Two Demosponges as Promising Bioremediators of a Potential Pathogenic Vibrio
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Bacterial Load Preparation
2.3. Experimental Design
2.4. Filtering Activity Estimation
2.5. Vibrio Parahaemolyticus Detection in Sponge Tissue
2.6. Statistical Analysis
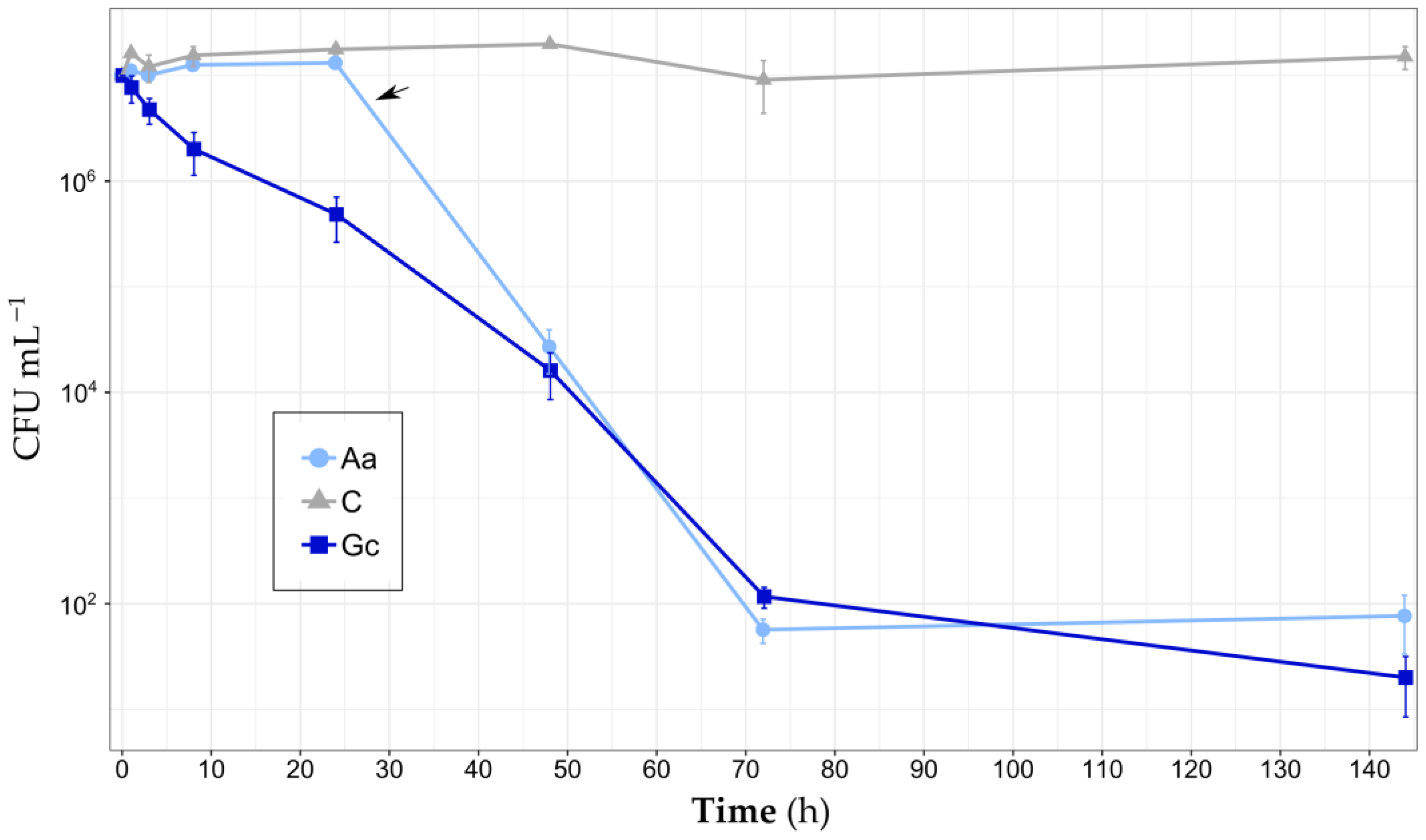
3. Results
3.1. Bacterial Load Bioremediation
3.2. Presence of Vibrio in Sponge Tissues
4. Discussion
| Subclass | MA | Species (n) | Mean CR mL·h−1·gDW−1 (SE) [min–max] | Mean Biomass gDW (SE) [min–max] | References |
|---|---|---|---|---|---|
| Heteroscleromorpha | LMA | Aaptos spp. (1) | 1872.53 (673.31) [3.57–12,857] | 8.19 (2.63) [0.001–34.59] | [93] |
| Axinella cannabina (3) | [63] | ||||
| Crambe crambe (1) | [94] | ||||
| Halichondria melanadocia (2) | [28] | ||||
| Halichondria panicea (2) | [59,95] | ||||
| Haliclona anonyma (1) | [96] | ||||
| Haliclona oculata (1) | [97] | ||||
| Haliclona tubifera (1) | [98] | ||||
| Hymeniacidon perlevis (5) | [32,33,37] | ||||
| Negombata magnifica (1) | [99] | ||||
| Pseudosuberites aff. Andrewsi (2) | [100] | ||||
| Tethya meloni (1) | [36] | ||||
| HMA | Agelas oroides (3) | 37.55 (20.15) [8.33–96.05] | 72 (60.6) [11.41–253.79] | [63] | |
| Geodia barretti (1) | [66] | ||||
| Geodia cydonium | 57.97 (5.41) | 9.63 (1.68) | Present paper | ||
| Keratosa | LMA | Dysidea avara (2) | 2925.9 (879.9) [2046–3805.8] | 1.29 (0.62) [0.67–1.91] | [17,94] |
| HMA | Aplysilla rosea (1) | 133.83 (87.05) [2.38–537.25] | 14.32 (2.07) [6.07–17.89] | [29] | |
| Sarcotragus foetidus (3) | [63] | ||||
| Sarcotragus spinosulus (1) | [35] | ||||
| Spongia officinalis (1) | [49] | ||||
| Verongimorpha | HMA | Chondrilla nucula (1) | 7.39 (1.82) [2.98–11.7] | 11.36 (2.79) [2.99–14.15] | [65] |
| Chondrosia reniformis (3) | [63] | ||||
| Aplysina aerophoba | 75.25 (9.58) | 5.44 (0.31) | Present paper |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Voogd, N.J.; Alvarez, B.; Boury-Esnault, N.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Goodwin, C.; Hajdu, E.; Hooper, J.N.A.; et al. World Porifera Database. Available online: https://www.marinespecies.org/porifera (accessed on 21 November 2024).
- Van Soest, R.W.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Hooper, J.N. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Larsen, P.S.; Riisgård, H.U. The Sponge Pump. J. Theor. Biol. 1994, 168, 53–63. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P.S. Filter-Feeding in Marine Macro-Invertebrates: Pump Characteristics, Modelling and Energy Cost. Biol. Rev. 1995, 70, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Pile, A.J.; Patterson, M.R.; Witman, J.D. In Situ Grazing on Plankton < 10 μm by the Boreal Sponge Mycale lingua. Mar. Ecol. Prog. Ser. 1996, 141, 95–102. [Google Scholar] [CrossRef]
- de Goeij, J.M.; Lesser, M.P.; Pawlik, J.R. Nutrient fluxes and ecological functions of coral reef sponges in a changing ocean. In Climate Change, Ocean Acidification and Sponges: Impacts Across Multiple Levels of Organization; Springer: Cham, Switzerland, 2017; pp. 373–410. [Google Scholar]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient Fluxes through Sponges: Biology, Budgets, and Ecological Implications. Adv. Mar. Biol. 2012, 62, 113–182. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Bayer, K.; López-Acosta, M. Nitrogen and Phosphorus Cycling Through Marine Sponges: Physiology, Cytology, Genomics, and Ecological Implications. In Frontiers in Invertebrate Physiology: A Collection of Reviews; Saleuddin, S., Leys, S.P., Roer, R.D., Wilkie, I.C., Eds.; Apple Academic Press: New York, NY, USA, 2024. [Google Scholar]
- de Goeij, J.M.; Van Oevelen, D.; Vermeij, M.J.; Osinga, R.; Middelburg, J.J.; De Goeij, A.F.; Admiraal, W. Surviving in a Marine Desert: The Sponge Loop Retains Resources within Coral Reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef] [PubMed]
- McMurray, S.E.; Stubler, A.D.; Erwin, P.M.; Finelli, C.M.; Pawlik, J.R. A Test of the Sponge-Loop Hypothesis for Emergent Caribbean Reef Sponges. Mar. Ecol. Prog. Ser. 2018, 588, 1–14. [Google Scholar] [CrossRef]
- Venkateswara Rao, J.; Srikanth, K.; Pallela, R.; Gnaneshwar Rao, T. The Use of Marine Sponge, Haliclona Tenuiramosa as Bioindicator to Monitor Heavy Metal Pollution in the Coasts of Gulf of Mannar, India. Environ. Monit. Assess. 2009, 156, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Tellini, K.; Nudi, A.H.; Massone, T.P.; Scofield, A.D.L.; Wagener, A.L.R. Marine Sponges as Bioindicators of Oil and Combustion Derived PAH in Coastal Waters. Mar. Environ. Res. 2013, 92, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Mahaut, M.L.; Basuyaux, O.; Baudinière, E.; Chataignier, C.; Pain, J.; Caplat, C. The Porifera Hymeniacidon perlevis (Montagu, 1818) as a Bioindicator for Water Quality Monitoring. Environ. Sci. Pollut. Res. 2013, 20, 2984–2992. [Google Scholar] [CrossRef]
- Celis-Hernández, O.; Ávila, E.; Ward, R.D.; Rodríguez-Santiago, M.A.; Aguirre-Téllez, J.A. Microplastic Distribution in Urban vs Pristine Mangroves: Using Marine Sponges as Bioindicators of Environmental Pollution. Environ. Pollut. 2021, 284, 117391. [Google Scholar] [CrossRef] [PubMed]
- Orani, A.M.; Vassileva, E.; Thomas, O.P. Marine Sponges as Coastal Bioindicators of Rare Earth Elements Bioaccumulation in the French Mediterranean Sea. Environ. Pollut. 2022, 304, 119172. [Google Scholar] [CrossRef] [PubMed]
- Reiswig, H.M. Particle feeding in natural populations of three marine demosponges. Biol. Bull. 1971, 141, 568–602. [Google Scholar] [CrossRef]
- Ribes, M.; Coma, R.; Gili, J.M. Natural Diet and Grazing Rate of the Temperate Marine Sponge Dysidea avara (Demospongiae, Dendroceratida) Throughout an Annual Cycle. Mar. Ecol. Prog. Ser. 1999, 176, 179–190. [Google Scholar] [CrossRef]
- Reiswig, H.M. Bacteria as Food for Temperate-Water Marine Sponges. Can. J. Zool. 1975, 53, 582–589. [Google Scholar] [CrossRef]
- Bagum, N.; Monir, M.S.; Khan, M.H. Present Status of Fish Diseases and Economic Losses Due to Incidence of Disease in Rural Freshwater Aquaculture of Bangladesh. J. Innov. Dev. Strategy 2013, 7, 48–53. [Google Scholar]
- Mishra, S.; Das, R.; Swain, P. Status of Fish Diseases in Aquaculture and Assessment of Economic Loss Due to Disease. In Contemporary Trends in Fisheries and Aquaculture; Rao, P., Pandey, B., Pandey, P., Joshi, B.D., Eds.; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2018. [Google Scholar]
- Liston, J. Microbial Hazards of Seafood Consumption. Food Technol. 1990, 44, 56–62. [Google Scholar]
- Su, Y.C.; Liu, C. Vibrio Parahaemolyticus: A Concern of Seafood Safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.G.; Son, K.T.; Yu, H.; Lee, T.S.; Lee, H.J.; Shin, S.; Kim, J. Antimicrobial Resistance of Vibrio parahaemolyticus and Vibrio alginolyticus Strains Isolated from Farmed Fish in Korea from 2005 through 2007. J. Food Prot. 2011, 74, 380–386. [Google Scholar] [CrossRef]
- Reilly, A.; Käferstein, F.J.A.R. Food Safety Hazards and the Application of the Principles of the Hazard Analysis and Critical Control Point (HACCP) System for Their Control in Aquaculture Production. Aquac. Res. 1997, 28, 735–752. [Google Scholar] [CrossRef]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.R.; Pomponi, S.A. Relative Importance of Bacteria, Microalgae and Yeast for Growth of the Sponge Halichondria melanadocia (De Laubenfels, 1936): A Laboratory Study. J. Exp. Mar. Biol. Ecol. 2005, 323, 151–159. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Tanner, J.E.; Barnett, S.J.; Bekker, J.; Franco, C.M.; Zhang, W. A Controlled Aquarium System and Approach to Study the Role of Sponge-Bacteria Interactions Using Aplysilla rosea and Vibrio natriegens. Sci. Rep. 2018, 8, 11801. [Google Scholar] [CrossRef]
- Maldonado, M.; Zhang, X.; Cao, X.; Xue, L.; Cao, H.; Zhang, W. Selective Feeding by Sponges On Pathogenic Microbes: A Reassessment of Potential For Abatement of Microbial Pollution. Mar. Ecol. Prog. Ser. 2010, 403, 75–89. [Google Scholar] [CrossRef]
- Wehrl, M.; Steinert, M.; Hentschel, U. Bacterial Uptake by the Marine Sponge Aplysina aerophoba. Microb. Ecol. 2007, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhang, J.; Zheng, C.; Liu, J.; An, Z.; Liu, H.; Zhang, W. Molecular Cloning of Partial 14-3-3 Genes in the Marine Sponge Hymeniacidon perleve and Its Role in Differentiating Infectious and Non-Infectious Bacteria. Chin. Sci. Bull. 2013, 58, 766–776. [Google Scholar] [CrossRef]
- Fu, W.; Sun, L.; Zhang, X.; Zhang, W. Potential of the Marine Sponge Hymeniacidon perleve as a Bioremediator of Pathogenic Bacteria in Integrated Aquaculture Ecosystems. Biotechnol. Bioeng. 2006, 93, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Corriero, G.; Licciano, M.; Stabili, L. Bacterial Accumulation by the Demospongiae Hymeniacidon perlevis: A Tool for the Bioremediation of Polluted Seawater. Mar. Pollut. Bull. 2010, 60, 1182–1187. [Google Scholar] [CrossRef]
- Trani, R.; Corriero, G.; de Pinto, M.C.; Mercurio, M.; Pazzani, C.; Pierri, C.; Scrascia, M.; Longo, C. Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. J. Mar. Sci. Eng. 2021, 9, 178. [Google Scholar] [CrossRef]
- Trani, R.; de Pinto, M.C.; Scrascia, M.; Ferriol, P.; Raso, A.G.; Longo, C. Tethya meloni (Porifera, Demospongiae): A Promising Bioremediator Species and Source of Marine Natural Products. In Proceedings of the IEEE International Workshop on Metrology for the Sea, Learning to Measure Sea Health Parameters. Milazzo, Italy, 3–5 October 2022; pp. 81–85. [Google Scholar] [CrossRef]
- Gentric, C.; Sauleau, P. Bacterial Load Mitigation of the Shellfish Magallana gigas by the Marine Sponge Hymeniacidon perlevis (Montagu 1818). Reg. Stud. Mar. Sci. 2024, 75, 103564. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Longo, C.; Corriero, G.; Mercurio, M. Evaluation of Microbiological Accumulation Capability of the Commercial Sponge Spongia officinalis var adriatica (Schmidt) (Porifera, Demospongiae). Water Res. 2008, 42, 2499–2506. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Xue, L.; Zhang, B.; Jin, M.; Fu, W. Bioremediation of Bacteria Pollution Using the Marine Sponge Hymeniacidon perlevis in the Intensive Mariculture Water System of Turbot Scophthalmus maximus. Biotechnol. Bioeng. 2010, 105, 59–68. [Google Scholar] [CrossRef]
- Longo, C.; Cardone, F.; Corriero, G.; Licciano, M.; Pierri, C.; Stabili, L. The Co-Occurrence of the Demosponge Hymeniacidon perlevis and the Edible Mussel Mytilus galloprovincialis as a New Tool for Bacterial Load Mitigation in Aquaculture. Environ. Sci. Pollut. Res. 2016, 23, 3736–3746. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Pierri, C.; Mercurio, M.; Trani, R.; Cardone, F.; Carbonara, P.; Stabili, L. Bioremediation Capabilities of Hymeniacidon perlevis (Porifera, Demospongiae) in a Land-Based Experimental Fish Farm. J. Mar. Sci. Eng. 2022, 10, 874. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Garrone, R.; Vacelet, J. Marine Sponges Discriminate Between Food Bacteria and Bacterial Symbionts: Electron Microscope Radioautography and In Situ Evidence. Proc. R. Soc. Lond. B Biol. Sci. 1984, 220, 519–528. [Google Scholar] [CrossRef]
- Bayer, K.; Schmitt, S.; Hentschel, U. Physiology, Phylogeny and In Situ Evidence for Bacterial and Archaeal Nitrifiers in the Marine Sponge Aplysina aerophoba. Environ. Microbiol. 2008, 10, 2942–2955. [Google Scholar] [CrossRef]
- Jiménez, E.; Ribes, M. Sponges as a Source of Dissolved Inorganic Nitrogen: Nitrification Mediated by Temperate Sponges. Limnol. Oceanogr. 2007, 52, 948–958. [Google Scholar] [CrossRef]
- Pfannkuchen, M.; Fritz, G.B.; Schlesinger, S.; Bayer, K.; Brümmer, F. In Situ Pumping Activity of the Sponge Aplysina aerophoba, Nardo 1886. J. Exp. Mar. Biol. Ecol. 2009, 369, 65–71. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Longo, C. An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Longo, C.; Trani, R.; Aguilo-Arce, J.; Ferriol, P.; Puthod, P.; Pierri, C. Porifera in the Remedia Life Integrated Multitrophic Aquaculture (IMTA) System. Biol. Mar. Mediterr. 2024, 28, 19–22. [Google Scholar]
- Martinez-Urtaza, J.; Lozano-Leon, A.; Viña-Feas, A.; De Novoa, J.; Garcia-Martin, O. Differences in the API 20E biochemical patterns of clinical and environmental Vibrio parahaemolyticus isolates. FEMS Microbiol. Lett. 2006, 255, 75–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coughlan, J. The Estimation of Filtering Rate from the Clearance of Suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering Activity of Spongia officinalis var adriatica (Schmidt) (Porifera, Demospongiae) on Bacterioplankton: Implications for Bioremediation of Polluted Seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Müller, W.E.; Wimmer, W.; Schatton, W.; Böhm, M.; Batel, R.; Filic, Z. Initiation of an Aquaculture of Sponges for the Sustainable Production of Bioactive Metabolites in Open Systems: Example, Geodia cydonium. Mar. Biotechnol. 1999, 1, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Aguilo-Arce, J.; Ferriol, P.; Trani, R.; Puthod, P.; Pierri, C.; Longo, C. Sponges as Emerging by-Product of Integrated Multitrophic Aquaculture (IMTA). J. Mar. Sci. Eng. 2023, 11, 80. [Google Scholar] [CrossRef]
- Aguilo-Arce, J.; Ferriol, P.; Trani, R.; Longo, C. The Remedia Life Integrated Multitrophic Aquaculture System as a Powerful Sponge Biomass Supply. Biol. Mar. Mediterr. 2024, 28, 87–89. [Google Scholar]
- Trani, R.; Aguilo-Arce, J.; Ferriol, P.; Puthod, P.; Pierri, C.; Longo, C. Metodologie Di Allevamento Di Poriferi in Un Sistema Di Acquacoltura Multi Trofica Integrata. Biol. Mar. Mediterr. 2024, 28, 94–97. [Google Scholar]
- Reiswig, H.M. In Situ Pumping Activities of Tropical Demospongiae. Mar. Biol. 1971, 9, 38–50. [Google Scholar] [CrossRef]
- Vogel, S. Current-Induced Flow Through Living Sponges in Nature. Proc. Natl. Acad. Sci. USA 1977, 74, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Gerrodette, T.; Flechsig, A.O. Sediment-Induced Reduction in the Pumping Rate of the Tropical Sponge Verongia lacunosa. Mar. Biol. 1979, 55, 103–110. [Google Scholar] [CrossRef]
- Kumala, L.; Riisgård, H.U.; Canfield, D.E. Osculum Dynamics and Filtration Activity in Small Single-Osclulum Explants of the Demosponge Halichondria panicea. Mar. Ecol. Prog. Ser. 2017, 572, 117–128. [Google Scholar] [CrossRef]
- Kumala, L.; Larsen, M.; Glud, R.N.; Canfield, D.E. Spatial and Temporal Anoxia in Single-Osclulum Halichondria panicea Demosponge Explants Studied with Planar Optodes. Mar. Biol. 2021, 168, 173. [Google Scholar] [CrossRef]
- Hoffmann, F.; Røy, H.; Bayer, K.; Hentschel, U.; Pfannkuchen, M.; Brümmer, F.; De Beer, D. Oxygen Dynamics and Transport in the Mediterranean Sponge Aplysina aerophoba. Mar. Biol. 2008, 153, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Ledda, F.D.; Pronzato, R.; Manconi, R. Mariculture for Bacterial and Organic Waste Removal: A Field Study of Sponge Filtering Activity in Experimental Farming. Aquac. Res. 2014, 45, 1389–1401. [Google Scholar] [CrossRef]
- Varamogianni-Mamatsi, D.; Anastasiou, T.I.; Vernadou, E.; Papandroulakis, N.; Kalogerakis, N.; Dailianis, T.; Mandalakis, M. A Multi-Species Investigation of Sponges’ Filtering Activity Towards Marine Microalgae. Mar. Drugs 2022, 20, 24. [Google Scholar] [CrossRef]
- Weisz, J.B.; Lindquist, N.; Martens, C.S. Do Associated Microbial Abundances Impact Marine Demosponge Pumping Rates and Tissue Densities? Oecologia 2008, 155, 367–376. [Google Scholar] [CrossRef]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sara, A.; Sidri, M.; Pronzato, R. The Marine Sponge Chondrilla nucula Schmidt, 1862 as an Elective Candidate for Bioremediation in Integrated Aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Leys, S.P.; Kahn, A.S.; Fang, J.K.H.; Kutti, T.; Bannister, R.J. Phagocytosis of Microbial Symbionts Balances the Carbon and Nitrogen Budget for the Deep-Water Boreal Sponge Geodia barretti. Limnol. Oceanogr. 2018, 63, 187–202. [Google Scholar] [CrossRef]
- Colwell, R.R.; Grimes, D.J. Vibrio Diseases of Marine Fish Populations. Helgol. Mar. Res. 1984, 37, 265–287. [Google Scholar] [CrossRef]
- Gloeckner, V.; Wehrl, M.; Moitinho-Silva, L.; Gernert, C.; Schupp, P.J.; Pawlik, J.R.; Lindquist, N.L.; Erpenbeck, D.; Wörheide, G.; Hentschel, U. The HMA-LMA dichotomy revisited: An electron microscopical survey of 56 sponge species. Biol. Bull. 2014, 227, 78–88. [Google Scholar] [CrossRef]
- Massaro, A.J.; Weisz, J.B.; Hill, M.S.; Webster, N.S. Behavioral and morphological changes caused by thermal stress in the Great Barrier Reef sponge Rhopaloeides odorabile. J. Exp. Mar. Biol. Ecol. 2012, 416, 55–60. [Google Scholar] [CrossRef]
- Goldstein, J.; Riisgård, H.U.; Larsen, P.S. Exhalant jet speed of single-osculum explants of the demosponge Halichondria panicea and basic properties of the sponge-pump. J. Exp. Mar. Biol. Ecol. 2019, 511, 82–90. [Google Scholar] [CrossRef]
- Morganti, T.M.; Ribes, M.; Yahel, G.; Coma, R. Size is the major determinant of pumping rates in marine sponges. Front. Physiol. 2019, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Giangrande, A.; Arduini, D.; Borghese, J.; Petrocelli, A.; Alabiso, G.; Longo, C. Environmental quality improvement of a mariculture plant after its conversion into a multi-trophic system. Sci. Total Environ. 2023, 884, 163846. [Google Scholar] [CrossRef]
- Fu, W.; Wu, Y.; Sun, L.; Zhang, W. Efficient bioremediation of total organic carbon (TOC) in integrated aquaculture system by marine sponge Hymeniacidon perleve. Biotechnol. Bioeng. 2007, 97, 1387–1397. [Google Scholar] [CrossRef]
- Gökalp, M.; Wijgerde, T.; Sarà, A.; De Goeij, J.M.; Osinga, R. Development of an integrated mariculture for the collagen-rich sponge Chondrosia reniformis. Mar. Drugs 2019, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Tramper, J.; Wijffels, R.H. Cultivation of marine sponges. Mar. Biotechnol. 1999, 1, 509–532. [Google Scholar] [CrossRef]
- Aguilo-Arce, J.; Schiavo, A.; Trani, R.; Longo, C. Do sustainably reared marine sponges represent a potential new product in aquariology? A citizen science-based approach. Sustainability 2024, 16, 1066. [Google Scholar] [CrossRef]
- Arduini, D.; Calabrese, C.; Borghese, J.; De Domenico, S.; Putignano, M.; Toso, A.; Giangrande, A. Perspectives for exploitation of Sabella spallanzanii’s biomass as a new integrated multi-trophic aquaculture (IMTA) by-product: Feeding trial on Amphiprion ocellaris using Sabella meal. J. Mar. Sci. Eng. 2023, 11, 123. [Google Scholar] [CrossRef]
- Stabili, L.; Cecere, E.; Licciano, M.; Petrocelli, A.; Sicuro, B.; Giangrande, A. Integrated multitrophic aquaculture by-products with added value: The polychaete Sabella spallanzanii and the seaweed Chaetomorpha linum as potential dietary ingredients. Mar. Drugs 2019, 17, 677. [Google Scholar] [CrossRef] [PubMed]
- Khailov, K.M.; Burlakova, Z.P. Release of dissolved organic matter by marine seaweeds and distribution of their total organic production to inshore communities. Limnol. Oceanogr. 1969, 14, 521–527. [Google Scholar] [CrossRef]
- Wang, X.; Andresen, K.; Handå, A.; Jensen, B.; Reitan, K.I.; Olsen, Y. Chemical composition and release rate of waste discharge from an Atlantic salmon farm with an evaluation of IMTA feasibility. Aquac. Environ. Interact. 2013, 4, 147–162. [Google Scholar] [CrossRef]
- Maxwell, K.H.; Gardner, J.P.; Heath, P.L. The effect of diet on the energy budget of the brown sea cucumber, Stichopus mollis (Hutton). J. World Aquac. Soc. 2009, 40, 157–170. [Google Scholar] [CrossRef]
- Zuppa, A.; Costantini, S.; Costantini, M. Comparative Sequence Analysis of Bacterial Symbionts from the Marine Sponges Geodia cydonium and Ircinia muscarum. Bioinformation 2014, 10, 196. [Google Scholar] [CrossRef][Green Version]
- Hentschel, U.; Schmid, M.; Wagner, M.; Fieseler, L.; Gernert, C.; Hacker, J. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol. Ecol. 2001, 35, 305–312. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, X.; Song, L.; Chang, Y.; Yang, G. Molecular Determination of Oxytetracycline-Resistant Bacteria and Their Resistance Genes from Mariculture Environments of China. J. Appl. Microbiol. 2007, 103, 2580–2592. [Google Scholar] [CrossRef]
- Baquiran, J.I.P.; Conaco, C. Sponge-microbe partnerships are stable under eutrophication pressure from mariculture. Mar. Pollut. Bull. 2018, 136, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Maslin, M.; Paix, B.; van der Windt, N.; Ambo-Rappe, R.; Debitus, C.; Gaertner-Mazouni, N.; de Voogd, N.J. Prokaryotic communities of the French Polynesian sponge Dactylospongia metachromia display a site-specific and stable diversity during an aquaculture trial. Antonie Van Leeuwenhoek 2024, 117, 65. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Giangrande, A.; Fanelli, G.; Cavallo, R.A. Sabella spallanzanii filter-feeding on bacterial community: Ecological implications and applications. Mar. Environ. Res. 2006, 61, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Longo, C.; Lezzi, M.; Giangrande, A. The Mediterranean non-indigenous ascidian Polyandrocarpa zorritensis: Microbiological accumulation capability and environmental implications. Mar. Pollut. Bull. 2015, 101, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Cardone, F.; Alifano, P.; Tredici, S.M.; Piraino, S.; Corriero, G.; Gaino, E. Epidemic Mortality of the Sponge Ircinia variabilis (Schmidt, 1862) Associated to Proliferation of a Vibrio Bacterium. Microb. Ecol. 2012, 64, 802–813. [Google Scholar] [CrossRef]
- Dinçtürk, E.; Öndes, F.; Leria, L.; Maldonado, M. Mass Mortality of the Keratose Sponge Sarcotragus foetidus in the Aegean Sea (Eastern Mediterranean) Correlates with Proliferation of Vibrio Bacteria in the Tissues. Front. Microbiol. 2023, 14, 1272733. [Google Scholar] [CrossRef]
- Sung, H.H.; Li, H.C.; Tsai, F.M.; Ting, Y.Y.; Chao, W.L. Changes in the Composition of Vibrio Communities in Pond Water During Tiger Shrimp (Penaeus monodon) Cultivation and in the Hepatopancreas of Healthy and Diseased Shrimp. J. Exp. Mar. Biol. Ecol. 1999, 236, 261–271. [Google Scholar] [CrossRef]
- Silva-Aciares, F.; Moraga, D.; Auffret, M.; Tanguy, A.; Riquelme, C. Transcriptomic and Cellular Response to Bacterial Challenge (Pathogenic Vibrio parahaemolyticus) in Farmed Juvenile Haliotis Rufescens Fed with or Without Probiotic Diet. J. Invertebr. Pathol. 2013, 113, 163–176. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Hewitt, J.E.; Thrush, S.F. Assessing Far-Field Effects of Terrigenous Sediment Loading in the Coastal Marine Environment. Mar. Ecol. Prog. Ser. 2006, 315, 13–18. [Google Scholar] [CrossRef]
- Turon, X.; Galera, J.; Uriz, M.J. Clearance Rates and Aquiferous Systems in Two Sponges with Contrasting Life-History Strategies. J. Exp. Zool. 1997, 278, 22–36. [Google Scholar] [CrossRef]
- Lüskow, F.; Riisgård, H.U.; Solovyeva, V.; Brewer, J.R. Seasonal Changes in Bacteria and Phytoplankton Biomass Control the Condition Index of the Demosponge Halichondria panicea in Temperate Danish Waters. Mar. Ecol. Prog. Ser. 2019, 608, 119–132. [Google Scholar] [CrossRef]
- Stuart, V.; Klumpp, D. Evidence for food-resource partitioning by kelp-bed filter feeders. Mar. Ecol. Prog. Ser. 1984, 16, 27–37. [Google Scholar] [CrossRef]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Growth Efficiency and Carbon Balance for the Sponge Haliclona oculata. Mar. Biotechnol. 2010, 12, 340–349. [Google Scholar] [CrossRef][Green Version]
- Echevarria, M.; Naar, J.P.; Tomas, C.; Pawlik, J.R. Effects of Karenia brevis on Clearance Rates and Bioaccumulation of Brevetoxins in Benthic Suspension Feeding Invertebrates. Aquat. Toxicol. 2012, 106, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hadas, E.; Shpigel, M.; Ilan, M. Particulate Organic Matter as a Food Source for a Coral Reef Sponge. J. Exp. Biol. 2009, 212, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Kleijn, R.; Groenendijk, E.; Niesink, P.; Tramper, J.; Wijffels, R.H. Development of in vivo sponge cultures: Particle feeding by the tropical sponge Pseudosuberites aff. andrewsi. Mar. Biotechnol. 2001, 3, 544–554. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilo-Arce, J.; Scrascia, M.; Trani, R.; Pazzani, C.; Ferriol, P.; Longo, C. Two Demosponges as Promising Bioremediators of a Potential Pathogenic Vibrio. Biology 2025, 14, 140. https://doi.org/10.3390/biology14020140
Aguilo-Arce J, Scrascia M, Trani R, Pazzani C, Ferriol P, Longo C. Two Demosponges as Promising Bioremediators of a Potential Pathogenic Vibrio. Biology. 2025; 14(2):140. https://doi.org/10.3390/biology14020140
Chicago/Turabian StyleAguilo-Arce, Joseba, Maria Scrascia, Roberta Trani, Carlo Pazzani, Pere Ferriol, and Caterina Longo. 2025. "Two Demosponges as Promising Bioremediators of a Potential Pathogenic Vibrio" Biology 14, no. 2: 140. https://doi.org/10.3390/biology14020140
APA StyleAguilo-Arce, J., Scrascia, M., Trani, R., Pazzani, C., Ferriol, P., & Longo, C. (2025). Two Demosponges as Promising Bioremediators of a Potential Pathogenic Vibrio. Biology, 14(2), 140. https://doi.org/10.3390/biology14020140










