Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) and Responses to Acute Cold Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Experimental Animals and Tissue Sample Collection
2.2. Identification and Characterization of the AQP Gene Family
2.3. Phylogenetic Analysis of the AQP Family
2.4. Structural Prediction of the AQP Proteins
2.5. RNA Extraction and cDNA Synthesis
2.6. Primer Design and Quantitative PCR (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Identification and Physicochemical Properties of the AQP Gene Family
3.2. Gene Structure Analysis and Conserved Motif Composition of AQP Gene Family
3.3. Phylogenetic Analysis of AQP Gene Family
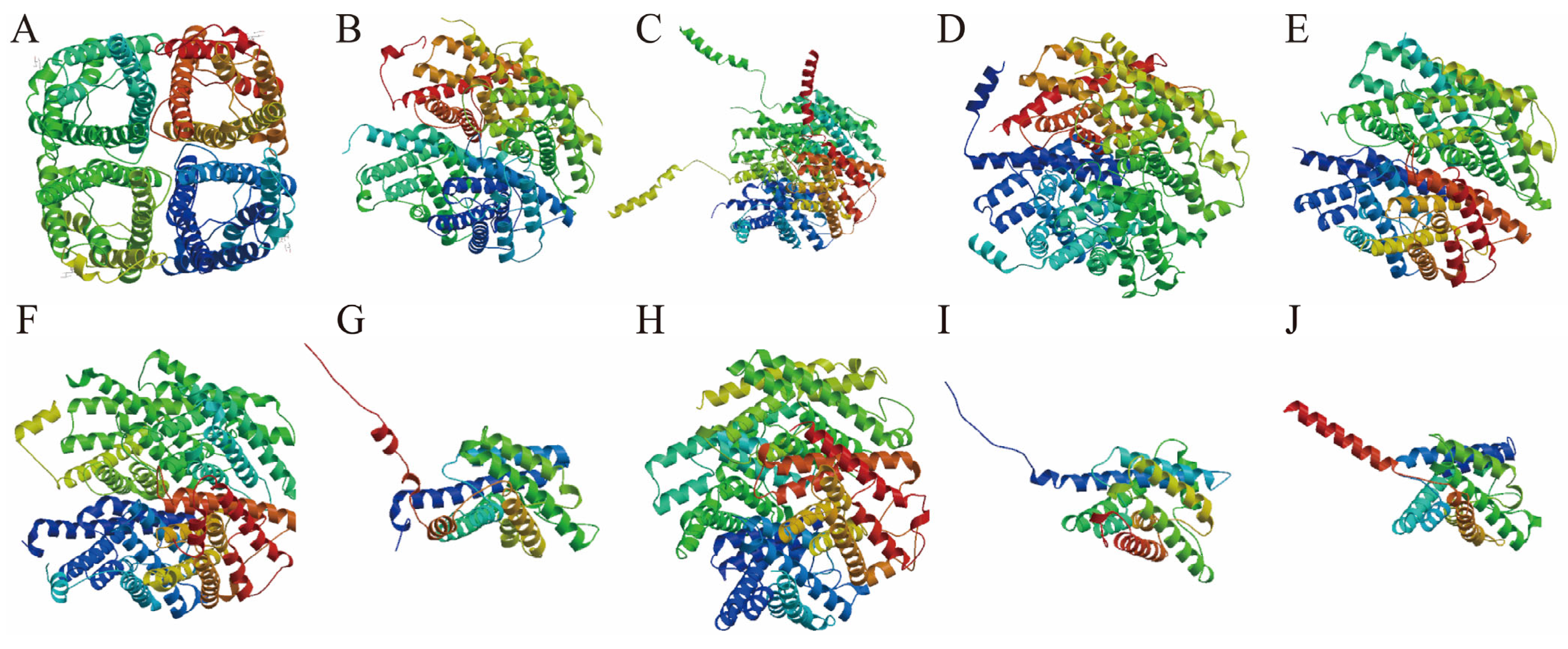
3.4. Predictive Analysis of Secondary and Tertiary Structures
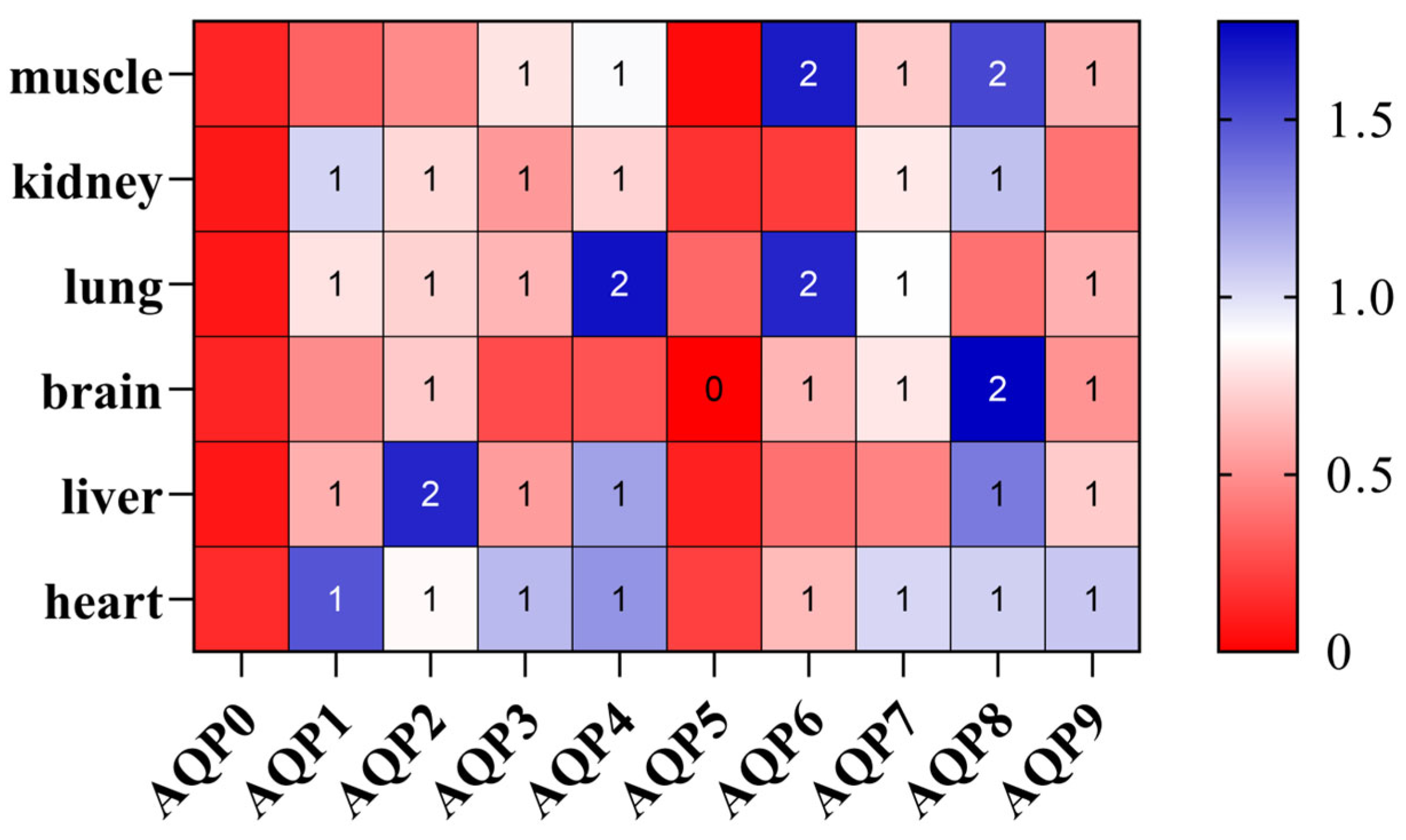
3.5. Expression Profile of AQP Genes in Different Tissues
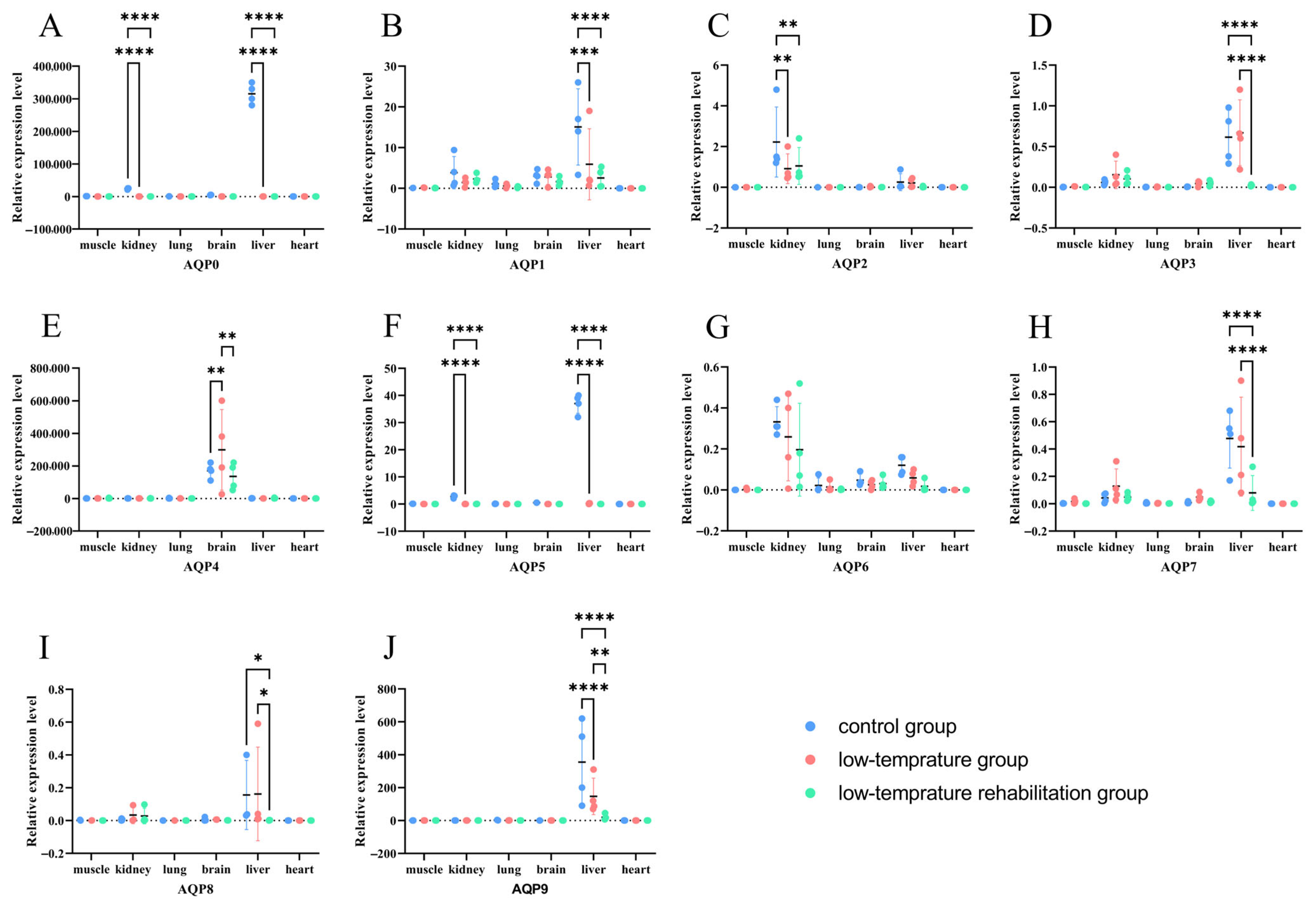
3.6. Tissue-Specific Remodeling of AQP Expression in Response to Cold Stress and Recovery
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, E. Roles of Aquaporins in Osmoregulation, Desiccation and Cold Hardiness in Insects. Entomol. Ornithol. Herpetol. Curr. Res. 2012, S1, 1. [Google Scholar] [CrossRef]
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Molecular Physiology of Freeze Tolerance in Vertebrates. Physiol. Rev. 2017, 97, 623–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lv, W.; Wang, Y.; Qian, Y.; Wang, C.; Sun, N.; Fang, C.; Irwin, D.M.; Gan, X.; He, S.; et al. Multi-omics Investigation of Freeze Tolerance in the Amur Sleeper, an Aquatic Ectothermic Vertebrate. Mol. Biol. Evol. 2023, 40, msad040. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bai, R.; Pei, T.; Meng, J.; Nwanade, C.F.; Zhang, Y.; Liang, X.; Tang, Y.; Liu, J.; Yu, Z. Aquaporins modulate the cold response of Haemaphysalis longicornis via changes in gene and protein expression of fatty acids. Parasit. Vectors 2025, 18, 70. [Google Scholar] [CrossRef]
- Rajput, S.; Gautam, D.; Vats, A.; Roshan, M.; Goyal, P.; Rana, C.; S M, P.; Ludri, A.; De, S. Aquaporin (AQP) gene family in Buffalo and Goat: Molecular characterization and their expression analysis. Int. J. Biol. Macromol. 2024, 280 Pt 4, 136145. [Google Scholar] [CrossRef]
- Bogner, B.; Schroedl, F.; Trost, A.; Kaser-Eichberger, A.; Runge, C.; Strohmaier, C.; Motloch, K.A.; Bruckner, D.; Hauser-Kronberger, C.; Bauer, H.C.; et al. Aquaporin expression and localization in the rabbit eye. Exp. Eye Res. 2016, 147, 20–30. [Google Scholar] [CrossRef]
- Chauvigné, F.; Zapater, C.; Stavang, J.A.; Taranger, G.L.; Cerdà, J.; Finn, R.N. The pH sensitivity of AQP0 channels in tetraploid and diploid teleosts. FASEB J. 2015, 29, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nishimura, H.; Yang, Y. Bird aquaporins: Molecular machinery for urine concentration. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183688. [Google Scholar] [CrossRef]
- Harvey, S.R.; O’Neale, C.; Schey, K.L.; Wysocki, V.H. Native Mass Spectrometry and Surface Induced Dissociation Provide Insight into the Post-Translational Modifications of Tetrameric AQP0 Isolated from Bovine Eye Lens. Anal. Chem. 2022, 94, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Chen, J.; Zhong, H.; Ren, J.; Zhao, W.; Man, Q.; Shang, S.; Tang, X. Genome-wide analysis of the Aquaporin gene family in reptiles. Int. J. Biol. Macromol. 2019, 126, 1093–1098. [Google Scholar] [CrossRef]
- Nishimura, H.; Yang, Y. Aquaporins in avian kidneys: Function and perspectives. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R1201–R1214. [Google Scholar] [CrossRef]
- Finn, R.N.; Chauvigné, F.; Hlidberg, J.B.; Cutler, C.P.; Cerdà, J. The Lineage-Specific Evolution of Aquaporin Gene Clusters Facilitated Tetrapod Terrestrial Adaptation. PLoS ONE 2014, 9, e113686. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Fan, Y.; Xie, J.; Ding, J.; Sha, L.; Shi, X.; Sun, X.; Hu, G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell Sci. 2008, 121, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.M.; Zheng, Y.H.; Wang, J.L. Seasonal flexibility of the gut structure and physiology in Eremias multiocellata. J. Comp. Physiol. B 2023, 193, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, J.; Li, B.; Yao, T.; Wang, S.; Li, S.; Cui, Z.; Wang, F.; Pan, B.; Fang, X.; et al. Uplift of the Qinghai-Xizang (Tibet) Plateau andeast Asia environmental change during late Cenozoic. Acta Geogr. Sin. 1999, 54, 10–20. [Google Scholar]
- Chen, Y.J.; Zhu, L.; Wu, Q.N.; Hu, C.C.; Qu, Y.F.; Ji, X. Geological and climatic influences on population differentiation of the Phrynocephalus vlangalii species complex (Sauria: Agamidae) in the northern Qinghai-Tibet Plateau. Mol. Phylogenet Evol. 2022, 169, 107394. [Google Scholar] [CrossRef]
- Zhang, W.; Li, N.; Tang, X.; Liu, N.; Zhao, W. Changes in intestinal microbiota across an altitudinal gradient in the lizard Phrynocephalus vlangalii. Ecol. Evol. 2018, 8, 4695–4703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Men, S.; Jia, L.; Tang, X.; Storey, K.B.; Niu, Y.; Chen, Q. Comparative metabolomics analysis reveals high-altitude adaptations in a toad-headed viviparous lizard, Phrynocephalus vlangalii. Front. Zool. 2023, 20, 35. [Google Scholar]
- Stogsdill, B.; Frisbie, J.; Krane, C.M.; Goldstein, D.L. Expression of the aquaglyceroporin HC-9 in a freeze-tolerant amphibian that accumulates glycerol seasonally. Physiological reports. 2017, 5, e13331. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.X.; Benoit, J.B.; Elnitsky, M.A.; Kaufmann, N.; Brodsky, J.L.; Zeidel, M.L.; Denlinger, D.L.; Lee, R.E., Jr. Function and immuno-localization of aquaporins in the Antarctic midge Belgica antarctica. J. Insect Physiol. 2011, 57, 1096–1105. [Google Scholar] [CrossRef]
- Philip, B.N.; Yi, S.X.; Elnitsky, M.A.; Lee, R.E., Jr. Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly, Eurosta solidaginis. J. Exp. Biol. 2008, 211 Pt 7, 1114–1119. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Krane, C.M.; Kishore, B.K. Aquaporins: Membrane water channels of the biological world. Biologist 2003, 50, 81–86. [Google Scholar]
- Mo, N.; Shao, S.; Yang, Y.; Bao, C.; Cui, Z. Identifying low salinity adaptation gene expression in the anterior and posterior gills of the mud crab (Scylla paramamosain) by transcriptomic analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101166. [Google Scholar] [CrossRef]
- Elwan, M.M.; Madkour, F.A.; Salem, M.M.; El-Nahass, E.E. Kidney Morphology in Marine and Terrestrial Birds and Its Phylogenetic Links to Mammals via Aquaporin (AQP) Genome Sequences. J. Exp. Zool. A Ecol. Integr. Physiol. 2025, 343, 1072–1089. [Google Scholar] [CrossRef]
- Martínez-Redondo, G.I.; Simón Guerrero, C.; Aristide, L.; Balart-García, P.; Tonzo, V.; Fernández, R. Parallel duplication and loss of aquaporin-coding genes during the “out of the sea” transition as potential key drivers of animal terrestrialization. Mol. Ecol. 2023, 32, 2022–2040. [Google Scholar] [CrossRef]
- Krane, C.M.; Goldstein, D.L. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm Genome 2007, 18, 452–462. [Google Scholar]
- Lorente-Martínez, H.; Agorreta, A.; Irisarri, I.; Zardoya, R.; Edwards, S.V.; San Mauro, D. Multiple Instances of Adaptive Evolution in Aquaporins of Amphibious Fishes. Biology 2023, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Redondo, G.I.; Simón Guerrero, C.; Aristide, L.; Balart-García, P.; Tonzo, V.; Fernández, R. Parallel duplication and loss of aquaporin-coding genes during the ‘out of the sea’ transition paved the way for animal terrestrialization. bioRxiv 2022. bioRxiv:2022.07.25.501387. [Google Scholar]
- Gelfman, S.; Burstein, D.; Penn, O.; Savchenko, A.; Amit, M.; Schwartz, S.; Pupko, T.; Ast, G. Changes in exon-intron structure during vertebrate evolution affect the splicing pattern of exons. Genome Res. 2012, 22, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, X. Polyploidization and pseudogenization in allotetraploid frog Xenopus laevis promote the evolution of aquaporin family in higher vertebrates. BMC Genom. 2020, 21, 525. [Google Scholar] [CrossRef]
- Campbell, E.M.; Ball, A.; Hoppler, S.; Bowman, A.S. Invertebrate aquaporins: A review. J. Comp. Physiol. B 2008, 178, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on AQP1 and AQP3 expression. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.P.; MacIver, B. Localization and Expression of Aquaporin 1 (AQP1) in the Tissues of the Spiny Dogfish (Squalus acanthias). Int. J. Mol. Sci. 2025, 26, 5593. [Google Scholar] [CrossRef]
- Kameya, N.; Sakai, I.; Saito, K.; Hamabe-Horiike, T.; Shinmyo, Y.; Nakada, M.; Okuda, S.; Kawasaki, H. Evolutionary changes leading to efficient glymphatic circulation in the mammalian brain. Nat. Commun. 2024, 15, 10048. [Google Scholar] [CrossRef]
- Florio, M.; Engfors, A.; Gena, P.; Larsson, J.; Massaro, A.; Timpka, S.; Reimer, M.K.; Kjellbom, P.; Beitz, E.; Johanson, U.; et al. Characterization of the Aquaporin-9 Inhibitor RG100204 In Vitro and in db/db Mice. Cells 2022, 11, 3118. [Google Scholar] [CrossRef]
- Hall, J.E.; Freites, J.A.; Tobias, D.J. Experimental and Simulation Studies of Aquaporin 0 Water Permeability and Regulation. Chem. Rev. 2019, 119, 6015–6039. [Google Scholar] [CrossRef]
- D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Hoogewijs, D.; Vogler, M.; Zwenger, E.; Krull, S.; Zieseniss, A. Oxygen-dependent regulation of aquaporin-3 expression. Hypoxia 2016, 4, 91–97. [Google Scholar] [CrossRef][Green Version]
- Ayasoufi, K.; Kohei, N.; Nicosia, M.; Fan, R.; Farr, G.W.; McGuirk, P.R.; Pelletier, M.F.; Fairchild, R.L.; Valujskikh, A. Aquaporin 4 blockade improves survival of murine heart allografts subjected to prolonged cold ischemia. Am. J. Transplant. 2018, 18, 1238–1246. [Google Scholar] [CrossRef]
- Hori, S.; Sakamoto, N.; Saitoh, O. Cloning and functional characterization of medaka TRPV4. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 267, 111182. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Liu, Y.; Zhan, Y. Identification and characterization of cold-responsive aquaporins from the larvae of a crambid pest Agriphila aeneociliella (Eversmann) (Lepidoptera: Crambidae). PeerJ 2023, 11, e16403. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.S.; Engelund, M.B.; Cutler, C.P. Water transport and functional dynamics of aquaporins in osmoregulatory organs of fishes. Biol. Bull. 2015, 229, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, R.; Laur, J. Aquaporins Respond to Chilling in the Phloem by Altering Protein and mRNA Expression. Cells 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.L.; Frisbie, J.; Goldstein, D.L.; West, J.; Rivera, K.; Krane, C.M. Excretion and conservation of glycerol, and expression of aquaporins and glyceroporins, during cold acclimation in Cope’s gray tree frog Hyla chrysoscelis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R544–R555. [Google Scholar] [CrossRef] [PubMed]
- Gotfryd, K.; Mósca, A.F.; Missel, J.W.; Truelsen, S.F.; Wang, K.; Spulber, M.; Krabbe, S.; Hélix-Nielsen, C.; Laforenza, U.; Soveral, G.; et al. Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat. Commun. 2018, 9, 4749. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Delporte, C. Involvement of aquaglyceroporins in energy metabolism in health and disease. Biochimie 2021, 188, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1–11. [Google Scholar]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of mammalian aquaporin water channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Mutyam, V.; Puccetti, M.V.; Frisbie, J.; Goldstein, D.L.; Krane, C.M. Dynamic regulation of aquaglyceroporin expression in erythrocyte cultures from cold- and warm-acclimated Cope’s gray treefrog, Hyla chrysoscelis. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 424–437. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequences | Tm/°C | Size/bp |
|---|---|---|---|
| PvlAQP0 | F:CCCGCAACTTCACCAACCT R:AGCTGCTCACCTTTCAAGATAGAG | 56.30 | 142 |
| PvlAQP1 | F:GCCACAACAGACAGGAGGAGA R:TACACAGCGGAGCCGAAAG | 60.10 | 143 |
| PvlAQP2 | F:TAGGTTCCCAAGTCTCCTTCCTT R:AGAACTTCCTCGGATGTGGTGT | 56.30 | 119 |
| PvlAQP3 | F:ATGCCTGGGAACCCTCGTA R:AATGCCAAGCGTCACAGCA | 55.40 | 130 |
| PvlAQP4 | F:TTCTTGCCGGAACCCTTTAT R:GGCTCCTGGTATCTTCTACTTCTATG | 43.90 | 132 |
| PvlAQP5 | F:TCTGCTCAATGGGTCTTCTGG R:GACTCGTAGGTGCCTTTCACG | 53.00 | 134 |
| PvlAQP6 | F:CTCGTATCTCCCTGGTGAAAGC R:GAGTTCTGAATCATGTTGATGCCTA | 58.80 | 136 |
| PvlAQP7 | F:GACTTGCACAATCGGTCTCG R:CGTCTTGCTTCCTACATTCCTC | 52.40 | 70 |
| PvlAQP8 | F:CTCTTGGTGCCTGGCTCATT R:TGCGACCGTCACAAACTGC | 63.50 | 137 |
| PvlAQP9 | F:AGCCCTGGGTGCGGTC R:GGTCAAGTTCGTGCTGGTTTC | 57.60 | 92 |
| ACTB | F:CACGGCATTATCACTAACTGGG R:GTAAGGTGGGATGTTCCTCTGG | 43.30 | 97 |
| ID | Gene Name | Number of Amino Acid | Molecular Weight (Da) | pI | GRAVY | Instability Index | Aliphatic Index |
|---|---|---|---|---|---|---|---|
| GWHPAAFC012688 | PvlAQP0 | 263 | 28227.04 | 6.97 | 0.645 | 33.50 | 112.74 |
| GWHPAAFC017642 | PvlAQP1 | 269 | 28459.04 | 6.06 | 0.556 | 36.04 | 109.85 |
| GWHPAAFC016973 | PvlAQP2 | 273 | 29846.73 | 6.83 | 0.428 | 47.92 | 106.15 |
| GWHPAAFC015342 | PvlAQP3 | 292 | 31452.68 | 6.70 | 0.498 | 24.51 | 104.25 |
| GWHPAAFC011293 | PvlAQP4 | 312 | 33801.56 | 6.81 | 0.446 | 28.63 | 108.75 |
| GWHPAAFC016972 | PvlAQP5 | 261 | 28240.04 | 6.95 | 0.548 | 39.51 | 98.62 |
| GWHPAAFC012707 | PvlAQP6 | 264 | 28168.94 | 7.77 | 0.688 | 39.03 | 120.53 |
| GWHPAAFC015346 | PvlAQP7 | 271 | 28776.54 | 6.40 | 0.552 | 37.05 | 107.31 |
| GWHPAAFC014848 | PvlAQP8 | 243 | 26088.66 | 5.61 | 0.572 | 35.38 | 111.98 |
| GWHPAAFC012538 | PvlAQP9 | 263 | 27874.82 | 6.22 | 0.543 | 37.68 | 109.13 |
| Gene ID | Protein Name | Alpha Helix (%) | Beta Turn (%) | Random Coil (%) | Extended Strand (%) |
|---|---|---|---|---|---|
| GWHPAAFC012688 | PvlAQP0 | 39.92 | 2.66 | 36.88 | 20.53 |
| GWHPAAFC017642 | PvlAQP1 | 38.29 | 1.86 | 40.52 | 19.33 |
| GWHPAAFC016973 | PvlAQP2 | 41.76 | 2.93 | 34.43 | 20.88 |
| GWHPAAFC015342 | PvlAQP3 | 38.01 | 2.74 | 36.99 | 22.26 |
| GWHPAAFC011293 | PvlAQP4 | 36.54 | 2.24 | 41.67 | 19.55 |
| GWHPAAFC016972 | PvlAQP5 | 41.76 | 3.07 | 36.40 | 18.77 |
| GWHPAAFC012707 | PvlAQP6 | 43.94 | 1.52 | 37.12 | 17.42 |
| GWHPAAFC015346 | PvlAQP7 | 38.01 | 2.74 | 36.99 | 22.26 |
| GWHPAAFC014848 | PvlAQP8 | 46.50 | 3.70 | 31.69 | 18.11 |
| GWHPAAFC012538 | PvlAQP9 | 32.32 | 3.80 | 38.02 | 25.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, P.; Li, X.; Wang, J. Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) and Responses to Acute Cold Stress. Biology 2025, 14, 1755. https://doi.org/10.3390/biology14121755
Zhang Y, Yang P, Li X, Wang J. Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) and Responses to Acute Cold Stress. Biology. 2025; 14(12):1755. https://doi.org/10.3390/biology14121755
Chicago/Turabian StyleZhang, Yurong, Ping Yang, Xinyang Li, and Jia Wang. 2025. "Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) and Responses to Acute Cold Stress" Biology 14, no. 12: 1755. https://doi.org/10.3390/biology14121755
APA StyleZhang, Y., Yang, P., Li, X., & Wang, J. (2025). Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) and Responses to Acute Cold Stress. Biology, 14(12), 1755. https://doi.org/10.3390/biology14121755





