HIV-Induced Apoptosis: Host Defense and Viral Strategy
Simple Summary
Abstract
1. Introduction
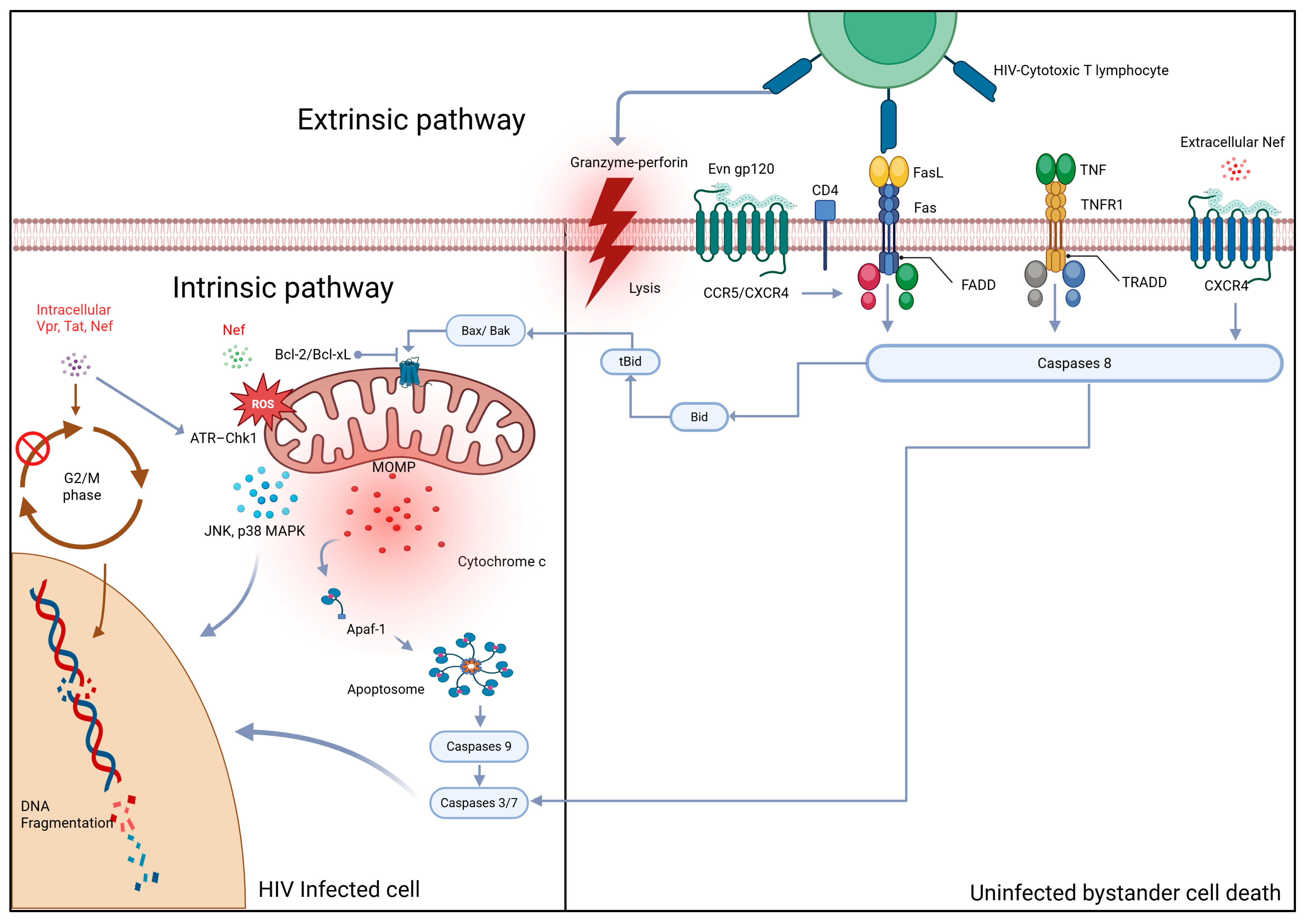
2. Mechanisms of HIV-Induced Apoptosis
2.1. Extrinsic Pathway Activation: Death Receptor Signaling
2.1.1. Fas/FasL Signaling Cascade
2.1.2. TNF/TNFR1 Signaling Cascade
2.1.3. Immune Cell-Mediated Cytotoxicity via Death Receptors
2.2. Direct Mechanisms: Viral Proteins and Intracellular Signaling
2.3. Role of Immune and Inflammatory Mediators
3. Apoptosis as a Host Defense Mechanism
3.1. Early Infection Response and Viral Containment
3.2. Cytotoxic Lymphocyte-Mediated Apoptosis
3.3. Controlled Apoptosis as an Immunoregulatory Tool
4. Apoptosis as a Viral Strategy
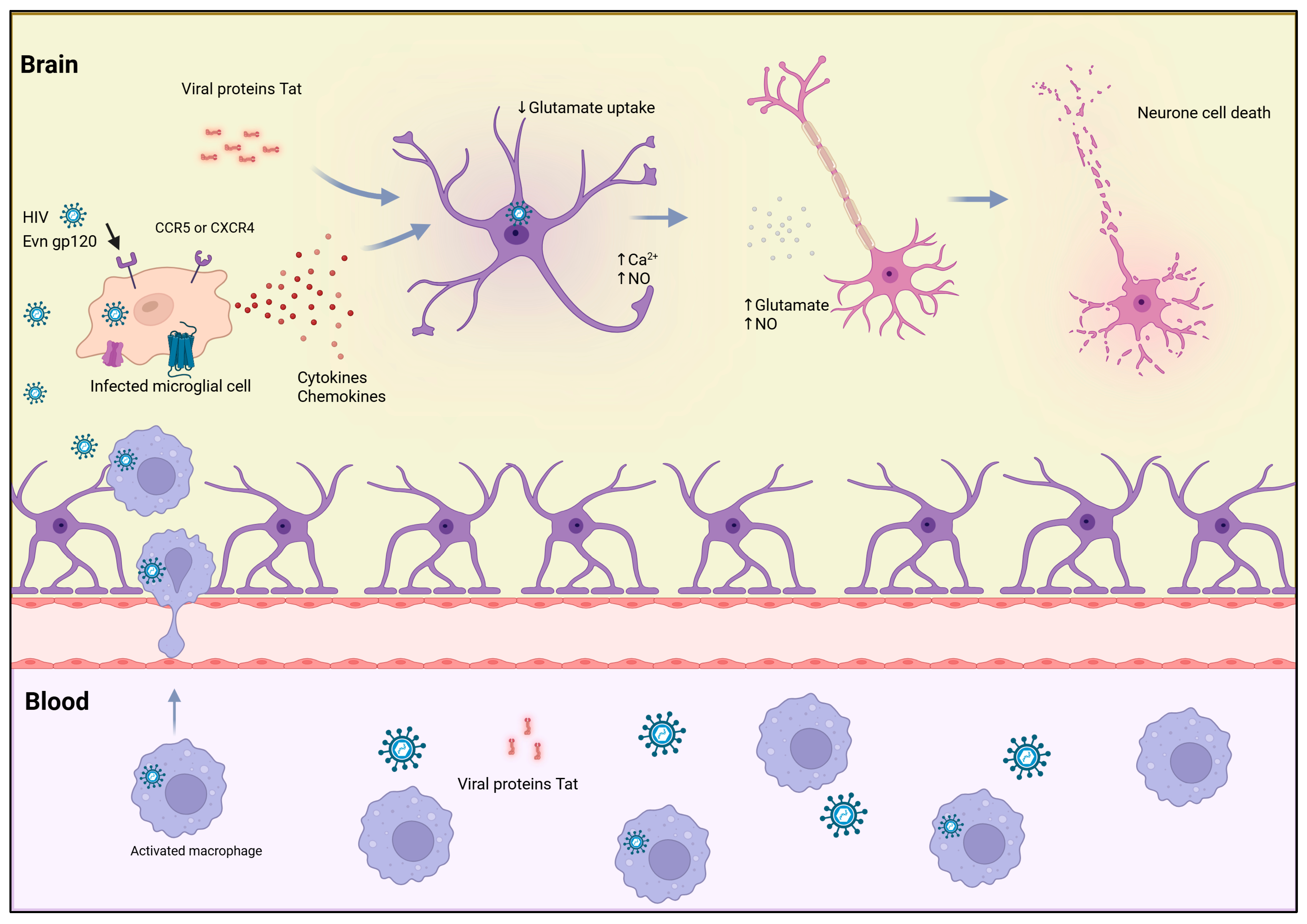
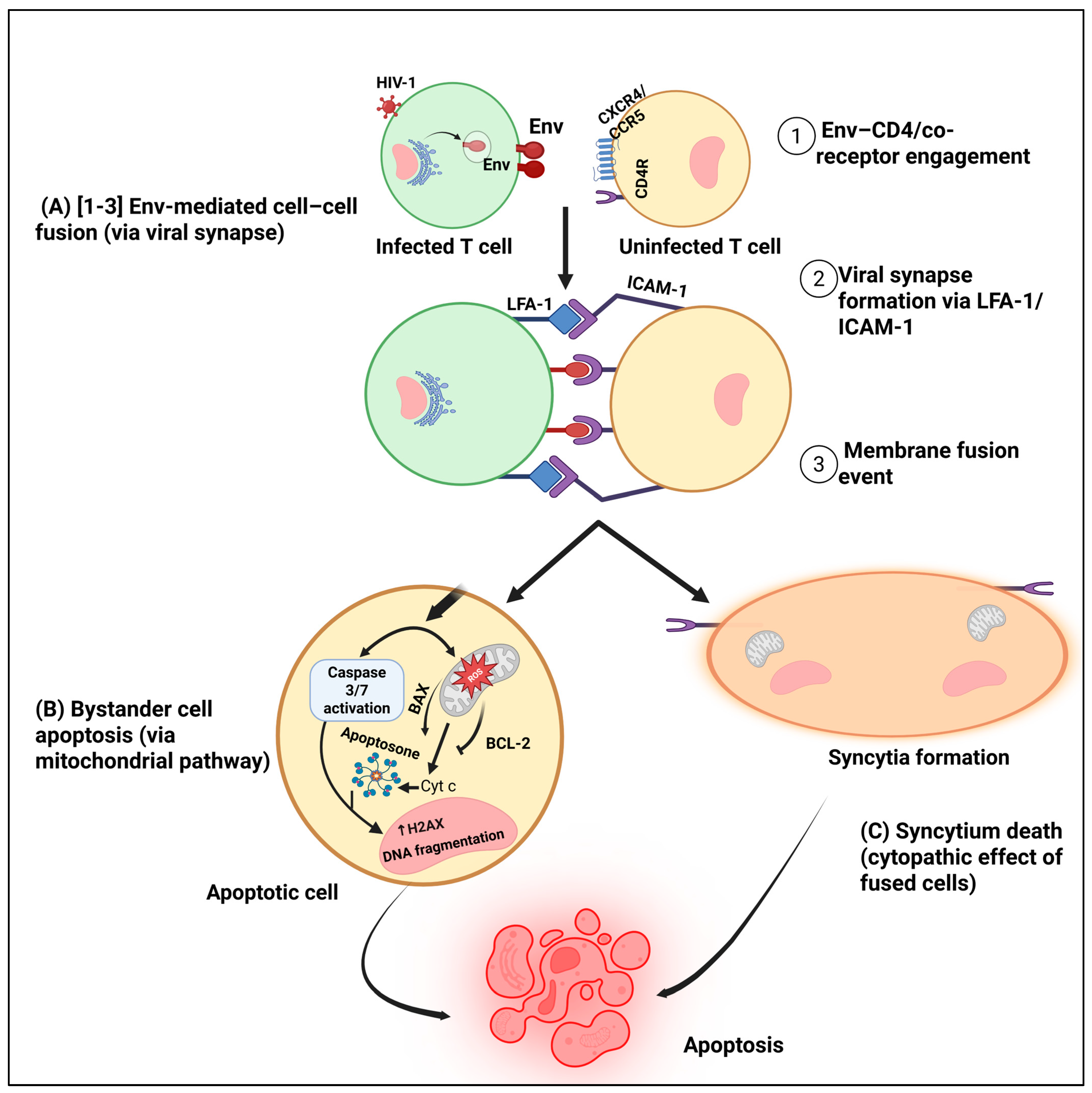
4.1. Bystander Apoptosis and Immune Collapse
4.2. Selective Killing of Immune Regulators
4.3. Hijacking Apoptotic Signaling to Promote Viral Persistence
5. Chronic Immune Activation and Pyroptosis
5.1. From Apoptosis to Pyroptosis
5.2. Role of Innate Immune Sensing and Inflammasomes
5.3. The Vicious Cycle: Immune Activation, Death, and Tissue Damage
6. Therapeutic Implications
6.1. Effects of Antiretroviral Therapy (ART) on Apoptotic Pathways
6.2. Targeting Apoptosis and Pyroptosis for Immune Preservation
6.3. Implications for HIV Cure Strategies
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyrer, C. A pandemic anniversary: 40 years of HIV/AIDS. Lancet 2021, 397, 2142–2143. [Google Scholar] [CrossRef]
- WHO. HIV Data and Statistics. 2024. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 20 October 2025).
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Cummins, N.W.; Badley, A.D. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010, 1, e99. [Google Scholar] [CrossRef] [PubMed]
- Yukl, S.A.; Shergill, A.K.; Ho, T.; Killian, M.; Girling, V.; Epling, L.; Li, P.; Wong, L.K.; Crouch, P.; Deeks, S.G.; et al. The Distribution of HIV DNA and RNA in Cell Subsets Differs in Gut and Blood of HIV-Positive Patients on ART: Implications for Viral Persistence. J. Infect. Dis. 2013, 208, 1212–1220. [Google Scholar] [CrossRef]
- Ta, T.M.; Malik, S.; Anderson, E.M.; Jones, A.D.; Perchik, J.; Freylikh, M.; Sardo, L.; Klase, Z.A.; Izumi, T. Insights into Persistent HIV-1 Infection and Functional Cure: Novel Capabilities and Strategies. Front. Microbiol. 2022, 13, 862270. [Google Scholar] [CrossRef]
- Prakash, P.; Swami Vetha, B.S.; Chakraborty, R.; Wenegieme, T.-Y.; Masenga, S.K.; Muthian, G.; Balasubramaniam, M.; Wanjalla, C.N.; Hinton, A.O.; Kirabo, A.; et al. HIV-Associated Hypertension: Risks, Mechanisms, and Knowledge Gaps. Circ. Res. 2024, 134, e150–e175. [Google Scholar] [CrossRef]
- Doitsh, G.; Galloway, N.L.K.; Geng, X.; Yang, Z.; Monroe, K.M.; Zepeda, O.; Hunt, P.W.; Hatano, H.; Sowinski, S.; Muñoz-Arias, I.; et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014, 505, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Joshi, A. Host and Viral Factors in HIV-Mediated Bystander Apoptosis. Viruses 2017, 9, 237. [Google Scholar] [CrossRef]
- Lamsira, H.K.; Sabatini, A.; Ciolfi, S.; Ciccosanti, F.; Sacchi, A.; Piacentini, M.; Nardacci, R. Autophagy and Programmed Cell Death Modalities Interplay in HIV Pathogenesis. Cells 2025, 14, 351. [Google Scholar] [CrossRef]
- Massanella, M.; Fromentin, R.; Chomont, N. Residual inflammation and viral reservoirs: Alliance against an HIV cure. Curr. Opin. HIV AIDS 2016, 11, 234–241. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Mueller, Y.M.; Rosa, S.C.D.; Hutton, J.A.; Witek, J.; Roederer, M.; Altman, J.D.; Katsikis, P.D. Increased CD95/Fas-Induced Apoptosis of HIV-Specific CD8+ T Cells. Immunity 2001, 15, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Jabea Ekabe, C.; Asaba Clinton, N.; Agyei, E.K.; Kehbila, J. Role of Apoptosis in HIV Pathogenesis. Adv. Virol. 2022, 2022, 8148119. [Google Scholar] [CrossRef]
- Green, D.R. The Death Receptor Pathway of Apoptosis. Cold Spring Harb. Perspect. Biol. 2022, 14, a041053. [Google Scholar] [CrossRef]
- Lavrik, I.N. Systems biology of death receptor networks: Live and let die. Cell Death Dis. 2014, 5, e1259. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Kaplan, D.; Sieg, S. Role of the Fas/Fas Ligand Apoptotic Pathway in Human Immunodeficiency Virus Type 1 Disease. J. Virol. 1998, 72, 6279–6282. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Vojdani, A.; Koksoy, S.; Vojdani, E.; Engelman, M.; Benzvi, C.; Lerner, A. Natural Killer Cells and Cytotoxic T Cells: Complementary Partners against Microorganisms and Cancer. Microorganisms 2024, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lu, J.; Fan, H.; Niu, C.; Han, Y.; Caiyin, Q.; Wu, H.; Qiao, J. FAS mediates apoptosis, inflammation, and treatment of pathogen infection. Front. Cell. Infect. Microbiol. 2025, 15, 1561102. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Tardivel, A.; Kovacsovics-Bankowski, M.; Hertig, S.; Gaide, O.; Martinon, F.; Tinel, A.; Deperthes, D.; Calderara, S.; Schulthess, T.; et al. Two Adjacent Trimeric Fas Ligands Are Required for Fas Signaling and Formation of a Death-Inducing Signaling Complex. Mol. Cell. Biol. 2003, 23, 1428–1440. [Google Scholar] [CrossRef]
- Ranjan, K.; Pathak, C. Cellular Dynamics of Fas-Associated Death Domain in the Regulation of Cancer and Inflammation. Int. J. Mol. Sci. 2024, 25, 3228. [Google Scholar] [CrossRef]
- Tourneur, L.; Chiocchia, G. FADD: A regulator of life and death. Trends Immunol. 2010, 31, 260–269. [Google Scholar] [CrossRef]
- Chang, D.W.; Xing, Z.; Capacio, V.L.; Peter, M.E.; Yang, X. Interdimer processing mechanism of procaspase–8 activation. EMBO J. 2003, 22, 4132–4142. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; O’Neill, K.L.; Gurumurthy, C.B.; Quadros, R.M.; Tu, Y.; Luo, X. Cleavage by Caspase 8 and Mitochondrial Membrane Association Activate the BH3-only Protein Bid during TRAIL-induced Apoptosis. J. Biol. Chem. 2016, 291, 11843–11851. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011, 18, 538–548. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Yosri, M.; Dokhan, M.; Aboagye, E.; Al Moussawy, M.; Abdelsamed, H.A. Mechanisms governing bystander activation of T cells. Front. Immunol. 2024, 15, 1465889. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Mohl, J.; Joshi, A. HIV-1 Induced Bystander Apoptosis. Viruses 2012, 4, 3020–3043. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, D.H. The multiple roles of Fas ligand in the pathogenesis of infectious diseases. Clin. Microbiol. Infect. 2003, 9, 766–779. [Google Scholar] [CrossRef]
- Pasquereau, S.; Kumar, A.; Herbein, G. Targeting TNF and TNF Receptor Pathway in HIV-1 Infection: From Immune Activation to Viral Reservoirs. Viruses 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Griewahn, L.; Köser, A.; Maurer, U. Keeping Cell Death in Check: Ubiquitylation-Dependent Control of TNFR1 and TLR Signaling. Front. Cell Dev. Biol. 2019, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-a from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, S.; Sanin, D.E.; Koss, C.K.; Willenborg, S.; Petzold, A.; Tanzer, M.C.; Dahl, A.; Kabat, A.M.; Lindenthal, L.; Zeitler, L.; et al. Gene-selective transcription promotes the inhibition of tissue reparative macrophages by TNF. Life Sci. Alliance 2022, 5, e202101315. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Goncharov, T.; Maecker, H.; Zobel, K.; Kömüves, L.G.; Deshayes, K.; Vucic, D. Cellular inhibitors of apoptosis are global regulators of NF-κB and MAPK activation by members of the TNF family of receptors. Sci. Signal. 2012, 5, ra22. [Google Scholar] [CrossRef]
- Li, Y.; Ye, R.; Dai, H.; Lin, J.; Cheng, Y.; Zhou, Y.; Lu, Y. Exploring TNFR1: From discovery to targeted therapy development. J. Transl. Med. 2025, 23, 71. [Google Scholar] [CrossRef]
- Witt, A.; Vucic, D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling. Cell Death Differ. 2017, 24, 1160–1171. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. TNF and TNF Receptor Superfamily Members in HIV infection: New Cellular Targets for Therapy? Mediat. Inflamm. 2013, 2013, 484378. [Google Scholar] [CrossRef]
- Schorn, F.; Werthenbach, J.P.; Hoffmann, M.; Daoud, M.; Stachelscheid, J.; Schiffmann, L.M.; Hildebrandt, X.; Lyu, S.I.; Peltzer, N.; Quaas, A.; et al. cIAPs control RIPK1 kinase activity-dependent and -independent cell death and tissue inflammation. EMBO J. 2023, 42, e113614. [Google Scholar] [CrossRef] [PubMed]
- Udawatte, D.J.; Rothman, A.L. Viral Suppression of RIPK1-Mediated Signaling. mBio 2021, 12, e0172321. [Google Scholar] [CrossRef]
- Zhao, J.; He, S.; Minassian, A.; Li, J.; Feng, P. Recent advances on viral manipulation of NF-κB signaling pathway. Curr. Opin. Virol. 2015, 15, 103–111. [Google Scholar] [CrossRef]
- Tang, Y.; Tu, H.; Zhang, J.; Zhao, X.; Wang, Y.; Qin, J.; Lin, X. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat. Commun. 2019, 10, 4157. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M.F. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Scheurich, P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011, 278, 862–876. [Google Scholar] [CrossRef]
- Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 2005, 12, 1417–1428. Available online: https://www.nature.com/articles/4401668#Sec8 (accessed on 15 October 2025). [CrossRef]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Decaudin, D.; Macho, A.; Hirsch, T.; Susin, S.A.; Petit, P.X.; Mignotte, B.; Kroemer, G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995, 182, 367–377. [Google Scholar] [CrossRef]
- Pramanik, D.; Chakrabarty, D.; Mondal, S.; Roy, A. A cross-sectional study on adherence to antiretroviral therapy among HIV-infected pediatric patients attending medical college and hospital, Kolkata. Asian J. Med. Sci. 2024, 15, 139–146. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Y.; Liu, S.; Li, C.; Dong, J.; Kong, X.; Ji, X.; Cheng, X.; Zhang, L. RIPK3 signaling and its role in regulated cell death and diseases. Cell Death Discov. 2024, 10, 200. [Google Scholar] [CrossRef]
- Beaudouin, J.; Liesche, C.; Aschenbrenner, S.; Hörner, M.; Eils, R. Caspase-8 cleaves its substrates from the plasma membrane upon CD95-induced apoptosis. Cell Death Differ. 2013, 20, 599–610. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K.-M. Necroptosis-independent signaling by the RIP kinases in inflammation. Cell Mol. Life Sci. 2016, 73, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; Khan, N.; Mildenhall, A.; Gerlic, M.; Croker, B.A.; D’Cruz, A.A.; Hall, C.; Kaur Spall, S.; Anderton, H.; Masters, S.L.; et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015, 6, 6282. [Google Scholar] [CrossRef] [PubMed]
- Sevilya, Z.; Chorin, E.; Gal-Garber, O.; Zelinger, E.; Turner, D.; Avidor, B.; Berke, G.; Hassin, D. Killing of Latently HIV-Infected CD4 T Cells by Autologous CD8 T Cells Is Modulated by Nef. Front. Immunol. 2018, 9, 2068. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef]
- McBrien, J.B.; Kumar, N.A.; Silvestri, G. Mechanisms of CD8+ T cell-mediated suppression of HIV/SIV replication. Eur. J. Immunol. 2018, 48, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Paim, A.C.; Badley, A.D.; Cummins, N.W. Mechanisms of Human Immunodeficiency Virus-Associated Lymphocyte Regulated Cell Death. AIDS Res. Hum. Retroviruses 2020, 36, 101–115. [Google Scholar] [CrossRef]
- Lopez, J.A.; Susanto, O.; Jenkins, M.R.; Lukoyanova, N.; Sutton, V.R.; Law, R.H.P.; Johnston, A.; Bird, C.H.; Bird, P.I.; Whisstock, J.C.; et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013, 121, 2659–2668. [Google Scholar] [CrossRef]
- Thiery, J.; Keefe, D.; Boulant, S.; Boucrot, E.; Walch, M.; Martinvalet, D.; Goping, I.S.; Bleackley, R.C.; Kirchhausen, T.; Lieberman, J. Perforin pores in the endosomal membrane trigger release of endocytosed granzyme B to the cytosol of target cells. Nat. Immunol. 2011, 12, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615–1626. [Google Scholar] [CrossRef]
- Hinton, A.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef]
- Barry, M.; Heibein, J.A.; Pinkoski, M.J.; Lee, S.-F.; Moyer, R.W.; Green, D.R.; Bleackley, R.C. Granzyme B Short-Circuits the Need for Caspase 8 Activity during Granule-Mediated Cytotoxic T-Lymphocyte Killing by Directly Cleaving Bid. Mol. Cell. Biol. 2000, 20, 3781–3794. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Zhang, Y.; Zhang, J.; Lu, W.; Chang, C.-H.; Jiang, S. HIV associated cell death: Peptide-induced apoptosis restricts viral transmission. Front. Immunol. 2023, 14, 1096759. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Babcock, I.W.; Minamide, L.S.; Shaw, A.E.; Bamburg, J.R.; Kuhn, T.B. Direct interaction of HIV gp120 with neuronal CXCR4 and CCR5 receptors induces cofilin-actin rod pathology via a cellular prion protein- and NOX-dependent mechanism. PLoS ONE 2021, 16, e0248309. [Google Scholar] [CrossRef] [PubMed]
- Mbita, Z.; Hull, R.; Dlamini, Z. Human Immunodeficiency Virus-1 (HIV-1)-Mediated Apoptosis: New Therapeutic Targets. Viruses 2014, 6, 3181–3227. [Google Scholar] [CrossRef]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV–Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef]
- Faivre, N.; Verollet, C.; Dumas, F. The chemokine receptor CCR5: Multi-faceted hook for HIV-1. Retrovirology 2024, 21, 2. [Google Scholar] [CrossRef]
- Shrestha, J.; Santerre, M.; Allen, C.N.; Arjona, S.P.; Hooper, R.; Mukerjee, R.; Kaul, M.; Shcherbik, N.; Soboloff, J.; Sawaya, B.E. HIV-1 gp120 protein promotes HAND through the calcineurin pathway activation. Mitochondrion 2023, 70, 31–40. [Google Scholar] [CrossRef]
- Kan, H.; Xie, Z.; Finkel, M.S. p38 MAP kinase-mediated negative inotropic effect of HIV gp120 on cardiac myocytes. Am. J. Physiol. Cell Physiol. 2004, 286, C1–C7. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Robinson, A.T.; Beach, L.B.; Lindsey, M.L.; Kirabo, A.; Hinton, A.; Erlandson, K.M.; Jenkins, N.D.M. Exercise to Prevent Accelerated Vascular Aging in People Living with HIV. Circ. Res. 2024, 134, 1607–1635. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, A.; Schietroma, I.; Sernicola, L.; Belli, R.; Campagna, M.; Mancini, F.; Farcomeni, S.; Pavone-Cossut, M.R.; Borsetti, A.; Monini, P.; et al. Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis. Int. J. Mol. Sci. 2024, 25, 1704. [Google Scholar] [CrossRef]
- Wu, J.J.; Bennett, A.M. Essential Role for Mitogen-activated Protein (MAP) Kinase Phosphatase-1 in Stress-responsive MAP Kinase and Cell Survival Signaling. J. Biol. Chem. 2005, 280, 16461–16466. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Andersen, J.L.; Le Rouzic, E.; Planelles, V. HIV-1 Vpr: Mechanisms of G2 Arrest and Apoptosis. Exp. Mol. Pathol. 2008, 85, 2–10. [Google Scholar] [CrossRef]
- Li, G.; Bukrinsky, M.; Zhao, R.Y. HIV-1 Viral Protein R (Vpr) and Its Interactions with the Host Cell. Curr. HIV Res. 2009, 7, 178–183. [Google Scholar] [CrossRef]
- Ryan, E.L.; Hollingworth, R.; Grand, R.J. Activation of the DNA Damage Response by RNA Viruses. Biomolecules 2016, 6, 2. [Google Scholar] [CrossRef]
- Petit, F.; Arnoult, D.; Viollet, L.; Estaquier, J. Intrinsic and extrinsic pathways signaling during HIV-1 mediated cell death. Biochimie 2003, 85, 795–811. [Google Scholar] [CrossRef]
- Chandrasekar, A.P.; Cummins, N.W.; Badley, A.D. The Role of the BCL-2 Family of Proteins in HIV-1 Pathogenesis and Persistence. Clin. Microbiol. Rev. 2019, 33, e00107-19. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-Ros Crosstalk in the Control of Cell Death and Aging. J. Signal. Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef]
- Hinton, A.O.; N’jai, A.U.; Vue, Z.; Wanjalla, C. Connection Between HIV and Mitochondria in Cardiovascular Disease and Implications for Treatments. Circ. Res. 2024, 134, 1581–1606. [Google Scholar] [CrossRef]
- Joseph, J.; Daley, W.; Lawrence, D.; Lorenzo, E.; Perrin, P.; Rao, V.R.; Tsai, S.-Y.; Varthakavi, V. Role of macrophages in HIV pathogenesis and cure: NIH perspectives. J. Leukoc. Biol. 2022, 112, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, A.; Spuch, C.; Penedo, M.A.; Olivares, J.M.; Agís-Balboa, R.C. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann. Med. 2021, 53, 43–69. [Google Scholar] [CrossRef]
- Gagliardi, S.; Hotchkin, T.; Hillmer, G.; Engelbride, M.; Diggs, A.; Tibebe, H.; Izumi, C.; Sullivan, C.; Cropp, C.; Lantz, O.; et al. Oxidative Stress in HIV-Associated Neurodegeneration: Mechanisms of Pathogenesis and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 6724. [Google Scholar] [CrossRef]
- Smith, L.K.; Kuhn, T.B.; Chen, J.; Bamburg, J.R. HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity. Curr. HIV Res. 2018, 16, 258–269. [Google Scholar] [CrossRef]
- Potter, M.C.; Figuera-Losada, M.; Rojas, C.; Slusher, B.S. Targeting the Glutamatergic System for the Treatment of HIV-Associated Neurocognitive Disorders. J. Neuroimmune Pharmacol. 2013, 8, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.A.; Pombo, C.; Betts, M.R. Cytokine production and dysregulation in HIV pathogenesis: Lessons for development of therapeutics and vaccines. Cytokine Growth Factor Rev. 2012, 23, 181–191. [Google Scholar] [CrossRef]
- Obare, L.M.; Priest, S.; Ismail, A.; Mashayekhi, M.; Zhang, X.; Stolze, L.K.; Sheng, Q.; Nthenge, K.; Vue, Z.; Neikirk, K.; et al. Cytokine and chemokine receptor profiles in adipose tissue vasculature unravel endothelial cell responses in HIV. J. Cell. Physiol. 2024, 239, e31415. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, M.; Jiang, P.; Wang, X.; Tong, X.; Wu, G. Unlocking Apoptotic Pathways: Overcoming Tumor Resistance in CAR-T-Cell Therapy. Cancer Med. 2024, 13, e70283. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Muthumani, K.; Shedlock, D.J.; Choo, D.K.; Fagone, P.; Kawalekar, O.U.; Goodman, J.; Bian, C.B.; Ramanathan, A.A.; Atman, P.; Tebas, P.; et al. HIV-Mediated Phosphatidylinositol 3-Kinase/Serine–Threonine Kinase Activation in APCs Leads to Programmed Death-1 Ligand Upregulation and Suppression of HIV-Specific CD8 T Cells. J. Immunol. 2011, 187, 2932–2943. [Google Scholar] [CrossRef]
- Making sense of how HIV kills infected CD4 T cells: Implications for HIV cure. Mol. Cell. Ther. 2014, 2, 20. Available online: https://molcelltherapies.biomedcentral.com/articles/10.1186/2052-8426-2-20 (accessed on 15 October 2025). [CrossRef] [PubMed]
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retroviruses 2017, 33, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, M.; Reay, E.; Sankaran, S.; Prindiville, T.; Flamm, J.; McNeil, A.; Dandekar, S. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration following Highly Active Antiretroviral Therapy. J. Virol. 2003, 77, 11708–11717. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, L.; Yang, H.-C.; Siliciano, R.F. Preferential Cytolysis of Peripheral Memory CD4+ T Cells by In Vitro X4-Tropic Human Immunodeficiency Virus Type 1 Infection before the Completion of Reverse Transcription. J. Virol. 2008, 82, 9154–9163. [Google Scholar] [CrossRef]
- Min, A.K.; Fortune, T.; Rodriguez, N.; Hedge, E.; Swartz, T.H. Inflammasomes as mediators of inflammation in HIV-1 infection. Transl. Res. 2023, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, M.L.; Laurent-Crawford, A.G.; Hovanessian, A.G.; Montagnier, L. Direct and indirect mechanisms mediating apoptosis during HIV infection: Contribution to In Vivo CD4 T cell depletion. Semin. Immunol. 1993, 5, 187–194. [Google Scholar] [CrossRef]
- Gougeon, M.-L.; Montagnier, L. Programmed Cell Death as a Mechanism of CD4 and CD8 T Cell Deletion in AIDS: Molecular Control and Effect of Highly Active Anti-retroviral Therapy. Ann. N. Y. Acad. Sci. 1999, 887, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Massanella, M.; Puig, J.; Pérez-Álvarez, N.; Gallego-Escuredo, J.M.; Villarroya, J.; Villarroya, F.; Molto, J.; Santos, J.R.; Clotet, B.; et al. Nadir CD4 T Cell Count as Predictor and High CD4 T Cell Intrinsic Apoptosis as Final Mechanism of Poor CD4 T Cell Recovery in Virologically Suppressed HIV-Infected Patients: Clinical Implications. Clin. Infect. Dis. 2010, 50, 1300–1308. [Google Scholar] [CrossRef]
- Okoye, A.A.; Picker, L.J. CD4+ T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Varbanov, M.; Espert, L.; Biard-Piechaczyk, M. Mechanisms of CD4 T-cell depletion triggered by HIV-1 viral proteins. AIDS Rev. 2006, 8, 221–236. [Google Scholar]
- Weller, M.; Frei, K.; Groscurth, P.; Krammer, P.H.; Yonekawa, Y.; Fontana, A. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J. Clin. Investig. 1994, 94, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Banki, K.; Hutter, E.; Gonchoroff, N.J.; Perl, A. Molecular Ordering in HIV-induced Apoptosis: Oxidative Stress, Activation of Caspases, and Cell Survival are Regulated by Transaldolase. J. Biol. Chem. 1998, 273, 11944–11953. [Google Scholar] [CrossRef]
- Finkel, T.H.; Tudor-Williams, G.; Banda, N.K.; Cotton, M.F.; Curiel, T.; Monks, C.; Baba, T.W.; Ruprecht, R.M.; Kupfer, A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1995, 1, 129–134. [Google Scholar] [CrossRef]
- Roggero, R.; Robert-Hebmann, V.; Harrington, S.; Roland, J.; Vergne, L.; Jaleco, S.; Devaux, C.; Biard-Piechaczyk, M. Binding of Human Immunodeficiency Virus Type 1 gp120 to CXCR4 Induces Mitochondrial Transmembrane Depolarization and Cytochrome c-Mediated Apoptosis Independently of Fas Signaling. J. Virol. 2001, 75, 7637–7650. [Google Scholar] [CrossRef]
- Doitsh, G.; Cavrois, M.; Lassen, K.G.; Zepeda, O.; Yang, Z.; Santiago, M.L.; Hebbeler, A.M.; Greene, W.C. Abortive HIV Infection Mediates CD4 T-Cell Depletion and Inflammation in Human Lymphoid Tissue. Cell 2010, 143, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Perfettini, J.-L.; Castedo, M.; Roumier, T.; Andreau, K.; Nardacci, R.; Piacentini, M.; Kroemer, G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005, 12, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Khanal, S.; Wang, L.; Li, Z.; Zhao, J.; Nguyen, L.N.; Nguyen, L.N.T.; Dang, X.; Schank, M.; Thakuri, B.K.C.; et al. A Matter of Life or Death: Productively Infected and Bystander CD4 T Cells in Early HIV Infection. Front. Immunol. 2021, 11, 626431. [Google Scholar] [CrossRef] [PubMed]
- Kell, L.; Simon, A.K.; Alsaleh, G.; Cox, L.S. The central role of DNA damage in immunosenescence. Front. Aging 2023, 4, 1202152. [Google Scholar] [CrossRef]
- Xie, M. Virus-Induced Cell Fusion and Syncytia Formation. In Syncytia: Origin, Structure, and Functions; Kloc, M., Uosef, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 283–318. ISBN 978-3-031-37936-9. [Google Scholar]
- Dimitrov, A.S.; Louis, J.M.; Bewley, C.A.; Clore, G.M.; Blumenthal, R. Conformational Changes in HIV-1 gp41 in the Course of HIV-1 Envelope Glycoprotein-Mediated Fusion and Inactivation. Biochemistry 2005, 44, 12471–12479. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Nguyen, H.T.; Chen, H.-C.; Chiu, T.-J.; Smith, A.B., III; Sodroski, J.G. Alterations in gp120 glycans or the gp41 fusion peptide-proximal region modulate the stability of the human immunodeficiency virus (HIV-1) envelope glycoprotein pretriggered conformation. J. Virol. 2023, 97, e00592-23. [Google Scholar] [CrossRef]
- Murooka, T.T.; Deruaz, M.; Marangoni, F.; Vrbanac, V.D.; Seung, E.; von Andrian, U.H.; Tager, A.M.; Luster, A.D.; Mempel, T.R. HIV-infected T cells are migratory vehicles for viral dissemination. Nature 2012, 490, 283–287. [Google Scholar] [CrossRef]
- Jolly, C.; Sattentau, Q.J. Retroviral spread by induction of virological synapses. Traffic 2004, 5, 643–650. [Google Scholar] [CrossRef]
- Keele, B.F.; Estes, J.D. Barriers to mucosal transmission of immunodeficiency viruses. Blood 2011, 118, 839–846. [Google Scholar] [CrossRef]
- Nie, Z.; Phenix, B.N.; Lum, J.J.; Alam, A.; Lynch, D.H.; Beckett, B.; Krammer, P.H.; Sekaly, R.P.; Badley, A.D. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ. 2002, 9, 1172–1184. [Google Scholar] [CrossRef]
- Yang, H.; Cheung, P.-H.H.; Wu, L. SAMHD1 enhances HIV-1-induced apoptosis in monocytic cells via the mitochondrial pathway. mBio 2025, 16, e00425-25. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Coyne, C.B. The Actin Cytoskeleton as a Barrier to Virus Infection of Polarized Epithelial Cells. Viruses 2011, 3, 2462–2477. [Google Scholar] [CrossRef]
- Sainski, A.M.; Dai, H.; Natesampillai, S.; Pang, Y.-P.; Bren, G.D.; Cummins, N.W.; Correia, C.; Meng, X.W.; Tarara, J.E.; Ramirez-Alvarado, M.; et al. Casp8p41 generated by HIV protease kills CD4 T cells through direct Bak activation. J. Cell Biol. 2014, 206, 867–876. [Google Scholar] [CrossRef]
- Ahr, B.; Robert-Hebmann, V.; Devaux, C.; Biard-Piechaczyk, M. Apoptosis of uninfected cells induced by HIV envelope glycoproteins. Retrovirology 2004, 1, 12. [Google Scholar] [CrossRef]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef]
- Younes, S.-A.; Yassine-Diab, B.; Dumont, A.R.; Boulassel, M.-R.; Grossman, Z.; Routy, J.-P.; Sekaly, R.-P. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 2003, 198, 1909–1922. [Google Scholar] [CrossRef]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 12866. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]
- Swingler, S.; Mann, A.M.; Zhou, J.; Swingler, C.; Stevenson, M. Apoptotic Killing of HIV-1–Infected Macrophages Is Subverted by the Viral Envelope Glycoprotein. PLoS Pathog. 2007, 3, e134. [Google Scholar] [CrossRef] [PubMed]
- Geleziunas, R.; Xu, W.; Takeda, K.; Ichijo, H.; Greene, W.C. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 2001, 410, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; van Bockel, D.; Kent, S.J.; Kelleher, A.D.; Zaunders, J.J.; Munier, C.M.L. Cytotoxic CD4 T Cells—Friend or Foe during Viral Infection? Front. Immunol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Wolf, D.; Witte, V.; Laffert, B.; Blume, K.; Stromer, E.; Trapp, S.; D’ALoja, P.; Schürmann, A.; Baur, A.S. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 2001, 7, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Diehl, N.; Schaal, H. Make Yourself at Home: Viral Hijacking of the PI3K/Akt Signaling Pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Kim, N.; Kukkonen, S.; Gupta, S.; Aldovini, A. Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells. PLoS Pathog. 2010, 6, e1001103. [Google Scholar] [CrossRef]
- Chugh, P.; Fan, S.; Planelles, V.; Maggirwar, S.B.; Dewhurst, S.; Kim, B. Infection of Human Immunodeficiency Virus and Intracellular Viral Tat Protein Exert a Pro-survival Effect in a Human Microglial Cell Line. J. Mol. Biol. 2007, 366, 67–81. [Google Scholar] [CrossRef]
- Lucas, A.; Kim, Y.; Rivera-Pabon, O.; Chae, S.; Kim, D.-H.; Kim, B. Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PLoS ONE 2010, 5, e13121. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Wherry, E.J.; Ahmed, R.; Sharpe, A.H. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J. Exp. Med. 2006, 203, 2223–2227. [Google Scholar] [CrossRef]
- West, E.E.; Jin, H.-T.; Rasheed, A.-U.; Penaloza-MacMaster, P.; Ha, S.-J.; Tan, W.G.; Youngblood, B.; Freeman, G.J.; Smith, K.A.; Ahmed, R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Investig. 2013, 123, 2604–2615. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, Y.A.; Narasipura, S.D.; Al-Harthi, L. Wnt/β-Catenin Protects Lymphocytes from HIV-Mediated Apoptosis via Induction of Bcl-xL. Viruses 2022, 14, 1469. [Google Scholar] [CrossRef]
- Pasquereau, S.; Herbein, G. CounterAKTing HIV: Toward a “Block and Clear” Strategy? Front. Cell. Infect. Microbiol. 2022, 12, 827717. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.-W.; Huang, H.-H.; Fan, X.; Huang, L.; Deng, J.-N.; Tu, B.; Wang, K.; Li, J.; Zhou, M.-J.; et al. NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1–infected patients. J. Clin. Investig. 2021, 131, e138861. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, X.; Harypursat, V.; Ouyang, J.; Chen, Y. The role of pyroptosis in incomplete immune reconstitution among people living with HIV: Potential therapeutic targets. Pharmacol. Res. 2023, 197, 106969. [Google Scholar] [CrossRef]
- Lupfer, C.R.; Rippee-Brooks, M.D.; Anand, P.K. Chapter Five—Common Differences: The Ability of Inflammasomes to Distinguish Between Self and Pathogen Nucleic Acids During Infection. In International Review of Cell and Molecular Biology; Vanpouille-Box, C., Galluzzi, L., Eds.; Nucleic Acid Sensing and Immunity, Part A.; Academic Press: Cambridge, MA, USA, 2019; Volume 344, pp. 139–172. [Google Scholar]
- Immunological recovery failure in cART-treated HIV-positive patients is associated with reduced thymic output and RTE CD4+ T cell death by pyroptosis. J. Leukoc. Biol. 2019, 107, 85–94. Available online: https://academic.oup.com/jleukbio/article-abstract/107/1/85/6884319?redirectedFrom=fulltext&login=false (accessed on 15 October 2025). [CrossRef]
- Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 2012, 13, 954–962. Available online: https://www.nature.com/articles/ni.2397 (accessed on 15 October 2025). [CrossRef] [PubMed]
- Xia, P.; Xing, X.-D.; Yang, C.-X.; Liao, X.-J.; Liu, F.-H.; Huang, H.-H.; Zhang, C.; Song, J.-W.; Jiao, Y.-M.; Shi, M.; et al. Activation-induced pyroptosis contributes to the loss of MAIT cells in chronic HIV-1 infected patients. Mil. Med. Res. 2022, 9, 24. [Google Scholar] [CrossRef]
- Pettersen, F.O.; Torheim, E.A.; Dahm, A.E.A.; Aaberge, I.S.; Lind, A.; Holm, M.; Aandahl, E.M.; Sandset, P.M.; Taskén, K.; Kvale, D. An Exploratory Trial of Cyclooxygenase Type 2 Inhibitor in HIV-1 Infection: Downregulated Immune Activation and Improved T Cell-Dependent Vaccine Responses. J. Virol. 2011, 85, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Z.; Zhou, J.; Li, Y.; Ning, C.; Su, Q.; Ye, L.; Ai, S.; Lai, J.; Pan, P.; et al. The altered metabolites contributed by dysbiosis of gut microbiota are associated with microbial translocation and immune activation during HIV infection. Front. Immunol. 2023, 13, 1020822. [Google Scholar] [CrossRef]
- Tuddenham, S.A.; Koay, W.L.A.; Zhao, N.; White, J.R.; Ghanem, K.G.; Sears, C.L. Impact of Human Immunodeficiency Virus Infection on Gut Microbiota α-Diversity: An Individual-level Meta-analysis. Clin. Infect. Dis. 2020, 70, 615–627. Available online: https://academic.oup.com/cid/article/70/4/615/5421718?login=false (accessed on 15 October 2025). [CrossRef] [PubMed]
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. Available online: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1004078 (accessed on 15 October 2025).
- Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Sci. Rep. 2017, 7, 6269. Available online: https://www.nature.com/articles/s41598-017-06675-1 (accessed on 15 October 2025). [CrossRef]
- Foli, Y.; Ghebremichael, M.; Li, M.; Paintsil, E. Upregulation of Apoptosis Pathway Genes in Peripheral Blood Mononuclear Cells of HIV-Infected Individuals with Antiretroviral Therapy-Associated Mitochondrial Toxicity. Antimicrob. Agents Chemother. 2017, 61, e00522-17. [Google Scholar] [CrossRef]
- Bose, E.; Paintsil, E.; Ghebremichael, M. Minimum redundancy maximal relevance gene selection of apoptosis pathway genes in peripheral blood mononuclear cells of HIV-infected patients with antiretroviral therapy-associated mitochondrial toxicity. BMC Med. Genom. 2021, 14, 285. [Google Scholar] [CrossRef]
- Lowe, D.M.; Bangani, N.; Goliath, R.; Kampmann, B.; Wilkinson, K.A.; Wilkinson, R.J.; Martineau, A.R. Effect of Antiretroviral Therapy on HIV-mediated Impairment of the Neutrophil Antimycobacterial Response. Ann. Am. Thorac. Soc. 2015, 12, 1627–1637. [Google Scholar] [CrossRef]
- Obeagu, E.I. Neutrophils in HIV pathogenesis: Dual roles, clinical implications, and therapeutic frontiers. Ann. Med. Surg. 2025, 87, 5578. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Araujo, H.F.; Guedes, M.C.S.; De Alencar, L.C.A.; Carvalho-Silva, W.H.V.; Montenegro, L.M.L.; Guimarães, R.L. The influence of extrinsic apoptosis gene expression on immunological reconstitution of male ART-treated PLHIV. BMC Infect. Dis. 2025, 25, 377. [Google Scholar] [CrossRef]
- Okine, T.; Hill, E.; Sheran, K.; Swartz, T.H. Beyond viral suppression: Decoding the mitochondrial-immune axis in HIV-associated inflammation and immune dysfunction. Front. Cell. Infect. Microbiol. 2025, 15, 1686785. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Pérez, L.; Dagenais-Lussier, X.; Mai, L.T.; Stögerer, T.; Swaminathan, S.; Isnard, S.; Rice, M.R.; Barnes, B.J.; Routy, J.-P.; van Grevenynghe, J.; et al. The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection. JCI Insight 2023, 8, e167329. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Chen, D.; Sun, Y.; Li, D.; Meng, Y.; Zhou, Q.; Zeng, F.; Deng, G.; Chen, X. Targeting regulated cell death: Apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis in anticancer immunity. J. Transl. Int. Med. 2025, 13, 10–32. [Google Scholar] [CrossRef]
- Feria, M.G.; Taborda, N.A.; Hernandez, J.C.; Rugeles, M.T. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS ONE 2018, 13, e0192845. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, K.; Ward, C.J.K.; Czubala, M.; Ferris, R.G.; Koppe, E.; Haffner, C.; Piguet, V.; Patel, V.K.; Amrine-Madsen, H.; Modis, L.K.; et al. Differential recognition of HIV-stimulated IL-1β and IL-18 secretion through NLR and NAIP signalling in monocyte-derived macrophages. PLoS Pathog. 2021, 17, e1009417. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Li, D.; Ma, Y.; Ishai, A.; Manion, M.; Nahrendorf, M.; Ganz, P.; Ridker, P.M.; Deeks, S.G.; Tawakol, A. IL-1β Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. JACC 2018, 72, 2809–2811. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Mei, X.; Zou, J.; Xiao, Q.; Ning, Q.; Xu, X.; Zhang, C.; Ji, L.; Deng, S.; Lu, B.; et al. Pyroptosis associated with immune reconstruction failure in HIV-1- infected patients receiving antiretroviral therapy: A cross-sectional study. BMC Infect. Dis. 2022, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Immune Activation and Inflammation Among People with HIV Receiving Antiretroviral Therapy; NIH: Bethesda, MD, USA, 2025. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/immune-activation-inflammation (accessed on 20 October 2025).
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “kill” into “shock and kill”: Strategies to eliminate latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, A.P.; Badley, A.D. Prime, shock and kill: BCL-2 inhibition for HIV cure. Front. Immunol. 2022, 13, 1033609. [Google Scholar] [CrossRef]
- Institute, T.D. Licence to Kill: Pro-Apoptotic Drugs in “Shock and Kill” HIV Cure Approach. Available online: https://www.doherty.edu.au/news-events/news/licence-to-kill-pro-apoptotic-drugs-in-shock-and-kill-hiv-cure-approach/ (accessed on 20 October 2025).
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef]
- Ward, A.R.; Mota, T.M.; Jones, R.B. Immunological approaches to HIV cure. Semin. Immunol. 2021, 51, 101412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisompola, D.; Mwansa, P.; Nzobokela, J.; Ameka, M.; Kirabo, A.; Hinton, A., Jr.; Masenga, S.K. HIV-Induced Apoptosis: Host Defense and Viral Strategy. Biology 2025, 14, 1680. https://doi.org/10.3390/biology14121680
Chisompola D, Mwansa P, Nzobokela J, Ameka M, Kirabo A, Hinton A Jr., Masenga SK. HIV-Induced Apoptosis: Host Defense and Viral Strategy. Biology. 2025; 14(12):1680. https://doi.org/10.3390/biology14121680
Chicago/Turabian StyleChisompola, David, Phinnoty Mwansa, John Nzobokela, Magdalene Ameka, Annet Kirabo, Antentor Hinton, Jr., and Sepiso K. Masenga. 2025. "HIV-Induced Apoptosis: Host Defense and Viral Strategy" Biology 14, no. 12: 1680. https://doi.org/10.3390/biology14121680
APA StyleChisompola, D., Mwansa, P., Nzobokela, J., Ameka, M., Kirabo, A., Hinton, A., Jr., & Masenga, S. K. (2025). HIV-Induced Apoptosis: Host Defense and Viral Strategy. Biology, 14(12), 1680. https://doi.org/10.3390/biology14121680









