The Mutation of myomiR miR499 Impacts the Intermuscular Bones in Zebrafish
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and Generation of miR499 Mutant Lines
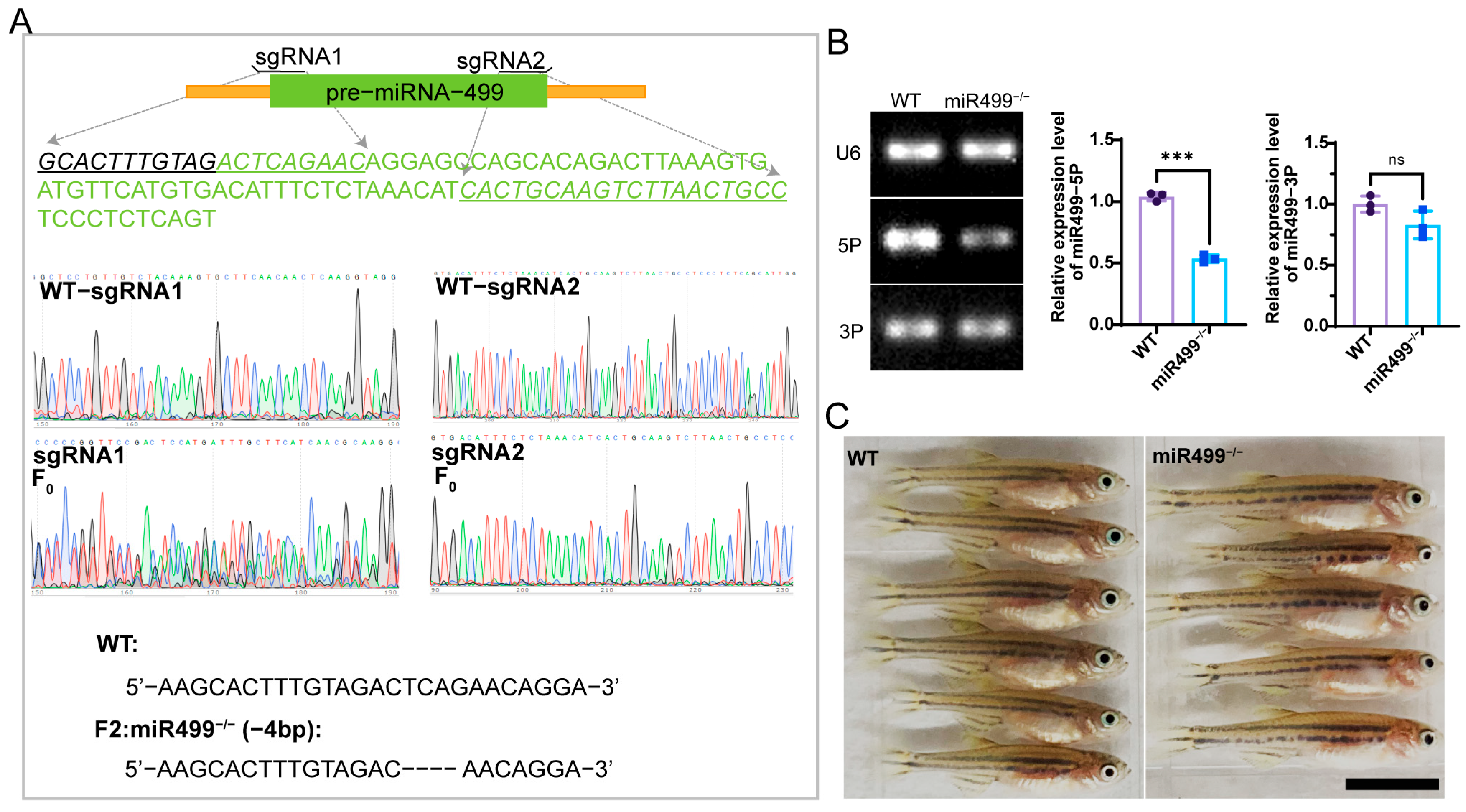
2.2. Analysis of miR499 Knockout Efficiency
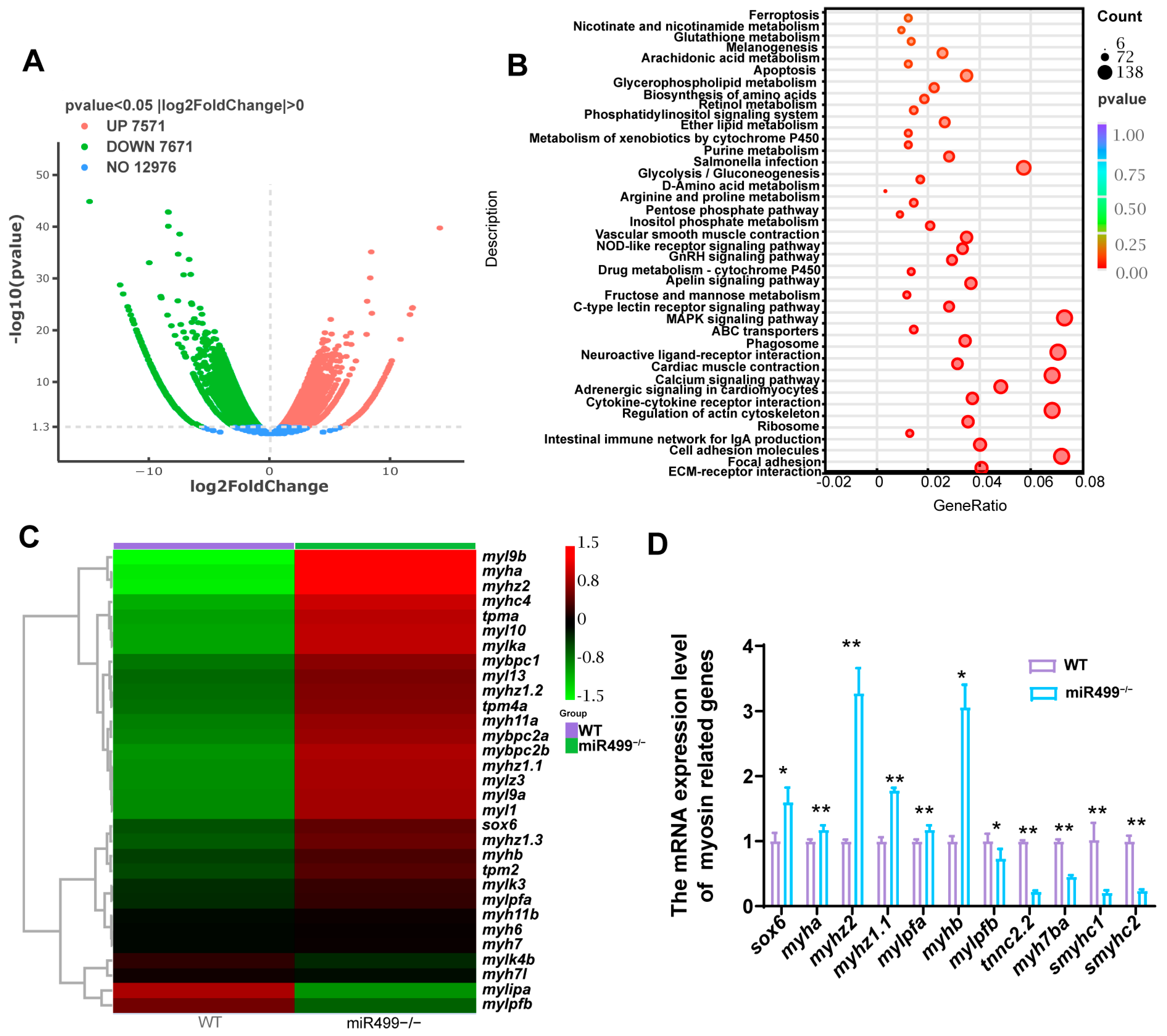
2.3. RNA Sequencing
2.4. Bone Staining and Morphometric Analysis
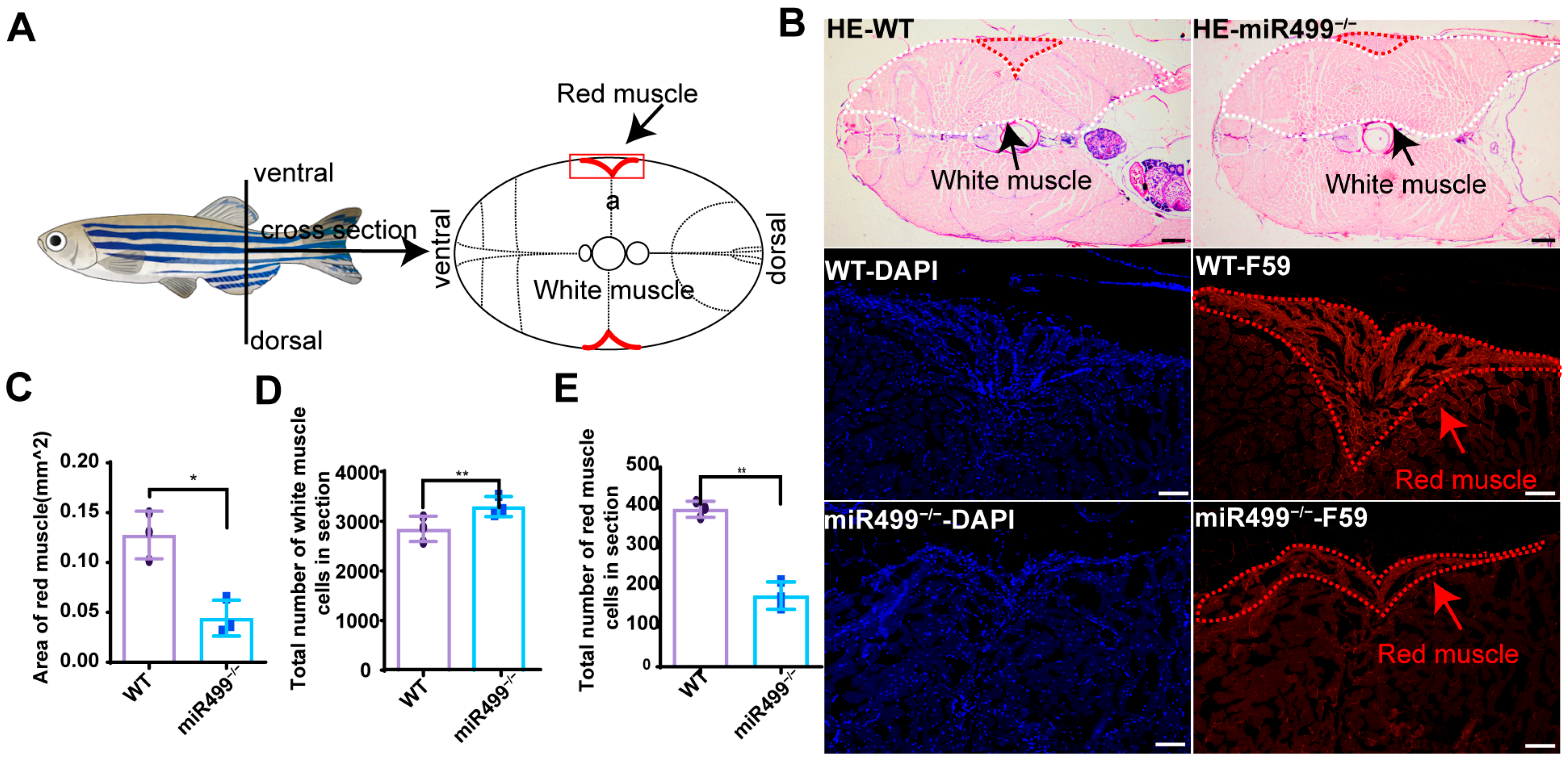
2.5. Skeletal Muscle Fiber Analysis
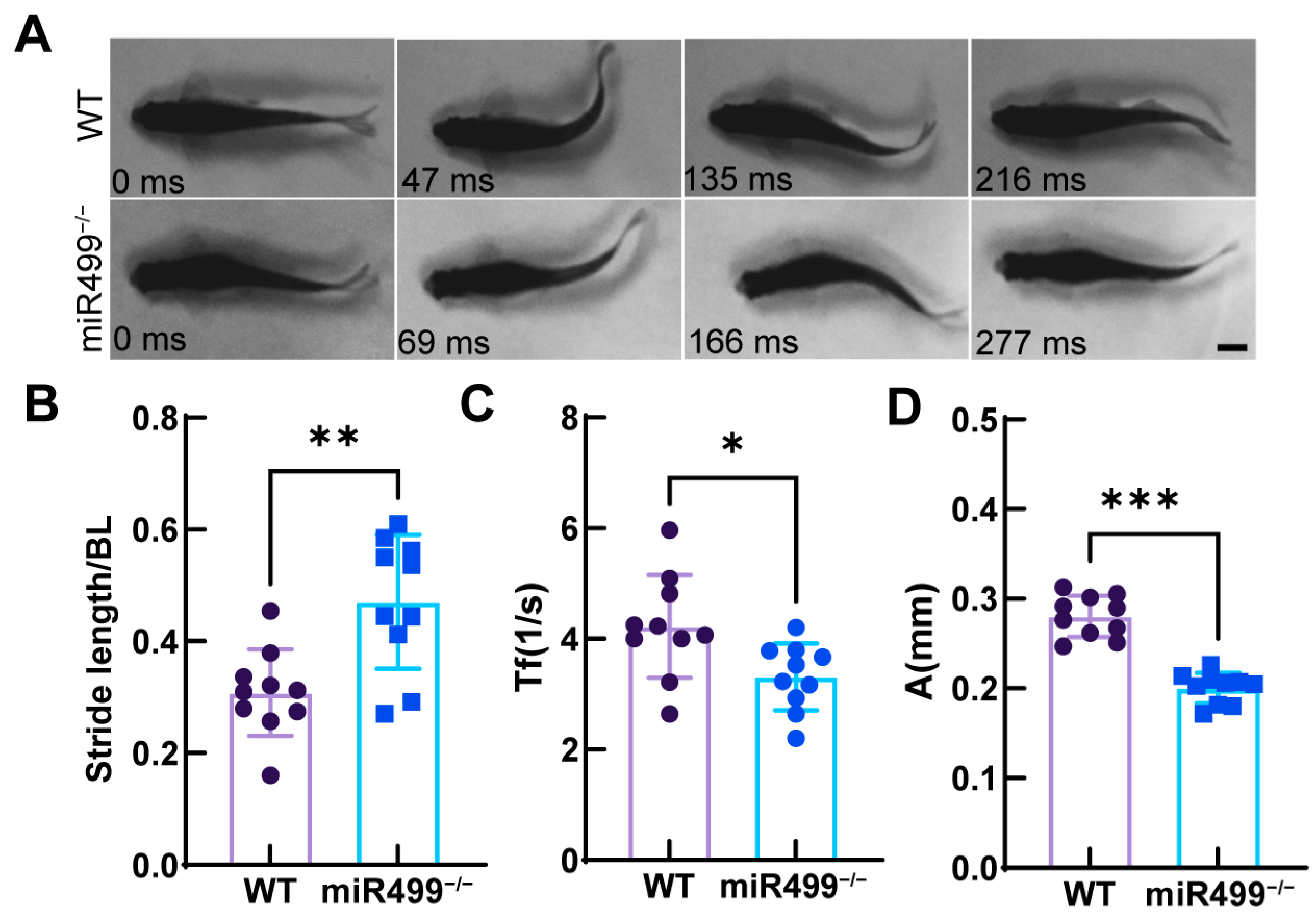
2.6. Locomotion Behavior Analysis
2.7. X-Ray Microtomography (Micro-CT)
2.8. Real Time Quantitative PCR (RT-qPCR)
2.9. Statistical Analysis
3. Results
3.1. Construction of miR499 Zebrafish Mutant by CRISPR/Cas9 Technology
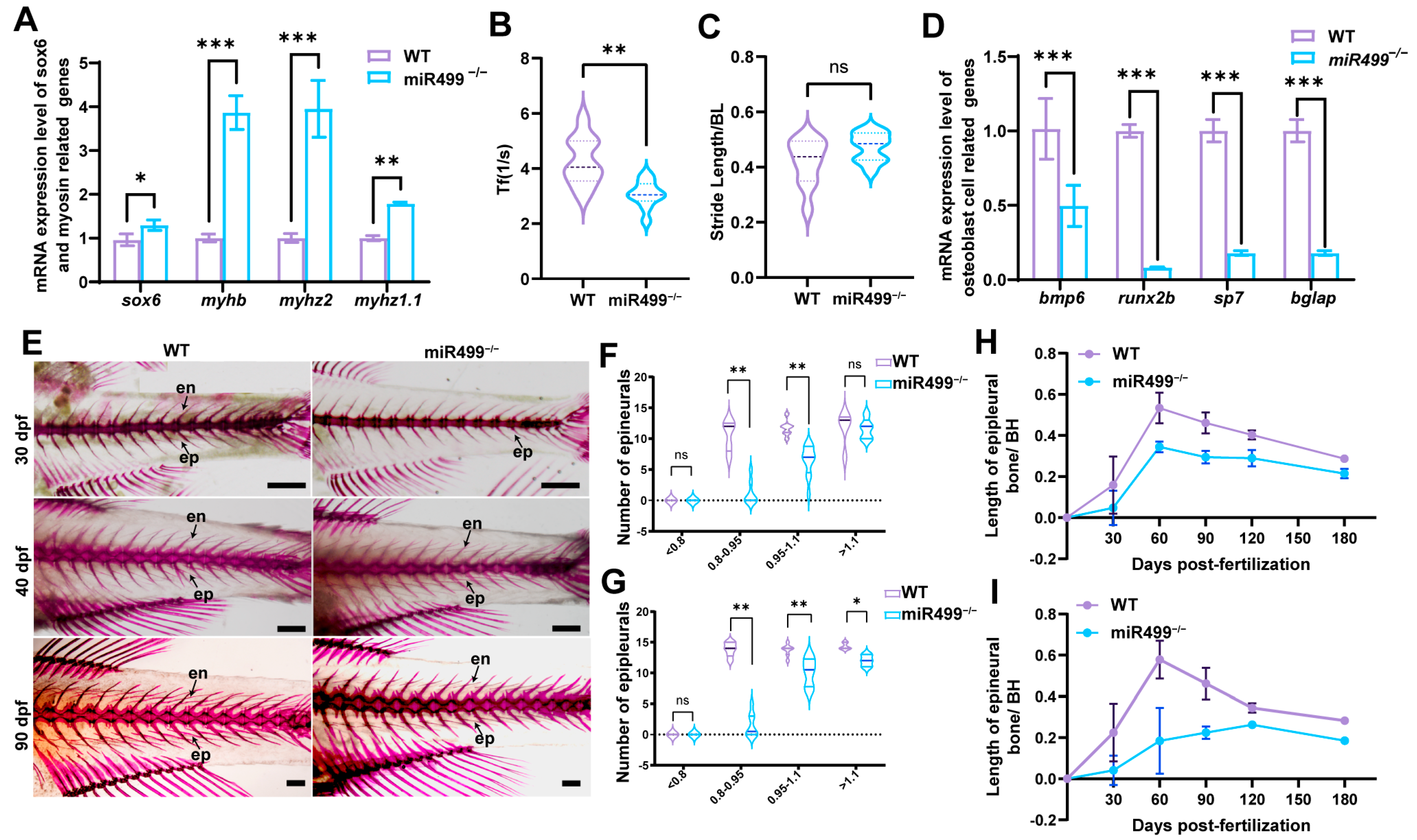
3.2. Regulation of sox6 and Myosin Heavy Chain-Related Genes by miR499 Knockout
3.3. Effects of miR499 Knockout on Zebrafish Muscle
3.4. Impacts of miR499 Knockout on Zebrafish Locomotion Capability
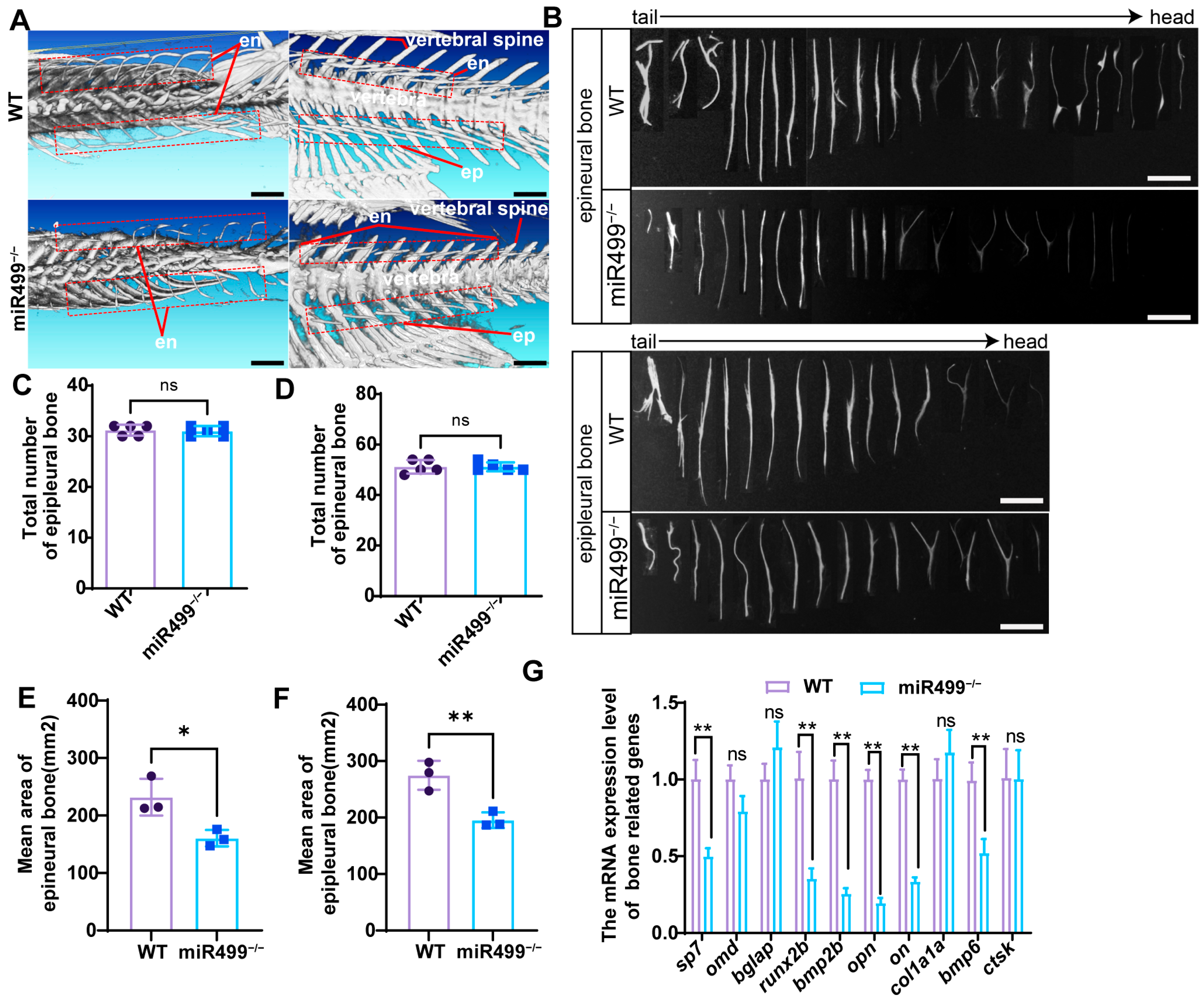
3.5. Role of miR499 in Intermuscular Bone (IB) Development in Zebrafish
3.6. Regulatory Function of miR499 in Intermuscular Bone (IB) Formation
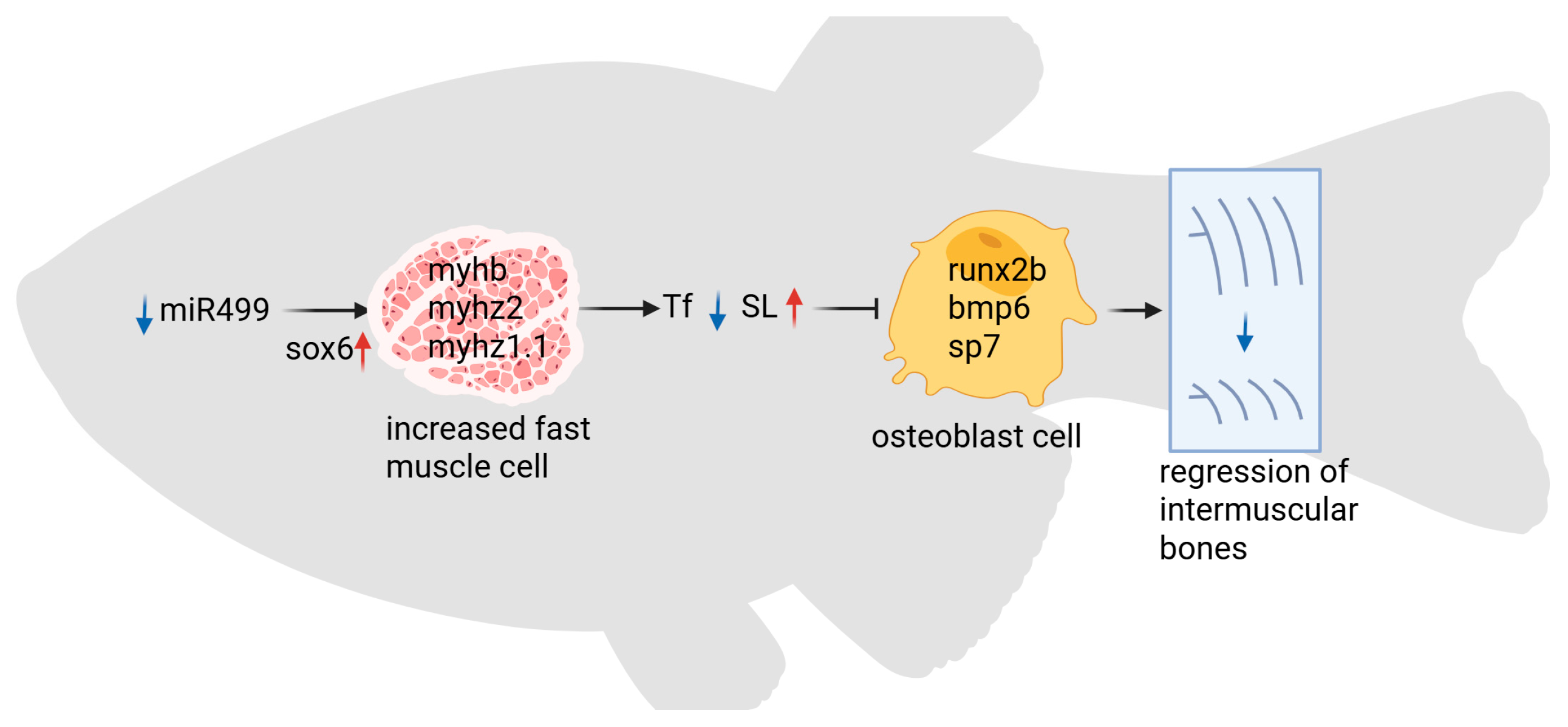
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Zhang, Y.W.; Liu, X.; Ma, L.; Yang, J.X. Molecular mechanisms of intermuscular bone development in fish: A review. Zool. Res. 2021, 42, 362–376. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, W.; Wang, X.; Zhang, Y.; Pan, X.; Yang, J. Evolution of the intermuscular bones in the Cyprinidae (Pisces) from a phylogenetic perspective. Ecol. Evol. 2019, 9, 8555–8566. [Google Scholar] [CrossRef]
- Fiedler, I.A.K.; Zeveleva, S.; Duarte, A.; Zhao, X.; Depalle, B.; Cardoso, L.; Jin, S.; Berteau, J.P. Microstructure, mineral and mechanical properties of teleost intermuscular bones. J. Biomech. 2019, 94, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mubango, E.; Tavakoli, S.; Liu, Y.Y.; Zheng, Y.Y.; Huang, X.R.; Benjakul, S.; Tan, Y.Q.; Luo, Y.K.; Hong, H. Intermuscular Bones in Asian Carps: Health Threats, Solutions, and Future Directions. Rev. Fish. Sci. Aquac. 2023, 31, 233–258. [Google Scholar] [CrossRef]
- Ronning, S.B.; Ostbye, T.K.; Krasnov, A.; Vuong, T.T.; Veiseth-Kent, E.; Kolset, S.O.; Pedersen, M.E. The role of extracellular matrix components in pin bone attachments during storage-a comparison between farmed Atlantic salmon (Salmo salar) and cod (Gadus morhua L.). Fish. Physiol. Biochem. 2017, 43, 549–562. [Google Scholar] [CrossRef]
- Cao, D.C.; Kuang, Y.Y.; Zheng, X.H.; Tong, G.X.; Li, C.T.; Sun, X.W. Comparative analysis of intermuscular bones in three strains of common carp. J. Appl. Ichthyol. 2015, 31, 32–36. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Z.; Zeng, M.; Liu, S.; Zhou, Y.; Xiao, J.; Wang, J.; Liu, Y. Comparative analysis of intermuscular bones in fish of different ploidies. Sci. China Life Sci. 2013, 56, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Perazza, C.A.; de Menezes, J.T.B.; Ferraz, J.B.S.; Pinaffi, F.L.V.; Silva, L.A.; Hilsdorf, A.S. Lack of intermuscular bones in specimens of: An unusual phenotype to be incorporated into genetic improvement programs. Aquaculture 2017, 472, 57–60. [Google Scholar] [CrossRef]
- Xiong, X.M.; Robinson, N.A.; Zhou, J.J.; Chen, Y.L.; Wang, W.M.; Wang, X.B.; Gao, Z.X. Genetic parameter estimates for intermuscular bone in blunt snout bream (Megalobrama amblycephala) based on a microsatellite-based pedigree. Aquaculture 2019, 502, 371–377. [Google Scholar] [CrossRef]
- Tang, G.P.; Lv, W.H.; Sun, Z.P.; Cao, D.C.; Zheng, X.H.; Tong, G.X.; Wang, H.L.; Zhang, X.F.; Kuang, Y.Y. Heritability and quantitative trait locus analyses of intermuscular bones in mirror carp (Cyprinus carpio). Aquaculture 2020, 515, 734601. [Google Scholar] [CrossRef]
- Wan, S.M.; Xiong, X.M.; Tomljanovic, T.; Chen, Y.L.; Liu, H.; Treer, T.; Gao, Z.X. Identification and mapping of SNPs associated with number of intermuscular bone in blunt snout bream. Aquaculture 2019, 507, 75–82. [Google Scholar] [CrossRef]
- Su, S.; Dong, Z. Comparative expression analyses of bone morphogenetic protein 4 (BMP4) expressions in muscles of tilapia and common carp indicate that BMP4 plays a role in the intermuscular bone distribution in a dose-dependent manner. Gene Expr. Patterns 2018, 27, 106–113. [Google Scholar] [CrossRef]
- Nie, C.H.; Wan, S.M.; Tomljanovic, T.; Treer, T.; Hsiao, C.D.; Wang, W.M.; Gao, Z.X. Comparative proteomics analysis of teleost intermuscular bones and ribs provides insight into their development. Bmc Genom. 2017, 18, 147. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Lan, T.; Nie, C.H.; Guan, N.N.; Gao, Z.X. Characterization and spatiotemporal expression analysis of nine bone morphogenetic protein family genes during intermuscular bone development in blunt snout bream. Gene 2018, 642, 116–124. [Google Scholar] [CrossRef]
- Niu, S.; Li, X.H.; Feng, C.; Zhang, Z.W.; Sha, H.; Luo, X.Z.; Zou, G.W.; Liang, H.W. Targeting and editing the second exon of bmp6 gene results in a silver carp with reduced intramuscular bones. Aquacult. Rep. 2025, 40, 102586. [Google Scholar] [CrossRef]
- Nie, C.H.; Wan, S.M.; Chen, Y.L.; Huysseune, A.; Wu, Y.M.; Zhou, J.J.; Hilsdorf, A.W.S.; Wang, W.M.; Witten, P.E.; Lin, Q.; et al. Single-cell transcriptomes and runx2b(-/-) mutants reveal the genetic signatures of intermuscular bone formation in zebrafish. Natl. Sci. Rev. 2022, 9, nwac152. [Google Scholar] [CrossRef]
- Xu, H.; Tong, G.; Yan, T.; Dong, L.; Yang, X.; Dou, D.; Sun, Z.; Liu, T.; Zheng, X.; Yang, J.; et al. Transcriptomic Analysis Provides Insights to Reveal the bmp6 Function Related to the Development of Intermuscular Bones in Zebrafish. Front. Cell Dev. Biol. 2022, 10, 821471. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef]
- Mizrahi, O.; Sheyn, D.; Tawackoli, W.; Kallai, I.; Oh, A.; Su, S.; Da, X.; Zarrini, P.; Cook-Wiens, G.; Gazit, D.; et al. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013, 20, 370–377. [Google Scholar] [CrossRef]
- Dong, Q.; Nie, C.H.; Wu, Y.M.; Zhang, D.Y.; Wang, X.D.; Tu, T.; Jin, J.; Tian, Z.Y.; Liu, J.Q.; Xiao, Z.Y.; et al. Generation of blunt snout bream without intermuscular bones by runx2b gene mutation. Aquaculture 2023, 567, 739263. [Google Scholar] [CrossRef]
- Azetsu, Y.; Inohaya, K.; Takano, Y.; Kinoshita, M.; Tasaki, M.; Kudo, A. The sp7 gene is required for maturation of osteoblast-lineage cells in medaka (Oryzias latipes) vertebral column development. Dev. Biol. 2017, 431, 252–262. [Google Scholar] [CrossRef]
- Zheng, J.; He, C.; Jiang, W.; Liu, S.; Li, F.; Chi, M.; Cheng, S.; Liu, Y. Screening for IBs-relative genes by transcriptome analysis and generation IBs-less mutants in Culter alburnus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 47, 101106. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, Y.; Cao, A.; Li, S.; He, C.; Song, L.; Gao, J.; Zhu, Y.; Cao, X. Transcriptome analyses of betta fish (Betta splendens) provide novel insights into fin regeneration and color-related genes. Gene 2023, 876, 147508. [Google Scholar] [CrossRef]
- Khan, M.F.; Parveen, S.; Sultana, M.; Zhu, P.; Xu, Y.; Safdar, A.; Shafique, L. Evolution and Comparative Genomics of the Transforming Growth Factor-beta-Related Proteins in Nile Tilapia. Mol. Biotechnol. 2025, 67, 3517–3531. [Google Scholar] [CrossRef]
- Danos, N.; Ward, A.B. The homology and origins of intermuscular bones in fishes: Phylogenetic or biomechanical determinants? Biol. J. Linn. Soc. 2012, 106, 607–622. [Google Scholar] [CrossRef]
- Bird, N.C.; Mabee, P.M. Homology, individuality, and developmental morphology of the axial skeleton of the zebrafish. In Proceedings of the Annual Meeting of the Society-for-Integrative-and-Comparative-Biology, Bonn, Germany, 26–31 August 2002; Volume 42, p. 1197. [Google Scholar]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, (Ostariophysi: Cyprinidae). Dev. Dynam. 2003, 228, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Gemballa, S.; Ebmeyer, L.; Hagen, K.; Hannich, T.; Hoja, K.; Rolf, M.; Treiber, K.; Vogel, F.; Weitbrecht, G. Evolutionary transformations of myoseptal tendons in gnathostomes. Proc. R. Soc. B-Biol. Sci. 2003, 270, 1229–1235. [Google Scholar] [CrossRef]
- Patterson, C.; Johnson, G.D. The Intermuscular Bones and Ligaments of Teleostean Fishes; Smithsonian Institution Press: Washington, DC, USA, 1995; pp. iv, 83p. [Google Scholar]
- Nie, C.H.; Hilsdorf, A.W.S.; Wan, S.M.; Gao, Z.X. Understanding the development of intermuscular bones in teleost: Status and future directions for aquaculture. Rev. Aquacult. 2020, 12, 759–772. [Google Scholar] [CrossRef]
- Li, G.; Liu, G.; Leng, D.; Fang, X.; Li, G.; Wang, W. Underwater Undulating Propulsion Biomimetic Robots: A Review. Biomimetics 2023, 8, 318. [Google Scholar] [CrossRef]
- Ke, Z.H.; Zhang, W.; Jiang, Y.; Bao, B.L. Developmental morphology of the intermuscular bone in Hypophthalmichthys molitrix. Chin. J. Zool. 2008, 43, 88–96. [Google Scholar]
- Wan, S.M.; Yi, S.K.; Zhong, J.; Nie, C.H.; Guan, N.N.; Zhang, W.Z.; Gao, Z.X. Dynamic mRNA and miRNA expression analysis in response to intermuscular bone development of blunt snout bream (Megalobrama amblycephala). Sci. Rep. 2016, 6, 31050. [Google Scholar] [CrossRef]
- Yao, W.; Lv, Y.; Gong, X.; Wu, J.; Bao, B. Different ossification patterns of intermuscular bones in fish with different swimming modes. Biol. Open 2015, 4, 1727–1732. [Google Scholar] [CrossRef]
- Danos, N.; Staab, K.L. Can mechanical forces be responsible for novel bone development and evolution in fishes? J. Appl. Ichthyol. 2010, 26, 156–161. [Google Scholar] [CrossRef]
- Lv, Y.P.; Yao, W.J.; Chen, J.; Bao, B.L. Newly identified gene muscle segment homeobox C may play a role in intermuscular bone development of Hemibarbus labeo. Genet. Mol. Res. 2015, 14, 11324–11334. [Google Scholar] [CrossRef]
- Nunes, J.R.S.; Pertille, F.; Andrade, S.C.S.; Perazza, C.A.; Villela, P.M.S.; Almeida-Val, V.M.F.; Gao, Z.X.; Coutinho, L.L.; Hilsdorf, A.W.S. Genome-wide association study reveals genes associated with the absence of intermuscular bones in tambaqui (Colossoma macropomum). Anim. Genet. 2020, 51, 899–909. [Google Scholar] [CrossRef]
- Ye, W.; Shi, M.; Cheng, Y.; Liu, Y.; Ren, K.; Fang, Y.; Younas, W.; Zhang, W.; Wang, Y.; Xia, X.Q. Integrated single-cell transcriptome and comparative genome analysis reveals the origin of intermuscular bones in zebrafish. Int. J. Biol. Macromol. 2025, 308, 142397. [Google Scholar] [CrossRef]
- Wang, X.; Ono, Y.; Tan, S.C.; Chai, R.J.; Parkin, C.; Ingham, P.W. Prdm1a and miR499 act sequentially to restrict Sox6 activity to the fast-twitch muscle lineage in the zebrafish embryo. Development 2011, 138, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Duran, B.; Dal-Pai-Silva, M.; Garcia de la Serrana, D. Rainbow trout slow myoblast cell culture as a model to study slow skeletal muscle, and the characterization of mir-133 and miR499 families as a case study. J. Exp. Biol. 2020, 223, jeb216390. [Google Scholar] [CrossRef]
- Siddique, B.S.; Kinoshita, S.; Wongkarangkana, C.; Asakawa, S.; Watabe, S. Evolution and Distribution of Teleost myomiRNAs: Functionally Diversified myomiRs in Teleosts. Mar. Biotechnol. 2016, 18, 436–447. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Jia, Z.; Cui, Q.; Zhang, C.; Wang, W.; Chen, P.; Ma, K.; Zhou, C. miR499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via Sox6 and cyclin D1. PLoS ONE 2013, 8, e74504. [Google Scholar] [CrossRef]
- Hasan, S.; Asakawa, S.; Watabe, S.; Kinoshita, S. Regulation of the Expression of the Myosin Heavy Chain (MYH) Gene myh14 in Zebrafish Development. Mar. Biotechnol. 2021, 23, 821–835. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.D.; Lv, M.Q.; Jia, L.; Liao, Y.G.; Xu, X.Y.; Meyer, A.; Sun, J.S.; Fan, G.Y.; Li, Y.M.; et al. Adaptive loss of shortwave-sensitive opsins during cartilaginous fish evolution. Nat. Commun. 2025, 16, 7684. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Shakur Ahammad, A.K.; Asakawa, S.; Kinoshita, S.; Watabe, S. 5′-flanking sequences of zebrafish fast myosin heavy chain genes regulate unique expression in the anterior, medial subsection and posterior tail somites of the skeletal muscle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 191, 1–12. [Google Scholar] [CrossRef]
- Nachtigall, P.G.; Dias, M.C.; Carvalho, R.F.; Martins, C.; Pinhal, D. MicroRNA-499 expression distinctively correlates to target genes sox6 and rod1 profiles to resolve the skeletal muscle phenotype in Nile tilapia. PLoS ONE 2015, 10, e0119804. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, M.; Shan, Y.J.; Pang, L.C.; Ji, G.G.; Ju, X.J.; Tu, Y.J.; Shi, S.Y.; Bai, H.; Zou, J.M.; et al. Transcriptome sequencing analysis of the role of miR499-5p and SOX6 in chicken skeletal myofiber specification. Front. Genet. 2022, 13, 1008649. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, X.L.; Huang, Z.Q.; Chen, D.W.; Yu, B.; He, J.; Luo, J.Q.; Luo, Y.H.; Chen, H.; Zheng, P.; et al. MicroRNA-499-5p regulates porcine myofiber specification by controlling Sox6 expression. Animal 2017, 11, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; He, J.; Zheng, P.; Luo, J.; Yu, J.; et al. Effects of active immunization against porcine Sox6 on meat quality and myosin heavy chain isoform expression in growing-finishing pigs. Anim. Biotechnol. 2019, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yue, B.; Lan, X.; Wang, Y.; Fang, X.; Ma, Y.; Bai, Y.; Qi, X.; Zhang, C.; Chen, H. miR499 regulates myoblast proliferation and differentiation by targeting transforming growth factor beta receptor 1. J. Cell Physiol. 2019, 234, 2523–2536. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J., Jr.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Bao, X.; Wang, X.; Wu, J.; Li, X.; Hong, W. MiR-499-5p protects cardiomyocytes against ischaemic injury via anti-apoptosis by targeting PDCD4. Oncotarget 2016, 7, 35607–35617. [Google Scholar] [CrossRef]
- Liu, J.; Liang, X.; Zhou, D.; Lai, L.; Xiao, L.; Liu, L.; Fu, T.; Kong, Y.; Zhou, Q.; Vega, R.B.; et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol. Med. 2016, 8, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, W.; Li, X.; Yuan, H.; Guo, J.; Wang, Y.; Shan, Z. MicroRNA-499-5p inhibits transforming growth factor-beta1-induced Smad2 signaling pathway and suppresses fibroblast proliferation and collagen synthesis in rat by targeting TGFbeta-R1. Mol. Biol. Rep. 2023, 50, 9757–9767. [Google Scholar] [CrossRef]
- Altringham, J.D.; Ellerby, D.J. Fish swimming: Patterns in muscle function. J. Exp. Biol. 1999, 202, 3397–3403. [Google Scholar] [CrossRef]
- Granzier, H.L.M.; Wiersma, J.; Akster, H.A.; Osse, J.W.M. Contractile properties of a white- and a red-fibre type of the m. hyohyoideus of the carp (Cyprinus carpio L.). J. Comp. Physiol. 1983, 149, 441–449. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Johnston, I.A. Muscle power output limits fast-start performance in fish. J. Exp. Biol. 1998, 201, 1505–1526. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.H.; Whang, H.; Wu, Z.C.; Jiang, S.W.; Chen, L.B. Deletion of Asb15b gene can lead to a significant decrease in zebrafish intermuscular bone. Gene 2024, 923, 148561. [Google Scholar] [CrossRef] [PubMed]
| Symbol | Forward Primer | Reverse Primer |
|---|---|---|
| miR499a-5P | GCGCGTTAAGACTTGCAGTGA | AGTGCAGGGTCCGAGGTATT |
| miR499a-3P | CGCGCGAACATCACTTTAAGTC | AGTGCAGGGTCCGAGGTATT |
| U6 | TGCTCGCTACGGTGGCACA | AAAACAGCAATATGGAGCGC |
| sox6 | AGTCACTTCCTTCGCTTCCG | CCAGGTTGGCCTGTACACAT |
| myha | CAAAAAGGCAAGGCTGAGGC | TTGAAGTGTCCTCAACGACGG |
| myhb | GAGTGGGGACCCAGAAATGG | TGCCACAGAGAGTTTGCACT |
| myhz2 | ATCTGGTCTCAAGGAACGCA | TTGGGAGGATTCATTGGGTGG |
| myhz1.1 | GACCTGAAACTGGCCCAAGA | CAGCTCGTTCAGCCTCGATT |
| mylpfb | GGATGTGCTGGCAACAATGG | GCGCCCTTTAGCTTTTCACC |
| mylpfa | GCAGAGCCAGATTCAGGAGTAC | GTGAAGTTGATTGGGCCGCT |
| tnnc2.2 | CGAGGAGGTCGATGAAGACG | CTGTCGATGTAGCCATCACCG |
| myh7ba | GACCAACAGGGAAACTGGCC | CGTTGTCACTCCTTGGGAGC |
| smyhc1 | CCTGGTGTCTCAGTTGACCA | TGTGCCAGGGCATTCTTT |
| smyhc2 | GACCGTCACTGTGAAGGAGG | CAGCCACTTGTAGGGGTTGAC |
| bmp6 | GCTGGAATCTCGCAGGTTGT | CCATCACGGCCTACTAACCC |
| runx2b | CGGCTCCTACCAGTTCTCCA | CCATCTCCCTCCACTCCTCC |
| cola1a1a | CTGTGCCAATCCCATCATTTC | ATATCGCCTGGTTCTCCTTTC |
| osteonectin | ACTAACAACAAGACCTAC | TCCGATGTAATCTATGTG |
| osteopontin | GCCTCCATCATCATCGTA | AATCACCAAGCACCAGTA |
| bmp4 | TTGTGCTGTGCATGTTTGAA | GGTCGCTTGGCTATGTGTTT |
| sp7 | GGCTATGCTAACTGCGACCTG | GCTTTCATTGCGTCCGTTTT |
| bglap | GTTTGTGAAGCGTGACGTGG | ATAGGCGGCGATGATTCCAG |
| omd | ACTTGGCCTCCGAGAGAGAT | GCGAAGGGATAAGACGAGGG |
| bmp2b | CGAGATCGACCGACGGAAAT | GACCACTGCCGATTTGCTTG |
| ctsk | CTATAAAGAGATTCCTCAGGG | ACACGGGTCCCACATTGG |
| eef1a1l1 | CTTCTCAGGCTGACTGTGC | CCGCTAGCATTACCCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, J.; Feng, Y.; Li, H.; Wang, Q.; Fan, C.; Bao, B. The Mutation of myomiR miR499 Impacts the Intermuscular Bones in Zebrafish. Biology 2025, 14, 1670. https://doi.org/10.3390/biology14121670
Che J, Feng Y, Li H, Wang Q, Fan C, Bao B. The Mutation of myomiR miR499 Impacts the Intermuscular Bones in Zebrafish. Biology. 2025; 14(12):1670. https://doi.org/10.3390/biology14121670
Chicago/Turabian StyleChe, Jinyuan, Yidong Feng, Haichuan Li, Qi Wang, Chunxin Fan, and Baolong Bao. 2025. "The Mutation of myomiR miR499 Impacts the Intermuscular Bones in Zebrafish" Biology 14, no. 12: 1670. https://doi.org/10.3390/biology14121670
APA StyleChe, J., Feng, Y., Li, H., Wang, Q., Fan, C., & Bao, B. (2025). The Mutation of myomiR miR499 Impacts the Intermuscular Bones in Zebrafish. Biology, 14(12), 1670. https://doi.org/10.3390/biology14121670





