Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives
Simple Summary
Abstract
1. Introduction
2. Extracellular Vesicle (EV) Characteristics
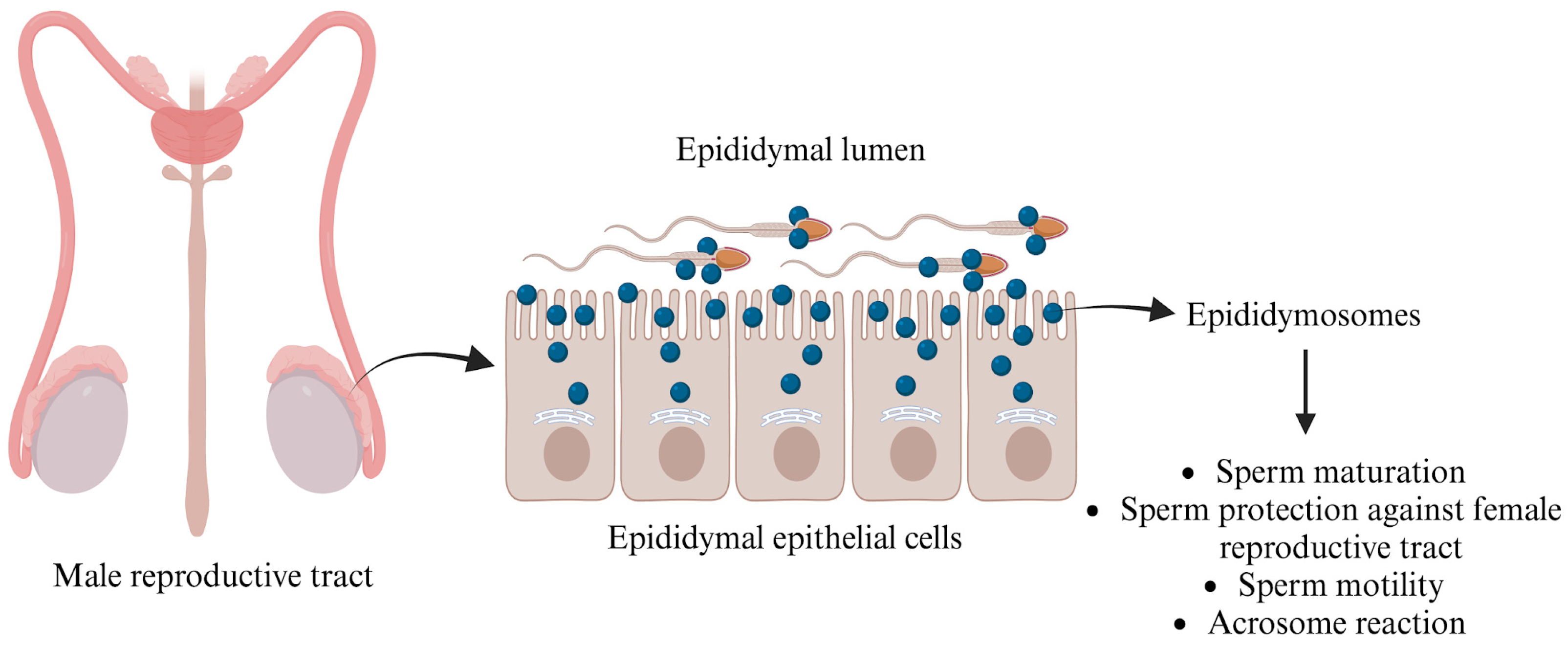
3. Extracellular Vesicles (EVs) in the Male Reproductive Tract
3.1. Detection
3.1.1. Epididymosomes
3.1.2. Prostasomes
3.2. Role of Seminal Plasma-EVs (SP-EVs) in Modulating Gamete Function
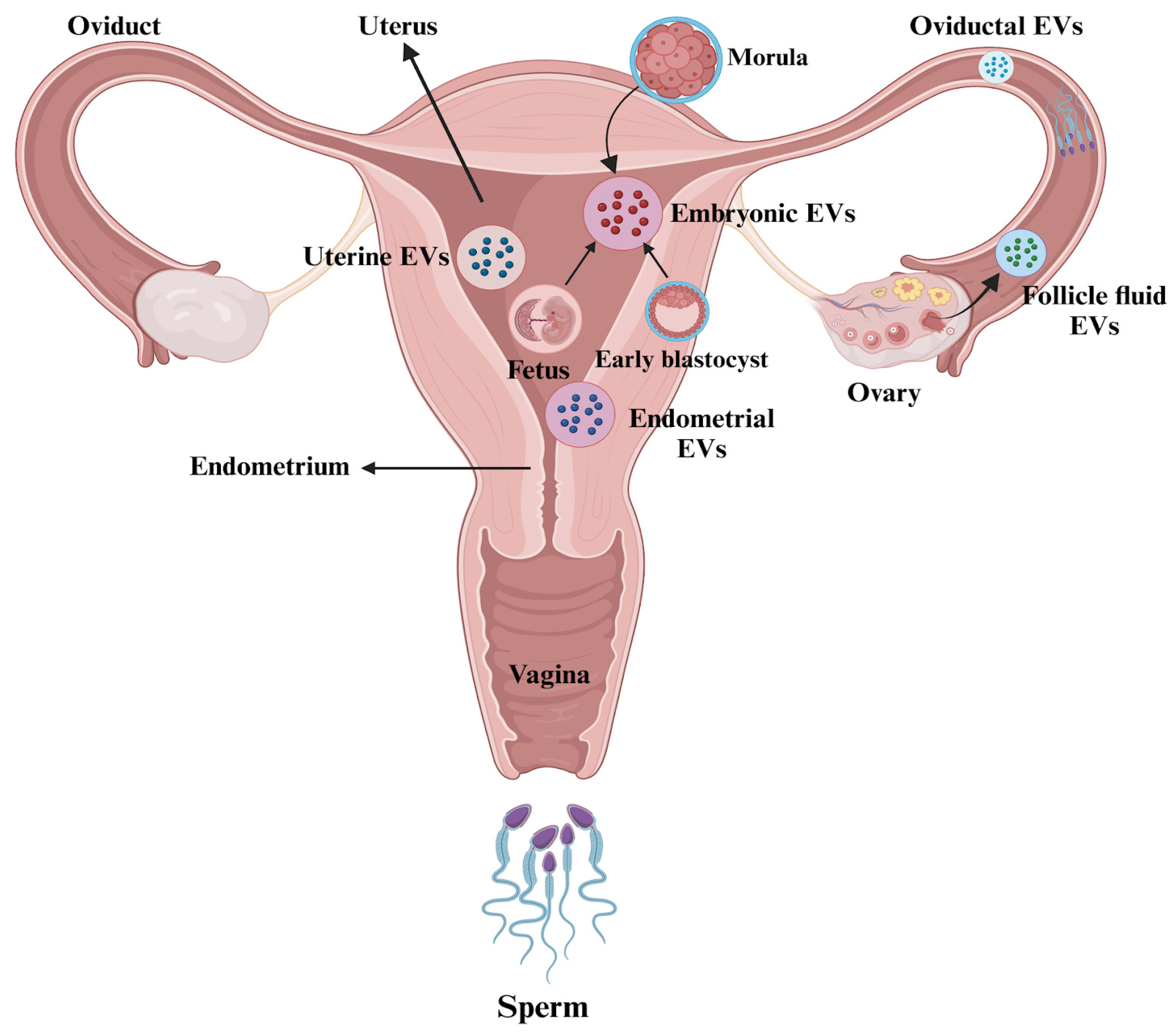
4. Extracellular Vesicles (EVs) in the Female Reproductive Tract
4.1. EVs in Follicular Fluid
4.2. Extracellular Vesicles (EVs) in Embryo–Maternal Interactions
4.2.1. Evidence from the Maternal Side
4.2.2. Evidence from the Embryo Side
4.3. Impacts of EVs on Sperm Function
4.4. Impacts of EVs on Embryo–Oviduct–Endometrium Interactions
4.4.1. Embryo-Secreted EVs
4.4.2. Embryo-Secreted EVs Influenced by Culture Conditions
5. Possible Therapeutic Applications of Extracellular Vesicles in Treating Infertility
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyt, P.; Maes, D.; Rijsselaere, T.; Dewulf, J.; de Kruif, A.; Van Soom, A. Semen handling in porcine AI centers: The Belgian situation. Vlaams Diergeneeskd. Tijdschr. 2007, 76, 195–200. [Google Scholar] [CrossRef]
- Maes, D.; Nauwynck, H.; Rijsselaere, T.; Mateusen, B.; Vyt, P.; de Kruif, A.; Van Soom, A. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology 2008, 70, 1337–1345. [Google Scholar] [CrossRef]
- Schulze, M.; Buder, S.; Rüdiger, K.; Beyerbach, M.; Waberski, D. Influences on semen traits used for selection of young AI boars. Anim. Reprod. Sci. 2014, 148, 164–170. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Roca, J.; Rodriguez-Martinez, H.; Padilla, L.; Lucas, X.; Barranco, I. Extracellular vesicles in seminal fluid and effects on male reproduction. An overview in farm animals and pets. Anim. Reprod. Sci. 2021, 16, 106853. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Roca, J. Extracellular vesicles in seminal plasma: A safe and relevant tool to improve fertility in livestock? Anim. Reprod. Sci. 2022, 244, 107051. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Callesen, H. In vivo versus in vitro produced bovine ova: Similarities and differences relevant for practical application. Reprod. Nutr. Dev. 1998, 38, 579–594. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-DeLeon, P.A. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Memili, E.; Dominko, T.; First, N.L. Onset of transcription in bovine oocytes and preimplantation embryos. Mol. Reprod. Dev. Inc. Gamete Res. 1998, 51, 36–41. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Veeramachaneni, D.N.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain miRNAs and Proteins: A Possible New Form of Cell Communication Within the Ovarian Follicle. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832. [Google Scholar] [CrossRef]
- Ruiz-González, I.; Xu, J.; Wang, X.; Burghardt, R.C.; Dunlap, K.A.; Bazer, F.W. Exosomes, endogenous retroviruses and toll-like receptors: Pregnancy recognition in ewes. Reproduction 2015, 149, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, A.C.F.C.M.; Bridi, A.; Andrade, G.M.; del Collado, M.; Sangalli, J.R.; Nociti, R.P.; da Silva Junior, W.A.; Bastien, A.; Robert, C.; Meirelles, F.V. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol. Reprod. 2020, 102, 362–375. [Google Scholar] [CrossRef]
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; O’Neil, E.V.; Hagen, D.E.; Behura, S.K.; Spencer, T.E. Progesterone effects on extracellular vesicles in the sheep uterus. Biol. Reprod. 2018, 98, 612–622. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltran-Brena, P.; Calle, A.; Redruello, A.; Lopez-Martin, S.; Gutierrez-Adan, A.; Yanez-Mo, M.; et al. Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef]
- Betteridge, K.; Fléchon, J.-E. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology 1988, 29, 155–187. [Google Scholar] [CrossRef]
- Spencer, T.E. Early pregnancy: Concepts, challenges, and potential solutions. Anim. Front. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- Guillomot, M. Cellular interactions during implantation in domestic ruminants. J. Reprod. Fertil.-Suppl. Only 1995, 49, 39–52. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Oh, H.J.; Lee, B.C. Embryonic–maternal cross-talk via exosomes: Potential implications. Stem Cells Cloning Adv. Appl. 2015, 8, 103–107. [Google Scholar]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular vesicles: Exosomes and microvesicles, integrators of homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, A.; Koenen, R.R. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and prostasomes: Their roles in posttesticular maturation of the sperm cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G.; Larsson, A.; Stavreus-Evers, A.; Ronquist, G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate 2012, 72, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes–oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Tamessar, C.T.; Trigg, N.A.; Nixon, B.; Skerrett-Byrne, D.A.; Sharkey, D.J.; Robertson, S.A.; Bromfield, E.G.; Schjenken, J.E. Roles of male reproductive tract extracellular vesicles in reproduction. Am. J. Reprod. Immunol. 2021, 85, e13338. [Google Scholar] [CrossRef] [PubMed]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.; Schwarzmann, G.; Mobius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Potential implications for their function and multivesicular body formation:Proteomic and biochemical analyses of human B cell-derived exosomes. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Caballero, J.; Frenette, G.; Sullivan, R. Post testicular sperm maturational changes in the bull: Important role of the epididymosomes and prostasomes. Vet. Med. Int. 2011, 2011, 757194. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.B.; Hinsch, G.W.; Anika, J.L. Ultrastructure and antigens of particles from rabbit semen. J. Reprod. Fertil. 1968, 17, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Mikamo, K.; Suzuki, F.; Yanagimachi, H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am. J. Anat. 1985, 172, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Girouard, J.; Frenette, G.; Sullivan, R. Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol. Reprod. 2009, 80, 965–972. [Google Scholar] [CrossRef][Green Version]
- Rejraji, H.; Vernet, P.; Drevet, J. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol. Reprod. Dev. 2002, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Frenette, G.; Lessard, C.; Sullivan, R. Polyol pathway along the bovine epididymis. Mol. Reprod. Dev. 2004, 69, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D.; Ruan, Y.C.; Wertheimer, E.V.; Battistone, M.A.; Pawlak, J.B.; Sanjay, A.; Pilder, S.H.; Cuasnicu, P.; Breton, S.; Visconti, P.E. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev. Biol. 2012, 369, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.S.; Suryawanshi, A.R.; Khan, S.A.; Balasinor, N.H.; Khole, V.V. Liprin α3: A putative estrogen regulated acrosomal protein. Histochem. Cell Biol. 2013, 139, 535–548. [Google Scholar] [CrossRef]
- Sullivan, R. Interaction between sperm and epididymal secretory proteins. In The Male Gamete: From Basic to Clinical Applications; Cache River Pr: Vienna, IL, USA, 1999; pp. 130–136. [Google Scholar]
- Légaré, C.; Bérubé, B.; Boué, F.; Lefièvre, L.; Morales, C.R.; El-Alfy, M.; Sullivan, R. Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol. Reprod. Dev. Inc. Gamete Res. 1999, 52, 225–233. [Google Scholar] [CrossRef]
- Frenette, G.; Sullivan, R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. Inc. Gamete Res. 2001, 59, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Han, C.; Cho, C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol. Cells 2009, 28, 441–446. [Google Scholar]
- Reilly, J.N.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 2015, 93, 1–20. [Google Scholar] [CrossRef]
- Belleannée, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015, 17, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G.; Brody, I.; Gottfries, A.; Stegmayr, B. An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid: Part I. Andrologia 1978, 10, 261–272. [Google Scholar] [CrossRef]
- Utleg, A.; Yi, E.C.; Xie, T.; Shannon, P.; White, J.T.; Goodlett, D.R.; Hood, L.; Lin, B. Proteomic analysis of human prostasomes. Prostate 2003, 56, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Nilsson, O.; Larsson, A.; Stridsberg, M.; Sahlen, G.; Ronquist, G. Antibacterial activity of human prostasomes. Prostate 2000, 44, 279–286. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Artonne, C.; Sion, B.; Brugnon, F.; Canis, M.; Janny, L.; Grizard, G. Prostasomes: Inhibitors of capacitation and modulators of cellular signalling in human sperm. Int. J. Androl. 2011, 34 Pt 1, 568–580. [Google Scholar] [CrossRef]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Carlsson, L.; Nilsson, O.; Larsson, A.; Stridsberg, M.; Sahlen, G.; Ronquist, G. Characteristics of human prostasomes isolated from three different sources. Prostate 2003, 54, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Ronquist, G. Association of some hydrolytic enzymes with the prostasome membrane and their differential responses to detergent and PIPLC treatment. Prostate 1995, 27, 95–101. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, B.J.; Kang, J.; Nam, T.S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.W.; Kim, U.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, 31. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J.J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Arienti, G.; Carlini, E.; Nicolucci, A.; Cosmi, E.V.; Santi, F.; Palmerini, C.A. The motility of human spermatozoa as influenced by prostasomes at various pH levels. Biol. Cell 1999, 91, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908.e2. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Rickard, J.P.; Pini, T.; Gadella, B.M.; de Graaf, S.P. Quantitative proteomic analysis of seminal plasma, sperm membrane proteins, and seminal extracellular vesicles suggests vesicular mechanisms aid in the removal and addition of proteins to the ram sperm membrane. Proteomics 2020, 20, 1900289. [Google Scholar] [CrossRef] [PubMed]
- Belleannée, C.; Calvo, É.; Caballero, J.; Sullivan, R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 2013, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Ntzouni, M.; Wright, D.; Khan, K.I.; Lopez-B´ejar, M.; Martinez, C.A.; Rodriguez-Martinez, H. Chicken seminal fluid lacks CD9- and CD44-bearing extracellular vesicles. Reprod. Domest. Anim. 2020, 55, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G. Expression pattern of seminal plasma extracellular vesicle small RNAs in boar semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, N.; Xie, S.; Ding, Y.; Huang, M.; Ding, X.; Jiang, L. Identification of crucial extracellular vesicle RNA molecules related to sperm motility and prostate cancer. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 104–126. [Google Scholar]
- Hessvik, N.P.; Phuyal, S.; Brech, A.; Sandvig, K.; Llorente, A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim. Biophys. Acta 2012, 1819, 1154–1163. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Hoshino, D.; Hong, N.H. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016, 15, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.C.; Torres, M.A.; Alkmin, D.V.; Pinzon, J.E.; Martins, S.M.M.K.; da Silveira, J.C.; de Andrade, A.F.C. Spermatozoa and seminal plasma small extracellular vesicles miRNAs as biomarkers of boar semen cryotolerance. Theriogenology 2021, 174, 60–72. [Google Scholar] [CrossRef]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of extracellular vesicle-coupled miRNA profiles in seminal plasma of boars with divergent semen quality status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, N.; Zhang, Y.; Xie, S.; Huang, M.; Ding, X.; Dong, W.; Zhang, Q.; Jiang, L. MicroRNA-222 Transferred From Semen Extracellular Vesicles Inhibits Sperm Apoptosis by Targeting BCL2L11. Front. Cell Dev. Biol. 2021, 9, 736864. [Google Scholar] [CrossRef] [PubMed]
- Werry, N.; Russell, S.J.; Gillis, D.J.; Miller, S.; Hickey, K.; Larmer, S.; Lohuis, M.; Librach, C.; LaMarre, J. Characteristics of miRNAs Present in Bovine Sperm and Associations with Differences in Fertility. Front. Endocrinol. 2022, 13, 874371. [Google Scholar] [CrossRef] [PubMed]
- Barceló, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Human Reproduction 2018, 33, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, J.; Sun, J.; He, J.; Sun, Y.; Yuan, R.; Li, Z. Motility-related microRNAs identified in pig seminal plasma exosomes by high-throughput small RNA sequencing. Theriogenology 2024, 215, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Almog, T.; Naor, Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Mol. Cell. Endocrinol. 2010, 314, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Lin, H.L.H.; Carvalho, A.V.; Grasseau, I.; Uzbekov, R.; Blesbois, E. First insights on seminal extracellular vesicles in chickens of contrasted fertility. Reproduction 2021, 161, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, L.; Guo, Y.; Yang, Y.; Gong, P.; Ye, S.; Wang, L.; Feng, Y. Identification of potential candidate miRNAs related to semen quality in seminal plasma extracellular vesicles and sperms of male duck (Anas Platyrhynchos). Poult. Sci. 2024, 103, 103928. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Z.; Zhang, Z.; Zhao, Y.; Jiang, X.; Yu, H.; Zhang, Y.; Zhao, R.; He, B. Extracellular ATPs produced in seminal plasma exosomes regulate boar sperm motility and mitochondrial metabolism. Theriogenology 2019, 139, 113–120. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: Implications for bovine oocyte developmental competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef] [PubMed]
- Navakanitworakul, R.; Hung, W.T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef]
- Martinez, R.M.; Liang, L.; Racowsky, C.; Dioni, L.; Mansur, A.; Adir, M.; Bollati, V.; Baccarelli, A.A.; Hauser, R.; Machtinger, R. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci. Rep. 2018, 8, 17036. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rosa, P.M.; Bridi, A.; de Ávila, F.G.; Nociti, R.P.; Dos Santos, A.C.; Cataldi, T.R.; Dos Santos, G.; Chiaratti, M.R.; Silva, L.A.; Pugliesi, G. Corpus luteum proximity alters molecular signature of the small extracellular vesicles and cumulus cells in the bovine ovarian follicle environment. Mol. Cell. Endocrinol. 2024, 592, 112347. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.T.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Avilés, M.; Gutiérrez-Adán, A.; Coy, P. Oviductal secretions: Will they be key factors for the future ARTs? Mol. Hum. Reprod. 2010, 16, 896–906. [Google Scholar] [CrossRef]
- Mazzarella, R.; Bastos, N.M.; Bridi, A.; Del Collado, M.; Andrade, G.M.; Pinzon, J.; Prado, C.M.; Silva, L.A.; Meirelles, F.V.; Pugliesi, G. Changes in oviductal cells and small extracellular vesicles miRNAs in pregnant cows. Front. Vet. Sci. 2021, 8, 639752. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.Á.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Schmitt, A.; Ott, T. The myxovirus-resistance protein, MX 1, is a component of exosomes secreted by uterine epithelial cells. Am. J. Reprod. Immunol. 2012, 67, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A. Maternal communication with gametes and embryo: A personal opinion. Reprod. Domest. Anim. 2011, 46, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol. Reprod. 2016, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef]
- Bridi, A.; Sangalli, J.R.; Nociti, R.P.; dos Santos, A.C.; Alves, L.; Bastos, N.M.; Ferronato, G.D.Á.; Rosa, P.M.D.S.; Fiorenza, M.F.; Pugliesi, G.; et al. Small extracellular vesicles derived from the crosstalk between early embryos and the endometrium potentially mediate corpus luteum function. Biol. Reprod. 2025, 112, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.; Andrade, G.M.; Del Collado, M.; Sangalli, J.R.; de Ávila, A.C.; Motta, I.G.; da Silva, J.C.; Pugliesi, G.; Silva, L.A.; Meirelles, F.V.; et al. Small extracellular vesicles derived from in vivo-or in vitro-produced bovine blastocysts have different miRNAs profiles—Implications for embryo-maternal recognition. Mol. Reprod. Dev. 2021, 88, 628–643. [Google Scholar] [CrossRef]
- Afzal, A.; Khan, M.; Gul, Z.; Asif, R.; Shahzaman, S.; Parveen, A.; Imran, M.; Khawar, M.B. Extracellular Vesicles: The Next Frontier in Pregnancy Research. Reprod. Sci. 2024, 31, 1204–1214. [Google Scholar] [CrossRef]
- da Silveira, J.; Carnevale, E.M.; Winger, Q.A.; Bouma, G. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod. Biol. Endocrinol. 2014, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Coy, P.; Cánovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; Matás, C.; Avilés, M. Oviduct specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef] [PubMed]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef] [PubMed]
- Alcântara-Neto, A.S.; Cuello, C.; Uzbekov, R.; Bauersachs, S.; Mermillod, P.; Almiñana, C. Oviductal extracellular vesicles enhance porcine in vitro embryo development by modulating the embryonic transcriptome. Biomolecules 2022, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kolakowska, J.; Souchelnytskyi, S.; Saini, R.K.R.; Franczak, A. Proteomic analysis of the endometrium during early pregnancy in the domestic pig. Reprod. Fertil. Dev. 2017, 29, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wu, Z.; He, C.; Lu, C.; He, D.; Li, X.; Duan, Z.; Zhao, H. Exosomes from uterine fluid promote capacitation of human sperm. PeerJ 2024, 12, e16875. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zang, X.; Ding, Y.; Gu, T.; Shi, J.; Li, Z.; Cai, G.; Liu, D.; Wu, Z.; Hong, L. Porcine uterine luminal fluid-derived extracellular vesicles improve conceptus-endometrial interaction during implantation. Theriogenology 2022, 178, 8–17. [Google Scholar] [CrossRef]
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Pavani, K.C.; Hendrix, A.; Van Den Broeck, W.; Couck, L.; Szymanska, K.; Lin, X.; De Koster, J.; Van Soom, A.; Leemans, B. Isolation and characterization of functionally active extracellular vesicles from culture medium conditioned by bovine embryos in vitro. Int. J. Mol. Sci. 2018, 20, 38. [Google Scholar] [CrossRef]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019, 31, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS ONE 2016, 11, e0158278. [Google Scholar] [CrossRef]
- Mellisho, E.A.; Velásquez, A.E.; Nuñez, M.J.; Cabezas, J.G.; Cueto, J.A.; Fader, C.; Castro, F.O.; Rodríguez-Álvarez, L. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE 2017, 12, e178306. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Nakamura, K.; Bai, R.; Nagaoka, K.; Sakurai, T.; Imakawa, K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem. Biophys. Res. Commun. 2018, 495, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.M.; Bomfim, M.M.; Del Collado, M.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium. Mol. Reprod. Dev. Inc. Gamete Res. 2019, 86, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Häusler, S.; Backes, C.; Fehlmann, T.; Staib, C.; Nestel, S.; Nazarenko, I.; Meese, E.; Keller, A. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing in vitro fertilization. Sci. Rep. 2017, 7, 13525. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.L.V.; Cañón-Beltrán, K.; Cajas, Y.N.; Hamdi, M.; Yaryes, A.; Millán de la Blanca, M.G.; Beltrán-Breña, P.; Mazzarella, R.; Da Silveira, J.C.; Gutiérrez-Adán, A. Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J. Anim. Sci. Biotechnol. 2022, 13, 116. [Google Scholar] [CrossRef]
- Mei, S.; Chen, P.; Lee, C.L.; Zhao, W.; Wang, Y.; Lam, K.K.W.; Ho, P.C.; Yeung, W.S.B.; Fang, C.; Chiu, P.C.N. The role of galectin-3 in spermatozoa-zona pellucida binding and its association with fertilization in vitro. Mol. Hum. Reprod. 2019, 25, 458–470. [Google Scholar] [CrossRef]
- Jena, S.R.; Nayak, J.; Kumar, S.; Kar, S.; Dixit, A.; Samanta, L. Paternal contributors in recurrent pregnancy loss: Cues from comparative proteome profiling of seminal extracellular vesicles. Mol. Reprod. Dev. 2021, 88, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Foot, N.J.; Gonzalez, M.B.; Gembus, K.; Fonseka, P.; Sandow, J.J.; Nguyen, T.T.; Tran, D.; Webb, A.I.; Mathivanan, S.; Robker, R.L. Arrdc4-dependent extracellular vesicle biogenesis is required for sperm maturation. J. Extracell. Vesicles 2021, 10, e12113. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jueraitetibaike, K.; Tang, T.; Wang, Y.; Jing, J.; Xue, T.; Ma, J.; Cao, S.; Lin, Y.; Li, X.; et al. Seminal plasma and seminal plasma exosomes of aged male mice affect early embryo implantation via immunomodulation. Front. Immunol. 2021, 12, 723409. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, L.; Marcianò, V.; Carpino, A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, K.G.; Ek, B.; Morrell, J.; Stavreus-Evers, A.; Holst, B.S.; Humblot, P.; Ronquist, G.; Larsson, A. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4604–4610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qu, P.; Luo, S.; Du, Y.; Zhang, Y.; Song, X.; Yuan, X.; Lin, Z.; Li, Y.; Liu, E. Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine. J. Pineal Res. 2020, 68, e12635. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.M.; Fernandez-Rufete, E.; Corbin, G.; Tsikis, R.; Uzbekov, A.S.; Garanina, P.; Coy, B.C.; Almiñana, A.F.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilization. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Bauersachs, S.; Mermillod, P.; Almiñana, C. The oviductal extracellular vesicles’ RNA cargo regulates the bovine embryonic transcriptome. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, Y.; Kanke, T.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS ONE 2019, 14, e0217760. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Navakanitworakul, R.; Khan, T.; Zhang, P.; Davis, J.S.; McGinnis, L.K.; Christenson, L.K. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol. Reprod. 2017, 97, 644–655. [Google Scholar] [CrossRef]
- da Silva Rosa, P.M.; Bridi, A.; de Ávila Ferronato, G.; Prado, C.M.; Bastos, N.M.; Sangalli, J.R.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: Consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J. Ovarian Res. 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells 2020, 9, 645. [Google Scholar] [CrossRef]
- Lv, C.; Yu, W.X.; Wang, Y.; Yi, D.J.; Zeng, M.H.; Xiao, H.M. MiR-21 in extracellular vesicles contributes to the growth of fertilized eggs and embryo development in mice. Biosci. Rep. 2018, 38, BSR20180036. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Qing, S.; Liu, R.; Qin, H.; Wang, W.; Qiao, F.; Ge, H.; Liu, J.; Zhang, Y.; Cui, W. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS ONE 2017, 12, e0174535. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Ge, H.; Ma, X.; Zhang, Y.; Zuo, Z.; Wang, M.; Zhang, Y.; Wang, Y. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology 2018, 114, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Munoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular vesicles in mammalian reproduction: A review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Song, H.; Kim, N.H.; Kim, J.H. The role of extracellular vesicles in animal reproduction and diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef]
- Menjivar, N.G.; Oropallo, J.; Gebremedhn, S.; Souza, L.A.; Gad, A.; Puttlitz, C.M.; Tesfaye, D. MicroRNA Nano-Shuttles: Engineering Extracellular Vesicles as a Cutting-Edge Biotechnology Platform for Clinical Use in Therapeutics. Biol. Proced. Online 2024, 26, 14. [Google Scholar] [CrossRef]
- Feugang, J.M.; Gad, A.; Menjivar, N.G.; Ishak, G.M.; Gebremedhn, S.; Gastal, M.O.; Dlamini, N.H.; Prochazka, R.; Gastal, E.L.; Tesfaye, D. Seasonal influence on miRNA expression dynamics of extracellular vesicles in equine follicular fluid. J. Anim. Sci. Biotechnol. 2024, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Gad, A.; Ishak, G.M.; Menjivar, N.G.; Gastal, M.O.; Feugang, J.M.; Prochazka, R.; Tesfaye, D.; Gastal, E.L. Dynamics of extracellular vesicle-coupled microRNAs in equine follicular fluid associated with follicle selection and ovulation. Mol. Hum. Reprod. 2023, 29, gaad009. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.; Navarro, A.; Al-Hendy, A. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- Candenas, L.; Chianese, R. Exosome composition and seminal plasma proteome: A promising source of biomarkers of male infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.; Srikumar, P.S.; Srinivasan, S.; Jeyanthi, P.; Anbarasu, K.; Thanigaivel, S.; Nibedita, D.; Rani, D.J.; Rohini, K. Seminal exosomes–an important biological marker for various disorders and syndrome in human reproduction. Saudi J. Biol. Sci. 2021, 28, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, T.; Zeng, Z.; Wu, J.; Tan, X.; Yan, J. The expression of small RNAs in exosomes of follicular fluid altered in human polycystic ovarian syndrome. PeerJ 2020, 8, e8640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; Zou, Y.; Yuan, Q.; Hu, X.; Chen, X.; Zhu, C.; Zhang, X.; Cui, H. Aberrant expression of long non-coding RNAs in exosomes in follicle fluid from PCOS patients. Front. Genet. 2021, 11, 608178. [Google Scholar] [CrossRef]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, G.; Jia, L.; Su, T.; Zhang, L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2019, 43, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lian, Y.; Jiang, J.; Wang, L.; Ren, L.; Li, Y.; Yan, X.; Chen, Q. Differential expression of microRNA in exosomes derived from endometrial stromal cells of women with endometriosis-associated infertility. Reprod. Biomed. Online 2020, 41, 170–181. [Google Scholar] [CrossRef]
- Baskaran, S.; Selvam, M.K.P.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [PubMed]
- Ghafourian, M.; Mahdavi, R.; Akbari Jonoush, Z.; Sadeghi, M.; Ghadiri, N.; Farzaneh, M.; Mousavi Salehi, A. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, S.M.; Mullen, J.B.; Jarvi, K.A.; Soosaipillai, A.; Diamandis, E.P.; Hamilton, R.J.; Lo, K.C. Seminal plasma lipocalin-type prostaglandin D synthase: A potential new marker for the diagnosis of obstructive azoospermia. J. Urol. 2008, 179, 1077–1080. [Google Scholar] [CrossRef]
- Ranjbaran, A.; Latifi, Z.; Nejabati, H.R.; Abroon, S.; Mihanfar, A.; Sadigh, A.R. Fattahi, A.; Nouri, M.; Raffel, N. Exosome-based intercellular communication in female reproductive microenvironments. J. Cell. Physiol. 2019, 234, 19212–19222. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef] [PubMed]

| Source of Extracellular Vesicles (EVs) | Species | Functional Role in the Female Reproduction |
|---|---|---|
| Seminal plasma | Human | |
| Mice | ||
| Porcine |
| |
| Oviductal fluid | Mice | |
| Porcine |
| |
| Bovine |
| |
| Follicular fluid | Porcine |
|
| Bovine |
| |
| Placental fluid | Human |
|
| Endometrium EVs | Mice |
|
| Embryonic EVs | Mice |
|
| Porcine |
| |
| Bovine |
| |
| Uterine luminal fluid | Ovine |
|
| Bovine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, N.H.; Bridi, A.; da Silveira, J.C.; Feugang, J.M. Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives. Biology 2025, 14, 198. https://doi.org/10.3390/biology14020198
Dlamini NH, Bridi A, da Silveira JC, Feugang JM. Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives. Biology. 2025; 14(2):198. https://doi.org/10.3390/biology14020198
Chicago/Turabian StyleDlamini, Notsile H., Alessandra Bridi, Juliano Coelho da Silveira, and Jean M. Feugang. 2025. "Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives" Biology 14, no. 2: 198. https://doi.org/10.3390/biology14020198
APA StyleDlamini, N. H., Bridi, A., da Silveira, J. C., & Feugang, J. M. (2025). Unlocking Gamete Quality Through Extracellular Vesicles: Emerging Perspectives. Biology, 14(2), 198. https://doi.org/10.3390/biology14020198









