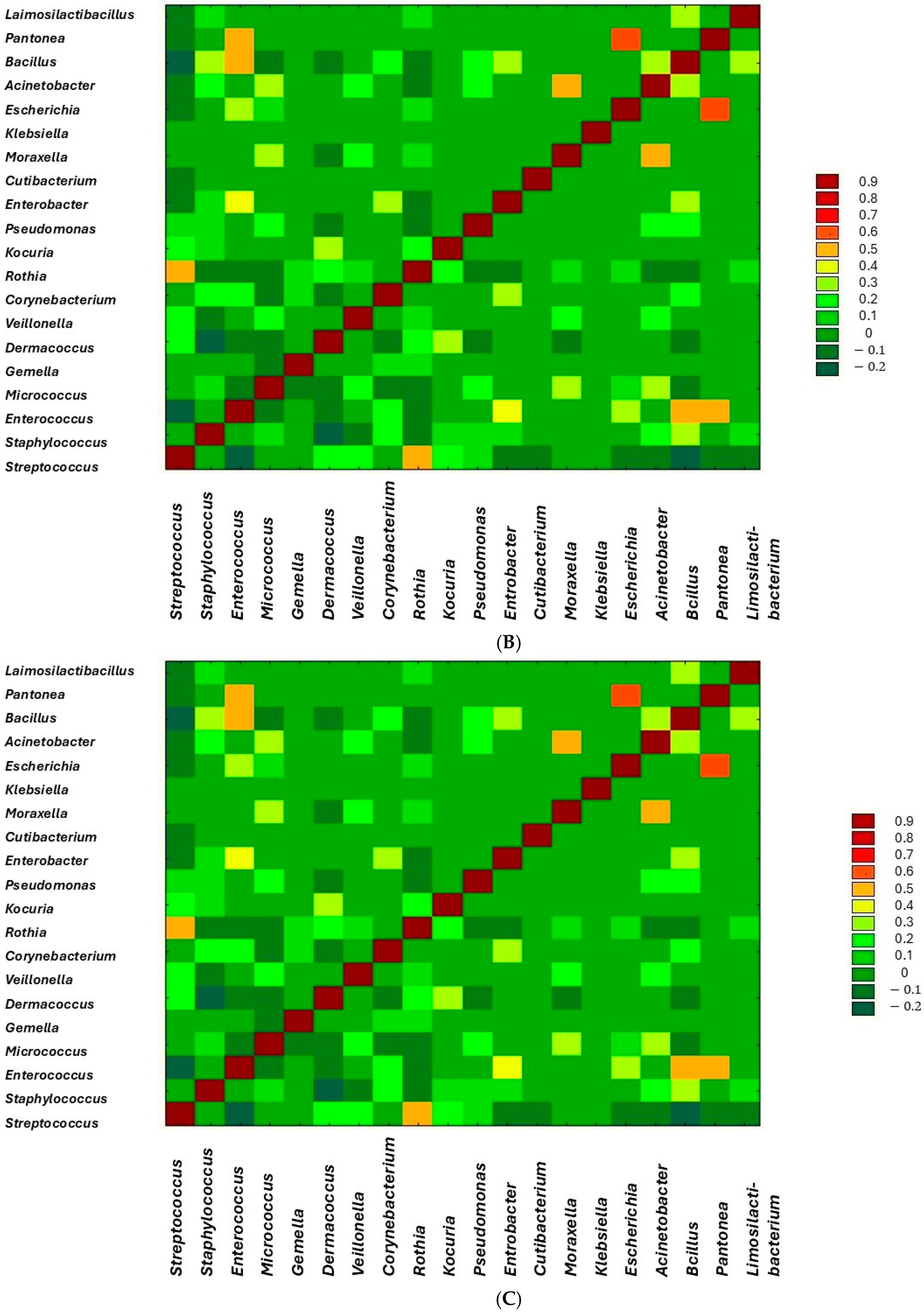
| Variable | Mean ± SE |
|---|---|
| Age [year] | 31.25 ± 3.54 |
| BMI [kg/m2] | 23.95 ± 4.18 |
| HBD | 39.39 ± 1.19 |
| Place of residence | N (%) |
| City | 52 (60%) |
| Village | 34 (40%) |
| Education | N (%) |
| Secondary | 9 (10.5%) |
| Higher | 77 (89.5%) |
| Parity | N (%) |
| Primiaraus | 50 (58%) |
| Multiparaus | 36 (42%) |
| Number of delivery—2 | 31 (86%) |
| Number of delivery—3 | 5 (14%) |
| Number of pregnancies | N (%) |
| 1 | 34 (39.5%) |
| 2 | 34 (39.5%) |
| 3 | 12 (14%) |
| 4 | 6 (7%) |
| Baby’s gender | N (%) |
| Woman | 36 (42%) |
| Man | 50 (58%) |
| Lactation period | N (%) |
| <6 months | 44 (51%) |
| 6–12 months | 22 (27%) |
| >6 months | 19 (22%) |
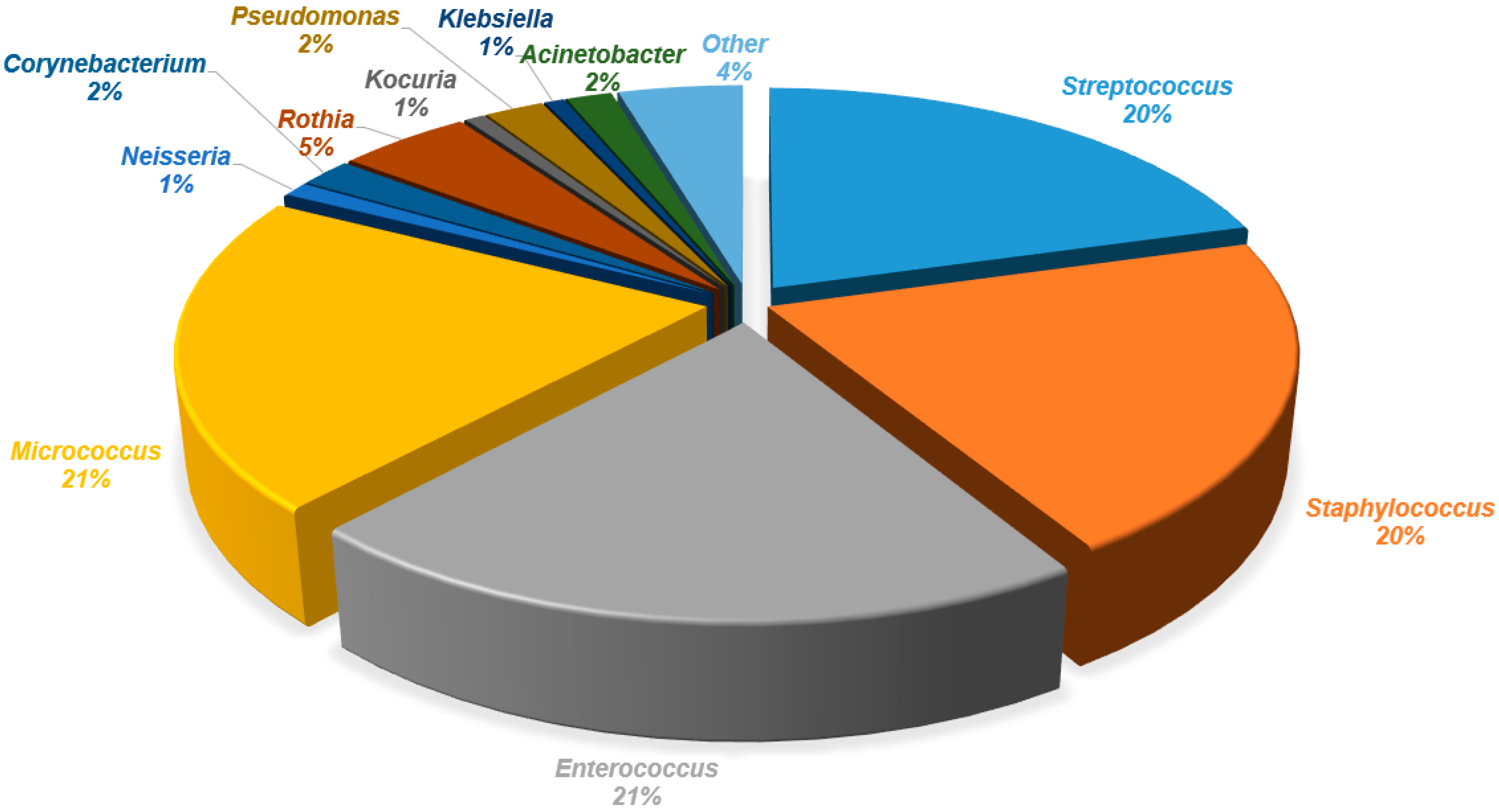
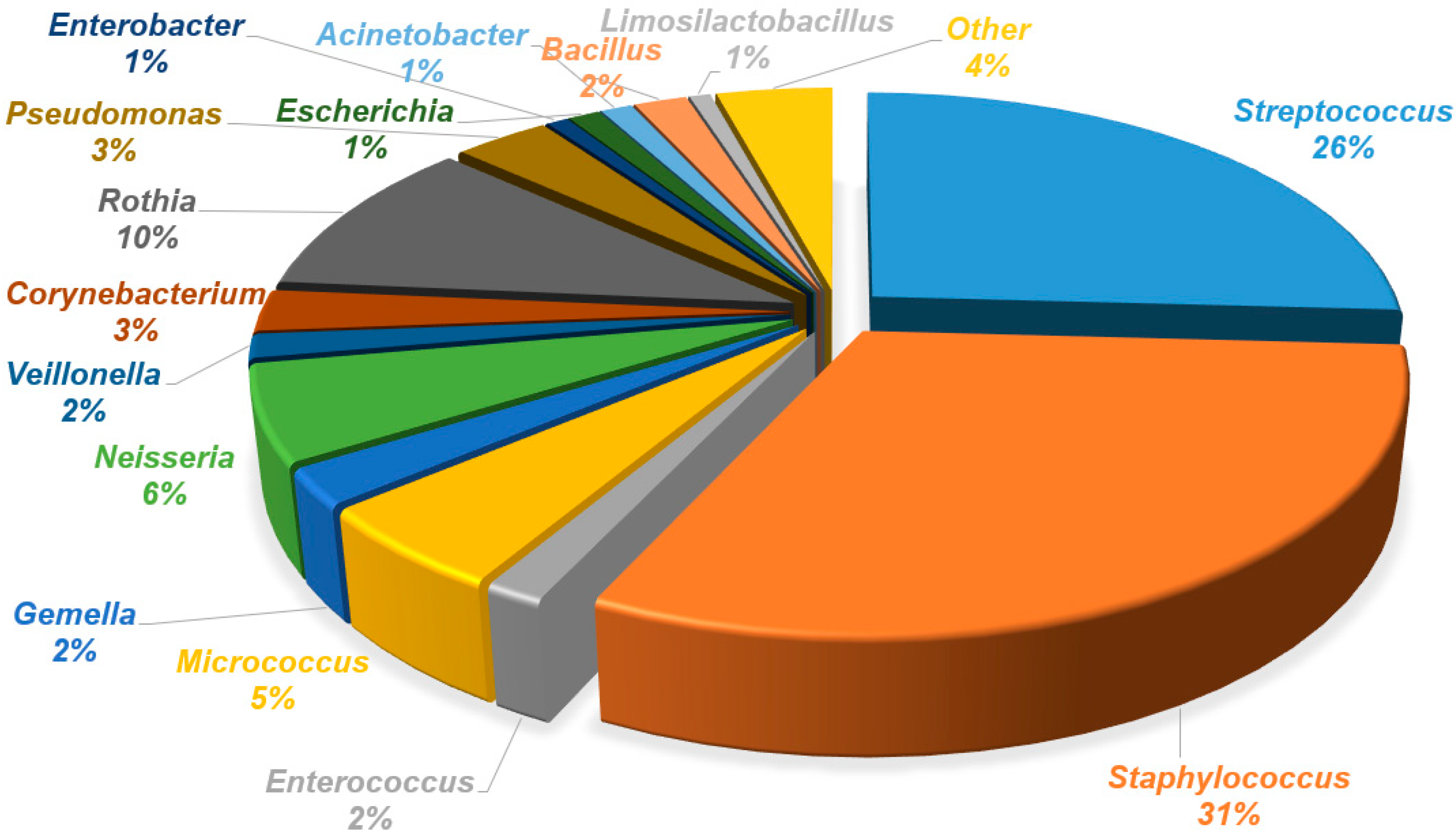
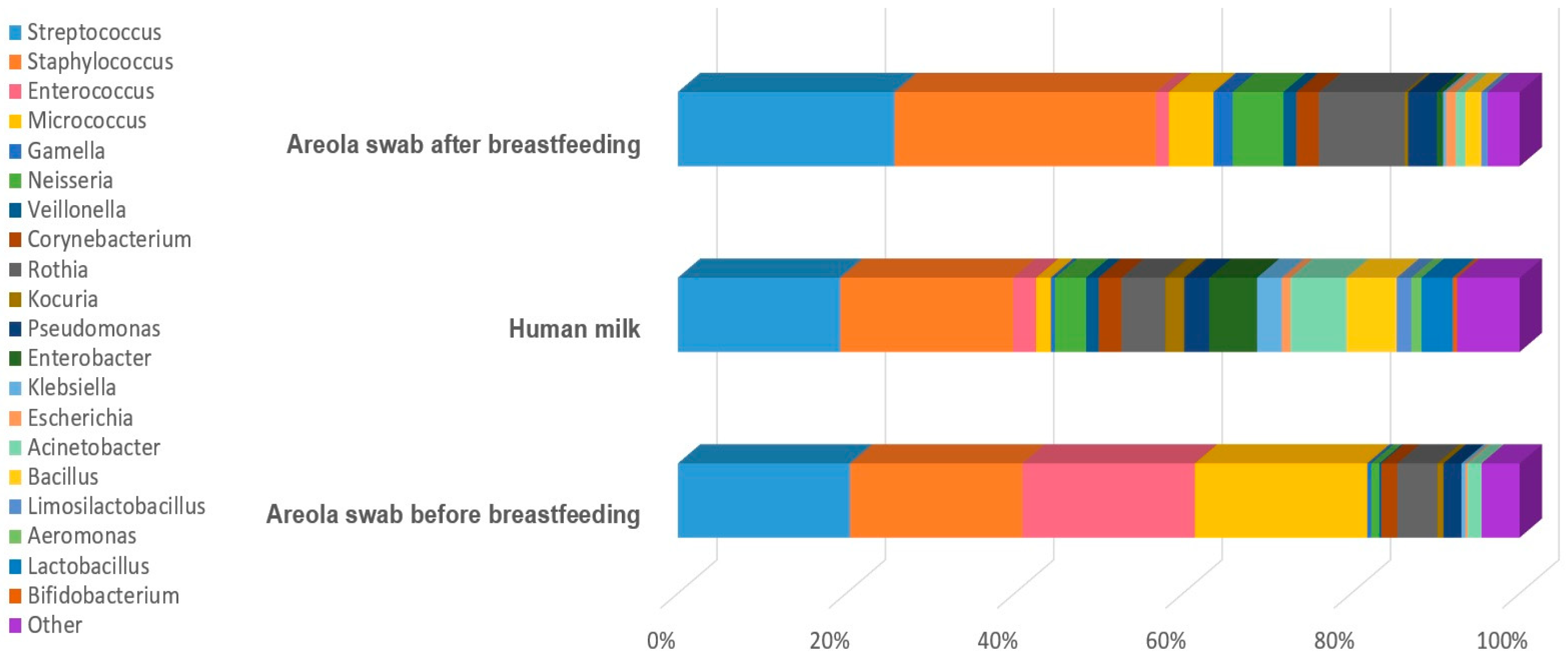
| Genera | Bacterial Strains Isolated from the Areola Before Breastfeeding | Bacterial Strains Isolated from Milk | Bacterial Strains Isolated from the Areola After Breastfeeding |
|---|---|---|---|
| Streptococcus | S. parasanguinis, S. mitis_orale, S. pneumoniae, S. sanguinis, S. vestibularis, S. gordonii, S. salivarius, S. pseudopneumoniae | S. parasanguinis, S. mitis_orale, S. pneumoniae, S. sanguinis, S. vestibularis, S. gordonii, S. salivarius, S. pseudopneumoniae, S. infantis, S. gallolyticus | S. mitis_orale, S. vestibularis, S. parasanguinis, S. pseudopneumoniae, S. salivarius, S. vestibularis, S. gordonii, S. pneumoniae |
| Staphylococcus | S. hominis, S. aureus, S. epidermidis, S. haemolyticus, S. caprae, S. petrasii, S. capitis, S. warneri | S. hominis, S. aureus, S. epidermidis, S. haemolyticus S. caprae, S. petrasii, S. capitis, S. warneri, S. saprophyticus, S. borealis, S. lugdunensis | S. hominis, S. aureus, S. epidermidis, S. haemolyticus S. caprae, S. capitis, S. warneri, S. lugdunensis |
| Enterococcus | E. faecalis | E. faecalis, E. faecium, E. italicus, E. durans | E. faecalis, E. faecium |
| Micrococcus | M. luteus | M. luteus | M. luteus |
| Gemella | G. haemolysans | G. sanguinis, G. haemolysans | G. sanguinis, G. haemolysans |
| Dermacoccus | D. nishinomiyaensis | D. nishinomiyaensis | |
| Neisseria | N. flavescens_subflava group, N. sicca, N. cinerea | N. flavescens_subflava group, N. sicca, N. cinerea | N. flavescens_subflava group, N. sicca, N. cinerea |
| Veillonella | V. dispar | V. parvula, V. atypica, V. dispar | V. atypica, V. dispar, V. parvula |
| Corynebacterium | C. mucifaciens, C. durum, C. argentoratense, C. simulans, C. tuberculostearicum | C. tuberculostearicum, C. mucifaciens, C. kroppenstedtii, C. bovis, C. lipophiloflavum, C. accolens, C. durum | C. argentoratense, C. tuberculostearicum, C. pseudodiphtheriticum, C. bovis, C. kroppenstedtii, C. amycolatum, C. propinquum |
| Rothia | R. deutocariosa, R. mucilaginosa, R. aeria, R. terrae, R. amarae, R. kristinae | R. mucilaginosa, R. deutocariosa, R. amarae, R. kristinae, R. terrae | R. mucilaginosa, R. aeria, R. dentocariosa |
| Kocuria | K. rhizophila | K. rhizophila | K. rhizophila |
| Actinomyces | A. naeslundii, A. oris, A. graevenitzii | A. naeslundii, A. graevenitzii | A. naeslundii |
| Pseudomonas | P. oryzihabitans, P. stutzeri, P. luteola, P. massiliensis, P. fulva, P. monteilli | P. oleovorans, P. oryzihabitans, P. stutzeri, P. cedrina, P. fulva, P. vulneris, P. luteola, P. monteteilli, P. putida, P. koreensis | P. oryzihabitans, P. fulva, P. massiliensis, P. luteola, P. vulneris, P. stutzeri |
| Enterobacter | E. hormaechei, E. kobei E. bugandensis, E. ludwigii, E. roggenkampii, E. asburiae | E. kobei | |
| Cutibacterium | C. avidum, C. flaccumfaciens | C. avidum, C. acnes | C. avidum |
| Moraxella | M. osloensis | M. osloensias | M. osloensis, M. catarrhalis |
| Klebsiella | K. pneumoniae, K. oxytoca | K. oxytoca, K. pneumoanie | K. oxytoca |
| Escherichia | E. coli | E. coli | E. coli |
| Acinetobacter | A. lwoffii, A. schindleri, A. johnsonii, A. junii, A. ursingii | A. ursingii, A. junii, A. baylyi, A. jonhsonii, A. lwoffii, A. parvus, A. pittii, A. baumannii | A. baylyi, A. lwoffii, A. lactucae, A. johnsonii, A. ursingii, A. junii, A. pittii, |
| Pantonea | P. agglomerans, P. septica | P. anthophila | |
| Bacillus | B. cereus | B. cereus | B. cereus |
| Limosilactobacillus | L. reuteri, L. fermentum, L. brevis | L. brevis | |
| Schaalia | S. odontolytica | S. odonolytica | |
| Leclercia | L. adecarboxylata | L. adecarboxylata | |
| Cytobacillus | C. firmus | C. firmus, C. horneckiae | |
| Lacticaseibacillus | L. rhamnosus | L. rhamnosus, L. paracasei | |
| Microbacterium | M. paraoxydans | ||
| Psychrobacter | P. sanguinis | ||
| Shewanella | S. oneidensis | ||
| Stenotrophomonas | S. maltophila | ||
| Raoultella | R. ornithinolytica | ||
| Aeromonas | A. caviae | ||
| Serratia | S. marcescens | ||
| Buttiauxella | B. gaviniae | ||
| Lactobacillus | L. gasseri, L. oris, L. crispatus, L. brevis, L. salivarius, L. rhamnosus | ||
| Bifidobacterium | B. breve | ||
| Empeadobacter | E. brevis |
| Genera | Areola Swab Before Breastfeeding/Human Milk p | Human Milk/Areola Swab After Breastfeeding p | Areola Swab After Breastfeeding/Areola Swab Before Breastfeeding p |
|---|---|---|---|
| Streptococcus | 0.006 | 0.800 | 0.014 |
| Staphylococcus | 0.119 | 0.033 | 0.028 |
| Enterococcus | 0.001 | 0.001 | 0.999 |
| Micrococcus | 0.001 | 0.958 | 0.705 |
| Gemella | 0.200 | 0.095 | 0.090 |
| Dermacoccus | 0.848 | 0.900 | 0.990 |
| Neisseria | 0.034 | 0.100 | 0.012 |
| Veillonella | 0.001 | 0.637 | 0.783 |
| Corynebacterium | 0.969 | 0.043 | 0.020 |
| Rothia | 0.074 | 0.100 | 0.010 |
| Kocuria | 0.184 | 0.730 | 0.100 |
| Actinomyces | 0.400 | >0.999 | >0.999 |
| Pseudomonas | 0.001 | 0.001 | <0.001 |
| Enterobacter | 0.027 | 0.700 | 0.999 |
| Cutibacterium | 0.848 | 0.001 | 0.913 |
| Moraxella | 0.782 | 0.802 | 0.238 |
| Klebsiella | 0.001 | 0.120 | 0.001 |
| Escherichia | 0.802 | 0.380 | 0.100 |
| Acinetobacter | 0.004 | 0.001 | 0.302 |
| Pantonea | 0.999 | 0.800 | 0.999 |
| Bacillus | 0.002 | 0.003 | 0.999 |
| Limosilactobacillus | 0.999 | 0.670 | 0.999 |
| Schaalia | 0.999 | 0.999 | 0.999 |
| Leclercia | 0.999 | 0.999 | 0.999 |
| Cytobacillus | 0.999 | 0.999 | 0.999 |
| Lacticaseibacillus | 0.999 | 0.999 | 0.999 |
| Microbacterium | 0.999 | 0.999 | - |
| Psychrobacter | 0.999 | 0.999 | - |
| Shewanella | 0.999 | 0.999 | - |
| Stenotrophomonas | 0.999 | 0.999 | - |
| Raoultella | 0.999 | 0.999 | - |
| Aeromonas | 0.999 | 0.999 | - |
| Serratia | 0.999 | 0.999 | - |
| Buttiauxella | 0.999 | 0.999 | - |
| Lactobacillus | 0.040 | 0.040 | - |
| Bifidobacterium | 0.999 | 0.999 | - |
| Empeadobacter | 0.999 | 0.999 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrustek, A.; Dombrowska-Pali, A.; Olszewska-Słonina, D.; Wiktorczyk-Kapischke, N.; Socha, M.W.; Budzyńska, A.; Sadowska-Krawczenko, I. Human Milk Microbiome from Polish Women Giving Birth via Vaginal Delivery—Pilot Study. Biology 2025, 14, 332. https://doi.org/10.3390/biology14040332
Chrustek A, Dombrowska-Pali A, Olszewska-Słonina D, Wiktorczyk-Kapischke N, Socha MW, Budzyńska A, Sadowska-Krawczenko I. Human Milk Microbiome from Polish Women Giving Birth via Vaginal Delivery—Pilot Study. Biology. 2025; 14(4):332. https://doi.org/10.3390/biology14040332
Chicago/Turabian StyleChrustek, Agnieszka, Agnieszka Dombrowska-Pali, Dorota Olszewska-Słonina, Natalia Wiktorczyk-Kapischke, Maciej W. Socha, Anna Budzyńska, and Iwona Sadowska-Krawczenko. 2025. "Human Milk Microbiome from Polish Women Giving Birth via Vaginal Delivery—Pilot Study" Biology 14, no. 4: 332. https://doi.org/10.3390/biology14040332
APA StyleChrustek, A., Dombrowska-Pali, A., Olszewska-Słonina, D., Wiktorczyk-Kapischke, N., Socha, M. W., Budzyńska, A., & Sadowska-Krawczenko, I. (2025). Human Milk Microbiome from Polish Women Giving Birth via Vaginal Delivery—Pilot Study. Biology, 14(4), 332. https://doi.org/10.3390/biology14040332







