Major Plant-Based Compounds for the Prevention and Treatment of Melanoma—A Mini Review
Simple Summary
Abstract
1. Introduction
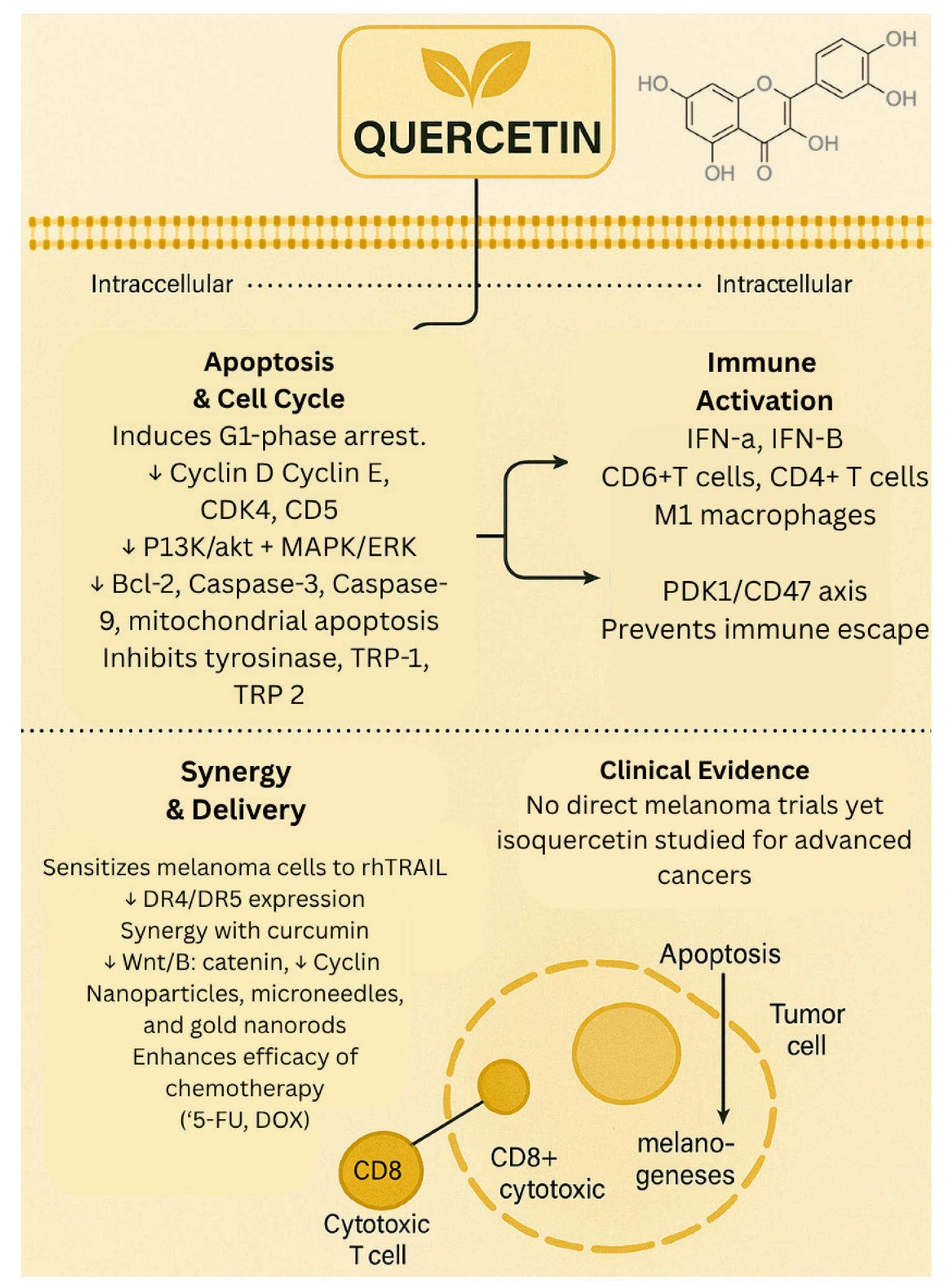
1.1. Quercetin
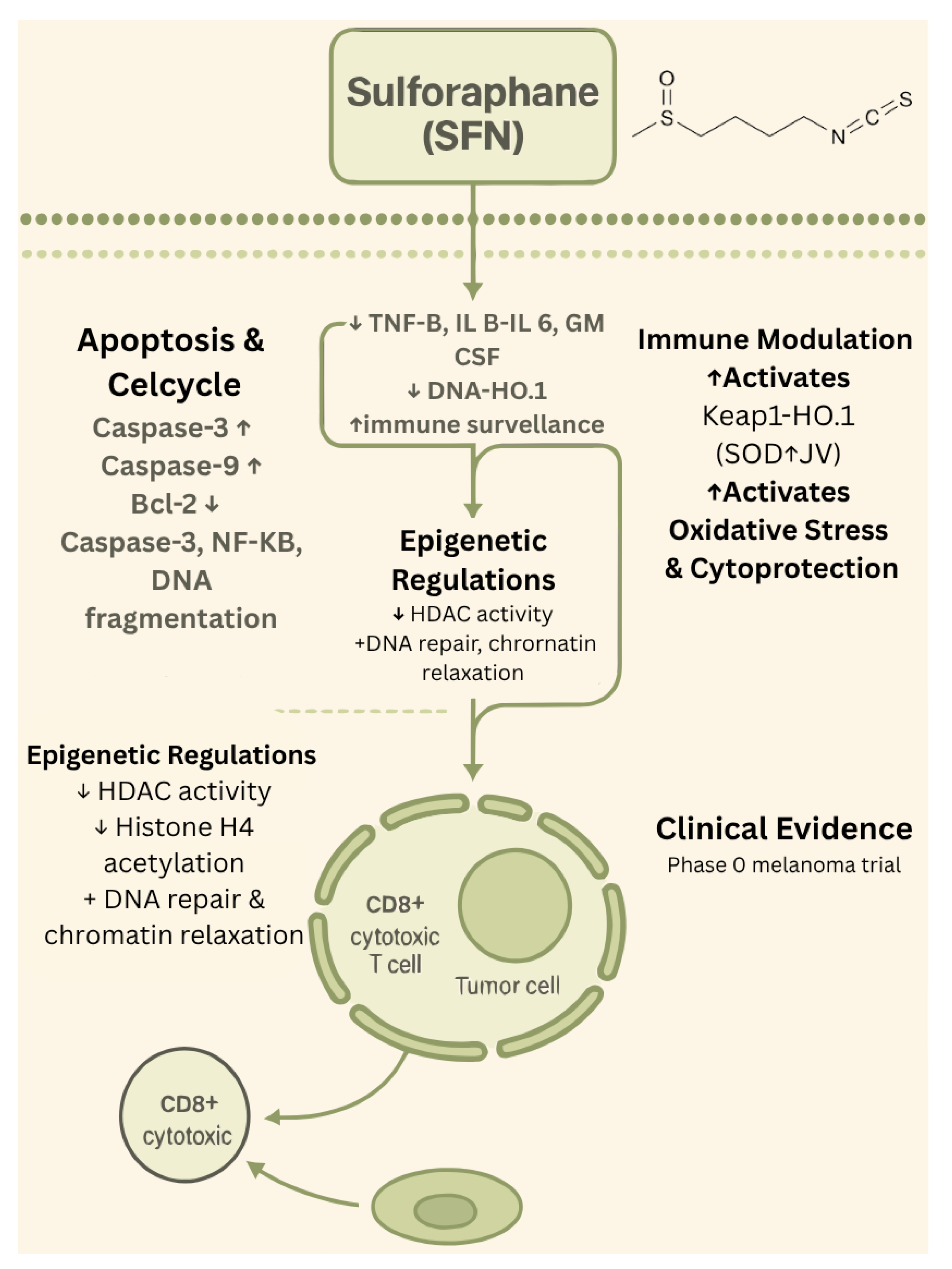
1.2. Sulforaphane
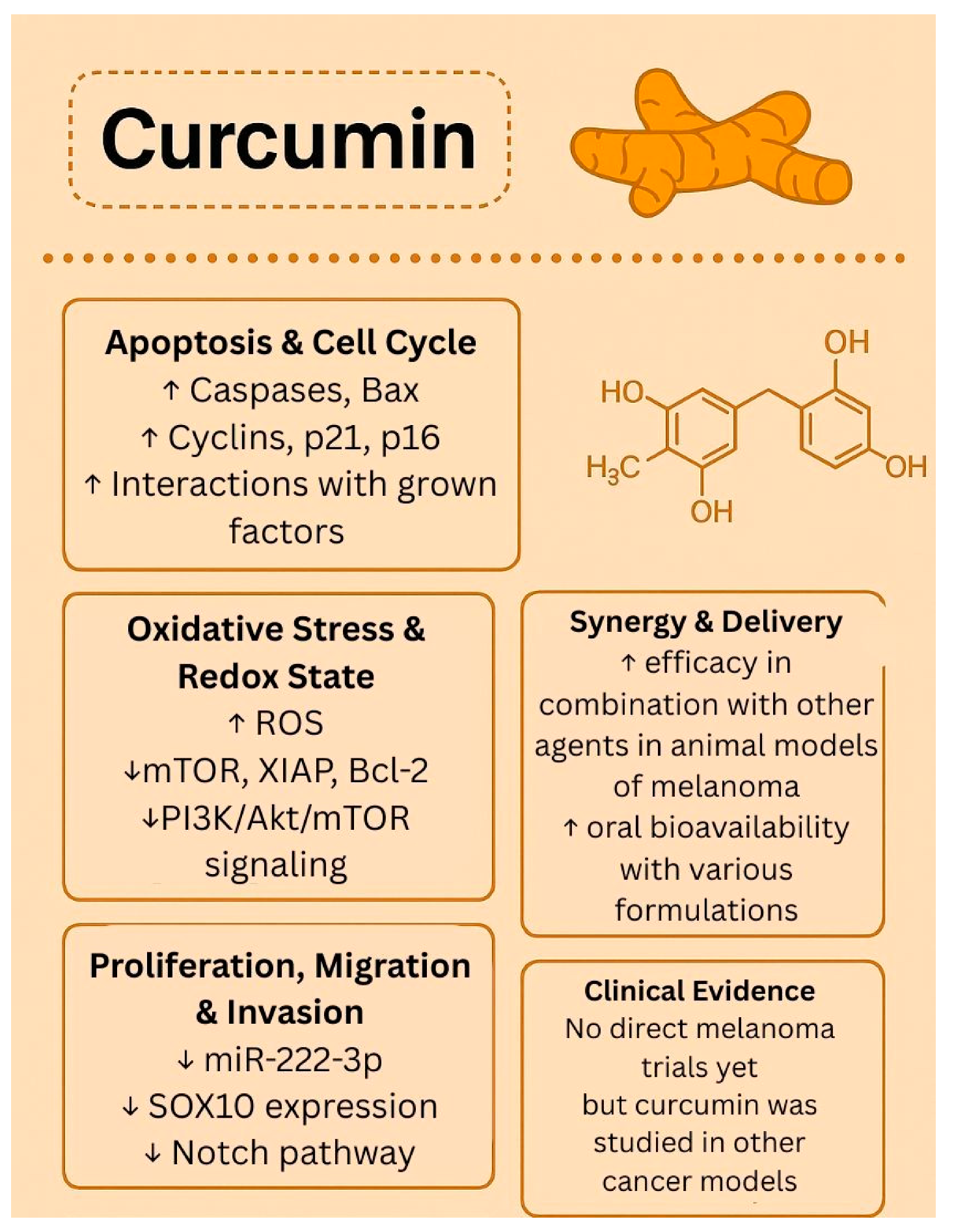
1.3. Curcumin
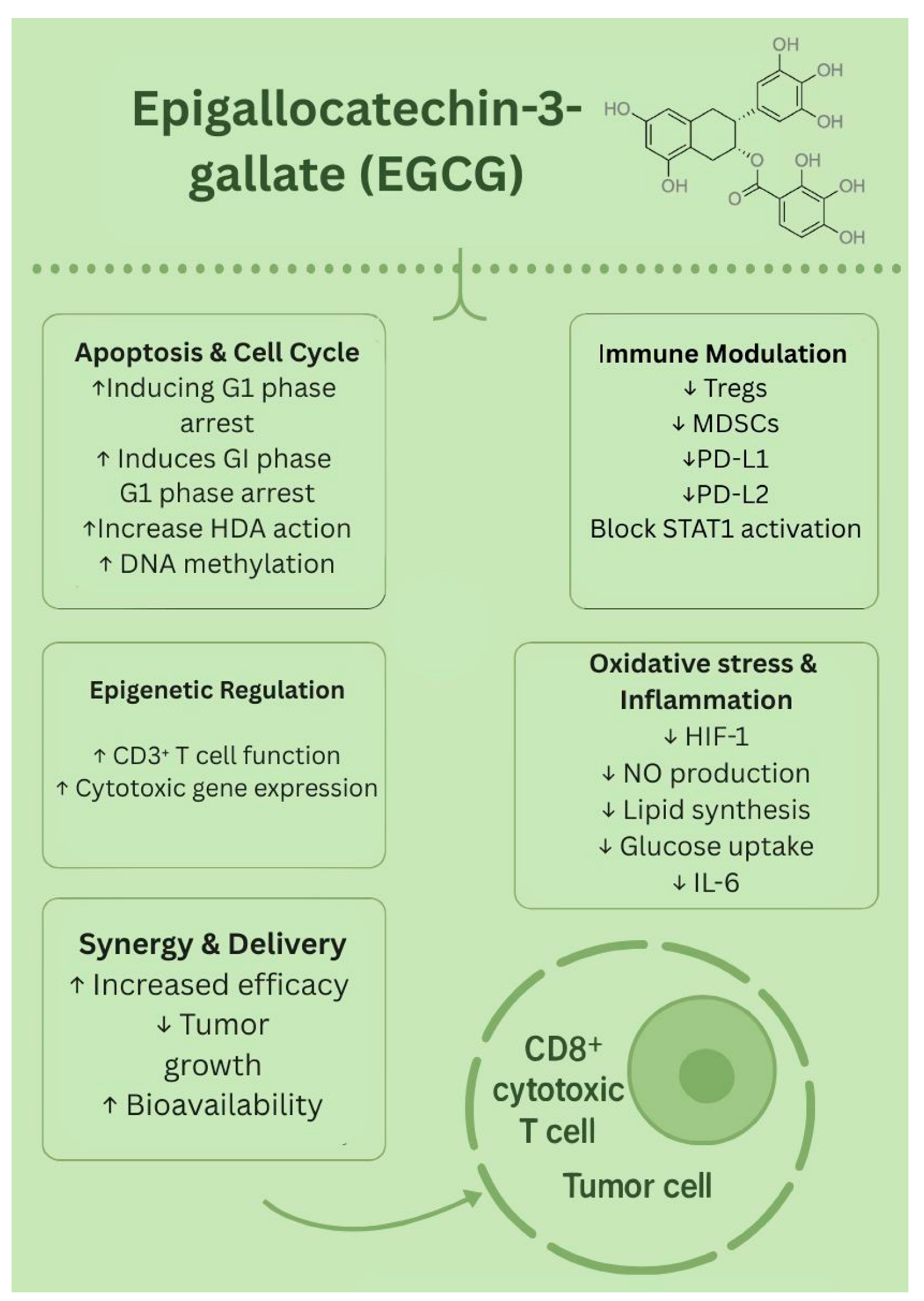
1.4. Epigallocatechin-3-Gallate (EGCG)
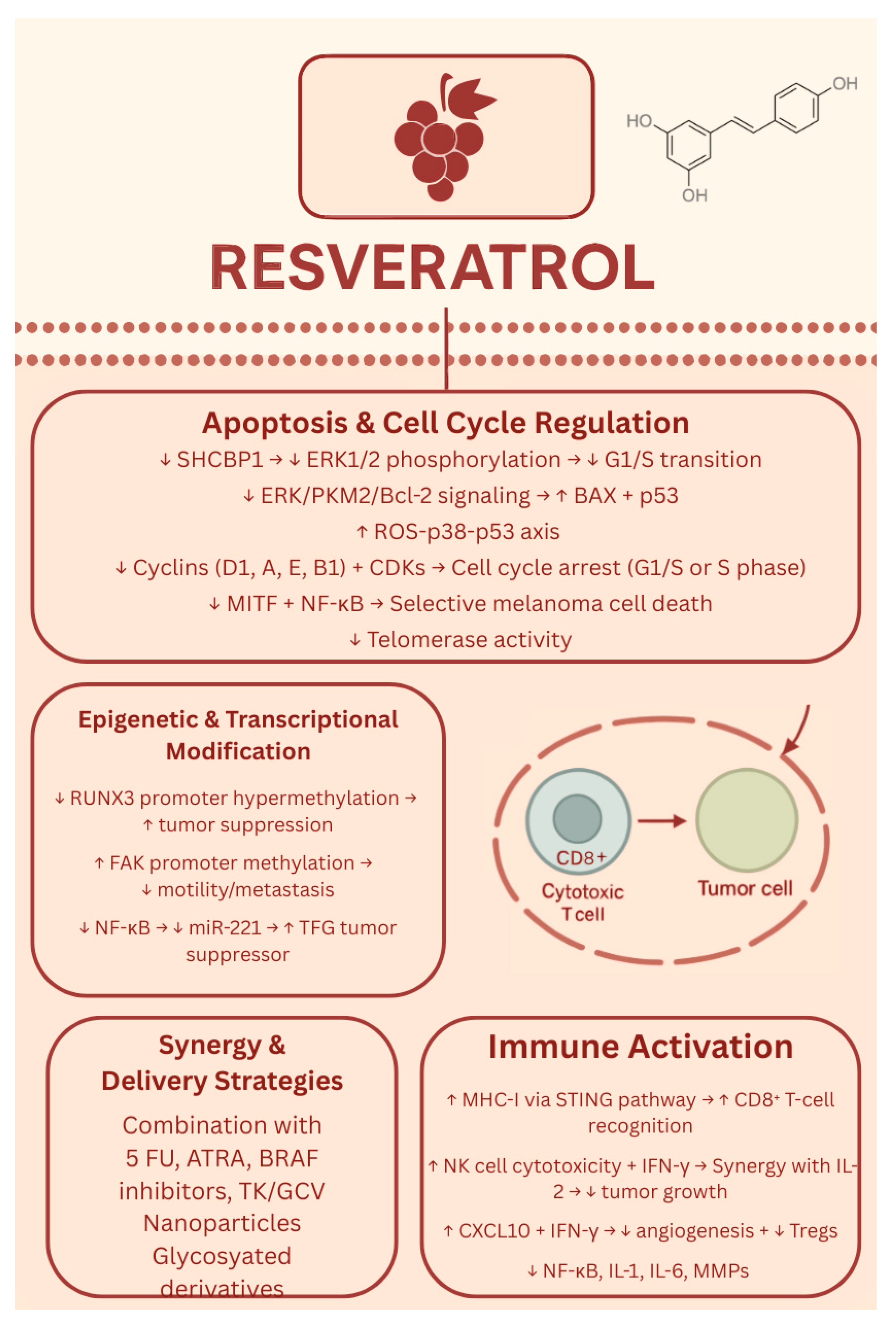
1.5. Resveratrol
1.6. Other Phytochemicals
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| 7,8-DHF | 7,8 dihydroxy flavone |
| AhR | aryl hydrocarbon receptor |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARE | antioxidant response element |
| ATRA | all-trans retinoic acid |
| Axin2 | axis inhibition protein 2 |
| BAX | Bcl-2–associated X protein [pro-apoptotic protein] |
| Bcl-2 B-cell lymphoma | 2 [anti-apoptotic protein] |
| B-RAF/BRAF V600E/BRAF V600E/K | B-Raf proto-oncogene mutations |
| CD4 | cluster of differentiation 4 |
| CD8 | cluster of differentiation 8 |
| CD8+ | cluster of differentiation 8 [cytotoxic T cell marker] |
| CDK6 | cyclin-dependent kinase 6 |
| CK1 | cysteine kinase 1 |
| COX-2 | cyclooxygenase-2 |
| CSC | cancer stem cells |
| CUR | curcumin |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCR4 | chemokine receptor type 4 |
| DATS | diallyl trisulfide |
| DC | destruction complex |
| DNA | deoxyribonucleic acid |
| DNMT3a | DNA methyltransferase 3 alpha |
| DR4 | death receptor 4 |
| DR5 | death receptor 5 |
| DVL2 | disheveled segment polarity protein 2 |
| EGCG | epigallocatechin gallate |
| EMT | epithelial–mesenchymal transition |
| ER | estrogen receptor |
| ERK/ERK1/2 | extracellular signal-regulated kinase [1 and 2] |
| FAK | focal adhesion kinase |
| GPCR | G-protein coupled receptor |
| GSK3β | glycogen synthase kinase 3β |
| HDAC | histone deacetylase |
| IFN-γ | interferon gamma |
| IGF-1R | insulin-like growth factor 1 receptor |
| IL1α, IL1β, IL6, IL8 | interleukin 1 alpha, 1 beta, 6, 8 |
| IL-2 | interleukin-2 |
| IL6R | interleukin-6 receptor |
| JAK | Janus kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MAPK/ERK | mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MDSC | myeloid-derived suppressor cell |
| MHC-I | major histocompatibility complex class I |
| miR-221 | microRNA-221 |
| MITF | microphthalmia-associated transcription factor |
| MMP2, MMP3 | matrix metalloproteinase 2 and 3 |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cells | natural killer cells |
| p21 | cyclin-dependent kinase inhibitor p21 |
| p38 | p38 mitogen-activated protein kinase |
| p53 | tumor suppressor protein p53 |
| PCOS | polycystic ovary syndrome |
| PD-L1 | programmed death-ligand 1 |
| PD-L2 | programmed death-ligand 2 |
| PI3K | phosphoinositide 3-kinase |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| PKM2 | pyruvate kinase M2 |
| rhTRAIL | recombinant human tumor necrosis factor-related apoptosis-inducing ligand |
| p62 | sequestosome-1 (autophagy adaptor protein) |
| ROS | reactive oxygen species |
| RUNX3 | runt-related transcription factor 3 |
| S phase | synthesis phase of DNA replication |
| SHCBP1 | SHC binding and spindle-associated 1 |
| SRT501 | micronized form of resveratrol [drug formulation] |
| STAT | signal transducer and activator of transcription |
| STING | stimulator of interferon genes |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TFG | tropomyosin receptor kinase fused gene [tumor suppressor gene] |
| TGFβ/TGFβ-R2 | transforming growth factor beta/receptor 2 |
| TK/GCV | thymidine kinase/ganciclovir [suicide gene therapy] |
| Treg | regulatory T cell |
| uPA/uPAR | urokinase-type plasminogen activator/receptor |
| UV | ultraviolet |
| VASP | vasodilator-stimulated phosphoprotein |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene 2003, 22, 3099–3112. [Google Scholar] [CrossRef]
- Garibyan, L.; Fisher, D.E. How sunlight causes melanoma. Curr. Oncol. Rep. 2010, 12, 319–326. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Rossi, M.; Pellegrini, C.; Cardelli, L.; Ciciarelli, V.; Di Nardo, L.; Fargnoli, M.C. Familial Melanoma: Diagnostic and Management Implications. Dermatol. Pract. Concept. 2019, 9, 10–16. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet Lond. Engl. 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin. Cancer Res. 2006, 12, 2366s–2370s. [Google Scholar] [CrossRef] [PubMed]
- Strickland, L.R.; Pal, H.C.; Elmets, C.A.; Afaq, F. Targeting drivers of melanoma with synthetic small molecules and phytochemicals. Cancer Lett. 2015, 359, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.S.; Kim, J.S.; Lee, H.A.; Koo, D.B.; Choo, Y.K. uercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Seo, Y.; Yang, J.; Lee, H.; Kong, C.S. Quercetin 3-O-Galactoside Isolated from Limonium tetragonum Inhibits Melanogenesis by Regulating PKA/MITF Signaling and ERK Activation. Int. J. Mol. Sci. 2023, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bai, J.; Li, F.; Liu, J.; Wang, Y.; Li, N.; Wang, Y.; Xu, J.; Liu, W.; Xu, L.; et al. Investigation of the mechanism of the anti-cancer effects of Astragalus propinquus Schischkin and Pinellia pedatisecta Schott [A&P] on melanoma via network pharmacology and experimental verification. Front. Pharmacol. 2022, 13, 895738. [Google Scholar] [CrossRef]
- Khuanekkaphan, M.; Netsomboon, K.; Fristiohady, A.; Asasutjarit, R. Development of Quercetin Solid Dispersion-Loaded Dissolving Microneedles and In Vitro Investigation of Their Anti-Melanoma Activities. Pharmaceutics 2024, 16, 1276. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Sun, Y.; Sun, L.; Yin, Q.; Deng, S.; Niu, L.; Lou, F.; Wang, Z.; Xu, Z.; et al. Melanoma suppression by quercein is correlated with RIG-I and type I interferon signaling. Biomed. Pharmacother. 2020, 125, 109984. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Lin, B.; Li, L.; Deng, Q.; Wang, C.; Zhang, J.; Chen, Y.; Zhao, J.; Li, X.; et al. Quercetin Limits Tumor Immune Escape through PDK1/CD47 Axis in Melanoma. Am. J. Chin. Med. 2024, 52, 541–563. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Esfahani, H.S.; Habibi, M.; Hosseinzadeh, G. Preparation of cerium-curcumin and cerium-quercetin complexes and their LEDs irradiation assisted anticancer effects on MDA-MB-231 and A375 cancer cell lines. Photodiagnosis Photodyn. Ther. 2021, 34, 102326. [Google Scholar] [CrossRef]
- Turner, K.A.; Manouchehri, J.M.; Kalafatis, M. Sensitization of recombinant human tumor necrosis factor-related apoptosis-inducing ligand-resistant malignant melanomas by quercetin. Melanoma Res. 2018, 28, 277–285. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. CATIQ Investigators11. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, e125851. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misiewicz, I.; Skupinska, K.; Kasprzycka-Guttman, T. Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells. Oncol. Rep. 2003, 10, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, T.P.; Thejass, P.; Kuttan, G. Induction of apoptosis by sulforaphane in highly metastatic B16F-10 melanoma cells. Drug Chem. Toxicol. 2011, 34, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Thejass, P.; Kuttan, G. Modulation of cell-mediated immune response in B16F-10 melanoma-induced metastatic tumor-bearing C57BL/6 mice by sulforaphane. Immunopharmacol. Immunotoxicol. 2007, 29, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Mitsiogianni, M.; Kyriakou, S.; Anestopoulos, I.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M.I. An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells. Antioxidants 2021, 10, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinkova-Kostova, A.T. Phytochemicals as protectors against ultraviolet radiation: Versatility of effects and mechanisms. Planta Medica 2008, 74, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Htut, N.W.; Onkoksoong, T.; Saelim, M.; Kueanjinda, P.; Sampattavanich, S.; Panich, U. Live-cell imaging Unveils stimulus-specific dynamics of Nrf2 activation in UV-exposed melanoma cells: Implications for antioxidant compound screening. Free Radic. Biol. Med. 2024, 211, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Do, D.P.; Pai, S.B.; Rizvi, S.A.; D’Souza, M.J. Development of sulforaphane-encapsulated microspheres for cancer epigenetic therapy. Int. J. Pharm. 2010, 386, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gursoy-Yuzugullu, O.; Parasuram, R.; Price, B.D. The tale of a tail: Histone H4 acetylation and the repair of DNA breaks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pradhan, S.J.; Mishra, R.; Sharma, P.; Kundu, G.C. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Exp. Ther. Med. 2010, 1, 915–920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiang, T.C.; Koss, B.; Su, L.J.; Washam, C.L.; Byrum, S.D.; Storey, A.; Tackett, A.J. Effect of Sulforaphane and 5-Aza-2’-Deoxycytidine on Melanoma Cell Growth. Medicines 2019, 6, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahata, S.; Singh, S.V.; Lin, Y.; Hahm, E.R.; Beumer, J.H.; Christner, S.M.; Rao, U.N.; Sander, C.; Tarhini, A.A.; Tawbi, H.; et al. Evaluation of Biodistribution of Sulforaphane after Administration of Oral Broccoli Sprout Extract in Melanoma Patients with Multiple Atypical Nevi. Cancer Prev. Res. 2018, 11, 429–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Q.H.; Jian, L.Y.; Hu, Y.; Wang, S. A comprehensive and systematic review of curcumin as a promising candidate for the inhibition of melanoma growth: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2024, 135, 156073. [Google Scholar] [CrossRef] [PubMed]
- Fança-Berthon, P.; Tenon, M.; Bouter-Banon, S.L.; Manfré, A.; Maudet, C.; Dion, A.; Chevallier, H.; Laval, J.; van Breemen, R.B. Pharmacokinetics of a single dose of turmeric curcuminoids depends on formulation: Results of a human crossover study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef]
- Liao, W.; Xiang, W.; Wang, F.F.; Wang, R.; Ding, Y. Curcumin inhibited growth of human melanoma A375 cells via inciting oxidative stress. Biomed. Pharmacother. 2017, 95, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via miR-222-3p/SOX10/Notch Axis. Dis. Markers 2022, 2022, 3129781. [Google Scholar] [CrossRef]
- Teng, L.; Li, W.; Shi, Y.; Qi, F. The Efficacy of Curcumin Application to Melanoma in Mice: A Systematic Review and Meta-analysis. Ann. Plast. Surg. 2024, 93, S75–S81. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Yang, J.S.; Tsai, F.J.; Chiu, H.Y.; Juan, Y.N.; Lo, Y.H.; Chiang, J.H. Curcumin suppresses cell proliferation and triggers apoptosis in vemurafenib-resistant melanoma cells by downregulating the EGFR signaling pathway. Environ. Toxicol. 2022, 37, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Heo, J.Y.; Kim, D.S.; Choi, Y.S.; Kim, S.; Nam, H.S.; Lee, S.H.; Cho, M.K. Curcumin Enhances the Anticancer Effects of Binimetinib on Melanoma Cells by Inducing Mitochondrial Dysfunction and Cell Apoptosis with Necroptosis. Ann. Dermatol 2023, 35, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Mohd, H.; Michniak-Kohn, B. Synergistic Anti-Cancer Effects of Curcumin and Thymoquinone Against Melanoma. Antioxidants 2024, 13, 1573. [Google Scholar] [CrossRef] [PubMed]
- Fontes, S.S.; Nogueira, M.L.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Vannier-Santos, M.A.; Bezerra, D.P. Combination Therapy of Curcumin and Disulfiram Synergistically Inhibits the Growth of B16-F10 Melanoma Cells by Inducing Oxidative Stress. Biomolecules 2022, 12, 1600. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef]
- Loch-Neckel, G.; Santos-Bubniak, L.; Mazzarino, L.; Jacques, A.V.; Moccelin, B.; Santos-Silva, M.C.; Lemos-Senna, E. Orally administered chitosan-coated poly-caprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J. Pharm. Sci. 2015, 104, 3524–3534. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective skin cancer treatment by topical co-delivery of curcumin and STAT3 siRNA using cationic liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Mardani, R.; Hamblin, M.R.; Taghizadeh, M.; Banafshe, H.R.; Nejati, M.; Mokhtari, M.; Borran, S.; Davoodvandi, A.; Khan, H.; Jaafari, M.R.; et al. Nanomicellar-Curcumin Exerts its Therapeutic Effects via Affecting Angiogenesis, Apoptosis, and T Cells in a Mouse Model of Melanoma Lung Metastasis. Pathol. Res. Pract. 2020, 216, 153082. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 6–10. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [PubMed]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Chopra, S.; Kaur, D.; Wang, H.; Li, M.; Chen, P.; Zhang, W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets 2020, 21, 1099–1104. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, C.; Liu, M.; Wang, J.; Qiao, S.; Jiang, P.; Sun, C.; Jiang, S. Epigallocatechin-3-gallate at the nanoscale: A new strategy for cancer treatment. Pharm. Biol. 2024, 62, 676–690. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Jang, M. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 445154, Resveratrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/resveratrol (accessed on 30 July 2025).
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019, 100, 1392–1404. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption But Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2010, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, Z.; Nie, S.; Zhang, T.; Lu, H. Effects of Resveratrol on Mouse B16 Melanoma Cell Proliferation through the SHCBP1-ERK1/2 Signaling Pathway. Molecules 2023, 28, 7614. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Li, L. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. OncoTargets Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.; Hwang, K.; Kang, J.; Choi, K. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Martin–Vega, A.; Cobb, M.H. ERK1/2-MAPK signaling: Metabolic, organellar, and cytoskeletal interactions. Curr. Opin. Cell Biol. 2025, 95, 102526. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; De Melo, J.; Tang, D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int. J. Cell Biol. 2013, 2013, 242513. [Google Scholar] [CrossRef]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory Effects of Oligostilbenoids from the Bark of Shorea roxburghii on Malignant Melanoma Cell Growth: Implications for Novel Topical Anticancer Candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef]
- Yang, H.Z.; Zhang, J.; Zeng, J.; Liu, S.; Zhou, F.; Zhang, F.; Giampieri, F.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Ansary, J.; et al. Resveratrol inhibits the proliferation of melanoma cells by modulating cell cycle. Int. J. Food Sci. Nutr. 2019, 71, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular analysis of differential antiproliferative activity of resveratrol, epsilon viniferin and labruscol on melanoma cells and normal dermal cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef]
- Mokhamatam, R.B.; Sahoo, B.K.; Manna, S.K. Suppression of microphthalmia-associated transcription factor, but not NF-kappa B sensitizes melanoma specific cell death. Apoptosis 2016, 21, 928–940. [Google Scholar] [CrossRef]
- Platella, C.; Raucci, U.; Rega, N.; D’Atri, S.; Levati, L.; Roviello, G.N.; Fuggetta, M.P.; Musumeci, D.; Montesarchio, D. Shedding light on the interaction of polydatin and resveratrol with G-quadruplex and duplex DNA: A biophysical, computational and biological approach. Int. J. Biol. Macromol. 2020, 151, 1163–1172. [Google Scholar] [CrossRef]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour activity of resveratrol on human melanoma cells: A possible mechanism related to its interaction with malignant cell telomerase. Biochim. Biophys. Acta [BBA] Gen. Subj. 2017, 1861, 2843–2851. [Google Scholar] [CrossRef]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef]
- Morehead, L.C.; Koss, B.; Fil, D.; Heflin, B.; Garg, S.; Wallis, K.F.; Tackett, A.J.; Miousse, I.R. Resveratrol induces major histocompatibility complex class I antigen presentation in a STING-dependent and independent manner in melanoma. Mol. Immunol. 2023, 163, 188–195. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, H.; Kim, J. In vivo Anti-Cancer Effects of Resveratrol Mediated by NK Cell Activation. J. Innate Immun. 2020, 13, 94–106. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Menicacci, B.; Laurenzana, A.; Chillà, A.; Margheri, F.; Peppicelli, S.; Tanganelli, E.; Fibbi, G.; Giovannelli, L.; Del Rosso, M.; Mocali, A. Chronic Resveratrol Treatment Inhibits MRC5 Fibroblast SASP-Related Protumoral Effects on Melanoma Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1187–1195. [Google Scholar] [CrossRef]
- Kang, S.; Wang, Z.; Li, B.; Gao, X.; He, W.; Cao, S.; Cai, Y.; Chen, H. Anti-tumor effects of resveratrol on malignant melanoma is associated with promoter demethylation of RUNX3 gene. Pharmazie 2019, 74, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Xu, C.; Wang, J.; Zhu, B.; Huang, Q.; Chen, D.; Sheng, J.; Zou, Y.; Lee, Y.M.; et al. Comparative profiling of analog targets: A case study on resveratrol for mouse melanoma metastasis suppression. Theranostics 2018, 8, 3504–3516. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cui, L. Resveratrol suppresses melanoma by inhibiting NF-κB/miR-221 and inducing TFG expression. Arch. Dermatol. Res. 2017, 309, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Katariya, M.; Patil, S.; Tatke, P.; Pillai, R. Skin delivery of resveratrol encapsulated lipidic formulation for melanoma chemoprevention. J. Microencapsul. 2019, 36, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.S.; Corrêa, M.A.; Ribeiro, C.A.; Dos Santos, J.L. Synthesis and evaluation of 1,3,5-triazine derivatives as sunscreens useful to prevent skin cancer. Bioorg. Med. Chem. Lett. 2019, 29, 126755. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Masayo Umebayashi Doi, K.; Morisaki, T.; Senji Shirasawa Tsunoda, T. Resveratrol Overcomes Cellular Resistance to Vemurafenib Through Dephosphorylation of AKT in BRAF-mutated Melanoma Cells. PubMed 2016, 36, 3585–3589. [Google Scholar]
- Corre, S.; Tardif, N.; Mouchet, N.; Leclair, H.M.; Boussemart, L.; Gautron, A.; Bachelot, L.; Perrot, A.; Soshilov, A.; Rogiers, A.; et al. Sustained activation of the Aryl hydrocarbon Receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat. Commun. 2018, 9, 4775. [Google Scholar] [CrossRef]
- Lee, S.H.; Koo, B.S.; Park, S.Y.; Kim, Y.M. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef]
- Kokoris, M.S.; Black, M.E. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. A Publ. Protein Soc. 2002, 11, 2267–2272. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, G.; Wu, Y.; Xiao, J.; Liu, J.; Qiu, P.; Liu, X.; Sun, L.; Du, B.; et al. Synergistic inhibitory effect of resveratrol and TK/GCV therapy on melanoma cells. J. Cancer Res. Clin. Oncol. 2020, 146, 1489–1499. [Google Scholar] [CrossRef]
- Kanai, M.; Shinagawa, A.; Ota, M.; Virgona, N.; Yano, T. Resveratrol Can Differentiate Human Melanoma Stem-like Cells from Spheroids Treated With All-trans Retinoic Acid. Anticancer. Res. 2024, 44, 5283–5292. [Google Scholar] [CrossRef]
- Al Hmada, Y.; Brodell, R.T.; Kharouf, N.; Flanagan, T.W.; Alamodi, A.A.; Hassan, S.Y.; Shalaby, H.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. Mechanisms of Melanoma Progression and Treatment Resistance: Role of Cancer Stem-like Cells. Cancers 2024, 16, 470. [Google Scholar] [CrossRef]
- Islam, S.U.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. PRPF overexpression induces drug resistance through actin cytoskeleton rearrangement and epithelial-mesenchymal transition. Oncotarget 2017, 8, 56659–56671. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The Role of BRAF V600 Mutation in Melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Sim, D.Y.; Sohng, J.K.; Jung, H.J. Anticancer activity of 7,8-dihydroxyflavone in melanoma cells via downregulation of α-MSH/cAMP/MITF pathway. Oncol. Rep. 2016, 36, 528–534. [Google Scholar] [CrossRef]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Dourado, D.; Batista, F.P.R.; Philadelpho, B.O.; de Souza, M.L.; de Cerqueira E Silva, M.B.; de Grandis, R.A.; Miranda, P.A.; Colauto, N.B.; Pereira, D.T.; Formiga, F.R.; et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 12112. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, H.; Zhang, W.; Rong, L.; Zhang, D. Resveratrol-Coated Gold Nanoflowers for CT Imaging and Apoptosis/Photothermal Synergistic Therapy of Malignant Melanoma. ACS Omega 2023, 8, 34629–34639. [Google Scholar] [CrossRef]
- Gao, F.; Li, L.; Liu, L.; Li, G.; Zhang, J.; Zhan, W.; You, W.; Lin, X.; Liu, Y.; Wang, J.; et al. Novel Silicon-Based Fluorescent Nanocomposite Drug Carriers for Natural Compound Delivery in Melanoma Treatment. J. Fluoresc. 2025, 35, 8755–8767. [Google Scholar] [CrossRef]
- Yee, Y.J.; Benson, H.A.E.; Dass, C.R.; Chen, Y. Evaluation of novel conjugated resveratrol polymeric nanoparticles in reduction of plasma degradation, hepatic metabolism and its augmentation of anticancer activity in vitro and in vivo. Int. J. Pharm. 2022, 615, 121499. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. Formulation and evaluation of resveratrol loaded cubosomal nanoformulation for topical delivery. Curr. Drug Deliv. 2020, 18, 607–619. [Google Scholar] [CrossRef]
- Bano, S.; Ahmed, F.; Khan, F.; Chaudhary, S.C.; Samim, M. Enhancement of the cancer inhibitory effect of the bioactive food component resveratrol by nanoparticle based delivery. Food Funct. 2020, 11, 3213–3226. [Google Scholar] [CrossRef]
- Wu, P.S.; Li, Y.S.; Kuo, Y.C.; Tsai, S.J.; Lin, C.C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600. [Google Scholar] [CrossRef]
- Moon, K.; Lee, S.; Park, H.; Cha, J. Enzymatic Synthesis of Resveratrol α-Glucoside by Amylosucrase of Deinococcus geothermalis. J. Microbiol. Biotechnol. 2021, 31, 1692–1700. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 [resveratrol] with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2012, 160, 714–717. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol [SRT501] in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
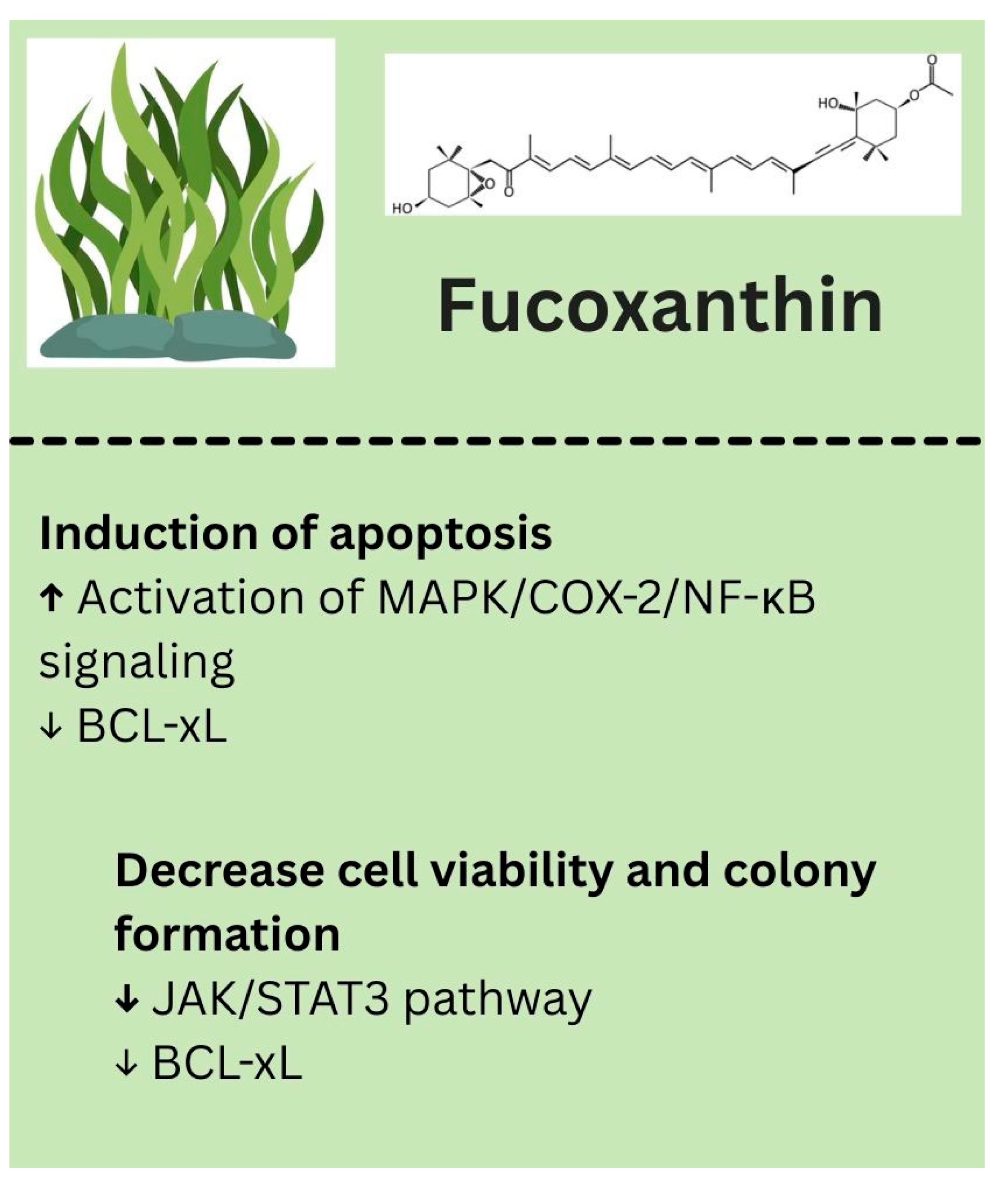
- Lu, Y.; Wang, Y.; Yao, T.; Dong, X.; Liu, Y.; Nakamura, Y.; Qi, H. Mechanism of inhibition of melanoma by fucoxanthin simulated in vitro digestion products in cell models constructed using human malignant melanoma cells (A375) and keratinocytes (HaCaT). Food Chem. 2025, 462, 141003. [Google Scholar] [CrossRef]
- Kuo, M.; Dai, W.; Chang, J.; Chang, J.; Lee, T.; Chang, C. Fucoxanthin induces human melanoma cytotoxicity by thwarting the JAK2/STAT3/BCL-xL signaling axis. Environ. Toxicol. 2024, 39, 3356–3366. [Google Scholar] [CrossRef]
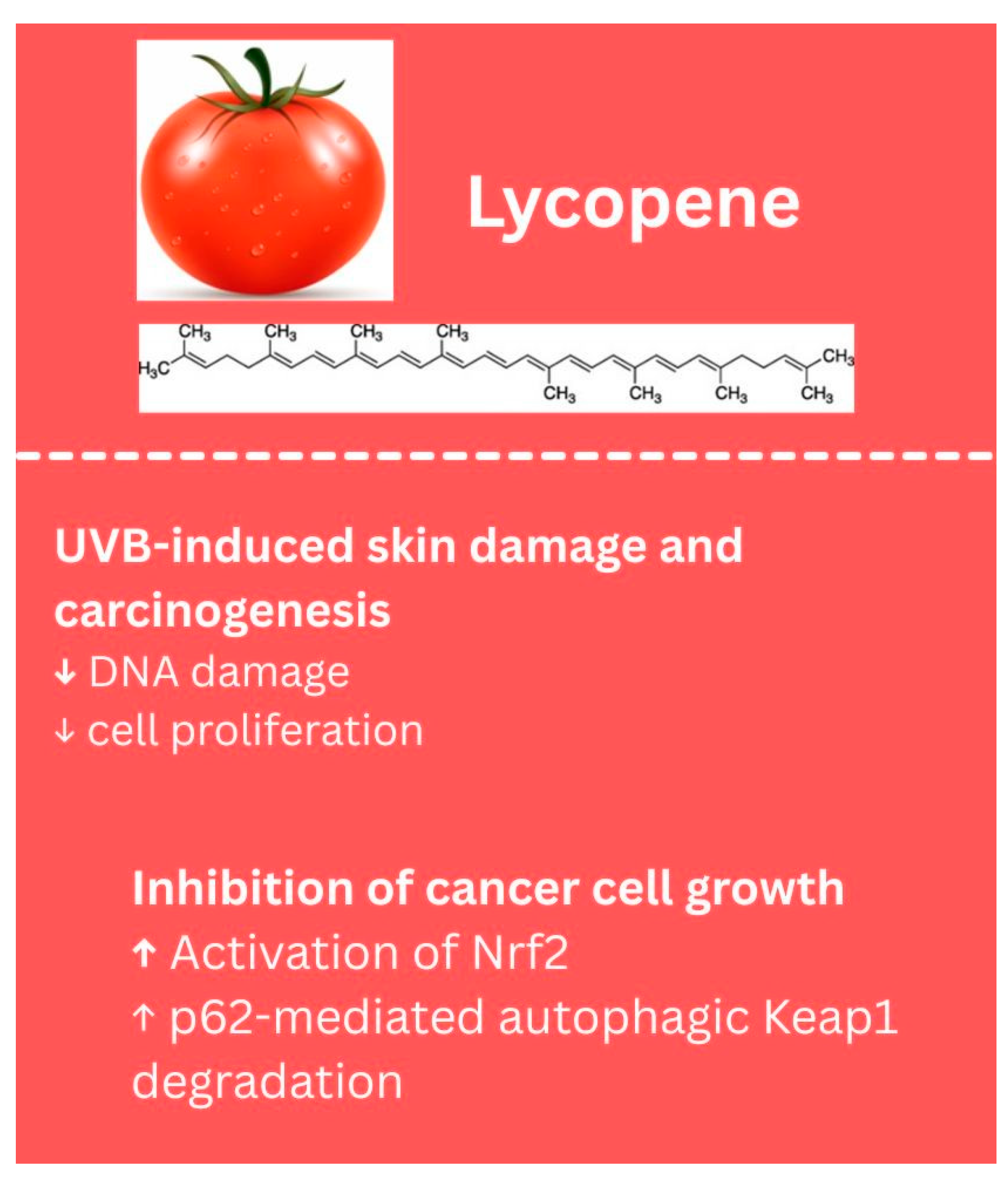
- Wang, S.; Wu, Y.Y.; Wang, X.; Shen, P.; Jia, Q.; Yu, S.; Wang, Y.; Li, X.; Chen, W.; Wang, A.; et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging 2020, 12, 8167–8190. [Google Scholar] [CrossRef]
- Zhou, X.; Burke, K.E.; Wang, Y.; Wei, H. Dietary Lycopene Protects SKH-1 Mice Against Ultraviolet B-Induced Photocarcinogenesis. J. Drugs Dermatol 2019, 18, 1244–1254. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirshteyn, I.; Srivastav, M.; Grace, K.; Cescato, V.; Bommareddy, A. Major Plant-Based Compounds for the Prevention and Treatment of Melanoma—A Mini Review. Biology 2025, 14, 1772. https://doi.org/10.3390/biology14121772
Kirshteyn I, Srivastav M, Grace K, Cescato V, Bommareddy A. Major Plant-Based Compounds for the Prevention and Treatment of Melanoma—A Mini Review. Biology. 2025; 14(12):1772. https://doi.org/10.3390/biology14121772
Chicago/Turabian StyleKirshteyn, Isabella, Megha Srivastav, Karen Grace, Victoria Cescato, and Ajay Bommareddy. 2025. "Major Plant-Based Compounds for the Prevention and Treatment of Melanoma—A Mini Review" Biology 14, no. 12: 1772. https://doi.org/10.3390/biology14121772
APA StyleKirshteyn, I., Srivastav, M., Grace, K., Cescato, V., & Bommareddy, A. (2025). Major Plant-Based Compounds for the Prevention and Treatment of Melanoma—A Mini Review. Biology, 14(12), 1772. https://doi.org/10.3390/biology14121772






