Primary Follicle Paces Fish Ovarian Maturation Developmental Progression via the Enhancement of Notch and mTOR
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Ethics Statement
2.3. Density Gradient Separation of Primary Follicles
2.4. Histological, Transmission Electron Microscope (TEM), Immunohistochemistry, and Immunofluorescence
2.5. Chromosome Spreading
2.6. Staining Nucleolar Organizer Region
2.7. DiOC6 and DAPI Staining
2.8. RNA-Sequencing
2.9. Methylation Sequencing and Analysis
2.10. Quantitative Real-Time PCR (qRT-PCR)
2.11. Inhibitor Treatment and Added Amino Acid Feeding
2.12. Western Blot
3. Results
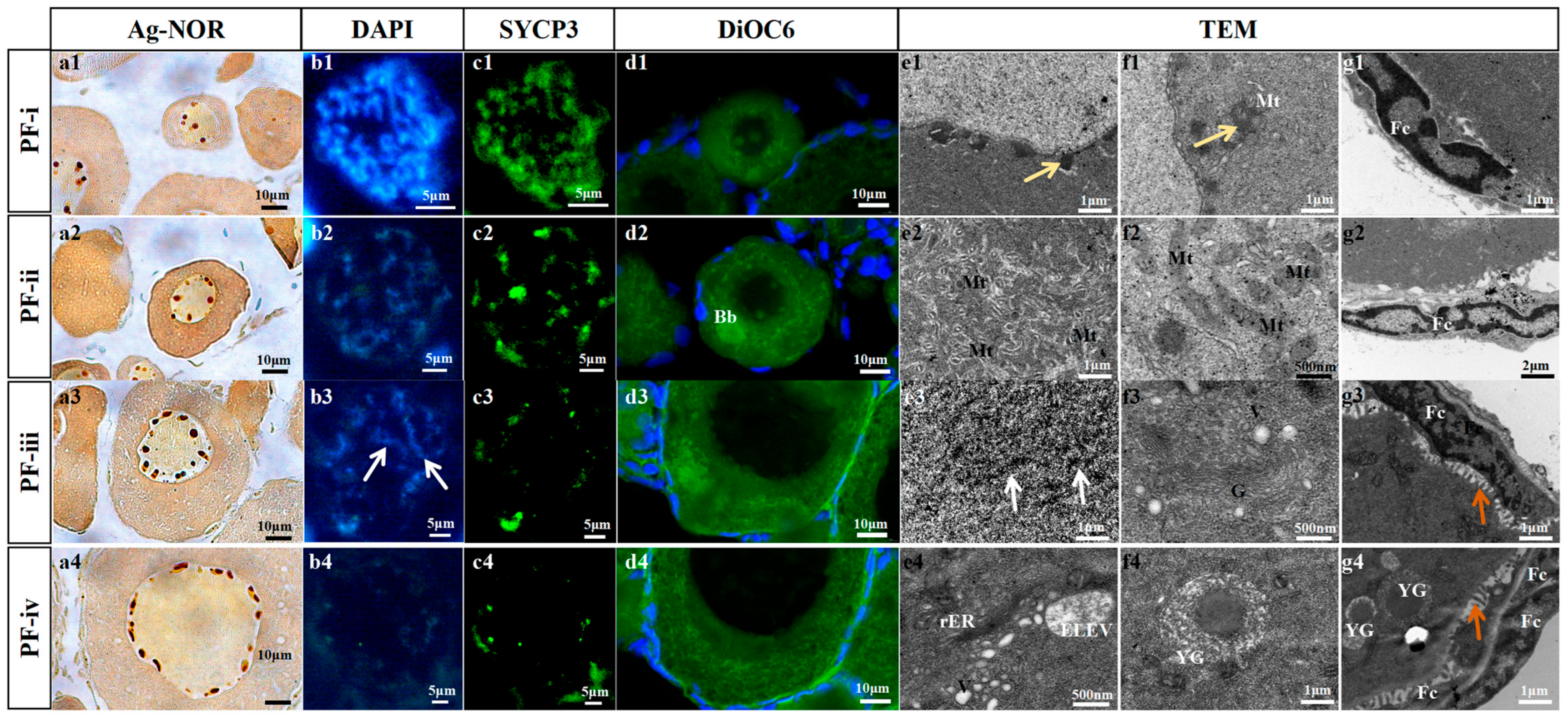
3.1. Classification of Primary Follicles and Their Cytological Characteristics in Zebrafish
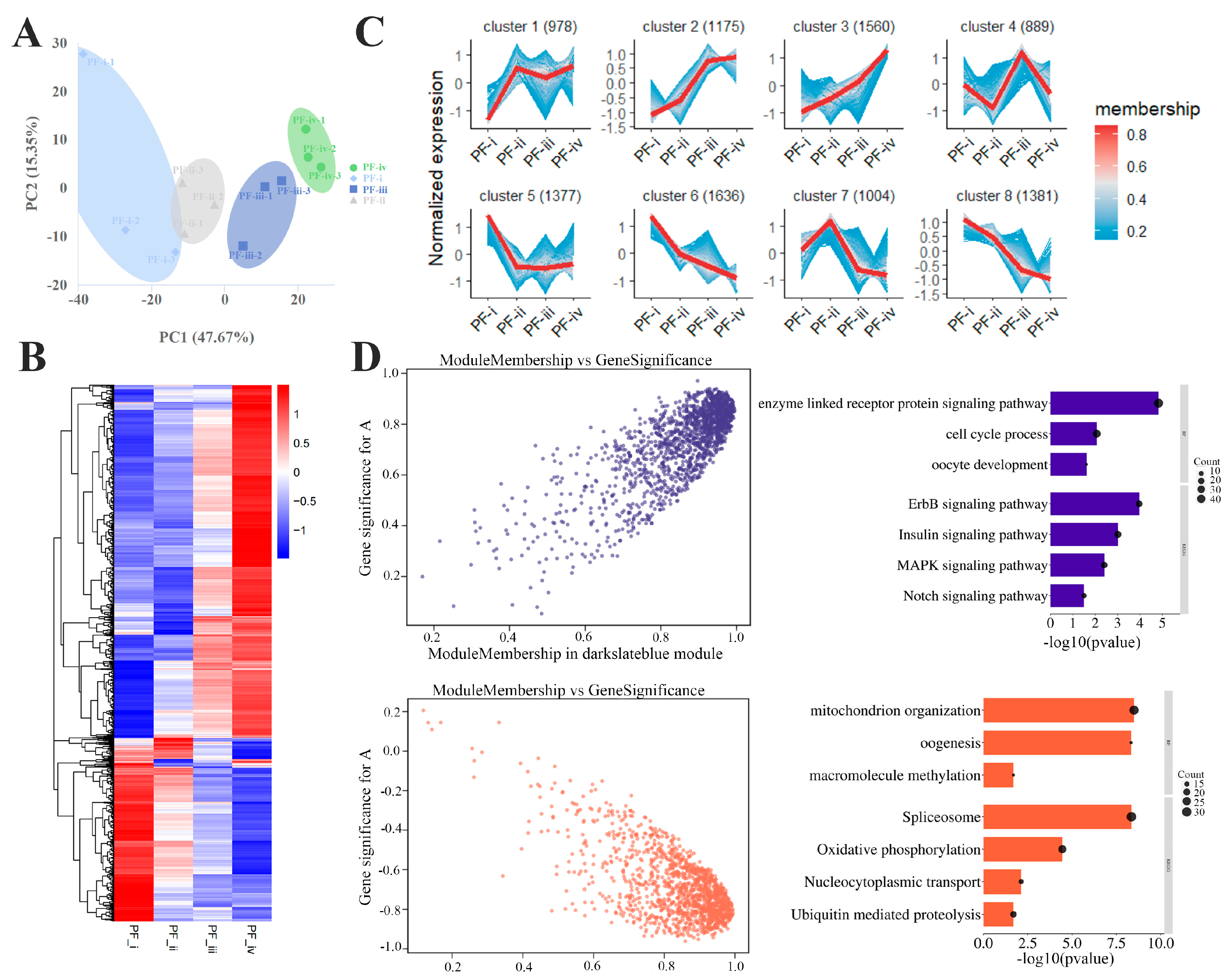
3.2. Gene Expression Profiles of Four Subtypes of PFs
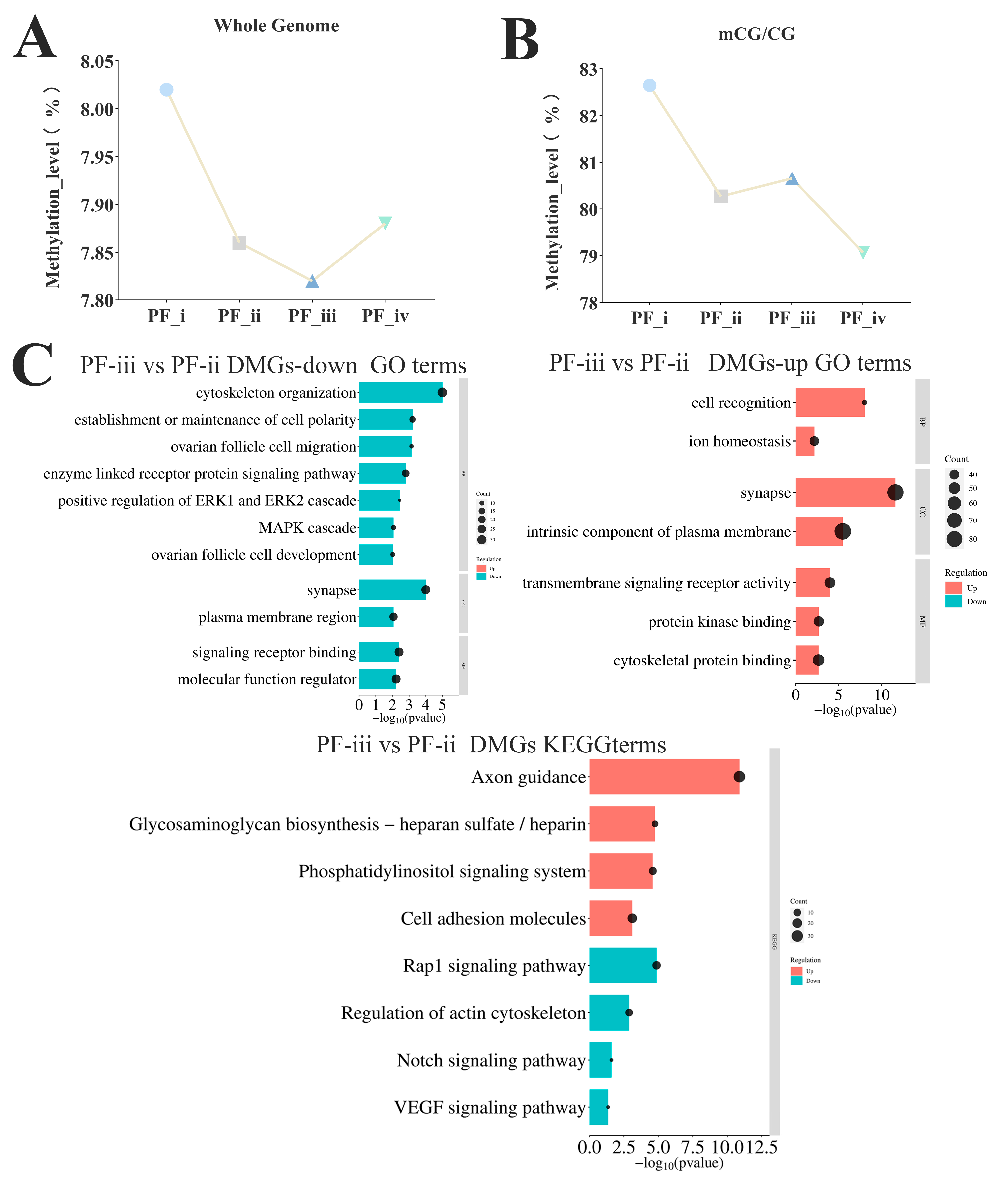
3.3. Analysis of Methylated Region Changes in Four Primary Follicle Subtypes
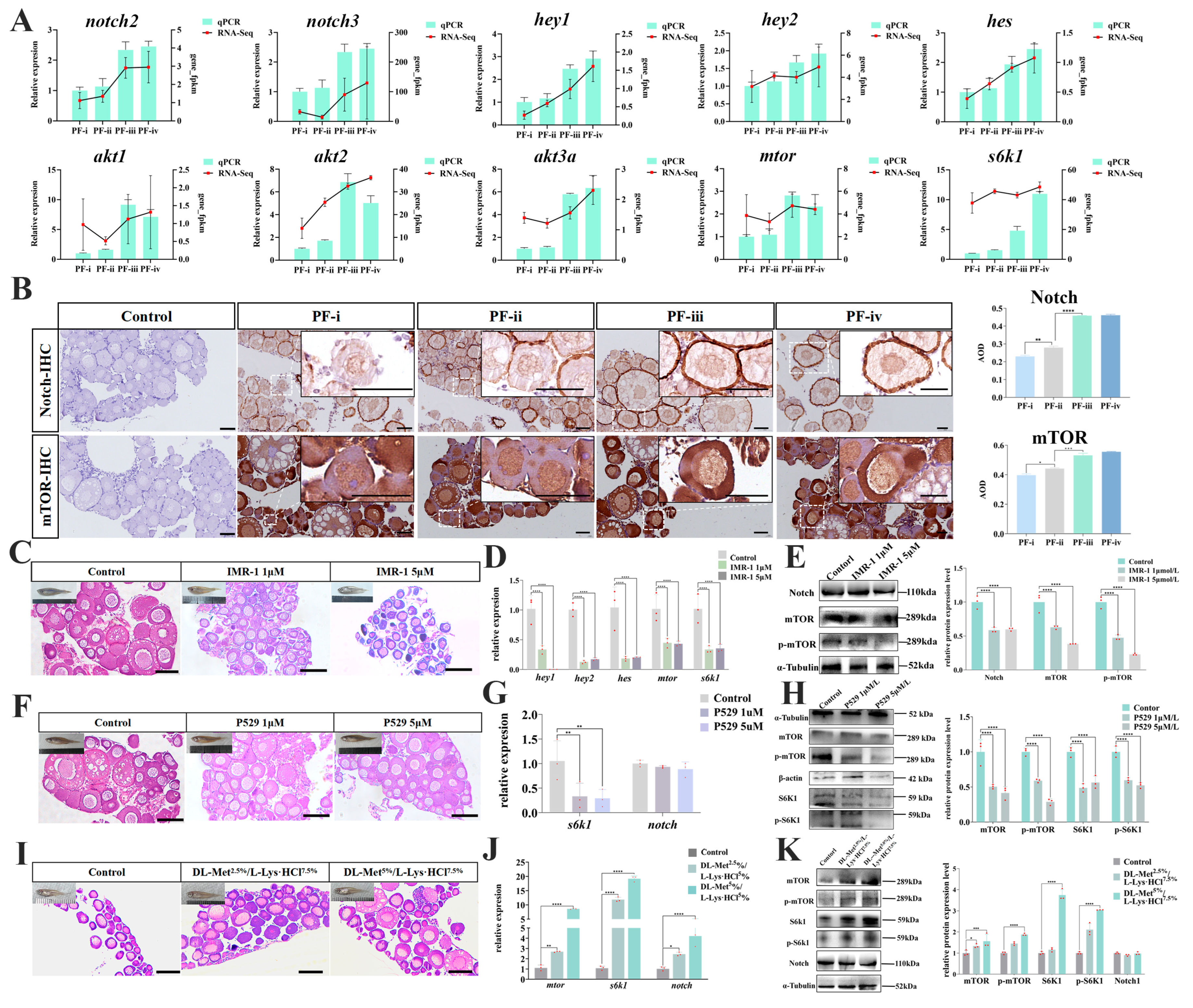
3.4. Regulatory Effect of Notch/mTOR on the Development of Primary Follicles in Zebrafish
4. Discussion
4.1. Isolation and Purification of PFs
4.2. Refined Classification of PFs
4.3. PF-ii to PF-iii Represents a Major Regulatory Transition
4.4. Coordinated Roles of Notch and mTOR in PF Progression
4.5. Integration of Endocrine and Nutritional Regulation and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grier, H.J.; Aranzdbal, M.C.U.; Patiño, R. The ovary, folliculogenesis, and oogenesis in teleosts. In Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes); CRC Press: Boca Raton, FL, USA, 2009; pp. 25–84. [Google Scholar]
- Mokhtar, D.M. Diversity and dynamics of fish ovaries: Insights into reproductive strategies, hormonal regulation, and ovarian development. Histol. Histopathol. 2025, 40, 283–295. [Google Scholar]
- Greenbaum, M.P.; Ma, L.; Matzuk, M.M. Conversion of midbodies into germ cell intercellular bridges. Dev. Biol. 2007, 305, 389–396. [Google Scholar] [CrossRef]
- Elkouby, Y.M.; Mullins, M.C. Methods for the analysis of early oogenesis in zebrafish. Dev. Biol. 2017, 430, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Matsuda, M.; Wang, D.S.; Nagahama, Y.; Shibata, N. Molecular cloning and analysis of gonadal expression of foxl2 in the medaka, oryzias latipes. Biochem. Biophys. Res. Commun. 2006, 344, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Reading, B.J.; Andersen, L.K.; Ryu, Y.-W.; Mushirobira, Y.; Todo, T.; Hiramatsu, N. Oogenesis and egg quality in finfish: Yolk formation and other factors influencing female fertility. Fishes 2018, 3, 45. [Google Scholar] [CrossRef]
- Elkouby, Y.M.; Jamieson-Lucy, A.; Mullins, M.C. Oocyte polarization is coupled to the chromosomal bouquet, a conserved polarized nuclear configuration in meiosis. PLoS. Biol. 2016, 14, e1002335. [Google Scholar] [CrossRef]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef]
- Ge, W. Intrafollicular paracrine communication in the zebrafish ovary: The state of the art of an emerging model for the study of vertebrate folliculogenesis. Mol. Cell. Eedocrinol. 2005, 237, 1–10. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocr. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Bogoch, Y.; Jamieson-Lucy, A.; Vejnar, C.E.; Levy, K.; Giraldez, A.J.; Mullins, M.C.; Elkouby, Y.M. Stage specific transcriptomic analysis and database for zebrafish oogenesis. Front. Cell Dev. Biol. 2022, 10, 826892. [Google Scholar] [CrossRef]
- Zhu, B.; Pardeshi, L.; Chen, Y.; Ge, W. Transcriptomic analysis for differentially expressed genes in ovarian follicle activation in the zebrafish. Front. Endocr. 2018, 9, 593. [Google Scholar] [CrossRef]
- Zhang, Z.; Lau, S.-W.; Zhang, L.; Ge, W. Disruption of zebrafish follicle-stimulating hormone receptor (fshr) but not luteinizing hormone receptor (lhcgr) gene by TALEN leads to failed follicle activation in females followed by sexual reversal to males. Endocrinology 2015, 156, 3747–3762. [Google Scholar] [CrossRef]
- Chu, L.; Li, J.; Liu, Y.; Cheng, C.H. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study. Mol. Endocrinol. 2015, 29, 1743–1758. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Zhu, J.H.; Lu, S.Q.; Cao, Z.M.; Ma, J.L.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 555, 738171. [Google Scholar] [CrossRef]
- Kamaszewski, M.; Skrobisz, M.; Wójcik, M.; Kawalski, K.; Szczepański, A.; Bujarski, P.; Szudrowicz, H.; Herman, A.P.; Martynow, J. The role of transcription factors in gonad development and sex differentiation of a teleost model Fish—Guppy (Poecilia reticulata). Animals 2020, 10, 2401. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yao, J.P.; Zhou, H.; Leng, X.q.; Wu, J.p.; He, S.; Luo, J.; Liang, X.f.; Wei, Q.w.; Tan, Q.s. Optimal dietary lipid level promoted ovary development of Chinese sturgeon (Acipenser sinensis) broodstocks. Aquaculture 2018, 495, 288–294. [Google Scholar] [CrossRef]
- George, A.E.; Chapman, D.C. Aspects of embryonic and larval development in bighead carp Hypophthalmichthys nobilis and silver carp Hypophthalmichthys molitrix. PLoS ONE 2013, 8, e73829. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Liu, Y. Reproductive Physiology of Farmed Fish; China Agricultural Press: Beijing, China, 1993. [Google Scholar]
- Doroshov, S.I.; Moberg, G.P.; Van Eenennaam, J.P. Observations on the reproductive cycle of cultures white sturgeon, Acipenser transmontanus. Environ. Biol. Fishes. 1997, 48, 265–278. [Google Scholar] [CrossRef]
- Xu, H.; Ban, W.; Tian, J.; Xu, J.; Tan, Z.; Li, S. The new roles of traf6gene involved in the development of zebrafish liver and gonads. Mar. Biotechnol. 2024, 26, 917–930. [Google Scholar] [CrossRef]
- Li, P.; Zhou, L.; Wei, S.; Yang, M.; Ni, S.; Yu, Y.; Cai, J.; Qin, Q. Establishment and characterization of a cell line from the head kidney of golden pompano Trachinotus ovatus and its application in toxicology and virus susceptibility. J. Fish. Biol. 2017, 90, 1944–1959. [Google Scholar] [CrossRef]
- Fu, W.; Chu, X.B.; Xiao, W.Q.; Shen, T.y.; Peng, L.y.; Wang, Y.d.; Liu, W.b.; Liu, J.h.; Luo, K.; Chen, B. Identification of gynogenetic Megalobrama amblycephala induced by red crucian carp sperm and establishment of a new hypoxia tolerance strain. Aquaculture 2022, 548, 737608. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Xu, W.; Jiang, M.; Li, H.; Long, M.; Liu, W.; Liu, J.; Zhao, X.; Xiao, Y. Generation of stable induced pluripotent stem-like cells from adult zebra fish fibroblasts. Int. J. Biol. Sci. 2019, 15, 2340. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, L.; Da Silva, T.G.; Wang, Z.; Han, X.; Jin, K.; VanWye, J.; Zhu, X.; Weaver, K.; Oashi, T.; Lopes, P.E. The small molecule IMR-1 inhibits the notch transcriptional activation complex to suppress tumorigenesis. Cancer Res. 2016, 76, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, K.; Zheng, D.; Liu, Y.; Li, L.; He, Z.; Sun, C.; Yu, C. RHBDL2 promotes the proliferation, migration, and invasion of pancreatic cancer by stabilizing the N1ICD via the OTUD7B and activating the Notch signaling pathway. Cell. Death Dis. 2022, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Hopkins, B.; Perruzzi, C.; Udayakumar, D.; Sherris, D.; Benjamin, L.E. Palomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res. 2008, 68, 9551–9557. [Google Scholar] [CrossRef]
- Xing, Z.-y.; Wang, Y.; Cheng, L.; Chen, J.; He, X.-z.; Xing, W. Bromodomain-containing protein 4 (BRD4) inhibition sensitizes palomid 529-induced anti-renal cell carcinoma cell activity in vitro and in vivo. Cell. Physiol. Biochem. 2018, 50, 640–653. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.-Q.; Chowdhury, M.K.; Chen, J.-N.; Leng, X.-J. Effects of protease supplementation in low fish meal pelleted and extruded diets on growth, nutrient retention and digestibility of gibel carp, Carassius auratus gibelio. Aquaculture 2016, 460, 37–44. [Google Scholar] [CrossRef]
- Melnik, V.; Ryabinina, E.; Rodionova, K.; Nalivayko, L.; Khimych, M. Chemical composition and amino acid profile of goose meat (ukrainian large gray and large white breeds) in semi-intensive system of growing. J. Microbiol. Biotechnol. 2023, 12, e9828. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Liu, W.; Ju, T.; Zhan, X. Effects of different methionine sources on production and reproduction performance, egg quality and serum biochemical indices of broiler breeders. Asian-Australas. J. Anim. Sci. 2017, 30, 828. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Ju, P.; Li, L.; Gong, Y.; Zhang, Q. The mechanism of maternal inheritance of glycolipid metabolism disorder in a zebrafish model of type 2 diabetes. Sci. Rep. 2025, 15, 15198. [Google Scholar] [CrossRef]
- Zheng, Q.; Xie, X.; Li, Y.; Ai, C.; Pu, S.; Chen, J. Rapid isolation of stage I oocytes in zebrafish devoid of granulosa cells. J. Vis. Exp. (JoVE) 2024, 2024, 66458. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Irving-Rodgers, H.F.; Russell, D.L. Extracellular matrix of the developing ovarian follicle. Reproduction 2003, 126, 415–424. [Google Scholar] [CrossRef]
- Berkholtz, C.B.; Lai, B.E.; Woodruff, T.K.; Shea, L.D. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem. Cell. Bio. 2006, 126, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.; Subramanian, G.N.; Ye, Y.; Homer, H. Isolation and in vitro culture of mouse oocytes. Bio. Protoc. 2021, 11, e4104. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, Y.; Akahoshi, M.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Isolation and purification of satellite cells from young rats by percoll density gradient centrifugation. In Myogenesis; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 81–93. [Google Scholar]
- Li, P.; Dzyuba, B.; Hulak, M.; Rodina, M.; Boryshpolets, S.; Li, Z.-H.; Linhart, O. Percoll gradient separation of cryopreserved common carp spermatozoa to obtain a fraction with higher motility, velocity and membrane integrity. Front. Vet. Sci. 2010, 74, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Sun, Y.Y.; Chen, Z.Y. An efficient and reproducible method for the isolation and culture of primary cardiomyocytes from adult zebrafish. Zebrafish 2023, 20, 113–121. [Google Scholar] [CrossRef]
- Tyler, C.; Sumpter, J. Oocyte growth and development in teleosts. Rev. Fish. Biol. Fisher. 1996, 6, 287–318. [Google Scholar] [CrossRef]
- Mohamedien, D.; Mokhtar, D.M.; Abdellah, N.; Awad, M.; Albano, M.; Sayed, R.K.A. Ovary of zebrafish during spawning season: Ultrastructure and immunohistochemical profiles of Sox9 and Myostatin. Animals 2023, 13, 3362. [Google Scholar] [CrossRef]
- Devesa, J.; Caicedo, D. The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Wong, A.O.L.; Zhou, H.; Jiang, Y.; Ko, W.K. Feedback regulation of growth hormone synthesis and secretion in fish and the emerging concept of intrapituitary feedback loop. Comp. Biochem. Physiol. A 2005, 144, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Clelland, E.; Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009, 312, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Dickey, J.; Beckman, B.; Young, G.; Pierce, A.; Fukada, H.; Swanson, P. Previtellogenic oocyte growth in salmon: Relationships among body growth, plasma insulin-like growth factor-1, estradiol-17beta, follicle-stimulating hormone and expression of ovarian genes for insulin-like growth factors, steroidogenic-acute regulatory protein and receptors for gonadotropins, growth hormone, and somatolactin. Biol. Reprod. 2006, 75, 34–44. [Google Scholar] [PubMed]
- Lim, J.S.; Ibaseta, A.; Fischer, M.M.; Cancilla, B.; O’Young, G.; Cristea, S.; Luca, V.C.; Yang, D.; Jahchan, N.S.; Hamard, C. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017, 545, 360–364. [Google Scholar] [CrossRef]
- Gershon, E.; Dekel, N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Dang, Y.; Luo, L.; Hu, B.; Wang, S.; Wang, H.; Zhang, K. NOTCH signaling pathway is required for bovine early embryonic development. Biol. Reprod. 2021, 105, 332–344. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Wang, L.; He, J.; Guo, Y.; Liu, Y.; Liu, X.; Lin, H. Fertility enhancement but premature ovarian failure in esr1-deficient female zebrafish. Front. Endocrinol. 2018, 9, 567. [Google Scholar] [CrossRef]
- Gu, X.; Orozco, J.M.; Saxton, R.A.; Condon, K.J.; Liu, G.Y.; Krawczyk, P.A.; Scaria, S.M.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 2017, 358, 813–818. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Wang, G.; Zhang, C.; Wang, F.; Shi, J.; Zhang, T.; Ding, J. Molecular mechanism of S-adenosylmethionine sensing by SAMTOR in mTORC1 signaling. Sci. Adv. 2022, 8, eabn3868. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, C.; Zhu, B.; Zhang, Z.; Ge, W. Loss of inhibin advances follicle activation and female puberty onset but blocks oocyte maturation in zebrafish. Endocrinology 2020, 161, bqaa184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Yuan, X.; Fu, W.; Wang, Y.; Huang, Z.; Peng, L.; Liu, J.; Liu, W.; Xiao, Y. Primary Follicle Paces Fish Ovarian Maturation Developmental Progression via the Enhancement of Notch and mTOR. Biology 2025, 14, 1752. https://doi.org/10.3390/biology14121752
Zhang G, Yuan X, Fu W, Wang Y, Huang Z, Peng L, Liu J, Liu W, Xiao Y. Primary Follicle Paces Fish Ovarian Maturation Developmental Progression via the Enhancement of Notch and mTOR. Biology. 2025; 14(12):1752. https://doi.org/10.3390/biology14121752
Chicago/Turabian StyleZhang, Guangjing, Xiudan Yuan, Wen Fu, Yujiao Wang, Zhen Huang, Liangyue Peng, Jinhui Liu, Wenbin Liu, and Yamei Xiao. 2025. "Primary Follicle Paces Fish Ovarian Maturation Developmental Progression via the Enhancement of Notch and mTOR" Biology 14, no. 12: 1752. https://doi.org/10.3390/biology14121752
APA StyleZhang, G., Yuan, X., Fu, W., Wang, Y., Huang, Z., Peng, L., Liu, J., Liu, W., & Xiao, Y. (2025). Primary Follicle Paces Fish Ovarian Maturation Developmental Progression via the Enhancement of Notch and mTOR. Biology, 14(12), 1752. https://doi.org/10.3390/biology14121752




