A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids
Simple Summary
Abstract
1. Introduction
- Cellular diversity, i.e., the presence of mucin-producing and secretory cells (1); a columnar (2), polarized (3) cellular monolayer and typical microanatomical features of enterocytes such as microvilli (4);
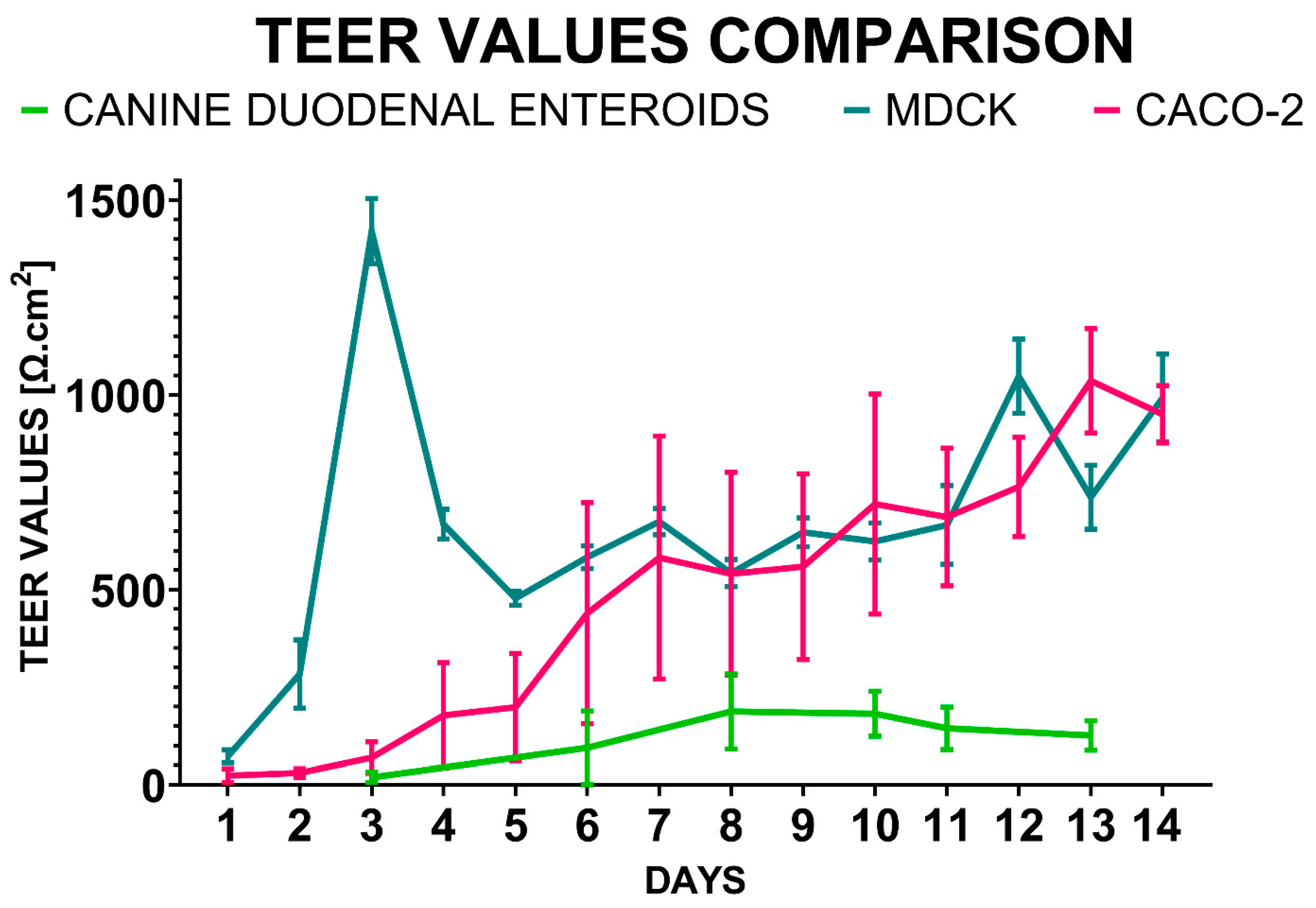
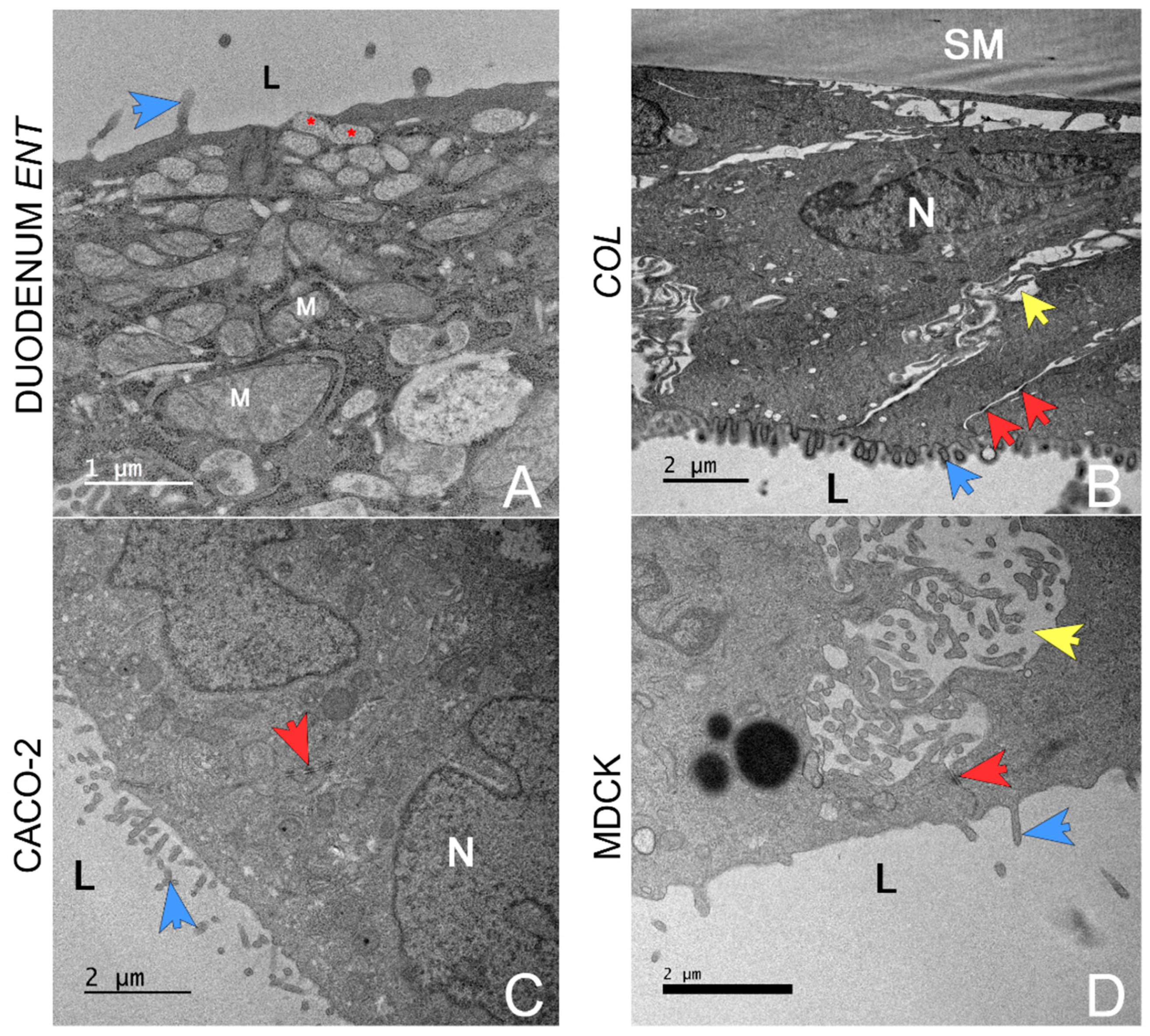
- The presence of tight junctions (5) as evaluated through transmission electron microscopy (TEM) and TEER measurements. TEER values should also replicate those measured in vivo (6), with minimal variations between replicates (7). The focus on TEER values can be justified as this method is the only quantifiable variable obtained in real-time during cell culture.
- Cell monolayers must be stable (8) for several days to allow for permeability assay experiments. Stability for these experiments was defined as a <10% decrease in TEER values in 2 days following the plateau state.
2. Materials and Methods
2.1. Canine 3D Enteroid/Colonoid Culture and Monolayer
2.2. 2D Cell Culture
2.3. Histochemical Staining
2.4. Cell Measurement
2.5. Transmission Electron Microscopy (TEM)
3. Statistical Analysis
4. Results
4.1. Canine Organoid Enterocyte Measurements
4.2. Cell Morphology
4.3. Transepithelial Electrical Resistance (TEER) Measurements
4.4. Transmission Electron Microscopy (TEM)
5. Discussion
Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; Macdonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Volpe, D.A. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J. Pharm. Sci. 2008, 97, 712–725. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. JNCI J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Gaush, C.R.; Hard, W.L. Characterization of an established line of canine kidney cells (MDCK). Proc. Soc. Exp. Biol. Med. 1966, 122, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A. Application of method suitability for drug permeability classification. AAPS J. 2010, 12, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.F.; Duivenvoorde, L.P.M.; Rijkers, D.; Nijssen, R.; Peijnenburg, A.A.C.M.; van der Zande, M.; Louisse, J. Cytochrome P450 expression, induction and activity in human induced pluripotent stem cell-derived intestinal organoids and comparison with primary human intestinal epithelial cells and Caco-2 cells. Arch. Toxicol. 2021, 95, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Anderle, P.; Niederer, E.; Rubas, W.; Hilgendorf, C.; Spahn-Langguth, H.; Wunderli-Allenspach, H.; Merkle, H.P.; Langguth, P. P-Glycoprotein (P-gp) Mediated Efflux in Caco-2 Cell Monolayers: The Influence of Culturing Conditions and Drug Exposure on P-gp Expression Levels. J. Pharm. Sci. 1998, 87, 757–762. [Google Scholar] [CrossRef]
- Mochel, J.P.; Jergens, A.E.; Kingsbury, D.; Kim, H.J.; Martin, M.G.; Allenspach, K. Intestinal Stem Cells to Advance Drug Development, Precision, and Regenerative Medicine: A Paradigm Shift in Translational Research. AAPS J. 2018, 20, 17. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Translational applications of adult stem cell-derived organoids. Development 2017, 144, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, V.; Zdyrski, C.; Sahoo, D.K.; Dao, K.; Bourgois-Mochel, A.; Atherly, T.; Martinez, M.N.; Volpe, D.A.; Kopper, J.; Allenspach, K.; et al. Canine Intestinal Organoids in a Dual-Chamber Permeable Support System. J. Vis. Exp. 2022, e63612. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, Z.; Sun, J.; Zhang, Y.; Li, D.; Zhang, X.; Liu, M.; Wang, X. A Novel Model of P-Glycoprotein Inhibitor Screening Using Human Small Intestinal Organoids. Basic Clin. Pharmacol. Toxicol. 2017, 120, 250–255. [Google Scholar] [CrossRef]
- Nagao, I.; Nakazawa, M.; Tachibana, Y.; Kawasaki, M.; Ambrosini, Y.M. Assessment of P-glycoprotein function using canine intestinal organoid-derived epithelial interfaces. Xenobiotica 2024, 54, 342–349. [Google Scholar] [CrossRef]
- Fleischer, D. Biological Transport Phenomena in the Gastrointestinal Tract: Cellular Mechanisms. In Transport Processes in Pharmaceutical Systems; CRC Press: Boca Raton, FL, USA, 1999; pp. 163–200. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. JALA J. Assoc. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Gabriel, V.; Zdyrski, C.; Sahoo, D.K.; Dao, K.; Bourgois-Mochel, A.; Kopper, J.; Zeng, X.-L.; Estes, M.K.; Mochel, J.P.; Allenspach, K. Standardization and Maintenance of 3D Canine Hepatic and Intestinal Organoid Cultures for Use in Biomedical Research. J. Vis. Exp. 2022, e63515. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest. 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zdyrski, C.; Gabriel, V.; Gessler, T.B.; Ralston, A.; Sifuentes-Romero, I.; Kundu, D.; Honold, S.; Wickham, H.; Topping, N.E.; Sahoo, D.K.; et al. Establishment and characterization of turtle liver organoids provides a potential model to decode their unique adaptations. Commun. Biol. 2024, 7, 218. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Gabriel, V.; Livania, V.; Liu, D.; Ahmed, B.H.; Lincoln, A.; Wickham, H.; Ralston, A.; Merodio, M.M.; Sahoo, D.K.; et al. Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies. Vet. Sci. 2023, 10, 417. [Google Scholar] [CrossRef]
- Thompson, F.M.; Catto-Smith, A.G.; Moore, D.; Davidson, G.; Cummins, A.G. Epithelial growth of the small intestine in human infants. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi-Ipadeola, G.O.; Hankins, J.D.; Kambal, A.; Zeng, X.-L.; Patil, K.; Poplaski, V.; Bomidi, C.; Nguyen-Phuc, H.; Grimm, S.L.; Coarfa, C.; et al. Infant and adult human intestinal enteroids are morphologically and functionally distinct. mBio 2024, 15, e0131624. [Google Scholar] [CrossRef]
- Pratscher, B.; Kuropka, B.; Csukovich, G.; Doulidis, P.G.; Spirk, K.; Kramer, N.; Freund, P.; Rodríguez-Rojas, A.; Burgener, I.A. Traces of Canine Inflammatory Bowel Disease Reflected by Intestinal Organoids. Int. J. Mol. Sci. 2024, 25, 576. [Google Scholar] [CrossRef]
- Kopper, J.J.; Iennarella-Servantez, C.; Jergens, A.E.; Sahoo, D.K.; Guillot, E.; Bourgois-Mochel, A.; Martinez, M.N.; Allenspach, K.; Mochel, J.P. Harnessing the Biology of Canine Intestinal Organoids to Heighten Understanding of Inflammatory Bowel Disease Pathogenesis and Accelerate Drug Discovery: A One Health Approach. Front. Toxicol. 2021, 3, 773953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, F.; Jin, Y.; Ma, Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct. Target. Ther. 2022, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J.W.; Selick, H.; Grove, J. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 1999, 88, 28–33. [Google Scholar] [CrossRef]
- Cereijido, M.; Robbins, E.; Dolan, W.; Rotunno, C.; Sabatini, D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Artursson, P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 305–330. [Google Scholar]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX Co-cultured Cell Lines: Permeabilities Via Diffusion, Inside- and Outside-Directed Carrier-Mediated Transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Sonnek, N.M.; Stappenbeck, T.S. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res. 2019, 37, 101430. [Google Scholar] [CrossRef] [PubMed]
- Penning, L.C.; Boom, R. Companion animal organoid technology to advance veterinary regenerative medicine. Front. Vet. Sci. 2023, 10, 1032835. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.; Novoa, C.V.; Wang, Y.; Allbritton, N.L. Fibroblasts modulate epithelial cell behavior within the proliferative niche and differentiated cell zone within a human colonic crypt model. Front. Bioeng. Biotechnol. 2024, 12, 1506976. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Martinez, M.N.; Dao, K.; Gabriel, V.; Zdyrski, C.; Jergens, A.E.; Atherly, T.; Iennarella-Servantez, C.A.; Burns, L.E.; Schrunk, D.; et al. Canine Intestinal Organoids as a Novel In Vitro Model of Intestinal Drug Permeability: A Proof-of-Concept Study. Cells 2023, 12, 1269. [Google Scholar] [CrossRef] [PubMed]



| Adult Duodenum | Juvenile Duodenum | Adult Ileum | Juvenile Ileum | Adult Colon | Juvenile Colon | Caco-2 | MDCK | |
|---|---|---|---|---|---|---|---|---|
| Number of values | 290 | 400 | 459 | 300 | 687 | 493 | 99 | 99 |
| Median | 14.2 d | 10.8 f | 17.4 c | 13.5 e | 19.7 a | 17.7 b | 13.8 de | 7.5 g |
| Interquartile range (IQR) | 6.2 | 3.6 | 5.9 | 7.2 | 6.1 | 5.4 | 6.6 | 2.6 |
| Mean ranks | 1149.0 | 582.1 | 1659.0 | 1078.0 | 2007.0 | 1715.0 | 1070.0 | 161.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbett, M.P.; Gabriel, V.; Livania, V.; Díaz-Regañón, D.; Ralston, A.; Zdyrski, C.; Liu, D.; Minkler, S.; Wickham, H.; Lincoln, A.; et al. A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids. Biology 2025, 14, 270. https://doi.org/10.3390/biology14030270
Corbett MP, Gabriel V, Livania V, Díaz-Regañón D, Ralston A, Zdyrski C, Liu D, Minkler S, Wickham H, Lincoln A, et al. A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids. Biology. 2025; 14(3):270. https://doi.org/10.3390/biology14030270
Chicago/Turabian StyleCorbett, Megan P., Vojtech Gabriel, Vanessa Livania, David Díaz-Regañón, Abigail Ralston, Christopher Zdyrski, Dongjie Liu, Sarah Minkler, Hannah Wickham, Addison Lincoln, and et al. 2025. "A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids" Biology 14, no. 3: 270. https://doi.org/10.3390/biology14030270
APA StyleCorbett, M. P., Gabriel, V., Livania, V., Díaz-Regañón, D., Ralston, A., Zdyrski, C., Liu, D., Minkler, S., Wickham, H., Lincoln, A., Paukner, K., Atherly, T., Merodio, M. M., Sahoo, D. K., Meyerholz, D. K., Allenspach, K., & Mochel, J. P. (2025). A Preclinical Model to Assess Intestinal Barrier Integrity Using Canine Enteroids and Colonoids. Biology, 14(3), 270. https://doi.org/10.3390/biology14030270










