Characteristics of Fungal Communities in Red Mud/Phosphogypsum-Based Artificial Soils
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Red Mud/Phosphogypsum-Based Artificial Soils
2.2. Sample Collection and Determination
2.3. Data Analysis
3. Results
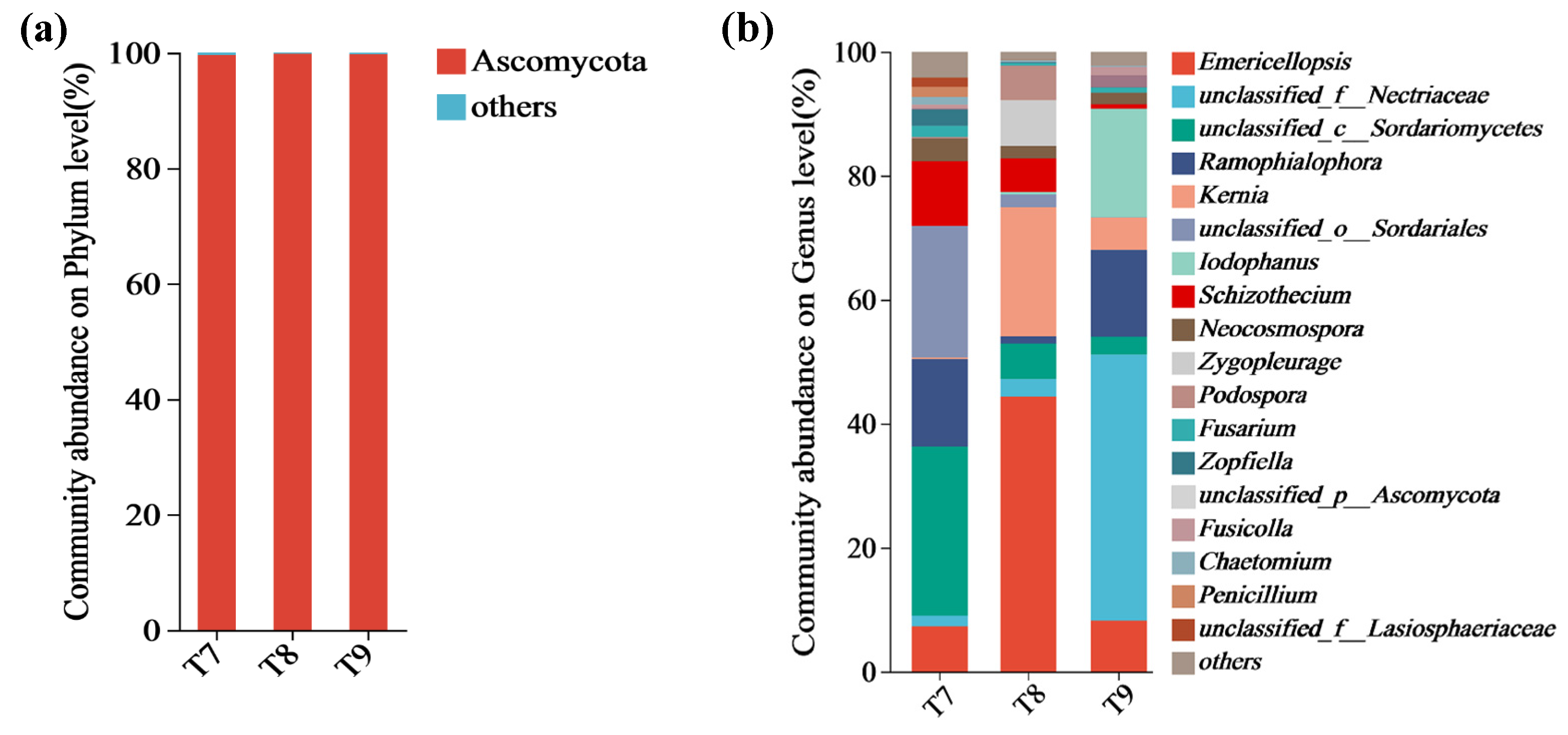
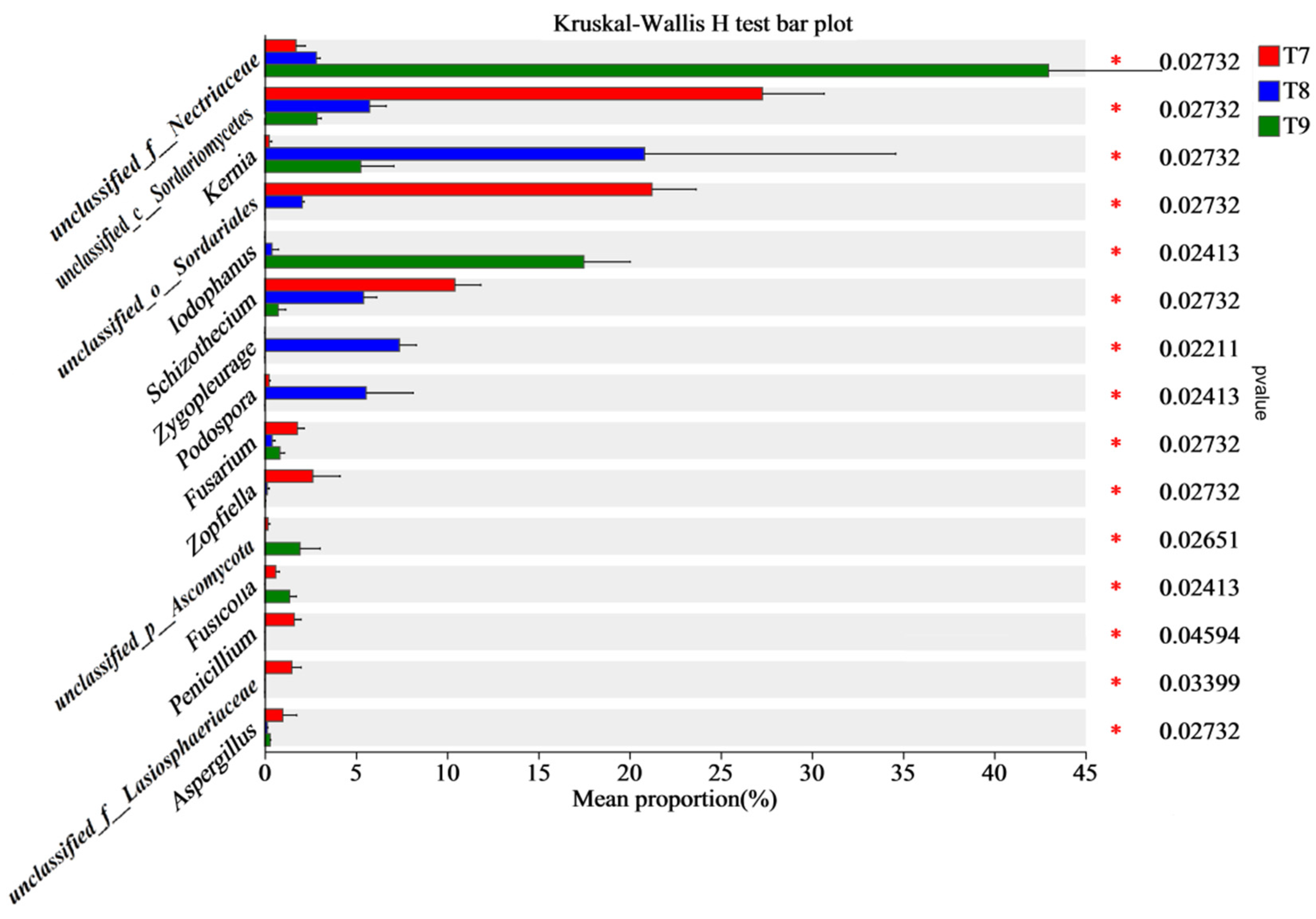
3.1. Composition Characteristics of Fungal Communities
3.2. Alpha Diversity Indices of Fungal Communities
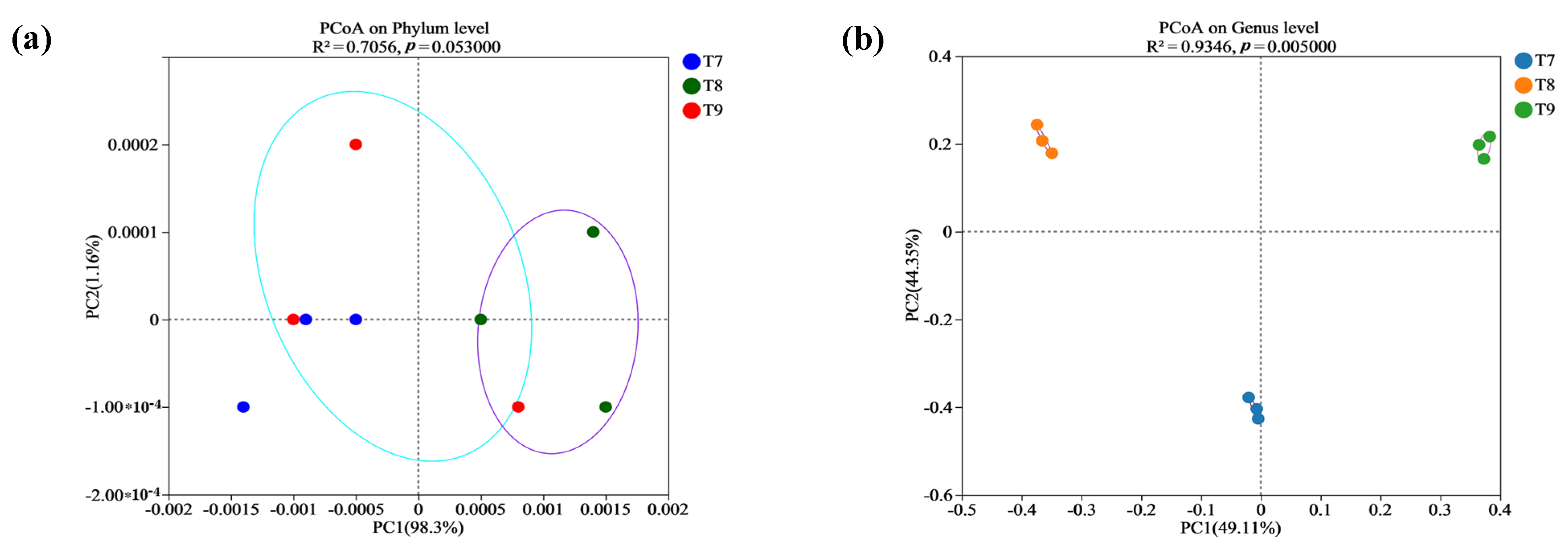
3.3. Beta Diversity of Fungal Communities
3.4. Functional Prediction of Fungal Communities
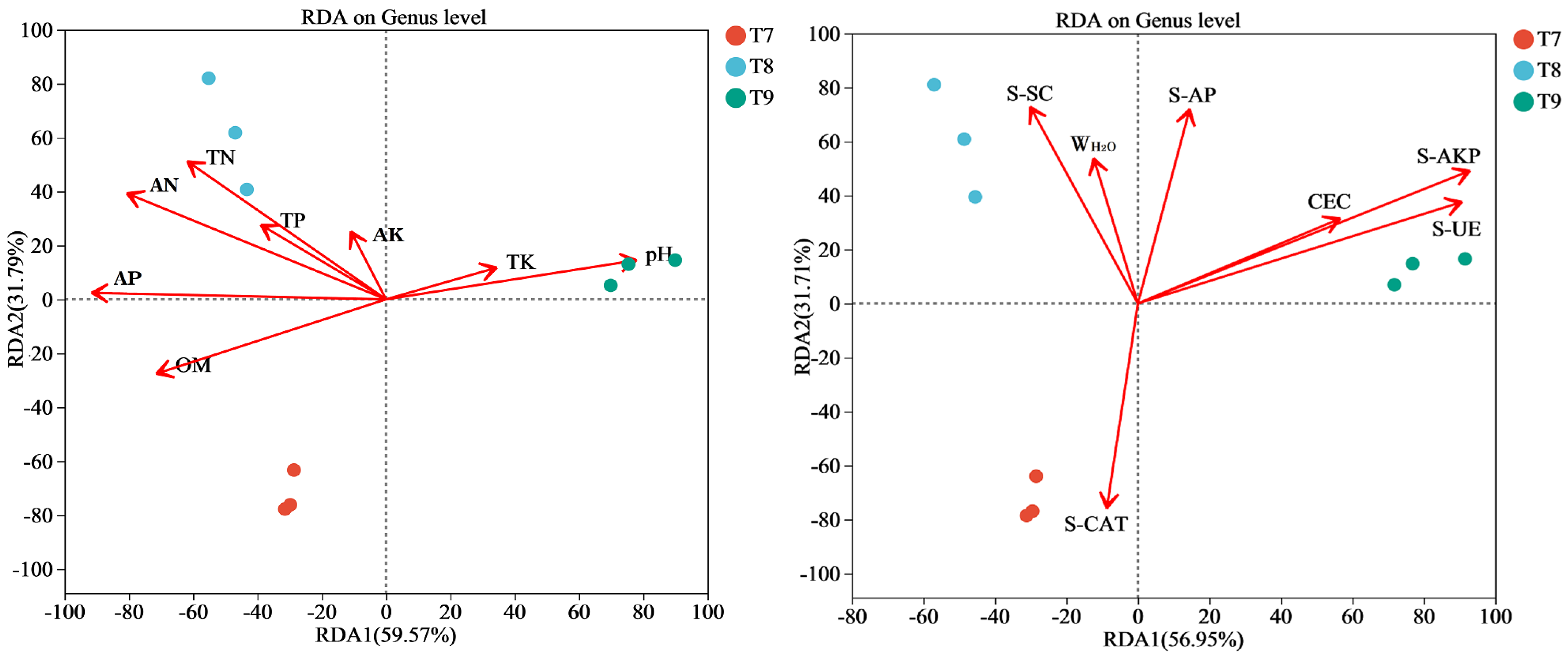
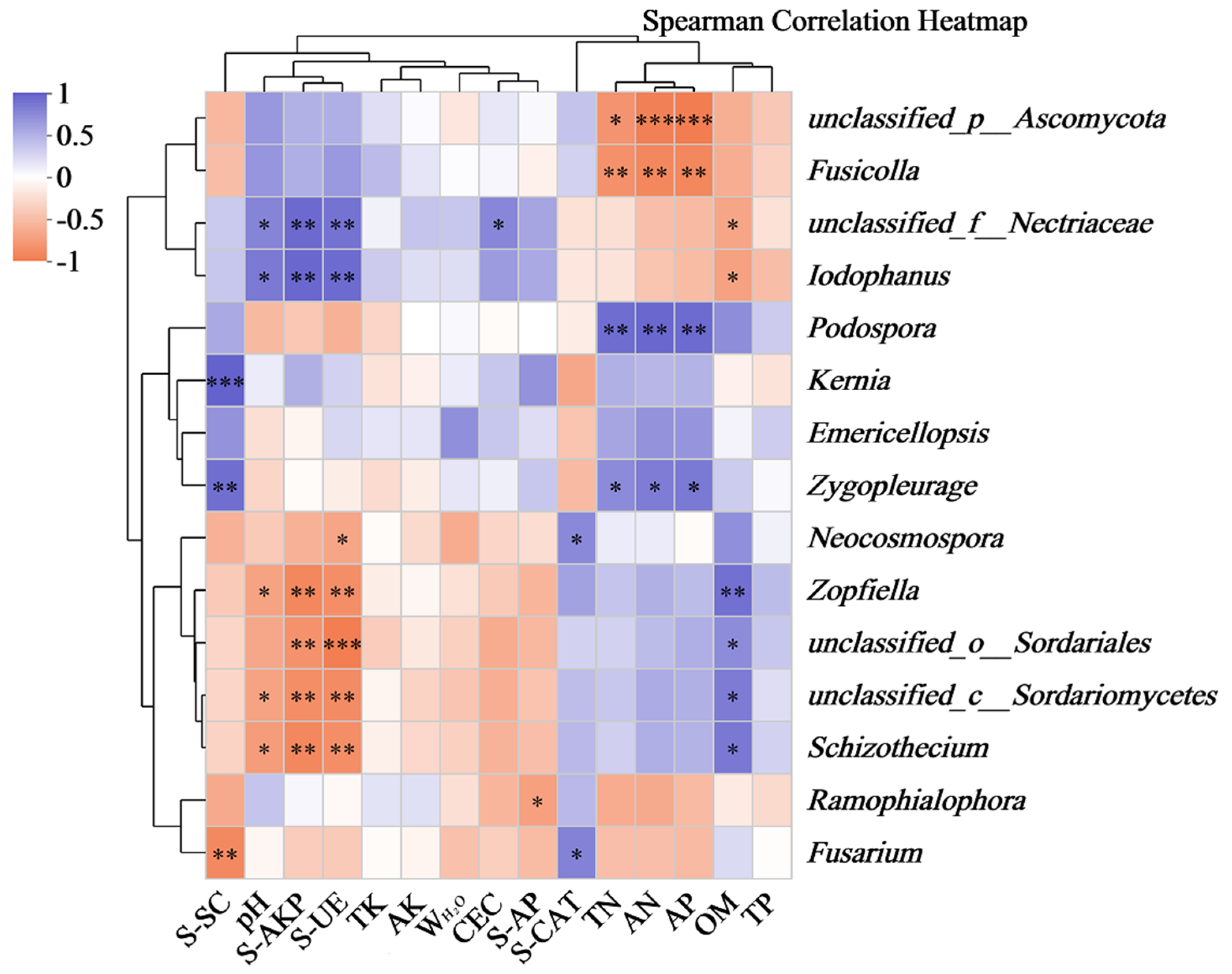
3.5. Relationships Between Fungal Communities and Different Environmental Factors
4. Discussion
4.1. Fungi—Key Drivers of Artificial Soil Development and Ecological Enhancement
4.2. Formation and Functional Characteristics of Fungal Communities in Artificial Soils
4.3. Relationship Between Fungal Communities and Different Environmental Factors in Artificial Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anagnostopoulos, A.; Navarro, M.E.; Stefanidou, M.; Ding, Y.; Gaidajis, G. Red mud-molten salt composites for medium-high temperature thermal energy storage and waste heat recovery applications. J. Hazard. Mater. 2021, 413, 125407. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xiang, L. Investigating the novel process for thorough removal of eutectic phosphate impurities from phosphogypsum. J. Mater. Res. Technol. 2023, 24, 5980–5990. [Google Scholar] [CrossRef]
- Cui, W.; Cui, Q.; Dong, X.; Liu, J.; Song, K.; Xie, M.; Yao, X. Current research status and emerging trends in utilization of red mud resources: A study based on bibliometric network analysis. Constr. Build. Mater. 2024, 442, 137605. [Google Scholar] [CrossRef]
- Yu, H.; Liu, L.; Liu, M.; Zhang, H.; Mao, R. Waste control by waste: Recovering iron from red mud with the effect of Phosphogypsum-included additive. Resour. Conserv. Recycl. 2024, 206, 107641. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Salo, M.; Mäkinen, J.; Yang, J.; Kurhila, M.; Koukkari, P. Continuous biological sulfate reduction from phosphogypsum waste leachate. Hydrometallurgy 2018, 180, 1–6. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Shi, Y.; Dong, T.; Lu, S.; Tian, H. A new route for separation and recovery of Fe, Al and Ti from red mud. Resour. Conserv. Recycl. 2021, 168, 105314. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Peng, J.; Li, G.; Yin, S. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances. Constr. Build. Mater. 2022, 318, 126021. [Google Scholar] [CrossRef]
- Yulikasari, A.; Tangahu, B.V.; Nurhayati, E.; Arliyani, I.; Mashudi Titah, H.S.; Lam, Y.M.; Wang, Y. A comprehensive review of integrated phytoremediation and nanoparticle methods for heavy metal in red mud. Ecotoxicol. Environ. Saf. 2024, 288, 117381. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyirő-Kósa, I.; Csákberényi-Malasics, D.; Tóth, Á.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Adeoye, C.; Gupta, J.; Demers, N.; Adhikari, A. Variations of radon and airborne particulate matter near three large phosphogypsum stacks in Florida. Environ. Monit. Assess. 2021, 193, 284. [Google Scholar] [CrossRef] [PubMed]
- Rékási, M.; Feigl, V.; Uzinger, N.; Gruiz, K.; Makó, A.; Anton, A. Effects of leaching from alkaline red mud on soil biota: Modelling the conditions after the Hungarian red mud disaster. Chem. Ecol. 2013, 29, 709–723. [Google Scholar] [CrossRef]
- Millán-Becerro, R.; Pérez-López, R.; Macías, F.; Cánovas, C.R. Design and optimization of sustainable passive treatment systems for phosphogypsum leachates in an orphan disposal site. J. Environ. Manag. 2020, 275, 111251. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Lazar, I.; Petrisor, I.G. Application of a vegetative cover on phosphogypsum stacks. Miner. Eng. 1999, 12, 175–185. [Google Scholar] [CrossRef]
- Moyo, A.; Parbhakar-Fox, A.; Meffre, S.; Cooke, D.R. Alkaline industrial wastes—Characteristics, environmental risks, and potential for mine waste management. Environ. Pollut. 2023, 323, 121292. [Google Scholar] [CrossRef]
- Kutepova, N.A.; Korobanova, T.N. Features of phosphogypsym dump deformation development. Min. Sci. Technol. 2017, 1, 31–39. [Google Scholar] [CrossRef][Green Version]
- Chang, S.; Dong, X.; Liu, X.; Xu, X.; Zhang, H.; Huang, Y. Study on the characteristics and evolution laws of seepage damage in red mud tailings dams. Water 2024, 16, 1487. [Google Scholar] [CrossRef]
- Chang, S.; Dong, X.; Liu, X.; Zhang, H.; Huang, Y. Experimental study on the evolutionary law of transient saturation zones in a red mud dam under rainfall conditions. Sustainability 2024, 16, 3903. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and comprehensive utilization of red mud research progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Tran, C.T.; Nguyen, A.T.N.; Nguyen, L.T.K.; Bach, N.H.; Nguyen, M.N. Effect of polyDADMAC on aggregation of clay-size particles in red mud: Implications for immobilization practices. Ecotoxicol. Environ. Saf. 2019, 168, 192–197. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Chen, L.; Xue, B.; Wang, G.; Zhu, G.; Gou, W.; Yang, D. Potential of artificial soil preparation for vegetation restoration using red mud and phosphogypsum. Sci. Total Environ. 2024, 941, 173553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Xue, B.; Chen, L.; Wang, G.; Wang, J.; Wan, H.; Lin, X.; Zhu, G. Simulation of red mud/phosphogypsum-based artificial soil engineering applications in vegetation restoration and ecological reconstruction. Sci. Total Environ. 2024, 951, 175656. [Google Scholar] [CrossRef] [PubMed]
- Knelman, J.E.; Schmidt, S.K.; Graham, E.B. Cyanobacteria in early soil development of deglaciated forefields: Dominance of non-heterocytous filamentous cyanobacteria and phosphorus limitation of N-fixing Nostocales. Soil Biol. Biochem. 2021, 154, 108127. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil microbiome: Diversity, benefits and interactions with plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zhang, L.; Wan, H.; Deng, F.; Zhao, Z.; Wang, J. Characteristics of Bacterial Community Structure and Function in Artificial Soil Prepared Using Red Mud and Phosphogypsum. Microorganisms 2024, 12, 1886. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Lim, A.; Atmaja, P.C.; Rustiani, S. Bio-mediated soil improvement of loose sand with fungus. J. Rock Mech. Geotech. Eng. 2020, 12, 180–187. [Google Scholar] [CrossRef]
- Borges, J.; Cardoso, P.; Lopes, I.; Figueira, E.; Venâncio, C. Exploring the potential of white-rot fungi exudates on the amelioration of salinized soils. Agriculture 2023, 13, 382. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Duan, Y.Y.; Sun, X.D.; Pang, X.P.; Guo, Z.G. Fungi contribute more than bacteria to the ecological uniqueness of soil microbial communities in alpine meadows. Glob. Ecol. Conserv. 2024, 55, e03246. [Google Scholar] [CrossRef]
- Huang, W.; van Bodegom, P.M.; Declerck, S.; Jussi, H.; Cosme, M.; Viskari, T.; Liski, J.; Soudzilovskaia, N.A. Mycelium chemistry differs markedly between ectomycorrhizal and arbuscular mycorrhizal fungi. Commun. Biol. 2022, 5, 398. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, J.; An, X.; Yang, J.; Wang, Y.; Dou, M.; Wang, M.; Huang, J.; Fu, Y. Insight of endophytic fungi promoting the growth and development of woody plants. Crit. Rev. Biotechnol. 2023, 44, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Izquierdo, L.; Rincón, A.; Lindahl, B.D.; Buée, M. Chapter 13—Fungal community of forest soil: Diversity, functions, and services. For. Microbiol. 2021, 1, 231–255. [Google Scholar] [CrossRef]
- Kalevitch, M.V.; Kefeli, V.I. Fungi in fabricated soils. Int. J. Environ. Pollut. 2007, 29, 424–434. [Google Scholar] [CrossRef]
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME J. 2017, 11, 1318. [Google Scholar] [CrossRef]
- Rogers, M.B.; Firek, B.; Shi, M.; Yeh, A.; Brower-Sinning, R.; Aveson, V.; Kohl, B.L.; Fabio, A.; Carcillo, J.A.; Morowitz, M.J. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome 2016, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.; Sun, W.Z.; Bumbie, G.Z.; Elokil, A.A.; Mohammed, K.A.F.; Zebin, R.; Hu, P.; Wu, L.; Tang, Z. Feeding Bacillus subtilis ATCC19659 to broiler chickens enhances growth performance and immune function by modulating intestinal morphology and cecum microbiota. Front. Microbiol. 2022, 12, 798350. [Google Scholar] [CrossRef]
- Song, C.; Wang, B.; Tan, J.; Zhu, L.; Lou, D.; Cen, X. Comparative analysis of the gut microbiota of black bears in China using high-throughput sequencing. Mol. Genet. Genom. 2017, 292, 407–414. [Google Scholar] [CrossRef]
- Huhe; Chen, X.; Hou, F.; Wu, Y.; Cheng, Y. Bacterial and fungal community structures in loess plateau grasslands with different grazing intensities. Front. Microbiol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, J.H.; Ross Friedman, C.; Wang, H.F. Variation of soil bacterial communities in a chronosequence of rubber tree (Hevea brasiliensis) plantations. Front. Plant Sci. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Ametrano, C.G.; Sterflinger, K.; Tesei, D. An overview of genomics, phylogenomics and proteomics approaches in Ascomycota. Life 2020, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Zheng, W.; Ryo, M.; Soutschek, K.; Roy, J.; Rongstock, R.; Maaß, S.; Rillig, M.C. Fungal traits important for soil aggregation. Front. Microbiol. 2019, 10, 2904. [Google Scholar] [CrossRef]
- Wang, J.; Fu, R.; Li, Y.; Tu, X.; Chen, Z.; Elrys, A.S.; Cheng, Y.; Zhang, J.; Cai, Z.; Müller, C.; et al. Fungal dominance of gross nitrogen mineralisation in an acidic upland soil amended with organic fertiliser. Eur. J. Soil Sci. 2022, 73, e13282. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Lian, L.; Wei, B.; Bi, Y.; Liu, N.; Yang, G.; Zhang, Y. Macrofungi promote SOC decomposition and weaken sequestration by modulating soil microbial function in temperate steppe. Sci. Total Environ. 2023, 899, 165556. [Google Scholar] [CrossRef]
- Qin, Y.; Yan, Y.; Cheng, L.; Lu, Y.; Chen, J.; Liu, F.; Tan, J. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen and phosphate availability in soybean/maize intercropping systems. J. Soil Sci. Plant Nutr. 2023, 23, 2723–2731. [Google Scholar] [CrossRef]
- Dong, K.; Tripathi, B.; Moroenyane, I.; Kim, W.; Li, N.; Chu, H.; Adams, J. Soil fungal community development in a high Arctic glacier foreland follows a directional replacement model, with a mid-successional diversity maximum. Sci. Rep. 2016, 6, 26360. [Google Scholar] [CrossRef]
- Sansupa, C.; Purahong, W.; Nawaz, A.; Wubet, T.; Suwannarach, N.; Chantawannakul, P.; Chairuangsri, S.; Disayathanoowat, T. Living fungi in an opencast limestone mine: Who are they and what can they do? J. Fungi 2022, 8, 987. [Google Scholar] [CrossRef]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Naumova, N.; Belanov, I.; Alikina, T.; Kabilov, M. Soil microbiome after nine years of fly ash dump spontaneous revegetation. Soil Res. 2021, 59, 673–683. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Sabata, D.D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Kuzikova, I.L.; Medvedeva, N.G. Long-chain alkylphenol biodegradation potential of soil Ascomycota. Dokl. Biol. Sci. 2023, 511, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Caputo, F.; Fornasier, F.; Paletto, A.; Ceotto, E.; Meo, I.D. Ascomycota and Basidiomycota fungal phyla as indicators of land use efficiency for soil organic carbon accrual with woody plantations. Ecol. Indic. 2024, 160, 111796. [Google Scholar] [CrossRef]
- Peng, S.L.; Shen, H.; Zhang, Y.T.; Guo, T. Compare different effect of arbuscular mycorrhizal colonization on soil structure. Acta Ecol. Sin. 2012, 32, 863–870. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Tian, D.; Cao, J.; Ren, S.; Zhu, Y.; Wang, H.; Wu, L.; Chen, L. Eucalyptus and native broadleaf mixed cultures boost soil multifunctionality by regulating soil fertility and fungal community dynamics. J. Fungi 2024, 10, 709. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Hilário, S.; Van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and metabolomic analyses of the marine fungus emericellopsis cladophorae: Insights into saltwater adaptability mechanisms and its biosynthetic potential. J. Fungi 2022, 8, 31. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Rossman, A.Y.; Rogers, J.D.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Gareth Jones, E.B.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Luo, Z.L.; Hyde, K.D.; Liu, J.K.; Maharachchikumbura, S.S.N.; Jeewon, R.; Bao, D.F.; Bhat, D.J.; Lin, C.G.; Li, W.L.; Yang, J.; et al. Freshwater Sordariomycetes. Fungal Divers. 2019, 99, 451–660. [Google Scholar] [CrossRef]
- Rissi, D.V.; Ijaz, M.; Baschien, C. Comparative genomics of fungi in nectriaceae reveals their environmental adaptation and conservation strategies. J. Fungi 2024, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zhao, X.; Zhang, X.; Zhao, Q.; Wang, X.; Li, Y. Restructured fungal community diversity and biological interactions promote metolachlor biodegradation in soil microbial fuel cells. Chemosphere 2019, 221, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Hannula, S.E.; van den Berg, M.; Korthals, G.; de Boer, W. The hidden potential of saprotrophic fungi in arable soil: Patterns of short-term stimulation by organic amendments. Appl. Soil Ecol. 2020, 147, 103434. [Google Scholar] [CrossRef]
- Peng, L.; Shan, X.; Yang, Y.; Wang, Y.; Druzhinina, I.S.; Pan, X.; Jin, W.; He, X.; Wang, X.; Zhang, X.; et al. Facultative symbiosis with a saprotrophic soil fungus promotes potassium uptake in American sweetgum trees. Plant Cell Environ. 2021, 44, 2793–2809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Du, C.; Geng, Z.; Wang, Q.; Zhang, T.; He, W.; Hou, L.; Wang, Y. Variations in bacterial and fungal communities through soil depth profiles in a Betula albosinensis forest. J. Microbiol. 2017, 55, 684–693. [Google Scholar] [CrossRef]
- Mushinski, R.M.; Gentry, T.J.; Bouttona, T.W. Organic matter removal associated with forest harvest leads to decade scale alterations in soil fungal communities and functional guilds. Soil Biol. Biochem. 2018, 127, 127–136. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; López-García, Á.; Domínguez, M.T.; Kjøller, R.; Navarro-Fernández, C.M.; Rosendahl, S.; Marañón, T. Soil fungal diversity and functionality are driven by plant species used in phytoremediation. Soil Biol. Biochem. 2021, 153, 108102. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Predominant microbial colonizers in the root endosphere and rhizosphere of turfgrass systems: Pseudomonas veronii, Janthinobacterium lividum, and Pseudogymnoascus spp. Front. Microbiol. 2021, 12, 643904. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Zhai, S.; Fang, C.; Liu, J.; Zhang, Q. Long term ditch-buried straw return affects soil fungal community structure and carbon-degrading enzymatic activities in a rice-wheat rotation system. Appl. Soil Ecol. 2020, 155, 103660. [Google Scholar] [CrossRef]
- Alimi, A.A.; Adeleke, R.; Moteetee, A. Soil environmental factors shape the rhizosphere arbuscular mycorrhizal fungal communities in South African indigenous legumes (Fabaceae). Biodiversitas 2021, 22, 2466–2476. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, Q.; Lei, P.; Xiang, W.; Ouyang, S.; Chen, L. Soil fungal communities and enzyme activities along local tree species diversity gradient in subtropical evergreen forest. Forests 2021, 12, 1321. [Google Scholar] [CrossRef]
- Liu, R.; Liang, B.; Zhao, H.; Zhao, Y. Impacts of various amendments on the microbial communities and soil organic carbon of coastal saline–alkali soil in the Yellow River Delta. Front. Microbiol. 2023, 14, 1239855. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. European J. Soil Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Teslya, A.V.; Gurina, E.V.; Poshvina, D.V.; Stepanov, A.A.; Iashnikov, A.V.; Vasilchenko, A.S. Fungal secondary metabolite gliotoxin enhances enzymatic activity in soils by reshaping their microbiome. Rhizosphere 2024, 32, 100960. [Google Scholar] [CrossRef]
- Mvila, B.G.; Pilar-Izquierdo, M.C.; Busto, M.D.; Perez-Mateos, M.; Ortega, N. Barley seed coating with urease and phosphatase to improve N and P uptake. Sci. Agric. 2020, 77, e20180227. [Google Scholar] [CrossRef]
- Zveushe, O.K.; Nkoh, J.N.; de Dios, V.R.; Manjoro, T.T.; Suanon, F.; Zhang, H.; Chen, W.; Lin, L.; Zhou, L.; Zhang, W.; et al. Enhancing hexavalent chromium stable reduction via sodium alginate encapsulation of newly isolated fungal and bacterial consortia. J. Hazard. Mater. 2025, 486, 136994. [Google Scholar] [CrossRef]
- Poljovka, A.; Musil, M.; Bednář, D.; Chovanová, K.; Bauerová-Hlinková, V.; Bellová, J.; Kohútová, L.; Baráth, P.; Zámocký, M. Comparison of fungal thermophilic and mesophilic catalase–peroxidases for their antioxidative properties. Antioxidants 2023, 12, 1382. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kumar, A.; Muddassar, M.; Matsuyama, A.; Yoshida, M.; Zhang, K.Y.J. Discovery of fungal denitrification inhibitors by targeting copper nitrite reductase from Fusarium Oxysporum. J. Chem. Inf. Model. 2017, 57, 203–213. [Google Scholar] [CrossRef]
- Zhou, J.; Gube, M.; Holz, M.; Song, B.; Shan, I.; Shi, L.; Kuzyakov, Y.; Dippold, M.A.; Pausch, J. Ectomycorrhizal and non-mycorrhizal rhizosphere fungi increase root-derived C input to soil and modify enzyme activities: A 14C pulse labelling of Picea abies seedlings. Plant Cell Environ. 2022, 45, 3122–3133. [Google Scholar] [CrossRef]
- Zi, H.; Hu, L.; Wang, C. Differentiate responses of soil microbial community and enzyme activities to nitrogen and phosphorus addition rates in an alpine meadow. Front. Plant Sci. 2022, 13, 829381. [Google Scholar] [CrossRef]



| Name | Red Mud–Phosphogypsum Substrate (g) | Rice Husk Powder (g) | Bentonite (g) | Fly Ash (g) | Polyacrylamide Flocculant (g) |
|---|---|---|---|---|---|
| T7 | 200 | 20 | 4 | 10 | 0.5 |
| T8 | 200 | 20 | 10 | 2 | 1 |
| T9 | 200 | 20 | 20 | 5 | 0.25 |
| Name | Seq-Optimized | Seq-Valid | OTUs |
|---|---|---|---|
| T7_1 | 38,517 | 20,998 | 66 |
| T7_2 | 33,173 | 20,998 | 74 |
| T7_3 | 47,092 | 20,998 | 63 |
| T8_1 | 33,306 | 20,998 | 67 |
| T8_2 | 66,568 | 20,998 | 67 |
| T8_3 | 58,221 | 20,998 | 59 |
| T9_1 | 32,293 | 20,998 | 60 |
| T9_2 | 37,599 | 20,998 | 72 |
| T9_3 | 31,873 | 20,998 | 72 |
| Name | pH | WH2O (%) | OM (%) | CEC (cmol/kg) | TN (mg/kg) | TP (mg/kg) | TK (g/kg) | AN (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| T7 | 8.4 ± 0.1 b | 30.1 ± 1.5 a | 5.5 ± 0.1 a | 11.1 ± 0.3 a | 445.5 ± 52.2 b | 2726.4 ± 196.8 a | 31.8 ± 1.8 a | 153.6 ± 22.0 b |
| T8 | 8.4 ± 0.0 b | 31.7 ± 2.1 a | 5.4 ± 0.3 a | 11.4 ± 0.4 a | 596.0 ± 83.8 a | 2842.9 ± 454.7 a | 32.4 ± 4.4 a | 211.4 ± 8.2 a |
| T9 | 8.7 ± 0.1 a | 30.9 ± 1.8 a | 5.0 ± 0.1 b | 12.3 ± 1.5 a | 395.2 ± 80.4 b | 2578.6 ± 108.3 a | 38.6 ± 11.9 a | 110.5 ± 8.3 c |
| Name | AP (mg/kg) | AK (mg/kg) | S-CAT (ml/g) | S-AKP (mg/g, 24 h) | S-UE (mg/g, 24 h) | S-AP (mg/g, 24 h) | S-SC (mg/g, 24 h) | |
| T7 | 482.7 ± 42.4 b | 14.2 ± 0.6 a | 1.285 ± 0.071 a | 0.329 ± 0.049 c | 0.318 ± 0.058 b | 0.028 ± 0.002 a | 1.020 ± 0.238 b | |
| T8 | 548.2 ± 19.5 a | 15.4 ± 4.3 a | 1.121 ± 0.121 a | 0.417 ± 0.039 b | 0.425 ± 0.072 b | 0.030 ± 0.002 a | 1.762 ± 0.385 a | |
| T9 | 180.8 ± 31.7 c | 14.5 ± 0.9 a | 1.162 ± 0.073 a | 0.561 ± 0.004 a | 0.789 ± 0.176 a | 0.029 ± 0.004 a | 1.253 ± 0.008 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, Z.; Zhang, L.; Deng, F.; Zhao, Z.; Xue, B.; Wang, J. Characteristics of Fungal Communities in Red Mud/Phosphogypsum-Based Artificial Soils. Biology 2025, 14, 285. https://doi.org/10.3390/biology14030285
Liu Y, Yang Z, Zhang L, Deng F, Zhao Z, Xue B, Wang J. Characteristics of Fungal Communities in Red Mud/Phosphogypsum-Based Artificial Soils. Biology. 2025; 14(3):285. https://doi.org/10.3390/biology14030285
Chicago/Turabian StyleLiu, Yong, Zhi Yang, Lishuai Zhang, Fang Deng, Zhiqiang Zhao, Binbin Xue, and Jingfu Wang. 2025. "Characteristics of Fungal Communities in Red Mud/Phosphogypsum-Based Artificial Soils" Biology 14, no. 3: 285. https://doi.org/10.3390/biology14030285
APA StyleLiu, Y., Yang, Z., Zhang, L., Deng, F., Zhao, Z., Xue, B., & Wang, J. (2025). Characteristics of Fungal Communities in Red Mud/Phosphogypsum-Based Artificial Soils. Biology, 14(3), 285. https://doi.org/10.3390/biology14030285







