Mechanobiological Approach for Intestinal Mucosal Immunology
Simple Summary
Abstract
1. Intestinal Microenvironment
1.1. Importance of the Intestine
1.2. Components of Intestinal Barrier
| Cell Type | Location | Key Functions | References |
|---|---|---|---|
| Enterocytes | Major cell type in intestinal villi | Nutrient absorption Immunomodulator | [44,45] |
| Goblet cells | Scattered in intestinal villi and crypts Prevalent in the large intestine | Mucus secretion Protection against bacterial invasion | [46,47] |
| Paneth cells | Crypts at the base of the small intestine | Production and secretion of AMP Performing phagocytosis, efferocytosis Preserving barrier integrity | [48,49,50] |
| Enteroendocrine cells | Relatively rare throughout the intestine | Release of hormones (GLP-1, GIP, CCK, secretin) | [51] |
| M cells | Follicle-associated epithelium of intestinal Peyer’s patches | Transporting antigen to mucosal lymphoid tissue | [52,53] |
| Tuft cells | Scattered in intestinal villi | Secretion of interleukin-25 after parasitic infection Initiating a type 2 immune response Sensing bacterial metabolite N-undecanoyl glycine | [54,55] |
| Stem cells | Bottom of the intestinal crypt between Paneth cells | Proliferation, and differentiation to replace intestinal epithelium Interacting with gut microbiota | [56,57,58] |
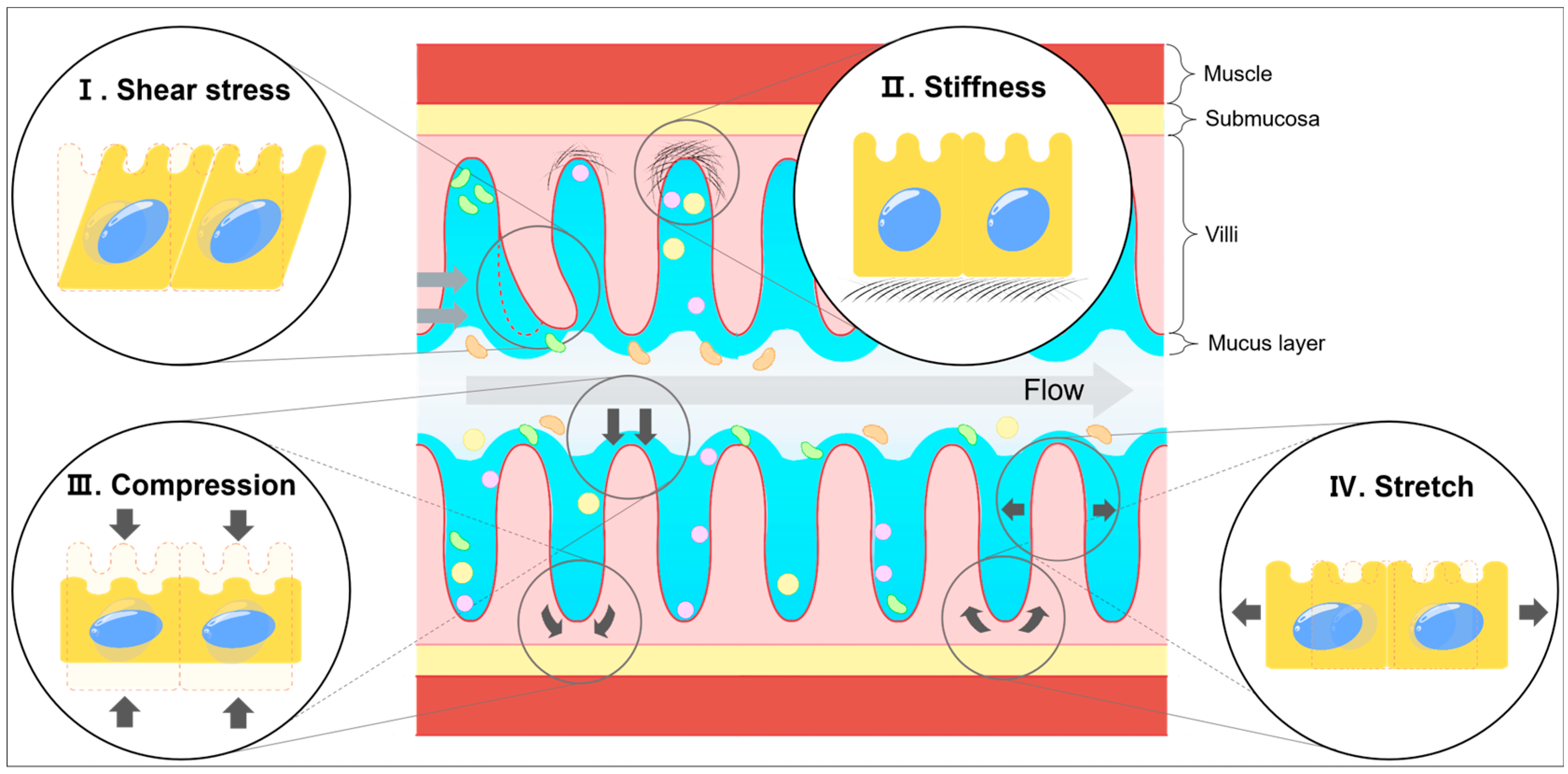
2. Mechanical Stress in the Intestine
2.1. Shear Stress
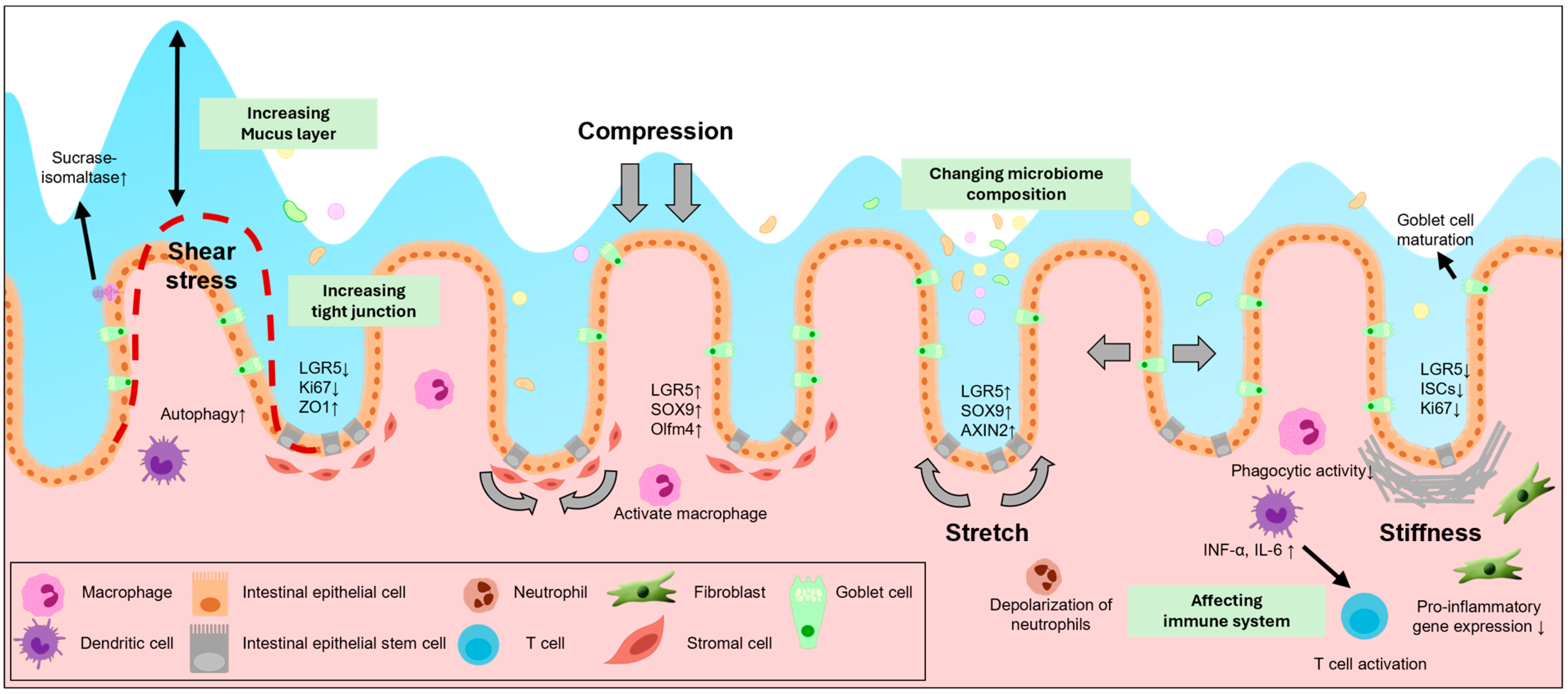
| Mechanical Force | Target Organ | Signaling Pathways | Effects on Cells | Reference |
|---|---|---|---|---|
| Shear stress | Small intestine | Unknown | Unknown | |
| Large intestine | Wnt/β-catenin signaling | Form villi-like epithelium | [63,65,66,67,75] | |
| Autophagy pathway | Form vacuole | |||
| Unknown | Increase alkaline phosphatase | |||
| Enhance barrier function | ||||
| Stiffness | Small intestine | YAP/TAZ signaling | Goblet cell differentiation | [76,77,78] |
| Wnt/β-catenin signaling | Decrease LGR5, Ki67 expression | |||
| Enhance crypt fission | ||||
| Large intestine | Ca2+ dependent | ISCs differentiation | [79,80,81,82] | |
| Hippo and YAP/TAZ signaling | Uncontrolled proliferation | |||
| FAK signaling | Enhance fibroblast density Reduce pro-inflammatory genes FAK-dependent proliferation | |||
| Compression | Small intestine | Wnt/β-catenin signaling | Increase LGR5, SOX9 expression | [61] |
| Large intestine | Unknown | Unknown | ||
| Stretch | Small intestine | Piezo1-mediated Ca2+ influx | Crypt fission | [83,84,85] |
| Wnt/β-catenin signaling | Increase Ki67, SOX9 expression | |||
| Increase LGR5, Olfm4 expression | ||||
| Piezo2-mediated Ca2+ influx | Release serotonin | |||
| Large intestine | Piezo2-mediated Ca2+ influx | Release serotonin | [86] |
2.2. Stiffness
2.3. Compression and Stretch Forces
2.4. Other Forces
3. Association Between Inflammatory Diseases and the Intestinal Environment
3.1. Importance of ECM
3.2. Remodeling of ECM in IBD
3.3. Mechanical Forces in Inflammation in the Intestine
3.4. ECM Remodeling in CRC
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IECs | intestinal epithelial cells |
| IBS | irritable bowel syndrome |
| IBD | inflammatory bowel disease |
| YAP | Yes-associated protein |
| ECM | extracellular matrix |
| IL | interleukin |
| CCK | cholecystokinin |
| IEC | intestinal epithelial cell |
| ZO | zonula occludens |
| BMP | bone morphogenic protein |
| TAZ | transcriptional co-activator with PDZ-binding motif |
| ISC | intestinal stem cell |
| AMP | antimicrobial peptide |
| TA | transit-amplifying zone |
| GAG | glycosaminoglycan |
| MMP | matrix metalloproteinase |
| CRC | colorectal cancer |
| ECM1 | extracellular matrix protein 1 |
| DSS | dextran sodium sulfate |
| MCP-1 | monocyte chemoattractant protein-1 |
References
- Ohara, T.E.; Colonna, M.; Stappenbeck, T.S. Adaptive differentiation promotes intestinal villus recovery. Dev. Cell 2022, 57, 166–179.e6. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.A.H.; Heyndrickx, M.; Jonkers, D.; Mackie, A.; Millet, S.; Naghibi, M.; Paerregaard, S.I.; Pot, B.; Saulnier, D.; Sina, C.; et al. Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration. Cell Rep. Med. 2023, 4, 101190. [Google Scholar] [CrossRef]
- Duca, F.A.; Waise, T.M.Z.; Peppler, W.T.; Lam, T.K.T. The metabolic impact of small intestinal nutrient sensing. Nat. Commun. 2021, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Xia, Z.S.; Zhong, Y.Q.; Zhang, S.N.; Wang, L.Y.; Shu, H.; Zhu, Z.H. Clinical analysis of primary small intestinal disease: A report of 309 cases. World J. Gastroenterol. 2004, 10, 2585–2587. [Google Scholar] [CrossRef]
- Burgueno, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef]
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.M.; Jorgensen, P.B.; Niss, K.; Rubin, S.J.S.; Morbe, U.M.; Riis, L.B.; Da Silva, C.; Plumb, A.; Vandamme, J.; Jakobsen, H.L.; et al. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity 2020, 52, 557–570.e6. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, S.; Wang, L.; Zhang, Y.; Chen, H.; Fu, Z.; Zhang, M.; Luo, H.; Liu, J. Reinforcement of the intestinal mucosal barrier via mucus-penetrating PEGylated bacteria. Nat. Biomed. Eng. 2024, 8, 823–841. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Burrello, C.; Garavaglia, F.; Cribiu, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91 e75. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Lin, Z.; Lv, C.; Shao, C.; Bi, X.; Deng, M.; Xu, J.; Guerrero-Juarez, C.F.; Li, M.; Wu, X.; et al. Cycling Stem Cells Are Radioresistant and Regenerate the Intestine. Cell Rep. 2020, 32, 107952. [Google Scholar] [CrossRef] [PubMed]
- He, X.C.; Zhang, J.; Tong, W.G.; Tawfik, O.; Ross, J.; Scoville, D.H.; Tian, Q.; Zeng, X.; He, X.; Wiedemann, L.M.; et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 2004, 36, 1117–1121. [Google Scholar] [CrossRef]
- Totaro, A.; Castellan, M.; Di Biagio, D.; Piccolo, S. Crosstalk between YAP/TAZ and Notch Signaling. Trends Cell Biol. 2018, 28, 560–573. [Google Scholar] [CrossRef]
- Ramadan, R.; van Driel, M.S.; Vermeulen, L.; van Neerven, S.M. Intestinal stem cell dynamics in homeostasis and cancer. Trends Cancer 2022, 8, 416–425. [Google Scholar] [CrossRef]
- Houtekamer, R.M.; van der Net, M.C.; Maurice, M.M.; Gloerich, M. Mechanical forces directing intestinal form and function. Curr. Biol. 2022, 32, R791–R805. [Google Scholar] [CrossRef] [PubMed]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, D.; Lee, K.H.; Park, S.J.; Park, S.; Kim, H.; Kim, T.; Joo, I.H.; Park, J.M.; Kang, Y.H.; et al. Biometabolites of Citrus unshiu Peel Enhance Intestinal Permeability and Alter Gut Commensal Bacteria. Nutrients 2023, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Davis, J.E.; Rappai, T.; McDonald, K.G.; Kulkarni, D.H.; Knoop, K.A.; Hogan, S.P.; Fitzpatrick, J.A.; Lencer, W.I.; Newberry, R.D. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. eLife 2021, 10, e67292. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.B.; Gribble, F.M.; Reimann, F. Free Fatty Acid Receptors in Enteroendocrine Cells. Endocrinology 2018, 159, 2826–2835. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef]
- Gonzalez, G.; Holman, A.R.; Nelson, A.C.; Engler, A.J. Engineering the niche to differentiate and deploy cardiovascular cells. Curr. Opin. Biotechnol. 2022, 74, 122–128. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction with the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Miron, N.; Cristea, V. Enterocytes: Active cells in tolerance to food and microbial antigens in the gut. Clin. Exp. Immunol. 2012, 167, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.E.; Harnik, Y.; Ben-Moshe, S.; Massasa, E.E.; Rozenberg, M.; Eilam, R.; Bahar Halpern, K.; Itzkovitz, S. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation Along the Intestinal Villus Axis. Cell 2018, 175, 1156–1167.e1115. [Google Scholar] [CrossRef]
- Naama, M.; Telpaz, S.; Awad, A.; Ben-Simon, S.; Harshuk-Shabso, S.; Modilevsky, S.; Rubin, E.; Sawaed, J.; Zelik, L.; Zigdon, M.; et al. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe 2023, 31, 433–446.e434. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, S.L.; Chase, D.G. Paneth cell function: Phagocytosis and intracellular digestion of intestinal microorganisms. II. Spiral microorganism. J. Ultrastruct. Res. 1972, 41, 319–333. [Google Scholar] [CrossRef]
- Bel, S.; Pendse, M.; Wang, Y.; Li, Y.; Ruhn, K.A.; Hassell, B.; Leal, T.; Winter, S.E.; Xavier, R.J.; Hooper, L.V. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 2017, 357, 1047–1052. [Google Scholar] [CrossRef]
- Shankman, L.S.; Fleury, S.T.; Evans, W.B.; Penberthy, K.K.; Arandjelovic, S.; Blumberg, R.S.; Agaisse, H.; Ravichandran, K.S. Efferocytosis by Paneth cells within the intestine. Curr. Biol. 2021, 31, 2469–2476.e2465. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Geurts, M.H.; Geurts, V.; Andersson-Rolf, A.; Akkerman, N.; Vollmy, F.; Krueger, D.; Busslinger, G.A.; Martinez-Silgado, A.; Boot, C.; et al. Description and functional validation of human enteroendocrine cell sensors. Science 2024, 386, 341–348. [Google Scholar] [CrossRef]
- Corr, S.C.; Gahan, C.C.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lelouard, H.; Fallet, M.; de Bovis, B.; Meresse, S.; Gorvel, J.P. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology 2012, 142, 592–601.e593. [Google Scholar] [CrossRef]
- Ndjim, M.; Gasmi, I.; Herbert, F.; Josephine, C.; Bas, J.; Lamrani, A.; Coutry, N.; Henry, S.; Zimmermann, V.S.; Dardalhon, V.; et al. Tuft cell acetylcholine is released into the gut lumen to promote anti-helminth immunity. Immunity 2024, 57, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhu, X.; Geng, J.; Xu, Y.; Wu, R.; Li, C.; Fan, D.; Qin, X.; Du, Y.; Tian, Y.; et al. Intestinal Tuft-2 cells exert antimicrobial immunity via sensing bacterial metabolite N-undecanoylglycine. Immunity 2022, 55, 686–700 e687. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Li, B.; Fei, L.; Horne, R.; Lee, D.; Loe, A.K.; Miyake, H.; Ayar, E.; Kim, D.K.; Surette, M.G.; et al. Gut microbiota promotes stem cell differentiation through macrophage and mesenchymal niches in early postnatal development. Immunity 2022, 55, 2300–2317.e2306. [Google Scholar] [CrossRef]
- Hou, Q.; Ye, L.; Huang, L.; Yu, Q. The Research Progress on Intestinal Stem Cells and Its Relationship with Intestinal Microbiota. Front. Immunol. 2017, 8, 599. [Google Scholar] [CrossRef]
- Choi, J.; Augenlicht, L.H. Intestinal stem cells: Guardians of homeostasis in health and aging amid environmental challenges. Exp. Mol. Med. 2024, 56, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.P.; Basson, M.D. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009, 21, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.L.; Olmedo, M.L. Mechanotransduction: Relevance to Physical Therapist Practice—Understanding Our Ability to Affect Genetic Expression Through Mechanical Forces. Phys. Ther. 2016, 96, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Hu, J.; Sheng, R.; Lin, Q.; He, X.; Guo, M. Volumetric Compression Induces Intracellular Crowding to Control Intestinal Organoid Growth via Wnt/beta-Catenin Signaling. Cell Stem Cell 2021, 28, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Ehrman, J.; Ahn, M.R.; Kondo, J.; Lopez, A.A.M.; Oh, Y.S.; Kim, X.H.; Crawley, S.W.; Goldenring, J.R.; Tyska, M.J.; et al. Shear stress induces noncanonical autophagy in intestinal epithelial monolayers. Mol. Biol. Cell 2017, 28, 3043–3056. [Google Scholar] [CrossRef]
- Fois, C.A.M.; Schindeler, A.; Valtchev, P.; Dehghani, F. Dynamic flow and shear stress as key parameters for intestinal cells morphology and polarization in an organ-on-a-chip model. Biomed. Microdevices 2021, 23, 55. [Google Scholar] [CrossRef]
- Yang, Q.; Xing, M.; Wang, K.; Wei, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Application of Fucoidan in Caco-2 Model Establishment. Pharmaceuticals 2022, 15, 418. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, J.; Su, W.; Li, A.; Zhang, W.; Li, H.; Hu, H.; Song, W.; Xu, C.; Chen, J. Gut-on-a-chip for exploring the transport mechanism of Hg(II). Microsyst. Nanoeng. 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C.; Bell, P.D.; Bodine, S.C.; Brooks, H.L.; Bunnett, N.; Joe, B.; Keehan, K.H.; Kleyman, T.R.; Marette, A.; Morty, R.E.; et al. An American Physiological Society cross-journal Call for Papers on “Deconstructing Organs: Single-Cell Analyses, Decellularized Organs, Organoids, and Organ-on-a-Chip Models”. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L266–L272. [Google Scholar] [CrossRef]
- Gong, J.; Nirala, N.K.; Chen, J.; Wang, F.; Gu, P.; Wen, Q.; Ip, Y.T.; Xiang, Y. TrpA1 is a shear stress mechanosensing channel regulating intestinal stem cell proliferation in Drosophila. Sci. Adv. 2023, 9, eadc9660. [Google Scholar] [CrossRef]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chen, J.; Gu, P.; Shang, Y.; Ruppell, K.T.; Yang, Y.; Wang, F.; Wen, Q.; Xiang, Y. Shear stress activates nociceptors to drive Drosophila mechanical nociception. Neuron 2022, 110, 3727–3742.e3728. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, K.; Yang, R.; Bousraou, Z.; Lee, Y.K.; Fasciano, S.; Wang, S. Notch signaling and fluid shear stress in regulating osteogenic differentiation. Front. Bioeng. Biotechnol. 2022, 10, 1007430. [Google Scholar] [CrossRef]
- Qiu, X.; Deng, Z.; Wang, M.; Feng, Y.; Bi, L.; Li, L. Piezo protein determines stem cell fate by transmitting mechanical signals. Hum. Cell 2023, 36, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Pu, L.; Qi, M.; Li, S.; Sun, B.; Wang, Y.; Liu, B.; Li, F. Laminar shear stress inhibits inflammation by activating autophagy in human aortic endothelial cells through HMGB1 nuclear translocation. Commun. Biol. 2022, 5, 425. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.S.; Choi, Y.Y.; Mo, S.J.; Ha, J.H.; Lee, Y.S.; Lee, H.U.; Park, S.D.; Shim, J.J.; Lee, J.L.; Chung, B.G. Contributions of the microbiome to intestinal inflammation in a gut-on-a-chip. Nano Converg. 2022, 9, 8. [Google Scholar] [CrossRef]
- Shin, W.; Hinojosa, C.D.; Ingber, D.E.; Kim, H.J. Human Intestinal Morphogenesis Controlled by Transepithelial Morphogen Gradient and Flow-Dependent Physical Cues in a Microengineered Gut-on-a-Chip. iScience 2019, 15, 391–406. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lei, P.; Kang, W.; Cheung, P.; Xu, T.; Mana, M.; Park, C.Y.; Wang, H.; Imada, S.; Russell, J.O.; et al. Stiffness Restricts the Stemness of the Intestinal Stem Cells and Skews Their Differentiation Toward Goblet Cells. Gastroenterology 2023, 164, 1137–1151 e1115. [Google Scholar] [CrossRef]
- Altay, G.; Larranaga, E.; Tosi, S.; Barriga, F.M.; Batlle, E.; Fernandez-Majada, V.; Martinez, E. Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci. Rep. 2019, 9, 10140. [Google Scholar] [CrossRef]
- Langlands, A.J.; Almet, A.A.; Appleton, P.L.; Newton, I.P.; Osborne, J.M.; Nathke, I.S. Paneth Cell-Rich Regions Separated by a Cluster of Lgr5+ Cells Initiate Crypt Fission in the Intestinal Stem Cell Niche. PLoS Biol. 2016, 14, e1002491. [Google Scholar] [CrossRef] [PubMed]
- Mestril, S.; Kim, R.; Hinman, S.S.; Gomez, S.M.; Allbritton, N.L. Stem/Proliferative and Differentiated Cells within Primary Murine Colonic Epithelium Display Distinct Intracellular Free Ca(2+) Signal Codes. Adv. Healthcare Mater. 2021, 10, e2101318. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Rodansky, E.S.; Sauder, K.L.; Horowitz, J.C.; Mih, J.D.; Tschumperlin, D.J.; Higgins, P.D. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef]
- Owen, K.A.; Abshire, M.Y.; Tilghman, R.W.; Casanova, J.E.; Bouton, A.H. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS ONE 2011, 6, e23123. [Google Scholar] [CrossRef]
- Rubi-Sans, G.; Nyga, A.; Mateos-Timoneda, M.A.; Engel, E. Substrate stiffness-dependent activation of Hippo pathway in cancer associated fibroblasts. Biomater. Adv. 2025, 166, 214061. [Google Scholar] [CrossRef] [PubMed]
- Tallapragada, N.P.; Cambra, H.M.; Wald, T.; Keough Jalbert, S.; Abraham, D.M.; Klein, O.D.; Klein, A.M. Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids. Cell Stem Cell 2021, 28, 1516–1532 e1514. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Shen, C.; Yang, L.; Ni, C.; Huang, J.; Lin, K.; Cao, Z.; Xu, S.; Cui, W.; Wang, X.; et al. Mechanical stretching boosts expansion and regeneration of intestinal organoids through fueling stem cell self-renewal. Cell Regen. 2022, 11, 39. [Google Scholar] [CrossRef]
- Wang, F.; Knutson, K.; Alcaino, C.; Linden, D.R.; Gibbons, S.J.; Kashyap, P.; Grover, M.; Oeckler, R.; Gottlieb, P.A.; Li, H.J.; et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 2017, 595, 79–91. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641. [Google Scholar] [CrossRef] [PubMed]
- Meran, L.; Baulies, A.; Li, V.S.W. Intestinal Stem Cell Niche: The Extracellular Matrix and Cellular Components. Stem Cells Int. 2017, 2017, 7970385. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Ghosh, K.; Pan, Z.; Guan, E.; Ge, S.; Liu, Y.; Nakamura, T.; Ren, X.D.; Rafailovich, M.; Clark, R.A. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 2007, 28, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef]
- Pinto, M.C.; Kihara, A.H.; Goulart, V.A.; Tonelli, F.M.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell Signal. 2015, 27, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 570195. [Google Scholar] [CrossRef]
- Chakraborty, M.; Chu, K.; Shrestha, A.; Revelo, X.S.; Zhang, X.; Gold, M.J.; Khan, S.; Lee, M.; Huang, C.; Akbari, M.; et al. Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell Rep. 2021, 34, 108609. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Chandra, V.; Avery, L.; Burkhardt, J.K. Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex. eLife 2020, 9, e55995. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure with Aging in Mice and Humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.U.; Sun, X.; El-Sawaf, M.; Haxhija, E.Q.; Brei, D.; Luntz, J.; Yang, H.; Teitelbaum, D.H. Enterogenesis in a clinically feasible model of mechanical small-bowel lengthening. Surgery 2006, 140, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Filzmayer, A.K.; Elfers, K.; Michel, K.; Buhner, S.; Zeller, F.; Demir, I.E.; Theisen, J.; Schemann, M.; Mazzuoli-Weber, G. Compression and stretch sensitive submucosal neurons of the porcine and human colon. Sci. Rep. 2020, 10, 13791. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, A.E.; Toepfner, N.; Fiddler, C.; Herbig, M.; Li, W.; Cojoc, G.; Summers, C.; Guck, J.; Chilvers, E.R. Mechanical deformation induces depolarization of neutrophils. Sci. Adv. 2017, 3, e1602536. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.K.; Fogle, L.N.; Aroom, K.R.; Gill, B.S.; Moore-Olufemi, S.D.; Jimenez, F.; Uray, K.S.; Walker, P.A.; Stewart, R.H.; Laine, G.A.; et al. Hydrostatic intestinal edema induced signaling pathways: Potential role of mechanical forces. Surgery 2010, 147, 772–779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.H.; Lv, X.; Liu, Y.L.; Zhao, Y.; Li, Q.; Chen, Y.J.; Zhang, M. Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: The roles of RhoA and Rac1. Stem Cell Res. 2015, 14, 283–296. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Bizios, R.; Medow, M.S.; Gerritsen, M.E. Exposure of human vascular endothelial cells to sustained hydrostatic pressure stimulates proliferation. Involvement of the alphaV integrins. Circ. Res. 1999, 84, 315–322. [Google Scholar] [CrossRef]
- Kourouklis, A.P.; Wahlsten, A.; Stracuzzi, A.; Martyts, A.; Paganella, L.G.; Labouesse, C.; Al-Nuaimi, D.; Giampietro, C.; Ehret, A.E.; Tibbitt, M.W.; et al. Control of hydrostatic pressure and osmotic stress in 3D cell culture for mechanobiological studies. Biomater. Adv. 2023, 145, 213241. [Google Scholar] [CrossRef] [PubMed]
- Poling, H.M.; Wu, D.; Brown, N.; Baker, M.; Hausfeld, T.A.; Huynh, N.; Chaffron, S.; Dunn, J.C.Y.; Hogan, S.P.; Wells, J.M.; et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nat. Biomed. Eng. 2018, 2, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.S.; Dunn, J.C.Y. Biomechanical Force Prediction for Lengthening of Small Intestine during Distraction Enterogenesis. Bioengineering 2020, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, S.; Gjorevski, N.; Lutolf, M.P. Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 2021, 276, 121020. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Kirov, S.; Sasson, A.; Zhang, C.; Chasalow, S.; Dongre, A.; Steen, H.; Stensballe, A.; Andersen, V.; Birkelund, S.; Bennike, T.B. Degradation of the extracellular matrix is part of the pathology of ulcerative colitis. Mol. Omics 2019, 15, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Porras, A.M.; Zhou, H.; Shi, Q.; Xiao, X.; Bank, J.R.I.L.C.; Longman, R.; Brito, I.L. Inflammatory Bowel Disease-Associated Gut Commensals Degrade Components of the Extracellular Matrix. mBio 2022, 13, e0220122. [Google Scholar] [CrossRef]
- Castaneda, F.E.; Walia, B.; Vijay-Kumar, M.; Patel, N.R.; Roser, S.; Kolachala, V.L.; Rojas, M.; Wang, L.; Oprea, G.; Garg, P.; et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: Central role of epithelial-derived MMP. Gastroenterology 2005, 129, 1991–2008. [Google Scholar] [CrossRef]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth Muscle Hyperplasia/Hypertrophy is the Most Prominent Histological Change in Crohn’s Fibrostenosing Bowel Strictures: A Semiquantitative Analysis by Using a Novel Histological Grading Scheme. J. Crohns Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Bland, P.W.; Tarlton, J.F.; Peters, I.; Moorghen, M.; Sylvester, P.A.; Probert, C.S.; Whiting, C.V. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: A role for innate lymphoid cells? PLoS ONE 2012, 7, e52332. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Muller, D.; Clark, A.G. Mechanosensory feedback loops during chronic inflammation. Front. Cell Dev. Biol. 2023, 11, 1225677. [Google Scholar] [CrossRef]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49 e37. [Google Scholar] [CrossRef]
- Dobre, M.; Milanesi, E.; Manuc, T.E.; Arsene, D.E.; Tieranu, C.G.; Maj, C.; Becheanu, G.; Manuc, M. Differential Intestinal Mucosa Transcriptomic Biomarkers for Crohn’s Disease and Ulcerative Colitis. J. Immunol. Res. 2018, 2018, 9208274. [Google Scholar] [CrossRef] [PubMed]
- Biel, C.; Faber, K.N.; Bank, R.A.; Olinga, P. Matrix metalloproteinases in intestinal fibrosis. J. Crohns Colitis 2024, 18, 462–478. [Google Scholar] [CrossRef]
- Koelink, P.J.; Overbeek, S.A.; Braber, S.; Morgan, M.E.; Henricks, P.A.; Abdul Roda, M.; Verspaget, H.W.; Wolfkamp, S.C.; te Velde, A.A.; Jones, C.W.; et al. Collagen degradation and neutrophilic infiltration: A vicious circle in inflammatory bowel disease. Gut 2014, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Engers, J.; Haque, M.; King, S.; Al-Omari, D.; Ma, T.Y. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-kappaB activation. PLoS ONE 2021, 16, e0249544. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Tolstanova, G.; Khomenko, T.; Chen, L.; Tarnawski, A.; Szabo, S.; Sandor, Z. Mesalamine restores angiogenic balance in experimental ulcerative colitis by reducing expression of endostatin and angiostatin: Novel molecular mechanism for therapeutic action of mesalamine. J. Pharmacol. Exp. Ther. 2009, 331, 1071–1078. [Google Scholar] [CrossRef]
- Wang, Y.D.; Mao, J.W. Expression of matrix metalloproteinase-1 and tumor necrosis factor-alpha in ulcerative colitis. World J. Gastroenterol. 2007, 13, 5926–5932. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.Q.; Yu, T.Y.; Yin, Y.Y.; Liu, Y.; Wang, X.D.; He, Z.G.; Yin, L.; Chen, C.Q.; Li, J.Y. Mast Cell Tryptase Promotes Inflammatory Bowel Disease-Induced Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Li, F.; Shi, X.Z. Mechanical stress is a pro-inflammatory stimulus in the gut: In vitro, in vivo and ex vivo evidence. PLoS ONE 2014, 9, e106242. [Google Scholar] [CrossRef] [PubMed]
- Roifman, I.; Sun, Y.C.; Fedwick, J.P.; Panaccione, R.; Buret, A.G.; Liu, H.; Rostom, A.; Anderson, T.J.; Beck, P.L. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2009, 7, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Geesala, R.; Zhang, K.; Lin, Y.M.; Johnson, J.C.; Cong, Y.; Cohn, S.; Shi, X.Z. Exclusive Enteral Nutrition Alleviates Th17-Mediated Inflammation via Eliminating Mechanical Stress-Induced Th17-Polarizing Cytokines in Crohn’s-like Colitis. Inflamm. Bowel Dis. 2024, 30, 429–440. [Google Scholar] [CrossRef]
- Johnson, J.C.; Geesala, R.; Zhang, K.; Lin, Y.M.; M’Koma, A.E.; Shi, X.Z. Smooth muscle dysfunction in the pre-inflammation site in stenotic Crohn’s-like colitis: Implication of mechanical stress in bowel dysfunction in gut inflammation. Front. Physiol. 2023, 14, 1215900. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, M.E.; Barbier, S.; Whitehead, J.; Bealle, G.; Michel, A.; Latorre-Ossa, H.; Rey, C.; Fouassier, L.; Claperon, A.; Brulle, L.; et al. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 2015, 523, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Avvisato, C.L.; Yang, X.; Shah, S.; Hoxter, B.; Li, W.; Gaynor, R.; Pestell, R.; Tozeren, A.; Byers, S.W. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J. Cell Sci. 2007, 120, 2672–2682. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Tsai, C.C.; Chen, P.C.; Lin, B.Y.; Hsu, S.C.N.; Huang, S.P.; Huang, B. Mechanical shear flow regulates the malignancy of colorectal cancer cells. Kaohsiung J. Med. Sci. 2024, 40, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Kanazawa, M.; Kano, M.; Sagami, Y.; Endo, Y.; Utsumi, A.; Nomura, T.; Hongo, M. Exaggerated motility of the descending colon with repetitive distention of the sigmoid colon in patients with irritable bowel syndrome. J. Gastroenterol. 2002, 37 (Suppl. S14), 145–150. [Google Scholar] [CrossRef]
- Brauchle, E.; Kasper, J.; Daum, R.; Schierbaum, N.; Falch, C.; Kirschniak, A.; Schaffer, T.E.; Schenke-Layland, K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018, 68–69, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Nebuloni, M.; Albarello, L.; Andolfo, A.; Magagnotti, C.; Genovese, L.; Locatelli, I.; Tonon, G.; Longhi, E.; Zerbi, P.; Allevi, R.; et al. Insight on Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci. Rep. 2016, 6, 22522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, A.S.; Seo, B.R.; Zhao, X.; Gupta, S.K.; Chen, M.; Han, Y.L.; Shih, T.Y.; Mooney, D.J.; Guo, M. Compression-induced dedifferentiation of adipocytes promotes tumor progression. Sci. Adv. 2020, 6, eaax5611. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Wang, J.; Weng, F.; Xiang, D.; Sun, G. Extracellular Matrix Protein 1 Regulates Colorectal Cancer Cell Proliferative, Migratory, Invasive and Epithelial-Mesenchymal Transition Activities Through the PI3K/AKT/GSK3beta/Snail Signaling Axis. Front. Oncol. 2022, 12, 889159. [Google Scholar] [CrossRef] [PubMed]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; de Paula, C.A.; Mader, A.M.; Waisberg, J.; Pinhal, M.A.; Friedl, A.; Toma, L.; Nader, H.B. Colorectal cancer desmoplastic reaction up-regulates collagen synthesis and restricts cancer cell invasion. Cell Tissue Res. 2011, 346, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, R.; Mo, L.; Tang, H.; Kuang, Y.; Fei, W.; He, C.; Hu, X. Expression of fibulin-1 predicted good prognosis in patients with colorectal cancer. Am. J. Transl. Res. 2015, 7, 339–347. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, S.-H.; Yang, J.-Y. Mechanobiological Approach for Intestinal Mucosal Immunology. Biology 2025, 14, 110. https://doi.org/10.3390/biology14020110
Kim H, Lee S-H, Yang J-Y. Mechanobiological Approach for Intestinal Mucosal Immunology. Biology. 2025; 14(2):110. https://doi.org/10.3390/biology14020110
Chicago/Turabian StyleKim, Hyeyun, Se-Hui Lee, and Jin-Young Yang. 2025. "Mechanobiological Approach for Intestinal Mucosal Immunology" Biology 14, no. 2: 110. https://doi.org/10.3390/biology14020110
APA StyleKim, H., Lee, S.-H., & Yang, J.-Y. (2025). Mechanobiological Approach for Intestinal Mucosal Immunology. Biology, 14(2), 110. https://doi.org/10.3390/biology14020110







