Ferroptosis in Podocytes: An Emerging Focus in Kidney Diseases
Simple Summary
Abstract
1. Introduction
2. Molecular Mechanisms of Ferroptosis
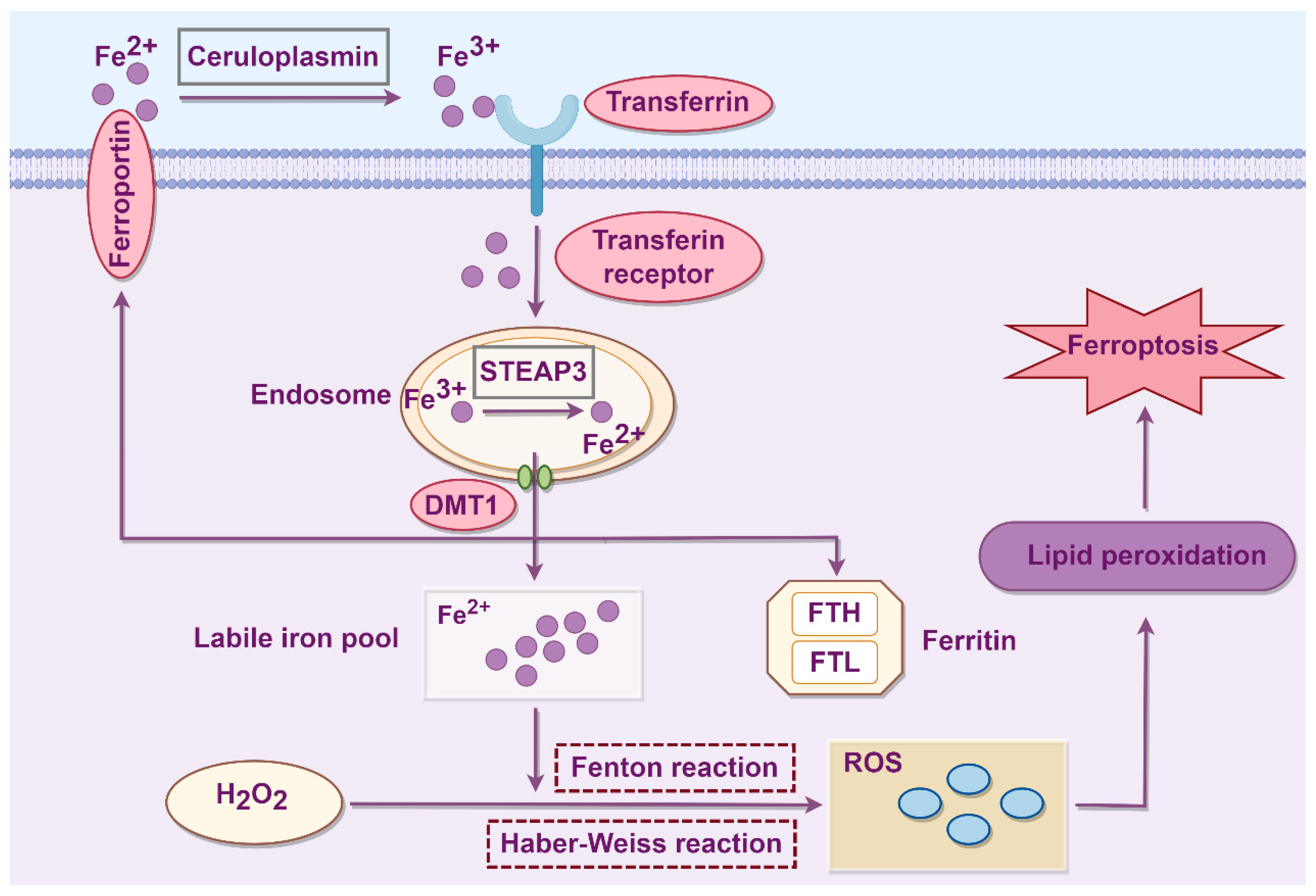
2.1. Iron Metabolism
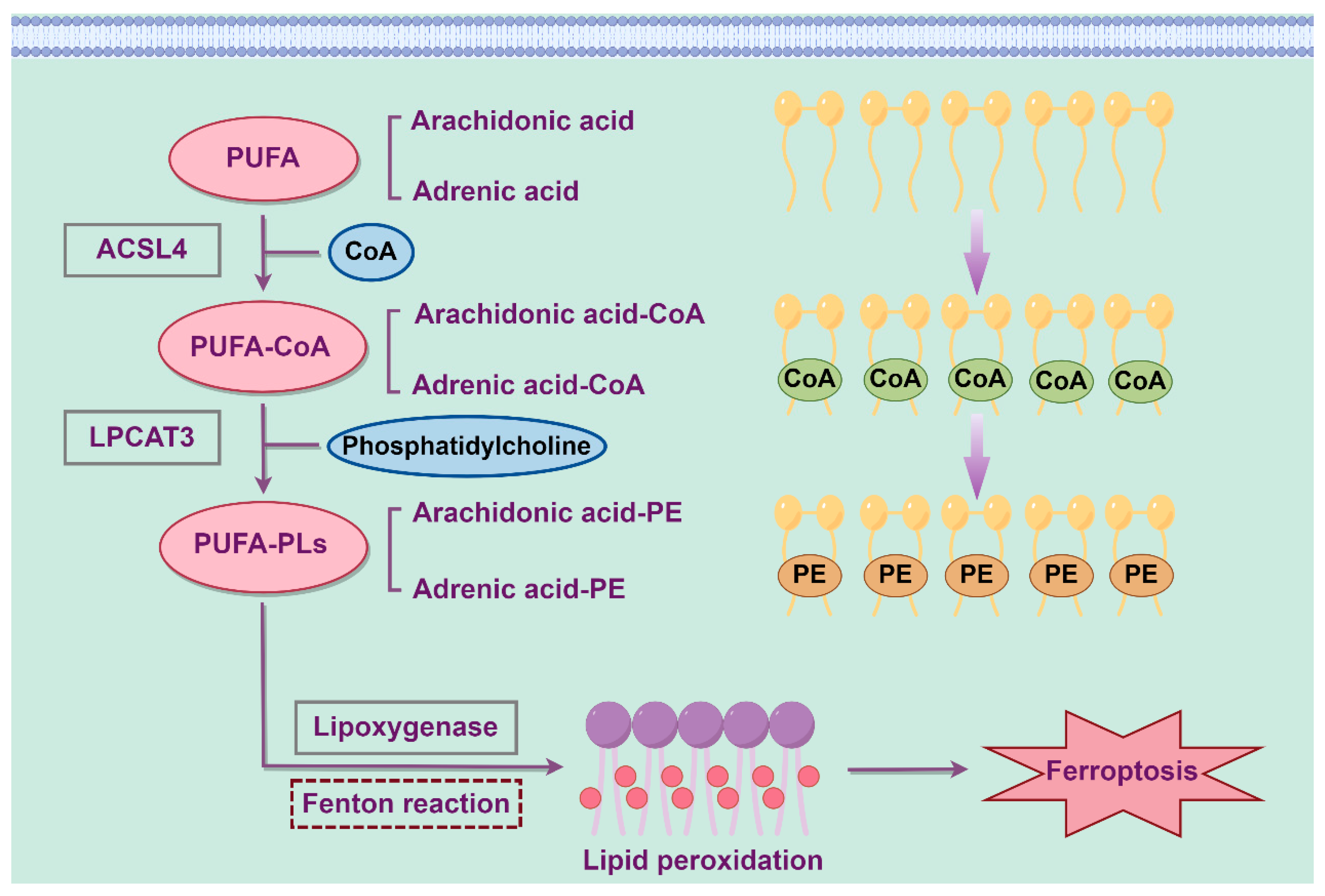
2.2. Lipid Metabolism
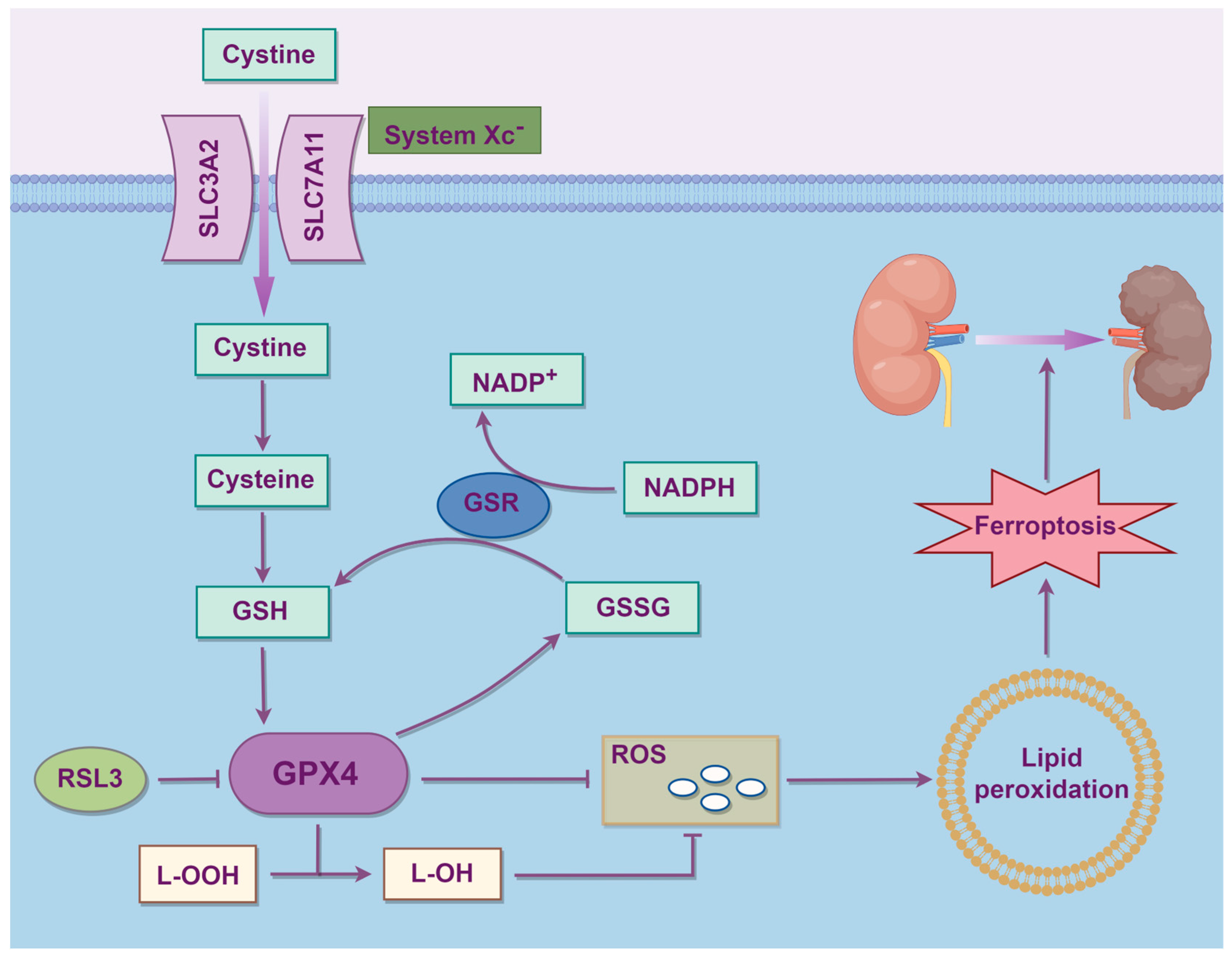
2.3. Amino Acid Metabolism
3. Podocyte Ferroptosis in Kidney Diseases
3.1. Diabetic Kidney Disease
3.2. Acute Kidney Injury
3.3. Hepatitis B Virus-Associated Glomerulonephritis
3.4. Lupus Nephritis
3.5. Focal Segmental Glomerulosclerosis
3.6. Fabry Disease
3.7. Cystinosis
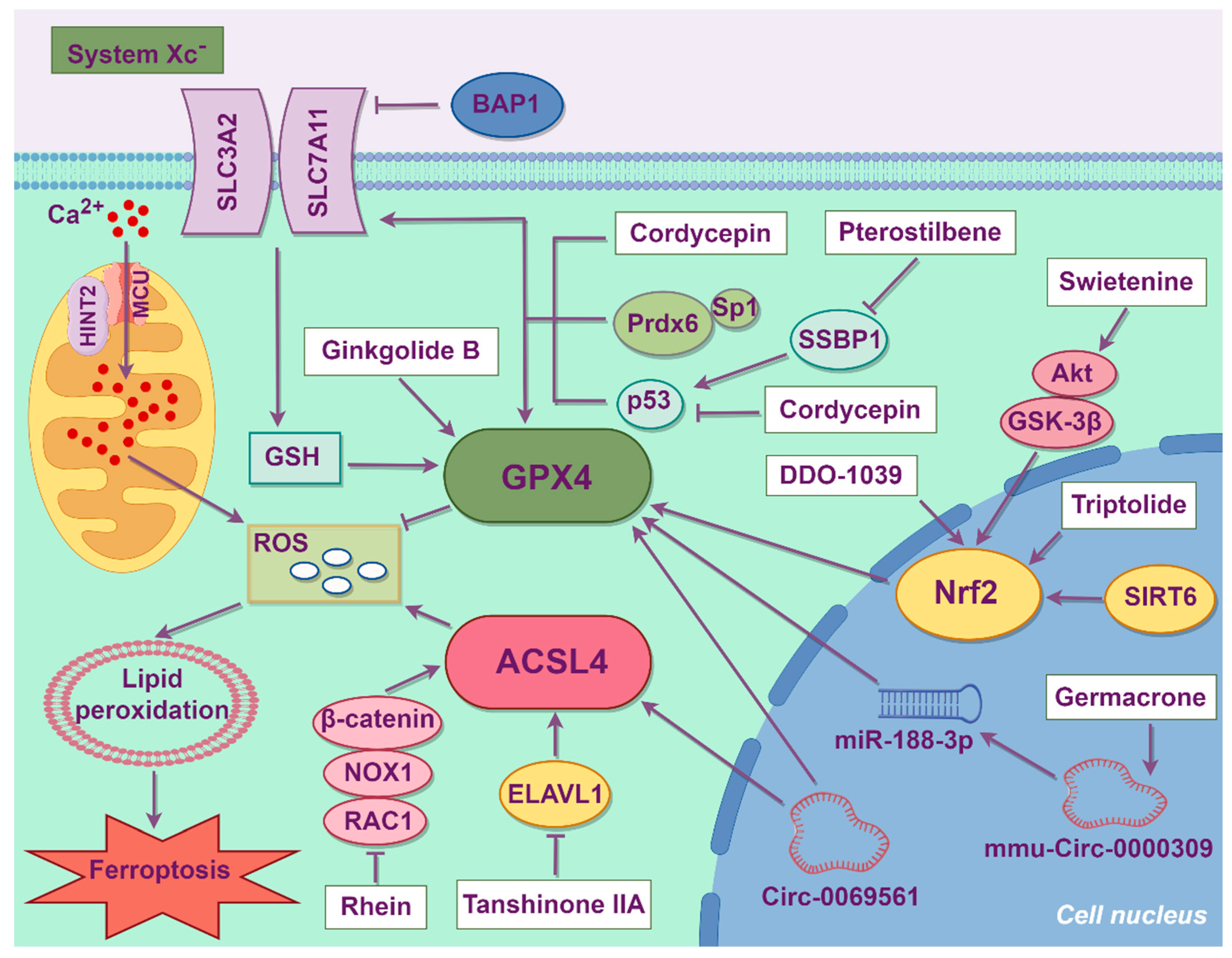
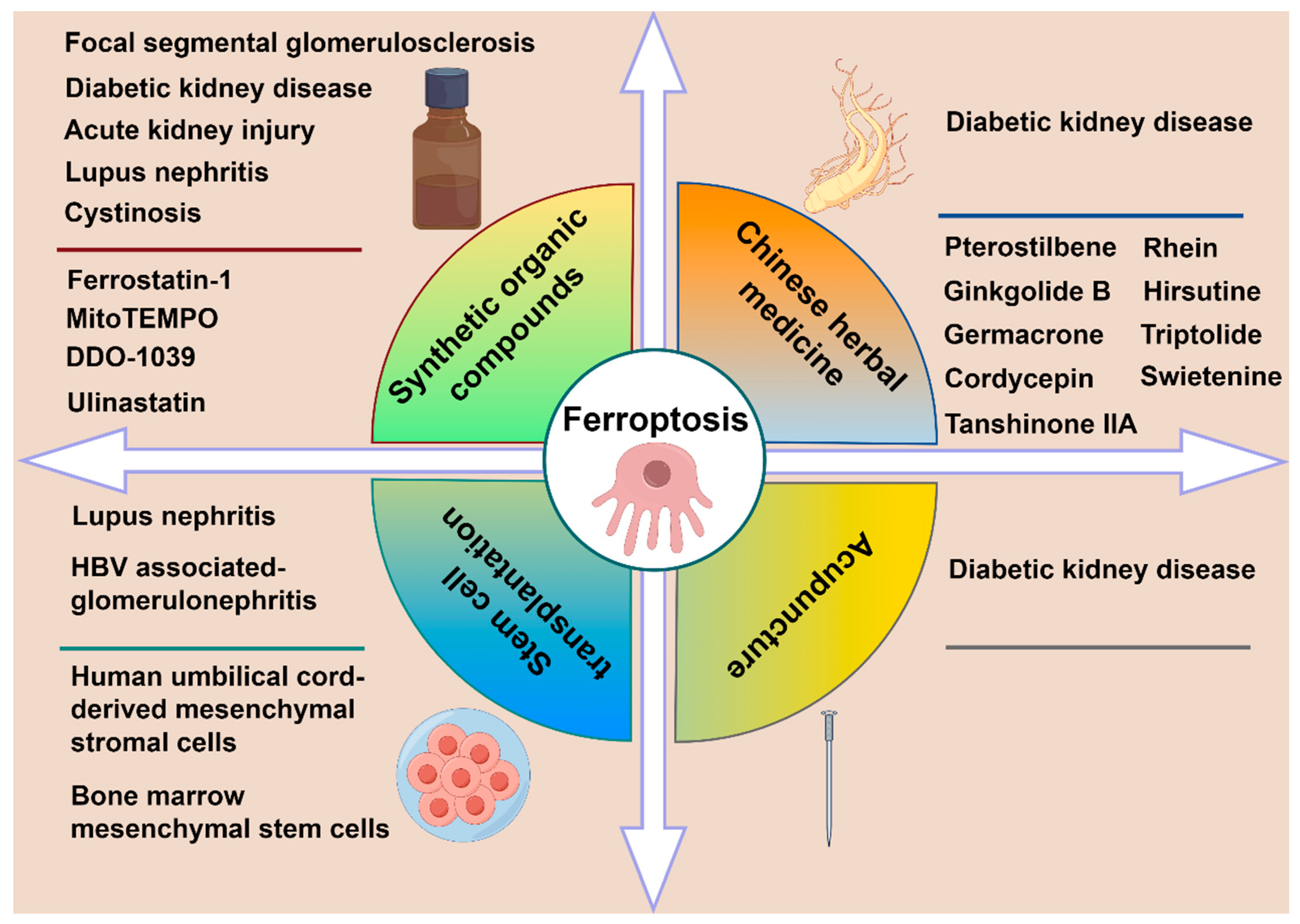
4. Treatment Strategies of Ferroptosis
4.1. Synthetic Organic Compounds
4.2. Stem Cell Transplantation
4.3. Chinese Herbal Medicine
4.4. Acupuncture
| Treatment | Medicine/Cells | Disease | Model | Target | Signaling Pathway | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Synthetic organic compounds | DDO-1039 | DKD | Mice | Nrf2 | Keap1/Nrf2 | Oxidative stress ↓ GPX4 ↑ | [64] |
| Cytochrome P450 substrate drugs | AKI | Mice | - | - | Lipid peroxyl radicals ↓ | [66] | |
| Ulinastatin | AKI | Mice/cell line | MiR-144-3p | MiR-144-3p/SLC7A11 | Fe2+, ROS ↓ | [65] | |
| Ferrostatin-1 | LN | Mice | OTUB1 | OTUB1/ SLC7A11 | Fe2+, ROS, MDA ↓ SLC7A11/GSH ↑ | [56] | |
| Ferrostatin-1 | FSGS | Mice | GPX4 | GPX4 | GSH ↑ | [58] | |
| MitoTEMPO | Cystinosis | Zebrafish | - | - | Mitochondrial function ↑ lipid peroxidation ↓ | [62] | |
| Stem cell transplantation | Bone marrow mesenchymal stem cells | HBV-GN | Human cell line | MiR-223-3p | HDAC2/STAT3 | Fe2+, ROS, MDA ↓ GPX4/SLC7A11/ACSL4 ↑ | [71] |
| Human umbilical cord-derived mesenchymal stromal cells | LN | Mice | Nrf2 | Nrf2/HO-1/GPX4 | ROS, MDA ↓ SOD, GSH ↑ | [73] | |
| Chinese herbal medicine | Hirsutine | DKD | Mice | GPX4 | p53/GPX4 | Fe2+, ROS, MDA ↓ Mitochondrial morphology | [82] |
| Triptolide | DKD | Mice | Nrf2 | Nrf2 | GPX4/FTH1/SLC7A11 ↑, mitochondrial function ↑, TFRC ↓, oxidative stress ↓ | [79] | |
| Tanshinone IIA | DKD | Mouse cell line | ELAVL1 | ELAVL1/ACSL4 | Fe2+, ROS, MDA ↓ GSH ↑ | [87] | |
| Rhein | DKD | Mice | Rac1 | Rac1/NOX1/β-catenin | Fe2+, ROS, MDA ↓ | [88] | |
| Pterostilbene | DKD | Rats | SSBP1 | DNA-PK/p53 | SLC7A11 ↑ | [85] | |
| Germacrone | DKD | Mice | mmu_circRNA_0000309 | miR-188-3p/GPX4 | GPX4, mitochondrial function ↑ | [84] | |
| Cordycepin | DKD | Mouse cell line | SLC7A11/GPX4 | - | Fe2+, ROS, MDA ↓ GSH ↑ | [86] | |
| Ginkgolide B | DKD | Mouse cell line | GPX4 | GPX4 | Fe2+, ROS, TFRC ↓ GPX4/FTH1 ↑ | [83] | |
| Swietenine | DKD | Rats | Akt | Akt/GSK-3β/Nrf2 | Fe2+, MDA, PTGS2 ↓ Oxidative stress ↓ GPX4/SLC7A11 ↑ | [89] | |
| Acupuncture | DKD | Rats | GPX4 and System Xc− | - | Oxidative stress ↓, iron homeostasis | [91] |
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AKI | Acute kidney injury |
| Akt | Protein kinase B |
| BAP1 | Brca1-associated protein 1 |
| DKD | Diabetic kidney disease |
| ELAVL1 | Embryonic lethal abnormal visual-like protein 1 |
| FSGS | Focal segmental glomerulosclerosis |
| FTH1 | Ferritin heavy chain 1 |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase 3β |
| HBV-GN | Hepatitis B virus-associated glomerulonephritis |
| HDAC2 | Histone deacetylase 2 |
| HINT2 | Histidine triad nucleotide-binding protein 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LN | Lupus nephritis |
| MCU | Mitochondrial calcium uniporter |
| MDA | Malondialdehyde |
| NOX1 | NADPH oxidase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OTUB1 | Ovarian tumor domain-containing ubiquitin aldehyde binding protein 1 |
| Prdx6 | Peroxiredoxin 6 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| ROS | Reactive oxygen species |
| SIRT6 | Sirtuin 6 |
| SLC3A2 | Solute carrier family 3 member 2 |
| SLC7A11 | Solute carrier family 7 member 11 |
| Sp1 | Specificity protein 1 |
| SSBP1 | Single-strand DNA-binding protein 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TFRC | Transferrin receptor |
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Lu, X.; Li, Z.; Wang, W.J.; Peng, K.; Liang, N.N.; Wang, Y.; Li, J.; Fu, L.; Zhao, H.; et al. Mitoquinone alleviates bleomycin-induced acute lung injury via inhibiting mitochondrial ros-dependent pulmonary epithelial ferroptosis. Int. Immunopharmacol. 2022, 113, 109359. [Google Scholar] [CrossRef]
- Wei, F.; Ruan, B.; Dong, J.; Yang, B.; Zhang, G.; Yeung, W.K.K.; Wang, H.; Cao, W.; Wang, Y. Asperosaponin vi inhibition of dnmt alleviates gpx4 suppression-mediated osteoblast ferroptosis and diabetic osteoporosis. J. Adv. Res. 2024, 75, 331–344. [Google Scholar] [CrossRef]
- Nan, P.; Wang, X.; Li, A.; Ge, Y.; Gu, Z.; Wang, Y.; Tao, R. Tspan15 sustains itgb1 stability to block gemcitabine-induced ferroptosis in pancreatic ductal adenocarcinoma through the fak/akt/mtor-gpx4 cascade. Redox Biol. 2025, 85, 103721. [Google Scholar] [CrossRef]
- Zha, X.; Liu, X.; Wei, M.; Huang, H.; Cao, J.; Liu, S.; Bian, X.; Zhang, Y.; Xiao, F.; Xie, Y.; et al. Microbiota-derived lysophosphatidylcholine alleviates alzheimer’s disease pathology via suppressing ferroptosis. Cell Metab. 2025, 37, 169–186. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, M.Y.; Mao, C.H.; Wang, X.; Xu, Y.Y.; Qian, X.J.; Ma, C.; Qiu, W.Y.; Zhu, Y.C. Structural changes in cerebral microvasculature induced by ferroptosis contribute to blood-brain barrier disruption in alzheimer’s disease: An autopsy study. Alzheimers Dement. 2025, 21, e70103. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Chen, S.; Zhang, J.; Cai, L.; Liu, C.; Zhang, Y.; Wu, X.; Li, N.; Ma, Z.; et al. Sting aggravates ferroptosis-dependent myocardial ischemia-reperfusion injury by targeting gpx4 for autophagic degradation. Signal Transduct. Target. Ther. 2025, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Tan, X.; Wang, T.; Shi, Y.; Xu, X.; Du, J.; Yu, Z.; Song, B. Targeting pim1 by bruceine d attenuates skin fibrosis via myofibroblast ferroptosis. Redox Biol. 2025, 82, 103619. [Google Scholar] [CrossRef]
- Wei, W.; Yang, L.; Wang, B.; Tang, L.; Li, J.; Liu, C.; Huang, Y.; Zhang, Z.; Zhang, D.; Zhang, L.; et al. Remote ischemic preconditioning attenuates mitochondrial dysfunction and ferroptosis of tubular epithelial cells by inhibiting nox4-ros signaling in acute kidney injury. Int. J. Biol. Sci. 2025, 21, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhou, S.; Wu, W.; Lin, Y.; Wang, T.; Sun, H.; A-Ni-Wan, A.S.; Li, Y.; Wang, C.; Li, X.; et al. Glp-1 receptor agonists alleviate diabetic kidney injury via beta-klotho-mediated ferroptosis inhibition. Adv. Sci. 2025, 12, e2409781. [Google Scholar] [CrossRef]
- Cheng, Q.; Mou, L.; Su, W.; Chen, X.; Zhang, T.; Xie, Y.; Xue, J.; Lee, P.Y.; Wu, H.; Du, Y. Ferroptosis of cd163+ tissue-infiltrating macrophages and cd10+ pc+ epithelial cells in lupus nephritis. Front. Immunol. 2023, 14, 1171318. [Google Scholar] [CrossRef] [PubMed]
- Helleux, A.; Davidson, G.; Lallement, A.; Hourani, F.A.; Haller, A.; Michel, I.; Fadloun, A.; Thibault-Carpentier, C.; Su, X.; Lindner, V.; et al. Tfe3 fusions drive oxidative metabolism and ferroptosis resistance in translocation renal cell carcinoma. EMBO Mol. Med. 2025, 17, 1041–1070. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, J.; Wu, J.; Mo, Z.; Ye, L.; Zhong, W.; Zhang, Y.; Lai, H.; Zhang, Y.; Qiu, J.; et al. Bioprinted mesenchymal stem cell microfiber-derived extracellular vesicles alleviate unilateral renal ischemia-reperfusion injury and fibrosis by inhibiting tubular epithelial cells ferroptosis. Bioact. Mater. 2024, 40, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Peng, Z.; Hu, H.; Cui, X.; Zhu, Z.; Qi, Y.; Chen, W.; Liu, H.; Liang, W.; et al. Piezo1-mediated calcium signaling and podocyte injury in diabetic kidney disease. J. Am. Soc. Nephrol. 2025, 36, 1310–1326. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.; Xiang, Y.; Mao, C.; Wang, Y.; Huang, J.; Liu, X.; Wang, C.; Liu, H.; Li, Z.; et al. A smart hydrogel dressing balances reactive oxygen species levels for effective treatment of bacteria-infected atopic dermatitis. Adv. Healthc. Mater. 2025, 14, e2501142. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Tian, Y.; Xu, X.; Wang, B.; Huang, Z.; Lou, S.; Kang, J.; Zhang, N.; Weng, J.; et al. Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice. Redox Biol. 2024, 73, 103195. [Google Scholar] [CrossRef]
- Zhong, P.; Li, L.; Feng, X.; Teng, C.; Cai, W.; Zheng, W.; Wei, J.; Li, X.; He, Y.; Chen, B.; et al. Neuronal ferroptosis and ferroptosis-mediated endoplasmic reticulum stress: Implications in cognitive dysfunction induced by chronic intermittent hypoxia in mice. Int. Immunopharmacol. 2024, 138, 112579. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Cao, J.; Wang, F.; Han, J.R.; Wu, T.W.; Li, L.; Yu, J.; Fan, Y.; Xie, G.; et al. Acsl4 and polyunsaturated lipids support metastatic extravasation and colonization. Cell 2025, 188, 412–429. [Google Scholar] [CrossRef]
- Samovich, S.N.; Mikulska-Ruminska, K.; Dar, H.H.; Tyurina, Y.Y.; Tyurin, V.A.; Souryavong, A.B.; Kapralov, A.A.; Amoscato, A.A.; Beharier, O.; Karumanchi, S.A.; et al. Strikingly high activity of 15-lipoxygenase towards di-polyunsaturated arachidonoyl/adrenoyl-phosphatidylethanolamines generates peroxidation signals of ferroptotic cell death. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314710. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. Acsl4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Huang, Q.; Ru, Y.; Luo, Y.; Luo, X.; Liu, D.; Ma, Y.; Zhou, X.; Linghu, M.; Xu, W.; Gao, F.; et al. Identification of a targeted acsl4 inhibitor to treat ferroptosis-related diseases. Sci. Adv. 2024, 10, k1200. [Google Scholar] [CrossRef] [PubMed]
- Shahtout, J.L.; Eshima, H.; Ferrara, P.J.; Maschek, J.A.; Cox, J.E.; Drummond, M.J.; Funai, K. Inhibition of the skeletal muscle lands cycle ameliorates weakness induced by physical inactivity. J. Cachexia Sarcopenia Muscle 2024, 15, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. Lpcat3 is transcriptionally regulated by yap/zeb/ep300 and collaborates with acsl4 and yap to determine ferroptosis sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef]
- Du, Y.; Guo, Z. Recent progress in ferroptosis: Inducers and inhibitors. Cell Death Discov. 2022, 8, 501. [Google Scholar] [CrossRef]
- Ye, Z.; Cheng, M.; Lian, W.; Leng, Y.; Qin, X.; Wang, Y.; Zhou, P.; Liu, X.; Peng, T.; Wang, R.; et al. Gpx4 deficiency-induced ferroptosis drives endometrial epithelial fibrosis in polycystic ovary syndrome. Redox Biol. 2025, 83, 103615. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, Y.; Huang, Z.; Wang, Y.; Wan, W.; Zou, H.; Wang, B.; Qiu, X.; Yang, X. Upregulation of gpx4 drives ferroptosis resistance in scleroderma skin fibroblasts. Free. Radic. Biol. Med. 2024, 221, 23–30. [Google Scholar] [CrossRef]
- Petrica, L. Special issue ijms-molecular mechanisms of diabetic kidney disease. Int. J. Mol. Sci. 2024, 25, 790. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Z.; Ma, Y.; Yang, X.; Zhu, Z.; Zhang, Z.; Hu, J.; Liang, W.; Ding, G. Akap1 contributes to impaired mtdna replication and mitochondrial dysfunction in podocytes of diabetic kidney disease. Int. J. Biol. Sci. 2022, 18, 4026–4042. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of sglt2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Peiyao, R.; Xueli, M.; Wenbo, S.; Danna, Z.; Jianguang, G.; Juan, J.; Qiang, H. High glucose induces podocyte ferroptosis through bap1/slc7a11 pathway. Heliyon 2025, 11, e40590. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.B.; Hong, Y.C.; Zhu, H.; He, Q.J.; Yang, B.; Ying, M.D.; Cao, J. Identification of prdx6 as a regulator of ferroptosis. Acta Pharmacol. Sin. 2019, 40, 1334–1342. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Hu, J.E.; Ding, Y.; Shen, Y.; Xu, H.; Chen, H.; Wu, N. Sp1-mediated upregulation of prdx6 expression prevents podocyte injury in diabetic nephropathy via mitigation of oxidative stress and ferroptosis. Life Sci. 2021, 278, 119529. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Zhu, S.; Wang, Y.; Ma, Y.; Hu, Z.; Wu, Y.; Jiang, L. Circ-0069561 as a novel diagnostic biomarker for progression of diabetic kidney disease. Ren. Fail. 2025, 47, 2490200. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Hao, J.L.; Li, L.; Ruan, Y.T.; An, X.N.; Huang, Q.L.; Dong, X.M.; Gao, P. Vstm2l protects prostate cancer cells against ferroptosis via inhibiting vdac1 oligomerization and maintaining mitochondria homeostasis. Nat. Commun. 2025, 16, 1160. [Google Scholar] [CrossRef]
- Yang, X.; Feng, J.; Liang, W.; Zhu, Z.; Chen, Z.; Hu, J.; Yang, D.; Ding, G. Roles of sirt6 in kidney disease: A novel therapeutic target. Cell. Mol. Life Sci. 2021, 79, 53. [Google Scholar] [CrossRef]
- Hao, Y.; Hu, J.; Zhang, Z.; Guan, Q.; Wang, J.; Tao, Y.; Cheng, J.; Fan, Y. Sirt6 deficiency exacerbates angiotensin ii-induced lipid nephrotoxicity by affecting pld6-derived cardiolipin metabolism in podocytes. Cell. Signal. 2025, 133, 111858. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Guo, C.; Zeng, S.; Yu, K.; Liu, M.; Li, Y. Sirt6 overexpression relieves ferroptosis and delays the progression of diabetic nephropathy via nrf2/gpx4 pathway. Ren. Fail. 2024, 46, 2377785. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Liu, M.; Chen, Y.; Wu, Y.; Li, Q.; Ma, T.; Gao, J.; Xia, Y.; Fan, M.; et al. Protective effect of hint2 on mitochondrial function via repressing mcu complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res. Cardiol. 2021, 116, 65. [Google Scholar] [CrossRef]
- Bai, M.; Lu, W.; Tan, J.; Mei, X. Hint2 may be one clinical significance target for patient with diabetes mellitus and reduced ros-induced oxidative stress and ferroptosis by mcu. Horm. Metab. Res. 2024, 56, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.; Roy, P.K.; Das, A.; Mukherjee, B.; Mandal, M. Glucose-6-phosphate dehydrogenase (g6pd) shields pancreatic cancer from autophagy-dependent ferroptosis by suppressing redox imbalance induced ampk/mtor signaling. Free. Radic. Biol. Med. 2025, 237, 195–209. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, Y.; Li, Y.; Bi, L.; Wang, L.; Chen, Q.; Lin, Y.; Jin, H.; Xu, X.; Peng, R.; et al. Snp alleviates mitochondrial homeostasis dysregulation-mediated developmental toxicity in diabetic zebrafish larvae. Biomed. Pharmacother. 2024, 177, 117117. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, X.; Shi, J.; An, J.; Yu, T.; Zou, G.; Li, W.; Zhuo, L. Unraveling the interplay of ferroptosis and immune dysregulation in diabetic kidney disease: A comprehensive molecular analysis. Diabetol. Metab. Syndr. 2024, 16, 86. [Google Scholar] [CrossRef]
- Baker, M.L.; Cantley, L.G. Adding insult to injury: The spectrum of tubulointerstitial responses in acute kidney injury. J. Clin. Investig. 2025, 135, e188358. [Google Scholar] [CrossRef]
- Wu, S.; Guo, M.; Wang, Y.; Zhou, Y.; Zhang, L.; Zhou, Y.; Xing, Y.; Sun, D.; Hu, X.; Ruan, Z.; et al. Relationship between podocyte injury and renal outcomes in patients with acute kidney injury: A report from a retrospective study in china. Am. J. Nephrol. 2025, 56, 595–604. [Google Scholar] [CrossRef]
- Gong, Q.; Lai, T.; Liang, L.; Jiang, Y.; Liu, F. Targeted inhibition of cx3cl1 limits podocytes ferroptosis to ameliorate cisplatin-induced acute kidney injury. Mol. Med. 2023, 29, 140. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, J.; Shi, M.; Huang, J.; Xu, Z. Ciapin1 attenuates ferroptosis via regulating pi3k/akt pathway in lps-induced podocytes. BMC Nephrol. 2025, 26, 201. [Google Scholar] [CrossRef] [PubMed]
- Yau, A.A.; Murugapandian, S.; Rizvi, A.W.; Gaddy, A. Viral nephropathies: Core curriculum 2024. Am. J. Kidney Dis. 2024, 84, 767–779. [Google Scholar] [CrossRef]
- Yu, P.; Jin, X.; Huang, W.; Wang, J.; Zhang, S.; Ren, L.; Zhang, H.; Shi, S. Characterization of immortalized human podocytes infected with lentivirus as an in vitro model of viral infection-associated podocytopathy. Am. J. Clin. Exp. Immunol. 2024, 13, 204–214. [Google Scholar] [CrossRef]
- Yang, Y.T.; Wang, X.; Zhang, Y.Y.; Yuan, W.J. The histone demethylase lsd1 promotes renal inflammation by mediating tlr4 signaling in hepatitis b virus-associated glomerulonephritis. Cell Death Dis. 2019, 10, 278. [Google Scholar] [CrossRef]
- Bian, L.; Niu, Y.; Yuan, W.; Du, H.; Yang, Y. Hbx promotes glomerular podocyte-induced immune cell responses. Ren. Fail. 2024, 46, 2373276. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Yang, W.; Guo, W.; Wan, X.; Li, J.; Zhang, Y.; Wang, B.; Liang, X.; Bai, O. Hbx induces chemoresistance in diffuse large b cell lymphoma by inhibiting intrinsic apoptosis via the nf-kappab/xiap pathway. Mol. Ther. Nucleic Acids 2024, 35, 102346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wan, S.; Luo, Y.; Liu, H.; Jiang, J.; Guo, Y.; Xiao, J.; Wu, B. Hepatitis b virus x protein induces aldh2 ubiquitin-dependent degradation to enhance alcoholic steatohepatitis. Gastroenterol. Rep. 2023, 11, d6. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Li, B.; Chen, Y.; Feng, M.; Hu, Y.; Jiang, W. Bone marrow mesenchymal stem cell-derived exosomes protect podocytes from hbx-induced ferroptosis. PeerJ 2023, 11, e15314. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, Z.; Zhang, M.; Li, H.; Lu, K.; Yang, C.; Li, M.; Liu, S. Antagonising yin yang 1 ameliorates the symptoms of lupus nephritis via modulating t lymphocyte signaling. Pharmacol. Res. 2024, 210, 107525. [Google Scholar] [CrossRef]
- Bhargava, R.; Upadhyay, R.; Zhao, C.; Katakam, P.; Wenderfer, S.; Chen, J.; He, H.; Cummings, R.; Tsokos, M.G.; Tsokos, G.C. Aberrant glycosylation of igg in children with active lupus nephritis alters podocyte metabolism and causes podocyte injury. Arthritis Rheumatol. 2025, 77, 1421–1432. [Google Scholar] [CrossRef]
- Liu, C.; Gan, Y.H.; Yong, W.J.; Xu, H.D.; Li, Y.C.; Hu, H.M.; Zhao, Z.Z.; Qi, Y.Y. Otub1 regulation of ferroptosis and the protective role of ferrostatin-1 in lupus nephritis. Cell Death Dis. 2024, 15, 791. [Google Scholar] [CrossRef]
- Schindler, M.; Siegerist, F.; Lange, T.; Simm, S.; Bach, S.M.; Klawitter, M.; Gehrig, J.; Gul, S.; Endlich, N. A novel high-content screening assay identified belinostat as protective in a fsgs-like zebrafish model. J. Am. Soc. Nephrol. 2023, 34, 1977–1990. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wang, M.; Zhang, P.; Wang, R.; Ji, D.; Gao, C.; Xia, Z. Targeting ferroptosis attenuates podocytes injury and delays tubulointerstitial fibrosis in focal segmental glomerulosclerosis. Biochem. Biophys. Res. Commun. 2023, 678, 11–16. [Google Scholar] [CrossRef]
- Braun, F.; Abed, A.; Sellung, D.; Rogg, M.; Woidy, M.; Eikrem, O.; Wanner, N.; Gambardella, J.; Laufer, S.D.; Haas, F.; et al. Accumulation of alpha-synuclein mediates podocyte injury in fabry nephropathy. J. Clin. Investig. 2023, 133, e157782. [Google Scholar] [CrossRef]
- Wise, A.F.; Krisnadevi, I.A.; Bruell, S.; Lee, H.C.; Bhuvan, T.; Kassianos, A.J.; Saini, S.; Wang, X.; Healy, H.G.; Qian, E.L.; et al. Fabry disease podocytes reveal ferroptosis as a potential regulator of cell pathology. Kidney Int. Rep. 2025, 10, 535–548. [Google Scholar] [CrossRef]
- Bellomo, F.; Pugliese, S.; Cairoli, S.; Krohn, P.; De Stefanis, C.; Raso, R.; Rega, L.R.; Taranta, A.; De Leo, E.; Ciolfi, A.; et al. Ketogenic diet and progression of kidney disease in animal models of nephropathic cystinosis. J. Am. Soc. Nephrol. 2024, 35, 1493–1506. [Google Scholar] [CrossRef]
- Berlingerio, S.P.; Bondue, T.; Tassinari, S.; Siegerist, F.; Ferrulli, A.; Lismont, C.; Cairoli, S.; Goffredo, B.M.; Ghesquiere, B.; Fransen, M.; et al. Targeting oxidative stress-induced lipid peroxidation enhances podocyte function in cystinosis. J. Transl. Med. 2025, 23, 206. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, G.; Sun, W.; Wu, C.; Yang, Q.; Xue, L.; Ye, S. Empagliflozin alleviates type 2 diabetic renal fibrosis by inhibiting slc7a7-mediated ferroptosis. Diabetol. Metab. Syndr. 2025, 17, 329. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, X.; Wang, X.; Zhu, X.; Wang, Z.; Jiang, Z.; Bao, H.; Chen, Z. Nuclear factor erythroid 2-related factor 2 activator ddo-1039 ameliorates podocyte injury in diabetic kidney disease via suppressing oxidative stress, inflammation, and ferroptosis. Antioxid. Redox Signal. 2025, 42, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, N. Ulinastatin ameliorates podocyte ferroptosis via regulating mir-144-3p/slc7a11 axis in acute kidney injury. Vitr. Cell Dev. Biol. Anim. 2023, 59, 697–705. [Google Scholar] [CrossRef]
- Mishima, E.; Sato, E.; Ito, J.; Yamada, K.I.; Suzuki, C.; Oikawa, Y.; Matsuhashi, T.; Kikuchi, K.; Toyohara, T.; Suzuki, T.; et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including aki, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 2020, 31, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, H.; Jia, H.; Wei, X.; Xue, J.; Li, W.; Zhang, J.; Wang, Y.; Yan, J.; Sun, H.; et al. Ferrostatin-1 reduces the inflammatory response of rheumatoid arthritis by decreasing the antigen presenting function of fibroblast-like synoviocytes. J. Transl. Med. 2025, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- Araoka, T.; Toyohara, K.; Ryosaka, M.; Inui, C.; Matsuura, M.; Ma, C.; Watahiki, J.; Li, Z.; Iwasaki, M.; Watanabe, A.; et al. Human ipsc-derived nephron progenitor cells treat acute kidney injury and chronic kidney disease in mouse models. Sci. Transl. Med. 2025, 17, t5553. [Google Scholar] [CrossRef]
- Campa-Carranza, J.N.; Capuani, S.; Joubert, A.L.; Hernandez, N.; Bo, T.; Sauceda-Villanueva, O.I.; Conte, M.; Franco, L.; Farina, M.; Rome, G.E.; et al. Immune and angiogenic profiling of mesenchymal stem cell functions in a subcutaneous microenvironment for allogeneic islet transplantation. Adv. Sci. 2025, 12, e2411574. [Google Scholar] [CrossRef]
- Huang, L.L.; Hou, Y.Y.; Yang, J.; Liao, X.N.; Ma, J.S.; Wang, W.C.; Quan, Y.X.; Jiang, H.Y.; Bai, Y.H. Mitigation of ferroptosis in diabetic kidney disease through mesenchymal stem cell intervention via the smad2/3/mettl3/s1pr1 axis. FASEB J. 2025, 39, e70714. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Feng, M.; Yu, Y.; Hu, Y.; Jiang, W. Exosomal mir-223-3p from bone marrow mesenchymal stem cells targets hdac2 to downregulate stat3 phosphorylation to alleviate hbx-induced ferroptosis in podocytes. Front. Pharmacol. 2024, 15, 1327149. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, L.; Tang, X.; Feng, R.; Yao, G.; Chen, W.; Li, W.; Feng, X.; Chen, H.; Sun, L. Mesenchymal stem cells prevent podocyte injury in lupus-prone b6.mrl-faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol. Dial. Transpl. 2019, 34, 597–605. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Wang, Y.; Yu, H.; Li, Q.; Zheng, Y.; Fu, Y.; Yao, G.; Sun, L. Mesenchymal stromal cells reduce ferroptosis of podocytes by activating the nrf2/ho-1/gpx4 pathway in lupus nephritis. Int. Immunopharmacol. 2025, 153, 114537. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lu, J.; Qian, H.; Xia, Z.; Mo, X.; An, M.; Yang, W.; Wang, S.; Che, D.; Wang, C.; et al. Immobilized protein strategies based on cell membrane chromatography and its application in discovering active and toxic substances in traditional Chinese medicine. Pharmacol. Res. 2024, 210, 107492. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.; Gao, R.; Yin, M.; Huang, F. Traditional Chinese medicine formulae and Chinese patent medicines for the treatment of diabetic kidney disease: Efficacies and mechanisms. Am. J. Chin. Med. 2025, 53, 675–707. [Google Scholar] [CrossRef]
- Gao, K.; Liu, Y.; Li, K.; Liu, L.; Cai, Y.; Zhang, X.; Zhao, Z. Nrf2-mediated ferroptosis is involved in berberine-induced alleviation of diabetic kidney disease. Phytother. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Y.; Song, J.; Wang, S.; Lu, J.; Wei, F.; Li, X. Urinary exosomes exacerbate diabetic kidney disease by promoting nlrp3 inflammasome activation via the microrna-516b-5p/sirt3/ampk pathway. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E911–E923. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Guo, Y.; Huang, M.; Liu, P.; Aikemu, A.; Mohammadtursun, N.; Pan, X.; Yang, X. Nuciferine restores autophagy via the pi3k-akt-mtor pathway to alleviate renal fibrosis in diabetic kidney disease. J. Agric. Food Chem. 2025, 73, 5223–5235. [Google Scholar] [CrossRef]
- Wang, H.Q.; Wu, H.X.; Shi, W.Q.; Yang, Y.; Lin, M.; Wang, K.; Bian, C.C.; An, X.F.; Wang, T.; Yan, M. Triptolide attenuates renal slit diagram to tight junction transition in diabetic kidney disease by regulating nrf2-ferroptosis pathway. Am. J. Chin. Med. 2024, 52, 2161–2185. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Wilairatana, P.; Ferdous, J.; Chowdhury, R.; Bappi, M.H.; Rahman, M.A.; Mubarak, M.S.; Islam, M.T. Hirsutine, an emerging natural product with promising therapeutic benefits: A systematic review. Molecules 2023, 28, 6141. [Google Scholar] [CrossRef]
- Hu, W.; Li, M.; Sun, W.; Li, Q.; Xi, H.; Qiu, Y.; Wang, R.; Ding, Q.; Wang, Z.; Yu, Y.; et al. Hirsutine ameliorates hepatic and cardiac insulin resistance in high-fat diet-induced diabetic mice and in vitro models. Pharmacol. Res. 2022, 177, 105917. [Google Scholar] [CrossRef]
- Pei, Z.; Chen, Y.; Zhang, Y.; Zhang, S.; Wen, Z.; Chang, R.; Ni, B.; Ni, Q. Hirsutine mitigates ferroptosis in podocytes of diabetic kidney disease by downregulating the p53/gpx4 signaling pathway. Eur. J. Pharmacol. 2025, 991, 177289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ou, Z.; Gao, T.; Yang, Y.; Shu, A.; Xu, H.; Chen, Y.; Lv, Z. Ginkgolide b alleviates oxidative stress and ferroptosis by inhibiting gpx4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 2022, 156, 113953. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, Y.; Zheng, D.; Liang, M.; He, Q. A novel identified circular rna, mmu_mmu_circrna_0000309, involves in germacrone-mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting mir-188-3p/gpx4 signaling axis. Antioxid. Redox Signal. 2022, 36, 740–759. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wang, Z.X.; Li, T.S.; Ding, X.Q.; Liu, Z.H.; Yang, J.; Fang, L.; Kong, L.D. Ssbp1 drives high fructose-induced glomerular podocyte ferroptosis via activating dna-pk/p53 pathway. Redox Biol. 2022, 52, 102303. [Google Scholar] [CrossRef]
- Wu, B.; Wang, J.; Yan, X.; Jin, G.; Wang, Q. Cordycepin ameliorates diabetic nephropathy injury by activating the slc7a11/gpx4 pathway. J. Diabetes Investig. 2025, 16, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Kang, Z.; Zhang, F. Tanshinone iia suppresses ferroptosis to attenuate renal podocyte injury in diabetic nephropathy through the embryonic lethal abnormal visual-like protein 1 and acyl-coenzyme a synthetase long-chain family member 4 signaling pathway. J. Diabetes Investig. 2024, 15, 1003–1016. [Google Scholar] [CrossRef]
- Xiong, D.; Hu, W.; Han, X.; Cai, Y. Rhein inhibited ferroptosis and emt to attenuate diabetic nephropathy by regulating the rac1/nox1/beta-catenin axis. Front. Biosci. 2023, 28, 100. [Google Scholar] [CrossRef]
- Duan, J.; Pei, F.; Miao, J.; Liu, S.; Tan, L.; Lu, M.; Liu, Y.; Zhang, C. Swietenine improved the progression of diabetic nephropathy through inhibiting ferroptosis via activating akt/gsk-3beta/nrf2 signaling pathway. J. Ethnopharmacol. 2025, 349, 119981. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Bai, Y.; Huang, L.; Zhong, Y.; Li, Y. Therapeutic effects of acupuncture therapy for kidney function and common symptoms in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2024, 46, 2301504. [Google Scholar] [CrossRef]
- Yue, J.I.; Xin-Yuan, Z.; Yun-Ming, X.; Zi-Hao, Z.; Xiao-Hui, Y.; Xin-Ju, L.I. Acupuncture improve proteinuria in diabetic kidney disease rats by inhibiting ferroptosis and epithelial-mesenchymal transition. Heliyon 2024, 10, e33675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Q.; Xia, C.; Zheng, H.; Jiang, W.; Wang, Y.; Sun, W. Qing-re-xiao-zheng-(yi-qi) formula attenuates the renal podocyte ferroptosis in diabetic kidney disease through ampk pathway. J. Ethnopharmacol. 2025, 351, 120157. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, Y.; Li, D.; Liang, Y. Single-cell multi-omics analysis reveals the mechanism of action of a novel antioxidant polyphenol nanoparticle loaded with stat3 agonist in mediating cardiomyocyte ferroptosis to ameliorate age-related heart failure. J. Nanobiotechnol. 2025, 23, 258. [Google Scholar]
- Lange, T.; Maron, L.; Weber, C.; Biedenweg, D.; Schluter, R.; Endlich, N. Efficient delivery of small rnas to podocytes in vitro by direct exosome transfection. J. Nanobiotechnol. 2025, 23, 373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Ma, Y.; Zhang, C. Ferroptosis in Podocytes: An Emerging Focus in Kidney Diseases. Biology 2025, 14, 1679. https://doi.org/10.3390/biology14121679
Feng J, Ma Y, Zhang C. Ferroptosis in Podocytes: An Emerging Focus in Kidney Diseases. Biology. 2025; 14(12):1679. https://doi.org/10.3390/biology14121679
Chicago/Turabian StyleFeng, Jun, Yiqiong Ma, and Chunyun Zhang. 2025. "Ferroptosis in Podocytes: An Emerging Focus in Kidney Diseases" Biology 14, no. 12: 1679. https://doi.org/10.3390/biology14121679
APA StyleFeng, J., Ma, Y., & Zhang, C. (2025). Ferroptosis in Podocytes: An Emerging Focus in Kidney Diseases. Biology, 14(12), 1679. https://doi.org/10.3390/biology14121679







