Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs
Simple Summary
Abstract
1. Introduction
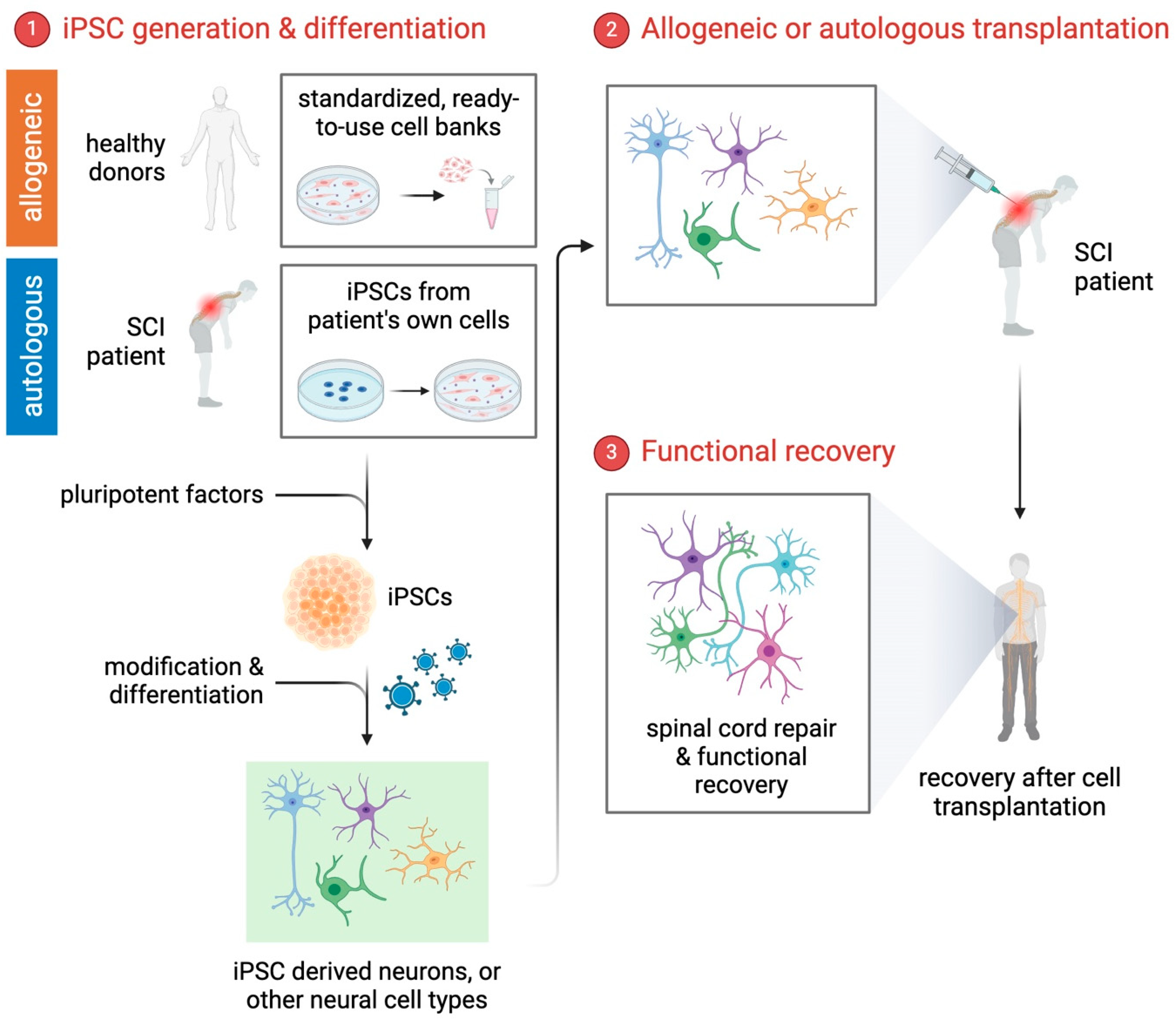
2. iPSCs: A Breakthrough in SCI Research
2.1. Patient-Specific Disease Modeling
2.2. Cellular Reprogramming and Differentiation
2.3. Drug Discovery and Screening
2.4. Precision Medicine and Personalized Therapies
2.5. Concerns Regarding Culture Media for iPSC Induction
3. Cell-Based Therapies for SCI
3.1. Neural Progenitor Cells
3.2. Oligodendrocyte Progenitor Cells
3.3. Astrocytes
3.4. Microglia
3.5. Combination Therapies
3.5.1. Biomaterial Scaffolds
3.5.2. Growth Factors
3.5.3. Electrical Stimulation
3.5.4. Therapeutic Potential of iPSC-Derived Exosomes
4. Clinical Research: Current Status and Prospects
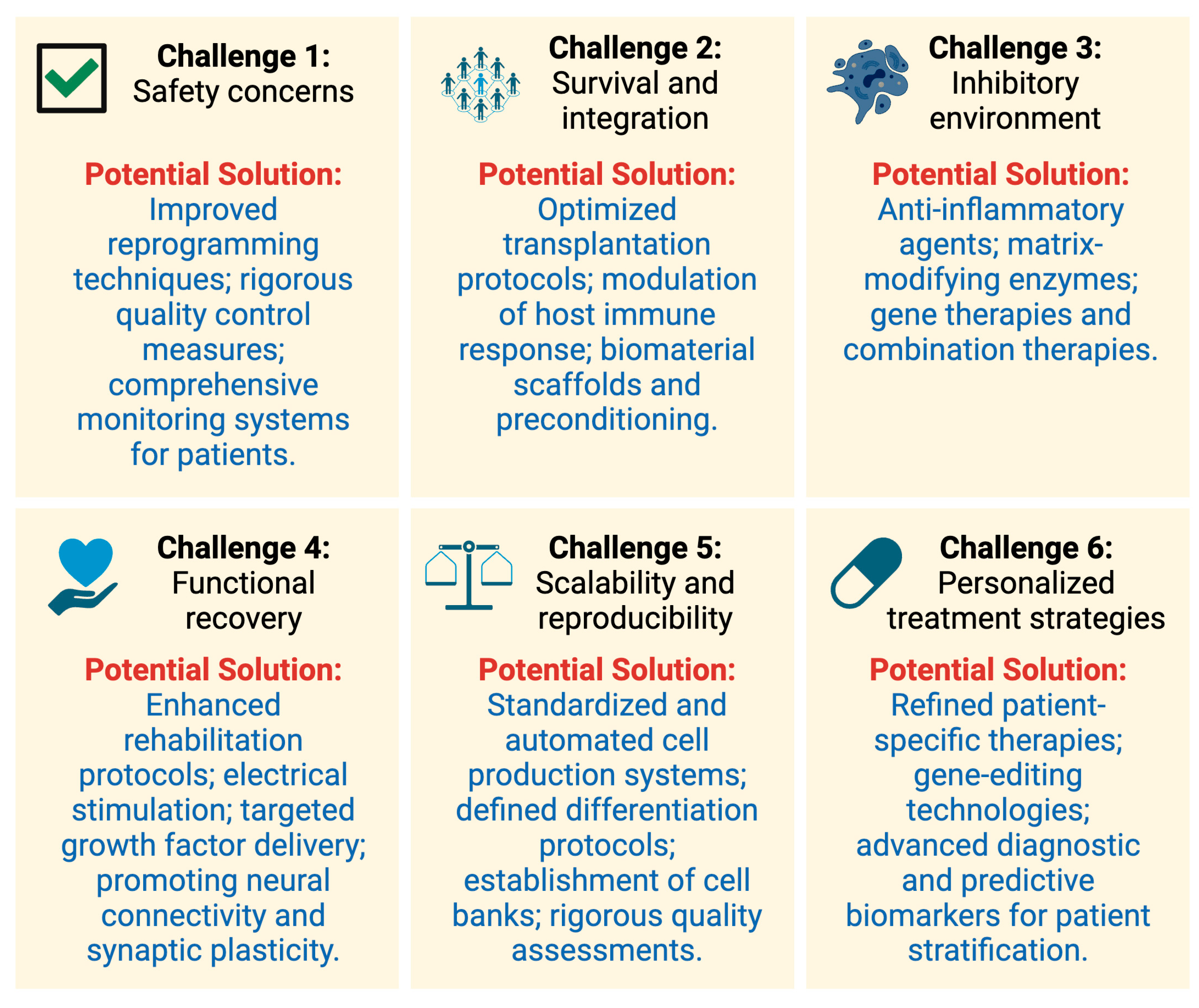
5. Challenges and the Road Ahead
5.1. Safety Concerns
5.2. Generation and Validation of Human iPSCs
5.3. Survival and Integration
5.4. Overcoming the Inhibitory Environment
5.5. Functional Recovery
5.6. Scalability and Reproducibility
5.7. Personalized Treatment Strategies
6. Conclusions
Funding
Conflicts of Interest
References
- Alhaffo, A.A.A.; Jubara, M.; Idan, G.F. Effect of Epidural Pulsed Radiofrequency with Neuro-Stimulation in Management of Thoracic Spinal Cord Injury. J. Popul. Ther. Clin. Pharmacol. 2023, 30, E273–E283. [Google Scholar] [CrossRef]
- Meyer, I.; E Richter, H. Impact of fecal incontinence and its treatment on quality of life in women. Women’s Health 2015, 11, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Kumar, R.; Gulia, K. The convergence of nanotechnology-stem cell, nanotopography-mechanobiology, and biotic-abiotic interfaces: Nanoscale tools for tackling the top killer, arteriosclerosis, strokes, and heart attacks. Nano Select. 2021, 2, 655–687. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflamm. 2019, 16, 178. [Google Scholar] [CrossRef]
- Zeng, C.-W. Multipotent mesenchymal stem cell-based therapies for spinal cord injury: current progress and future prospects. Biology 2023, 12, 653. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2016, 16, 115–130. [Google Scholar] [CrossRef]
- Liang, X.; Kristiansen, C.K.; Vatne, G.H.; Hong, Y.; Bindoff, L.A. Patient-specific neural progenitor cells derived from induced pluripotent stem cells offer a promise of good models for mitochondrial disease. Cell Tissue Res. 2020, 380, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2019, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xia, K.; Xu, A.; Yu, C.; Wang, C.; Zhu, J.; Huang, X.; Chen, Q.; Li, F.; Liang, C. Stem Cell Transplantation: A Promising Therapy for Spinal Cord Injury. Curr. Stem Cell Res. Ther. 2020, 15, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, Z.; Feng, S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022, 10, 35. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 2017, 94, 278–293. [Google Scholar] [CrossRef]
- Fermini, B.; Coyne, S.T.; Coyne, K.P. clinical trials in a dish: A perspective on the coming revolution in drug development. SLAS Discov. Adv. Sci. Drug Discov. 2018, 23, 765–776. [Google Scholar] [CrossRef]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic instability of iPSCs: Challenges towards their clinical applications. Stem Cell Rev. Rep. 2016, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pendse, S.; Vaidya, A.; Kale, V. Clinical applications of pluripotent stem cells and their derivatives: Current status and future perspectives. Regen. Med. 2022, 17, 677–690. [Google Scholar] [CrossRef]
- Bragança, J.; Lopes, J.A.; Mendes-Silva, L.; Santos, J.M.A. Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J. Stem Cells 2019, 11, 421. [Google Scholar] [CrossRef]
- Vanneaux, V. Induced pluripotent stem cells for clinical use. In Update on Mesenchymal and Induced Pluripotent Stem Cells; IntechOpen: London, UK, 2019. [Google Scholar]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Sharma, A.; Blériot, C.; Currenti, J.; Ginhoux, F. Oncofetal reprogramming in tumour development and progression. Nat. Rev. Cancer 2022, 22, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, F.; Pan, S.; Liu, Z. Stem cells: A potential treatment option for kidney diseases. Stem Cell Res. Ther. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Sada, T.S. The Potential of Stem Cells in Regenerative Medicine, Diseases Therapeutics and Research. Cell Biol. 2022, 10, 1. [Google Scholar]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying human neurological disorders using induced pluripotent stem cells: from 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 2019, 9, 565. [Google Scholar] [PubMed]
- Mertens, J.; Marchetto, M.C.; Bardy, C.; Gage, F.H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 2016, 17, 424–437. [Google Scholar] [CrossRef]
- Schweizer, P.A.; Darche, F.F.; Ullrich, N.D.; Geschwill, P.; Greber, B.; Rivinius, R.; Seyler, C.; Müller-Decker, K.; Draguhn, A.; Utikal, J.; et al. Subtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cells. Stem Cell Res. Ther. 2017, 8, 229. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Parrotta, E.I.; Scalise, S.; Scaramuzzino, L.; Cuda, G. Stem cells: The game changers of human cardiac disease modelling and regenerative medicine. Int. J. Mol. Sci. 2019, 20, 5760. [Google Scholar] [CrossRef]
- Petit, G.H.; Olsson, T.T.; Brundin, P. The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol. Appl. Neurobiol. 2014, 40, 60–70. [Google Scholar] [CrossRef]
- E Simonson, O.; Domogatskaya, A.; Volchkov, P.; Rodin, S. The safety of human pluripotent stem cells in clinical treatment. Ann. Med. 2015, 47, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.S.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Current approaches and future perspectives on strategies for the development of personalized tissue engineering therapies. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Nagoshi, N.; Okano, H. Applications of induced pluripotent stem cell technologies in spinal cord injury. J. Neurochem. 2017, 141, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. Recent progress in the regeneration of spinal cord injuries by induced pluripotent stem cells. Int. J. Mol. Sci. 2019, 20, 3838. [Google Scholar] [CrossRef]
- Cho, I.K.; Yang, B.; Forest, C.; Qian, L.; Chan, A.W.S. Amelioration of Huntington’s disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington’s disease monkeys. PLoS ONE 2019, 14, e0214156. [Google Scholar] [CrossRef]
- Barak, M.; Fedorova, V.; Pospisilova, V.; Raska, J.; Vochyanova, S.; Sedmik, J.; Hribkova, H.; Klimova, H.; Vanova, T.; Bohaciakova, D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022, 18, 792–820. [Google Scholar] [CrossRef]
- Nagoshi, N.; Okano, H. iPSC-derived neural precursor cells: Potential for cell transplantation therapy in spinal cord injury. Cell. Mol. Life Sci. 2017, 75, 989–1000. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, D.; Shen, H.; Zhao, Y.; Xu, B.; Fan, Y.; Sun, Z.; Chen, B.; Xue, W.; Shi, Y.; et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 2020, 8, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef]
- Ottoboni, L.; De Feo, D.; Merlini, A.; Martino, G. Commonalities in immune modulation between mesenchymal stem cells (MSCs) and neural stem/precursor cells (NPCs). Immunol. Lett. 2015, 168, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, W.-W.; Jiang, Z.; Feng, M.-J. Advances in treatment of neurodegenerative diseases: Perspectives for combination of stem cells with neurotrophic factors. World J. Stem Cells 2020, 12, 323–338. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jiang, M.Q.; Jesson, K.; Gu, X.; Logun, M.T.; Wu, A.; Gonsalves, N.; Karumbaiah, L.; Yu, S.P.; Wei, L. Glycosaminoglycan scaffolding and neural progenitor cell transplantation promotes regenerative immunomodulation in the mouse ischemic brain. Exp. Neurol. 2022, 357, 114177. [Google Scholar] [CrossRef] [PubMed]
- Bammidi, S.; Bali, P.; Kalra, J.; Anand, A. Transplantation efficacy of human ciliary epithelium cells from fetal eye and linve stem cells from umbilical cord blood in the murine retinal degeneration model of laser injury. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Czopka, T. Insights into mechanisms of central nervous system myelination using zebrafish. Glia 2015, 64, 333–349. [Google Scholar] [CrossRef]
- Irfan, M.; Evonuk, K.S.; DeSilva, T.M. Microglia phagocytose oligodendrocyte progenitor cells and synapses during early postnatal development: Implications for white versus gray matter maturation. FEBS J. 2021, 289, 2110–2127. [Google Scholar] [CrossRef]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Thiruvalluvan, A.; Czepiel, M.; Kap, Y.A.; Mantingh-Otter, I.; Vainchtein, I.; Kuipers, J.; Bijlard, M.; Baron, W.; Giepmans, B.; Brück, W.; et al. Survival and functionality of human induced pluripotent stem cell-derived oligodendrocytes in a nonhuman primate model for multiple sclerosis. Stem Cells Transl. Med. 2016, 5, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Fessler, R.G.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.D.; McKenna, S.L. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J. Neurosurg. Spine 2022, 37, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Jung, S.J.; Lee, J.S.; Lim, B.Y.; Kim, H.A.; Yoo, J.-E.; Kim, D.-W.; Leem, J.W. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Exp. Mol. Med. 2017, 49, e361. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lopez, M.; Fernandez-Muñoz, B.; Canovas, S. Pluripotent Stem Cells for Spinal Cord Injury Repair. Cells 2021, 10, 3334. [Google Scholar] [CrossRef]
- Yao, Z.-F.; Wang, Y.; Lin, Y.-H.; Wu, Y.; Zhu, A.-Y.; Wang, R.; Shen, L.; Xi, J.; Qi, Q.; Jiang, Z.-Q.; et al. Transplantation of PDGF-AA-overexpressing oligodendrocyte precursor cells promotes recovery in rat following spinal cord injury. Front. Cell. Neurosci. 2017, 11, 79. [Google Scholar] [CrossRef]
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No Longer underappreciated: The emerging concept of astrocyte heterogeneity in neuroscience. Brain Sci. 2020, 10, 168. [Google Scholar] [CrossRef]
- Hayashi, K.; Hashimoto, M.; Koda, M.; Naito, A.T.; Murata, A.; Okawa, A.; Takahashi, K.; Yamazaki, M. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell–derived astrocytes in a rat spinal cord injury model. J. Neurosurg. Spine 2011, 15, 582–593. [Google Scholar] [CrossRef]
- Strnadel, J.; Carromeu, C.; Bardy, C.; Navarro, M.; Platoshyn, O.; Glud, A.N.; Marsala, S.; Kafka, J.; Miyanohara, A.; Kato, T.; et al. Survival of syngeneic and allogeneic iPSC–derived neural precursors after spinal grafting in minipigs. Sci. Transl. Med. 2018, 10, eaam6651. [Google Scholar] [CrossRef]
- Arranz, A.M.; De Strooper, B. The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 2019, 18, 406–414. [Google Scholar] [CrossRef]
- Lukacova, N.; Kisucka, A.; Bimbova, K.K.; Bacova, M.; Ileninova, M.; Kuruc, T.; Galik, J. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int. J. Mol. Sci. 2021, 22, 13577. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of microglia in modulating neuroinflammation after spinal cord injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.R.; Wilems, T.S.; Sakiyama-Elbert, S.E. Stem cells for spinal cord injury: Strategies to inform differentiation and transplantation. Biotechnol. Bioeng. 2016, 114, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Pang, Q.-M.; Chen, S.-Y.; Xu, Q.-J.; Fu, S.-P.; Yang, Y.-C.; Zou, W.-H.; Zhang, M.; Liu, J.; Wan, W.-H.; Peng, J.-C.; et al. Neuroinflammation and scarring after spinal cord injury: Therapeutic roles of mscs on inflammation and glial scar. Front. Immunol. 2021, 12, 751021. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, A.M.; D’angelo, A.; Ionescu, R.-B.; Krzak, G.; Willis, C.M.; Pluchino, S. The role of neural stem cells in regulating glial scar formation and repair. Cell Tissue Res. 2021, 387, 399–414. [Google Scholar] [CrossRef]
- Raspa, A.; Carminati, L.; Pugliese, R.; Fontana, F.; Gelain, F. Self-assembling peptide hydrogels for the stabilization and sustained release of active Chondroitinase ABC in vitro and in spinal cord injuries. J. Control. Release 2021, 330, 1208–1219. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the neurovascular Unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.W. Immune Cell-NSPC Interactions: Friend or Foe in CNS Injury and Repair? Differentiation 2025, 143, 100855. [Google Scholar] [CrossRef]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2015, 75, 13–24. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Amo-Aparicio, J.; Martinez-Muriana, A.; López-Vales, R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 2016, 64, 2079–2092. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, R.; Wang, H.; Hou, Y.; Li, Y.; Zhu, J.; Xu, F.; Fu, C. Biomaterials delivery strategies to repair spinal cord injury by modulating macrophage phenotypes. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Niskanen, J.; Kälvälä, S.; Lehtonen, Š. Utilising induced pluripotent stem cells in neurodegenerative disease research: focus on glia. Int. J. Mol. Sci. 2021, 22, 4334. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. the contribution of microglia to neuroinflammation in parkinson’s disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Reich, M.; Paris, I.; Ebeling, M.; Dahm, N.; Schweitzer, C.; Reinhardt, D.; Schmucki, R.; Prasad, M.; Köchl, F.; Leist, M.; et al. Alzheimer’s risk gene TREM2 determines func-tional properties of new type of human iPSC-derived microglia. Front. Immunol. 2021, 11, 617860. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Lu, Y.; Do, K.; Mize, T.; Wu, X.; Chen, X.; Chen, J. Validation of induced microglia-like cells (iMG cells) for future studies of brain diseases. Front. Cell. Neurosci. 2021, 15, 629279. [Google Scholar] [CrossRef]
- Qu, W.; Li, L. Microglial TREM2 at the Intersection of brain aging and Alzheimer’s disease. Neuroscientist 2021, 29, 302–316. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, Z.; Qie, S. The Implications of Microglial Regulation in Neuroplasticity-Dependent Stroke Recovery. Biomolecules 2023, 13, 571. [Google Scholar] [CrossRef]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef]
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef]
- Beliën, H.; Evens, L.; Hendrikx, M.; Bito, V.; Bronckaers, A. Combining stem cells in myocardial infarction: The road to superior repair? Med. Res. Rev. 2022, 42, 343–373. [Google Scholar]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 2015, 95, 255–266. [Google Scholar] [CrossRef]
- Eugenis, I.; Wu, D.; Rando, T.A. Cells, scaffolds, and bioactive factors: Engineering strategies for improving regeneration following volumetric muscle loss. Biomaterials 2021, 278, 121173. [Google Scholar] [CrossRef]
- Hosseini, M.; Shafiee, A. Engineering Bioactive Scaffolds for Skin Regeneration. Small 2021, 17, 2101384. [Google Scholar] [CrossRef]
- Assunção-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells Int. 2015, 2015, 948040. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.A.; Windebank, A.J.; Moore, M.J.; Spinner, R.J.; Currier, B.L.; Yaszemski, M.J. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery 2002, 51, 742–752. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent advancements in bone tissue engineering: integrating smart scaffold technologies and bio-responsive systems for enhanced regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2020, 9, 567–584. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, K.; Luo, S.; Li, F.; Zuo, X.; Fan, C.; Li, Q. Programmable DNA hydrogels as artificial extracellular matrix. Small 2022, 18, 2107640. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, L.; Khakbiz, M.; Moghaddam, M.M.; Bonakdar, S. A biomaterials approach to Schwann cell development in neural tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2425–2446. [Google Scholar] [CrossRef] [PubMed]
- Idrisova, K.F.; Zeinalova, A.K.; Masgutova, G.A.; Bogov, A.A.; Allegrucci, C.; Syromiatnikova, V.Y.; Salafutdinov, I.I.; Garanina, E.E.; Andreeva, D.I.; Kadyrov, A.A.; et al. Application of neurotrophic and proangiogenic factors as therapy after peripheral nervous system injury. Neural Regen. Res. 2022, 17, 1240. [Google Scholar]
- Zhang, H.; Guo, J.; Wang, Y.; Shang, L.; Chai, R.; Zhao, Y. Natural Polymer-Derived Bioscaffolds for Peripheral Nerve Re-generation. Adv. Funct. Mater. 2022, 32, 2203829. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2022, 234, 108043. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Li, J.; Xiang, X.; Xu, H.; Shi, Y. Cilostazol Promotes Angiogenesis and Increases Cell Proliferation After Myocardial Ischemia–Reperfusion Injury Through a cAMP-Dependent Mechanism. Cardiovasc. Eng. Technol. 2019, 10, 638–647. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Sun, J.-H.; Shi, L.-Y.; Huang, M.-Y.; Huang, L.-J.; Lin, Z.-J.; Lin, Q.-Y.; Lai, B.-Q.; Ma, Y.-H.; et al. An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact. Mater. 2021, 6, 3766–3781. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Fooladi, A.A.I. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.H. Biomaterials and neural regeneration. Neural Regen. Res. 2020, 15, 1243. [Google Scholar] [PubMed]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Pluripotent Stem Cells in Clinical Setting—New Developments and Overview of Current Status. Stem Cells 2022, 40, 791–801. [Google Scholar] [CrossRef]
- Pizzolato, C.; Gunduz, M.A.; Palipana, D.; Wu, J.; Grant, G.; Hall, S.; Dennison, R.; Zafonte, R.D.; Lloyd, D.G.; Teng, Y.D. Non-invasive approaches to functional recovery after spinal cord injury: Therapeutic targets and multimodal device interventions. Exp. Neurol. 2021, 339, 113612. [Google Scholar] [CrossRef]
- Fang, J.; Li, J.J.; Zhong, X.; Zhou, Y.; Lee, R.J.; Cheng, K.; Li, S. Engineering stem cell therapeutics for cardiac repair. J. Mol. Cell. Cardiol. 2022, 171, 56–68. [Google Scholar] [CrossRef]
- Tsuji, O.; Sugai, K.; Yamaguchi, R.; Tashiro, S.; Nagoshi, N.; Kohyama, J.; Iida, T.; Ohkubo, T.; Itakura, G.; Isoda, M.; et al. Concise review: Laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells 2018, 37, 6–13. [Google Scholar] [CrossRef]
- Zhang, W.; Ross, P.J.; Ellis, J.; Salter, M.W. Targeting NMDA receptors in neuropsychiatric disorders by drug screening on human neurons derived from pluripotent stem cells. Transl. Psychiatry 2022, 12, 243. [Google Scholar] [CrossRef]
- Bao, S.C.; Khan, A.; Song, R.; Tong, R.K.Y. Rewiring the lesioned brain: Electrical stimulation for post-stroke motor resto-ration. J. Stroke 2020, 22, 47. [Google Scholar] [CrossRef]
- Lai, B.-Q.; Zeng, X.; Han, W.-T.; Che, M.-T.; Ding, Y.; Li, G.; Zeng, Y.-S. Stem cell-derived neuronal relay strategies and functional electrical stimulation for treatment of spinal cord injury. Biomaterials 2021, 279, 121211. [Google Scholar] [CrossRef] [PubMed]
- Krut, Z.; Pelled, G.; Gazit, D.; Gazit, Z. Stem cells and exosomes: New therapies for intervertebral disc degeneration. Cells 2021, 10, 2241. [Google Scholar] [CrossRef]
- Zeng, C.-W. Advancing spinal cord injury treatment through stem cell therapy: A comprehensive review of cell types, challenges, and emerging technologies in regenerative medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, H.-S.; Hong, I.-S. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.-T.; Guo, H.-D. Small extracellular vesicles derived from induced pluripotent stem cells in the treatment of myocardial injury. Int. J. Mol. Sci. 2023, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.-F.; Wu, J.-H.; Cao, J.; Ma, T.; Li, L.-M.; Feng, S.-Q.; Gao, J.-Q. Rapid and effective treatment of traumatic spinal cord injury using stem cell derived exosomes. Asian J. Pharm. Sci. 2021, 16, 806–815. [Google Scholar] [CrossRef]
- Ahmed, I.; Johnston, R.J., Jr.; Singh, M.S. Pluripotent stem cell therapy for retinal diseases. Ann. Transl. Med. 2021, 9, 1279. [Google Scholar] [CrossRef]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef]
- Dahlke, J.; Schott, J.W.; Barbosa, P.V.; Klatt, D.; Selich, A.; Lachmann, N.; Morgan, M.; Moritz, T.; Schambach, A. Efficient genetic safety switches for future application of iPSC-derived cell transplants. J. Pers. Med. 2021, 11, 565. [Google Scholar] [CrossRef]
- Masuda, K.; Kawamoto, H. Possible NK cell-mediated immune responses against iPSC-derived cells in allogeneic trans-plantation settings. Inflamm. Regen. 2021, 41, 2. [Google Scholar] [PubMed]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef]
- Bassett, A.R. Editing the genome of hiPSC with CRISPR/Cas9: Disease models. Mamm. Genome 2017, 28, 348–364. [Google Scholar] [CrossRef]
- Kawamata, S.; Kanemura, H.; Sakai, N.; Takahashi, M.; Go, M.J. Design of a tumorigenicity test for induced pluripotent stem cell (iPSC)-derived cell products. J. Clin. Med. 2015, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous induced pluripotent stem cell–based cell therapies: Promise, progress, and challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, R.; Li, W.; Lin, J.; Stamm, C.; Steinhoff, G.; Ma, N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell. Mol. Life Sci. 2019, 76, 1681–1695. [Google Scholar] [CrossRef]
- Ahmadian-Moghadam, H.; Sadat-Shirazi, M.S.; Zarrindast, M.R. Therapeutic potential of stem cells for treatment of neu-rodegenerative diseases. Biotechnol. Lett. 2020, 42, 1073–1101. [Google Scholar]
- Lingam, S.; Liu, Z.; Yang, B.; Wong, W.; Parikh, B.H.; Ong, J.Y.; Goh, D.; Wong, D.S.L.; Tan, Q.S.W.; Tan, G.S.W.; et al. cGMP-grade human iPSC-derived retinal photoreceptor precursor cells rescue cone photoreceptor damage in non-human primates. Stem Cell Res. Ther. 2021, 12, 464. [Google Scholar] [CrossRef]
- Attwood, S.W.; Edel, M.J. iPS-cell technology and the problem of genetic instability—Can it ever be safe for clinical use? J. Clin. Med. 2019, 8, 288. [Google Scholar] [CrossRef]
- Choudhury, S.; Surendran, N.; Das, A. Recent advances in the induced pluripotent stem cell-based skin regeneration. Wound Repair Regen. 2021, 29, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; DeVine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M.; et al. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, W.; Zhu, S.; Zhu, J.; Shi, Y.; Lin, T.; Hao, E.; Hayek, A.; Deng, H.; Ding, S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 2009, 4, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A more efficient method to generate integrationfree human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef]
- Pozner, T.; Grandizio, C.; Mitchell, M.W.; Turan, N.; Scheinfeldt, L. Human iPSC Reprogramming Success: The Impact of Approaches and Source Materials. Stem Cells Int. 2025, 2025, 2223645. [Google Scholar] [CrossRef]
- Barbuti, P.A.; Barker, R.A.; Brundin, P.; Przedborski, S.; Papa, S.M.; Kalia, L.V.; Mochizuki, H.; MDS Scientific Issues Committee. Recent advances in the development of stem-cell-derived dopaminergic neuronal transplant therapies for Parkinson’s disease. Mov. Disord. 2021, 36, 1772–1780. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Jiang, A.Y.; Eufrásio-Da-Silva, T.; Dolatshahi-Pirouz, A.; Langer, R.; Orive, G.; Grattoni, A. Emerging immunomodulatory strategies for cell therapeutics. Trends Biotechnol. 2022, 41, 358–373. [Google Scholar] [CrossRef]
- Layrolle, P.; Payoux, P.; Chavanas, S. Message in a Scaffold: Natural Biomaterials for Three-Dimensional (3D) Bioprinting of Human Brain Organoids. Biomolecules 2022, 13, 25. [Google Scholar] [CrossRef]
- Abati, E.; Bresolin, N.; Comi, G.P.; Corti, S. Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol. Neurobiol. 2018, 56, 3356–3367. [Google Scholar] [CrossRef]
- Kwon, H.; Paschos, N.K.; Hu, J.C.; Athanasiou, K. Articular cartilage tissue engineering: The role of signaling molecules. Cell. Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, G.; Shi, B.; Ni, H.; Wang, J.; Qiu, Y.; Yang, L.; Zhu, Z.; Yi, X.; Du, X.; et al. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv. Health Mater. 2023, 12, e2300123. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-modulating hydrogels for osteoarthritis cartilage tissue engineering. Cells 2020, 9, 419. [Google Scholar] [CrossRef]
- Heider, J.; Vogel, S.; Volkmer, H.; Breitmeyer, R. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int. J. Mol. Sci. 2021, 22, 10254. [Google Scholar] [CrossRef]
- Makuloluwa, A.K.; Hamill, K.J.; Rauz, S.; Bosworth, L.; Haneef, A.; Romano, V.; Williams, R.L.; Dartt, D.A.; Kaye, S.B. The conjunctival extracellular matrix, related disorders and development of substrates for conjunctival restoration. Ocul. Surf. 2023, 28, 322–335. [Google Scholar] [CrossRef]
- Zavvarian, M.-M.; Toossi, A.; Khazaei, M.; Hong, J.; Fehlings, M. Novel innovations in cell and gene therapies for spinal cord injury. F1000Research 2020, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, J.A.; Przepiorski, A.; D’souza, R.F.; Sreebhavan, S.; Wolvetang, E.J.; Harrison, P.T.; Davidson, A.J.; Holm, T.M. Use of human induced pluripotent stem cells and kidney organoids to develop a cysteamine/mtor inhibition combination therapy for cystinosis. J. Am. Soc. Nephrol. 2020, 31, 962–982. [Google Scholar] [CrossRef] [PubMed]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C 2020, 110, 110741. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, H.; Zhu, Y.; Yan, L.; Liu, R.; Wang, G.; Wang, B.; Zhao, B. Animal models for treating spinal cord injury using biomaterials-based tissue engineering strategies. tissue Eng. Part B Rev. 2022, 28, 79–100. [Google Scholar] [CrossRef]
- Onitsuka, T.; Hirano, Y.; Nakazawa, T.; Ichihashi, K.; Miura, K.; Inada, K.; Mitoma, R.; Yasui-Furukori, N.; Hashimoto, R. Toward recovery in schizophrenia: Current concepts, findings, and future research directions. Psychiatry Clin. Neurosci. 2022, 76, 282–291. [Google Scholar] [CrossRef]
- Kitagawa, T.; Nagoshi, N.; Okano, H.; Nakamura, M. A Narrative Review of Advances in Neural Precursor Cell Transplantation Therapies for Spinal Cord Injury. Neurospine 2022, 19, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Eaves, E.; Dams-O’connor, K.; Ho, L.; Leung, E.; Wong, E.; Carpenter, D.; Ng, J.; Gordon, W.; Pasinetti, G. Diffuse disconnectivity in traumatic brain injury: A resting state fMRI and DTI study. Transl. Neurosci. 2012, 3, 9–14. [Google Scholar] [CrossRef]
- Sun, P.; Murphy, R.K.J.; Gamble, P.; George, A.; Song, S.-K.; Ray, W.Z. Diffusion assessment of cortical changes, induced by traumatic spinal cord injury. Brain Sci. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Colter, J.; Murari, K.; Biernaskie, J.; Kallos, M.S. Induced pluripotency in the context of stem cell expansion bioprocess de-velopment, optimization, and manufacturing: A roadmap to the clinic. NPJ Regen. Med. 2021, 6, 72. [Google Scholar] [PubMed]
- Dashtban, M.; Panchalingam, K.M.; Shafa, M.; Baghbaderani, B.A. Addressing manufacturing challenges for commercialization of iPSC-based therapies. Stem Cells Good Manuf. Pract. Methods Protoc. Regul. 2021, 2286, 179–198. [Google Scholar]
- Abraham, E.; Ahmadian, B.B.; Holderness, K.; Levinson, Y.; McAfee, E. Platforms for manufacturing allogeneic, autologous and iPSC cell therapy products: An industry perspective. Adv. Biochem. Eng. Biotechnol. 2018, 165, 323–350. [Google Scholar]
- Borys, B.S.; So, T.; Colter, J.; Dang, T.; Roberts, E.L.; Revay, T.; Larijani, L.; Krawetz, R.; Lewis, I.; Argiropoulos, B.; et al. Optimized serial expansion of human induced pluripotent stem cells using low-density inoculation to generate clinically relevant quantities in vertical-wheel bioreactors. Stem Cells Transl. Med. 2020, 9, 1036–1052. [Google Scholar] [CrossRef]
- Okano, H.; Sipp, D. New trends in cellular therapy. Development 2020, 147, dev192567. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Hsiao, Y.-J.; Chang, K.-J.; Foustine, S.; Ko, Y.-L.; Tsai, Y.-C.; Tai, H.-Y.; Ko, Y.-C.; Chiou, S.-H.; Lin, T.-C.; et al. Pluripotent Stem Cells in Clinical Cell Transplantation: Focusing on Induced Pluripotent Stem Cell-Derived RPE Cell Therapy in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 13794. [Google Scholar] [CrossRef]
| Cell Type | Research Methods | Therapeutic Potential | References |
|---|---|---|---|
| iPSC-derived neural progenitor cells (NPCs) | Differentiation, transplantation, functional recovery assessment | Promote axonal regeneration, remyelination, synapse formation, modulate local immune response | [38,39,40,41,42] |
| iPSC-derived oligodendrocyte progenitor cells (OPCs) | Differentiation, transplantation, remyelination assessment | Promote remyelination, improve neuronal function, secrete neurotrophic factors, modulate inflammation | [49,50,51,52,53,54,55,56] |
| iPSC-derived astrocytes | Differentiation, transplantation, functional recovery assessment | Modulate inflammation, promote tissue repair, provide structural support to regenerating axons, regulate glial scar formation | [57,58,59,60,61,62,63,64,65,66,67] |
| iPSC-derived microglia | Differentiation, transplantation, functional recovery assessment | Modulate inflammation, regulate immune response, investigate signaling pathways | [68,69,70,71,72,73,74,75,76,77,78,79,80] |
| Combination therapies | Co-transplantation of different cell types, incorporation of other therapeutic strategies (biomaterial scaffolds, growth factors, electrical stimulation) | Enhance neuroprotection, promote cell survival, support functional recovery through combinatorial approaches | [81,82,83,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.-W. Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs. Biology 2025, 14, 314. https://doi.org/10.3390/biology14030314
Zeng C-W. Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs. Biology. 2025; 14(3):314. https://doi.org/10.3390/biology14030314
Chicago/Turabian StyleZeng, Chih-Wei. 2025. "Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs" Biology 14, no. 3: 314. https://doi.org/10.3390/biology14030314
APA StyleZeng, C.-W. (2025). Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs. Biology, 14(3), 314. https://doi.org/10.3390/biology14030314






