1. Introduction
Scarab beetles (Coleoptera) represent economically significant pests that often inflict damage on a broad spectrum of agricultural crops, forests, and fruit trees, particularly during their larval stage when they affect the underground portions of plants [
1]. Accurate species identification is crucial for agricultural researchers and scientists to develop effective control methods or management strategies [
2,
3,
4,
5,
6]. Traditional insect classification methods, which rely on external features such as the head, thorax, abdomen, and propodites, present considerable challenges for non-specialists [
7,
8,
9].
Fortunately, numerical taxonomy [
10,
11] has advanced significantly in recent decades. Today, it serves as a vital tool in modern systematics, especially in the study of fossil organisms within the Dicksoniaceae family [
12]. With advancements in computer technology and image processing, geometric morphometrics—a statistical method for quantitatively describing organisms—has emerged as an effective and scientific approach in entomology, applicable to various insect species [
13,
14].
In Coleoptera taxonomy, interspecies differences or sexual dimorphisms are commonly studied using landmark analysis or morphological data such as hind wing shape [
15,
16]. At higher taxonomic levels, landmark data from the metendosternites of Scarabaeoidea beetles have been extracted and analyzed, demonstrating the utility of this structure for beetle systematists [
17]. Through three-dimensional reconstructions, morphological variations in mandibles among three feeding types of beetles (omnivorous, phytophagous, and coprophagous) have been examined, leading to insights into the relationship between feeding habits and mandible structure [
18]. Based on 19 landmarks of hind wings and 119 morphological characters from 81 beetle species, phylogenetic relationships have been investigated [
15].
Numerous studies have predominantly focused on sister species or pairs of species. For instance, shape and size differences between polyphenic sister species within the genus
Onthophagus Latreille, 1802 were studied using landmarks on the head, pronotum, and elytra [
19]. However, these external traits alone were insufficient to distinguish between the two species unless primary sexual characteristics were considered [
20]. In
Colophon Gray, 1832 beetles, significant intersexual and interspecific variations in size and shape in the mandibles, heads, pronota, and elytra between two sympatric species (
Colophon haughtoni Barnard, 1929 and
C. kawaii Mizukami, 1997) showed significant sexual dimorphism. These variations allow for the accurate identification of males based on mandibles, heads, and pronota [
21]. In two ground beetles (
Carabus auronitens Fabricius, 1792 and
C. nemoralis Müller, 1764), significant size differences were noted between sexes, with positive correlations observed between sexes and shapes [
22]. Nikola et al. (2019) [
23] found that allometric components significantly contributed to body shape differences between sexes, while taxonomic differences between two ground beetle species (
Carabus coriaceus cerisyi Dejean, 1826 and
C. kollari praecellens Palliardi, 1825) were influenced by additional factors and processes. For cantharid beetles (Cantharinae), hind wing shape differences among the genera appear more variable than within each genus, with geometric morphometrics revealing distinct variations for each species [
24].
In the study of sexual dimorphism in insect species, scientists typically examine external morphological characteristics. For instance, in the case of the scarab beetle (
Adoretus sinicus Burmeister, 1855), sexual differences were determined by the morphology of the terminal sternite and the length-to-width ratio of certain protarsomere segments [
16]. The shape and size patterns of horns in
Ceratophyus rossii Jekel, 1865 have been identified and modeled using both traditional and geometric morphometric methods [
25]. Regarding ambrosia beetles (
Euwallacea fornicatus Eichhoff, 1868 and
Euwallacea sp. Bright, 2014), adult males of
E. sp. exhibit significantly shorter body lengths, narrower head widths, and smaller pronotal widths compared to females. Additionally, the females of
E. sp. have significantly narrower head widths and pronotal widths than female
E. fornicates, while demonstrating significantly greater body lengths and ratios of body length to pronotal width [
26].
In the field of ecology, the differences between insect ecotypes have been utilized to evaluate the relationship between morphological traits and ecological factors. For instance, morphological measurements were employed to compare intraspecific variations among dung beetles from different land use types, thereby elucidating the connection between morphological traits and land use patterns. The study demonstrated that intraspecific differences in dung beetle morphological traits vary across different land use types, and changes in land use can induce phenotypic plasticity [
27]. By analyzing shape variation in 495 specimens from 38 species of dung beetles, it was found that taxonomic diversity is positively correlated with morphometric diversity [
28]. Additionally, the shape variation and fluctuating asymmetry levels observed in the carabid beetle
Pterostichus melas melas Creutzer, 1799 can serve as indicators of environmental stability [
29]. In
Carabus aeruginosus Fischer, 1822, certain environmental factors such as anthropogenic press and biotope vegetation significantly influenced body size variation, leading to sexual size and shape dimorphism [
30]. Furthermore, latitudinal variation was evident in the sexual traits of 22 populations of the false blister beetle (
Oedemera sexualis Marseul, 1877) [
31]. However, the relationship between morphological diversity and species richness is not always straightforward; for example, studies on Lucanidae indicate that their morphological diversity does not necessarily correlate with species richness [
32].
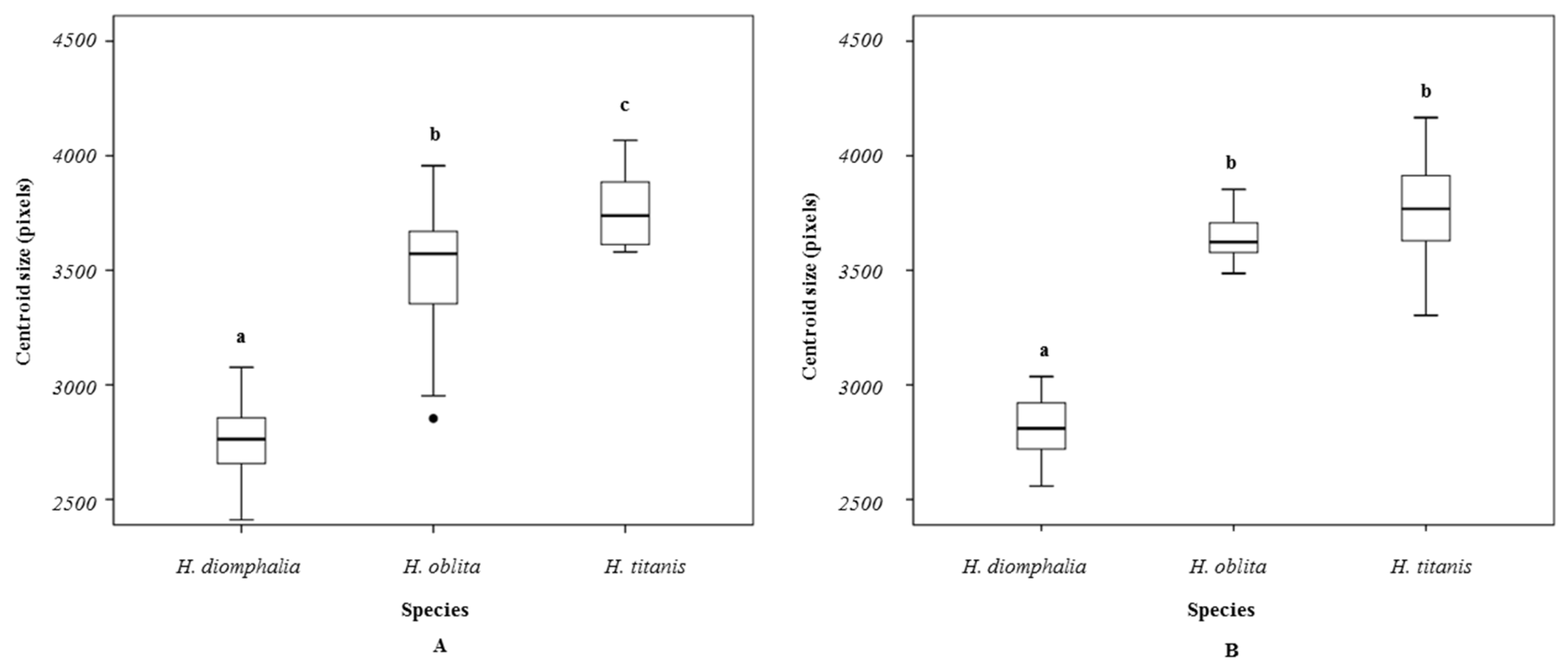
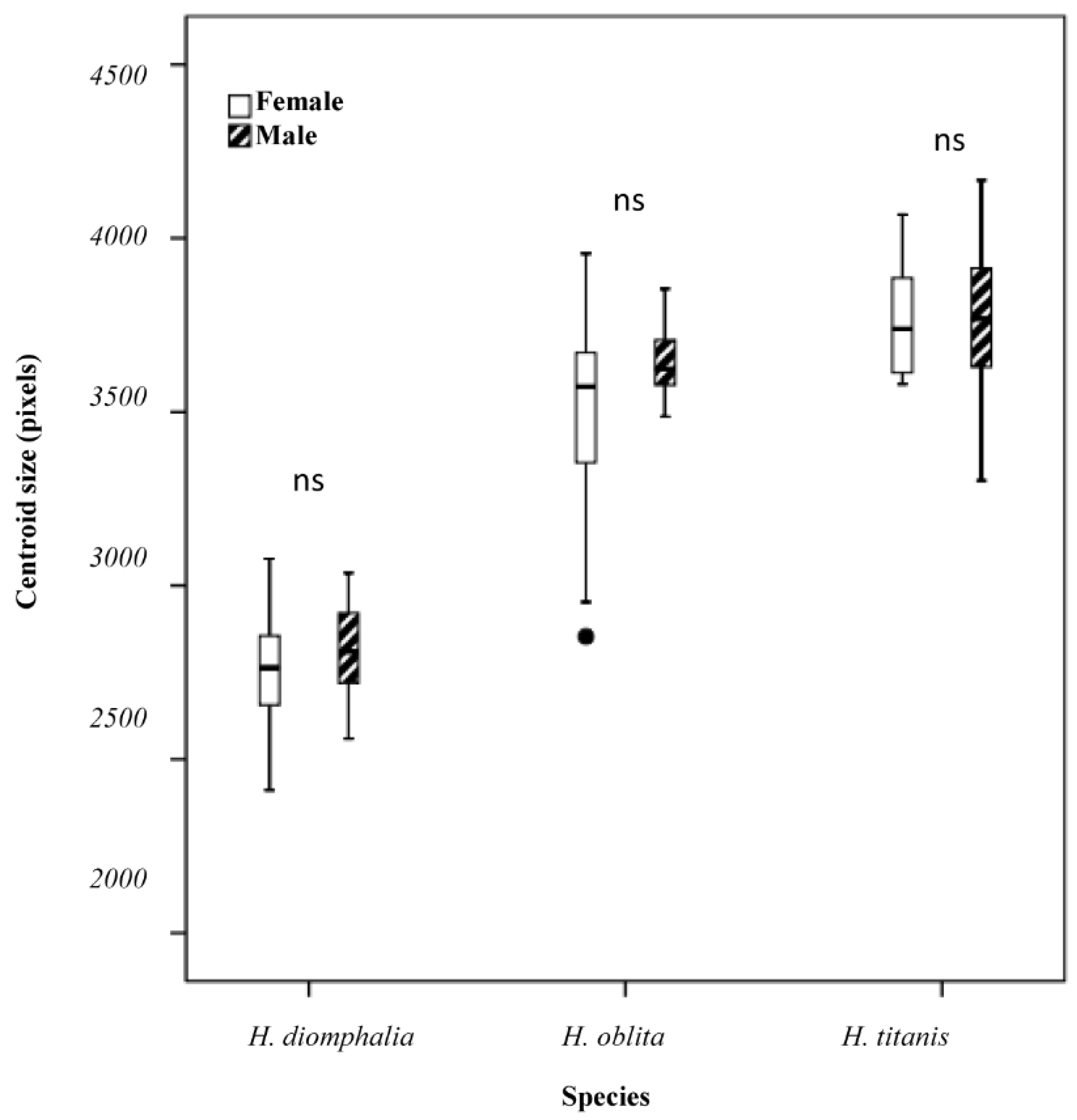
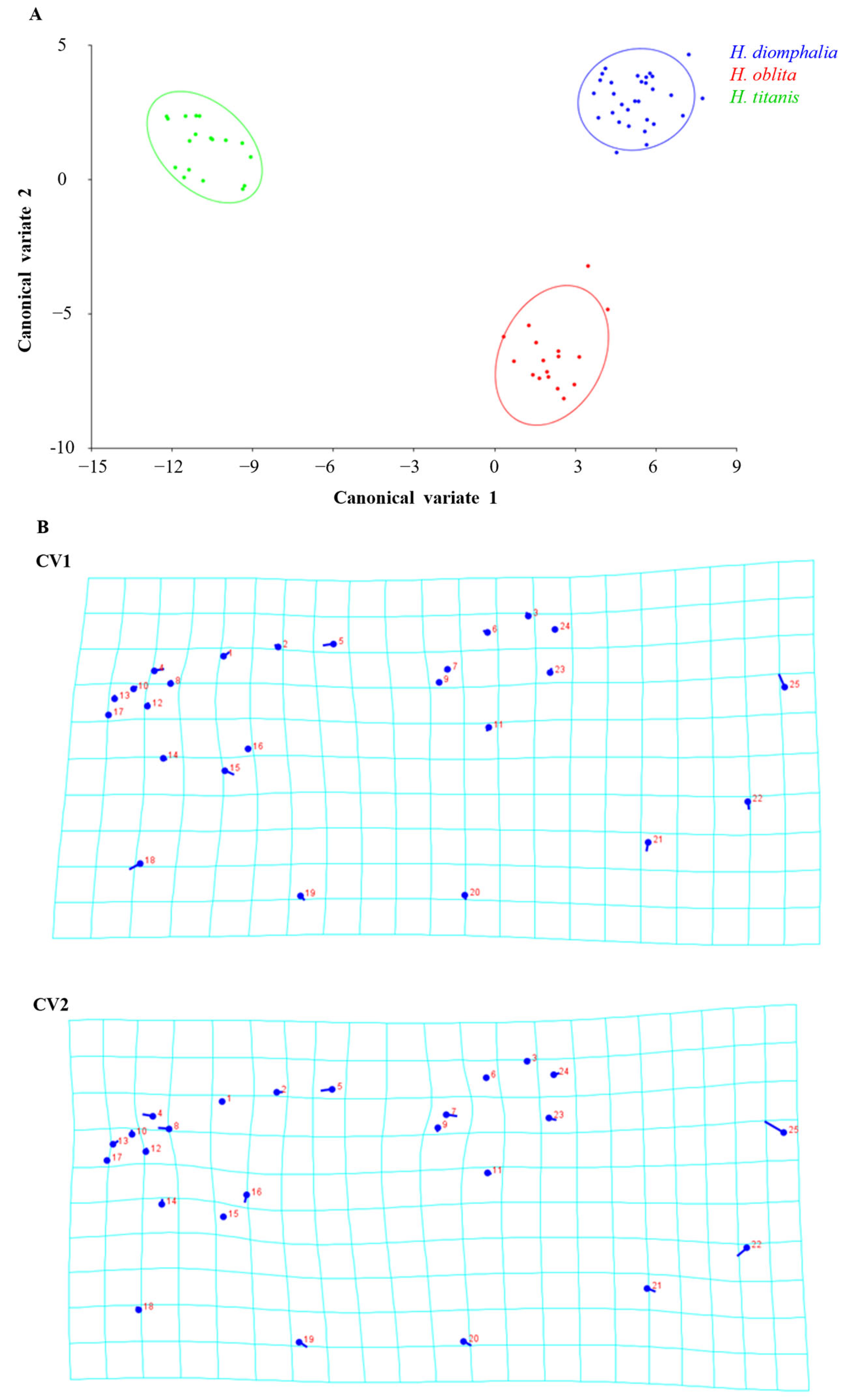
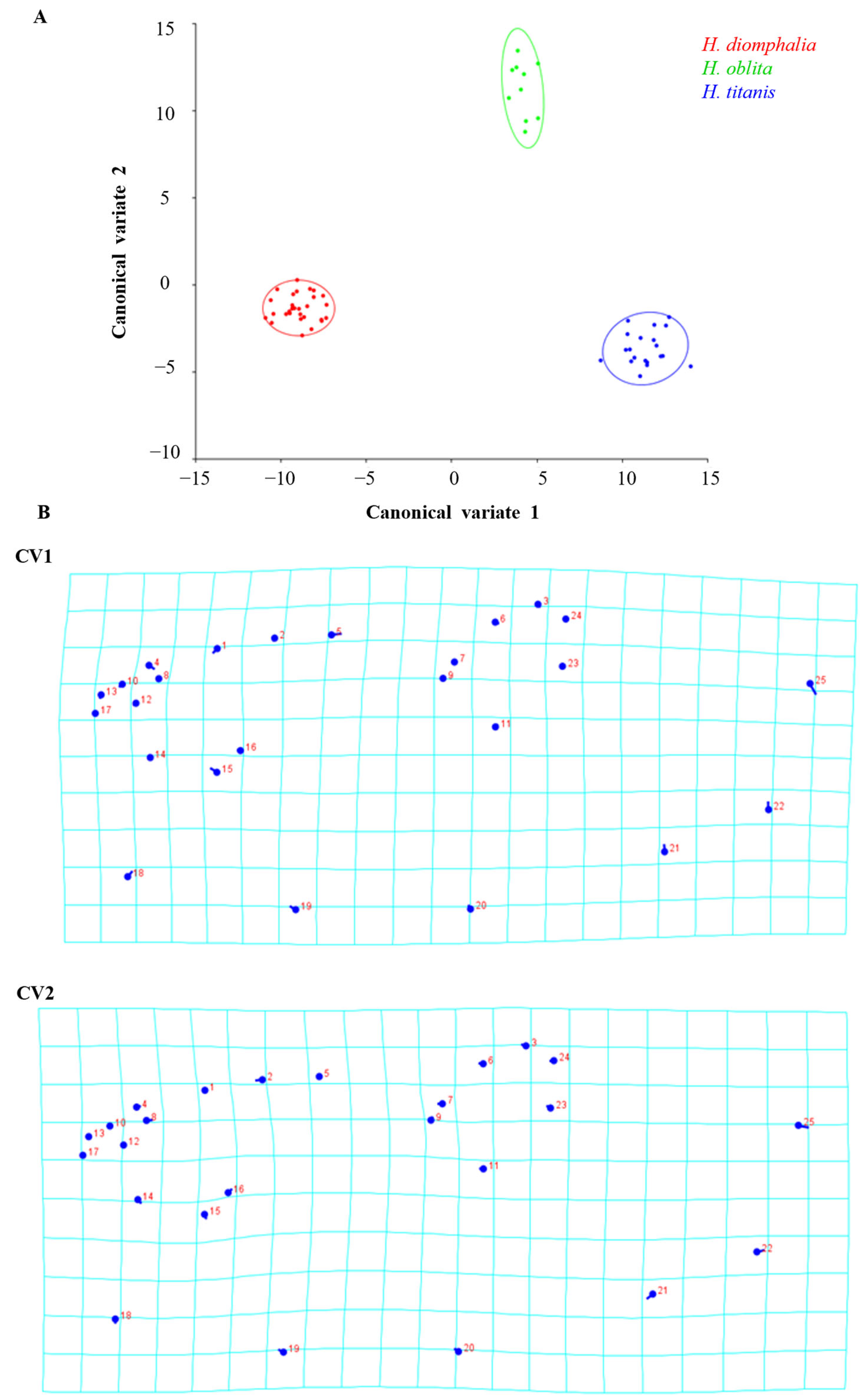
Species within the genus
Holotrichia exhibit a distribution spanning the Palaearctic and Oriental regions. To date, over 500 species have been taxonomically recognized worldwide, among which 73 are documented in China [
33]. The females and males of the three beetle species (
Holotrichia diomphalia Bates, 1888,
H. titanis Reitter, 1902, and
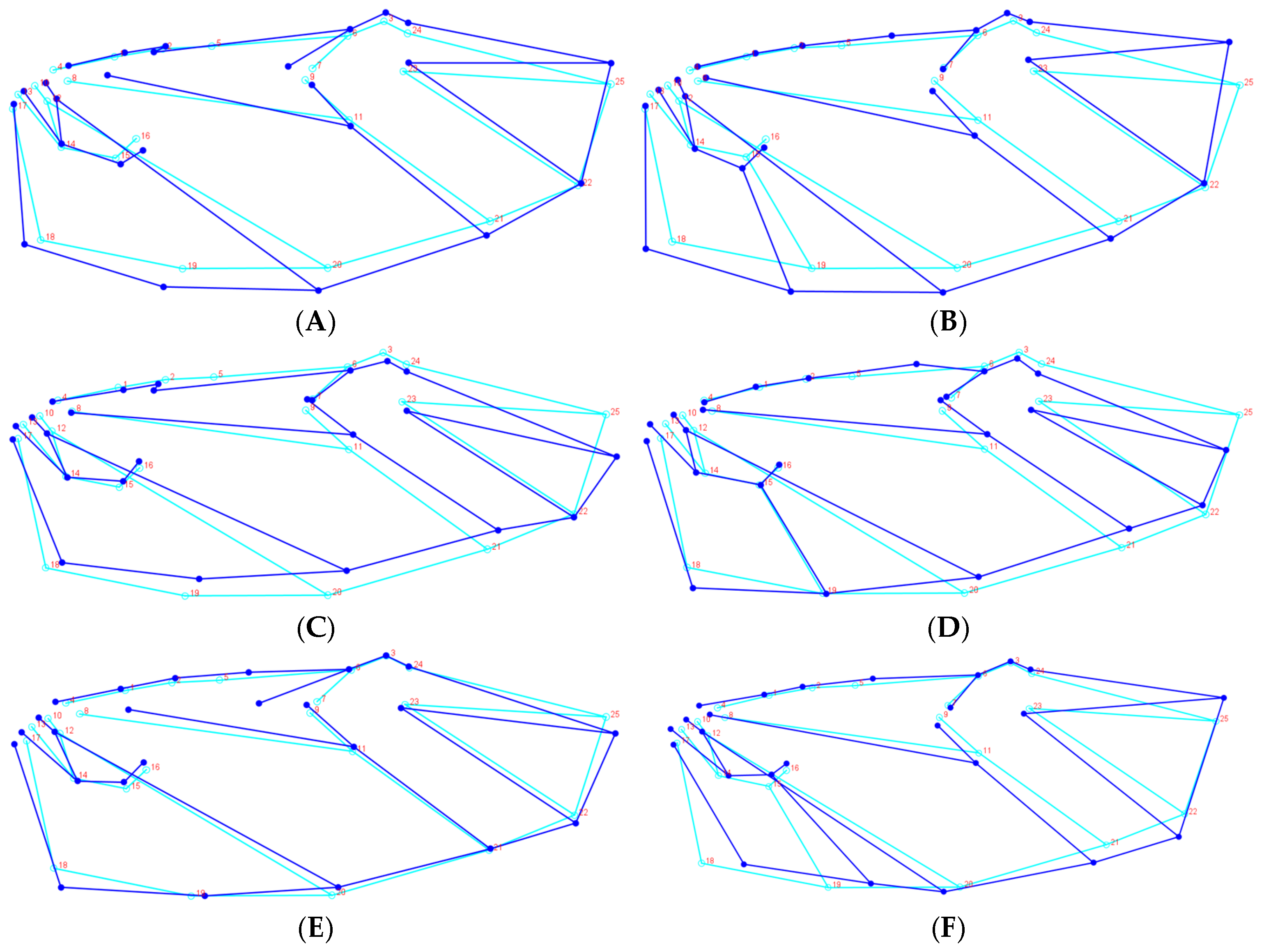
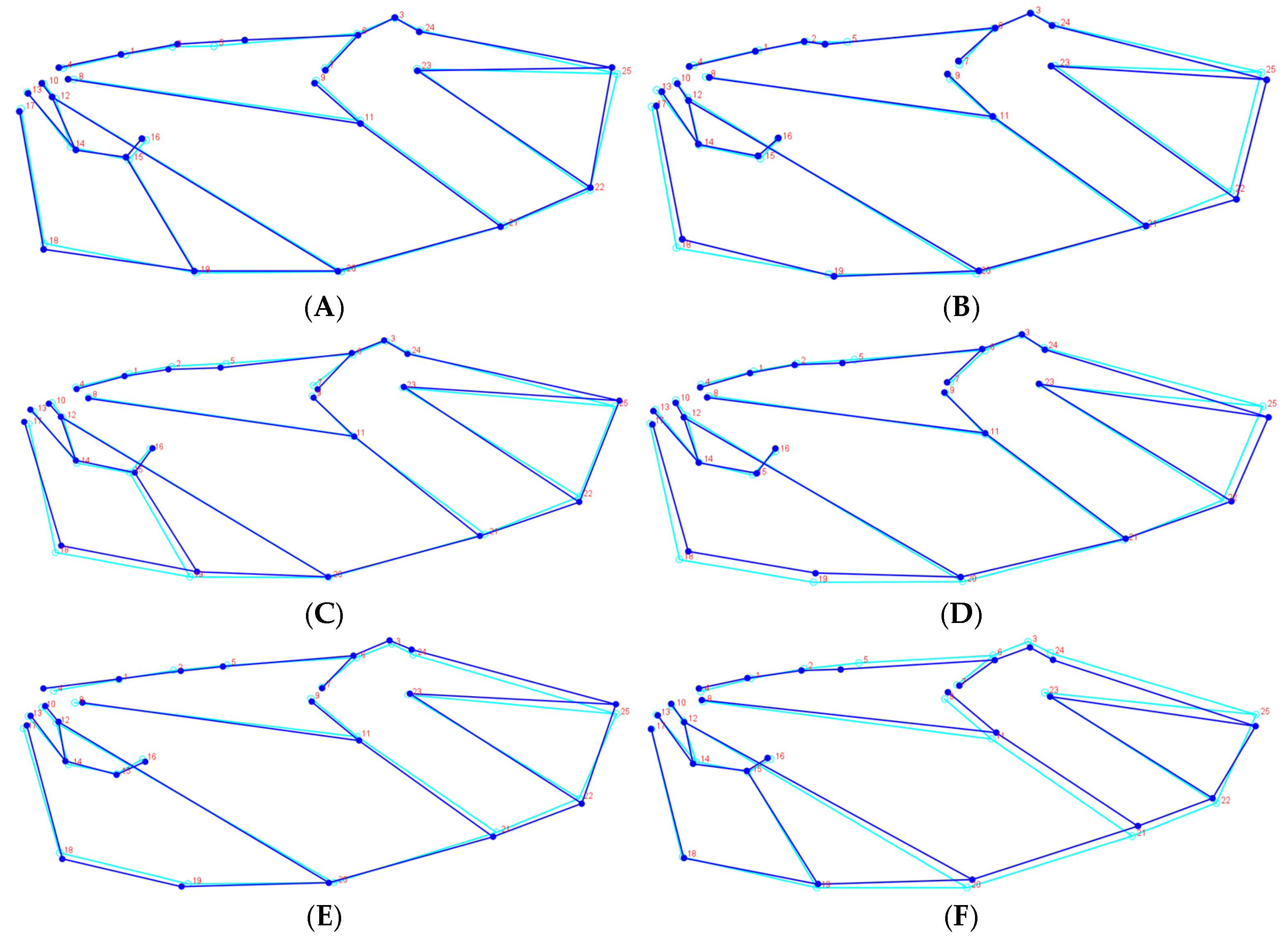
H. oblita Faldermann, 1835) examined in this study exhibit no clear sexual dimorphism and possess a high degree of morphological similarity, making visual sex determination challenging. The hind wings of these beetles have traditionally not been considered a key diagnostic feature for species identification. Therefore, the objective of this study is to evaluate the utility of hind-wing morphology in distinguishing between these beetle species or their sexes through the extraction and analysis of landmarks on hind wings, which is a fast method that does not require taxonomic expertise.