Age-Related Human Adaptation to Extreme Climatic Factors and Environmental Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
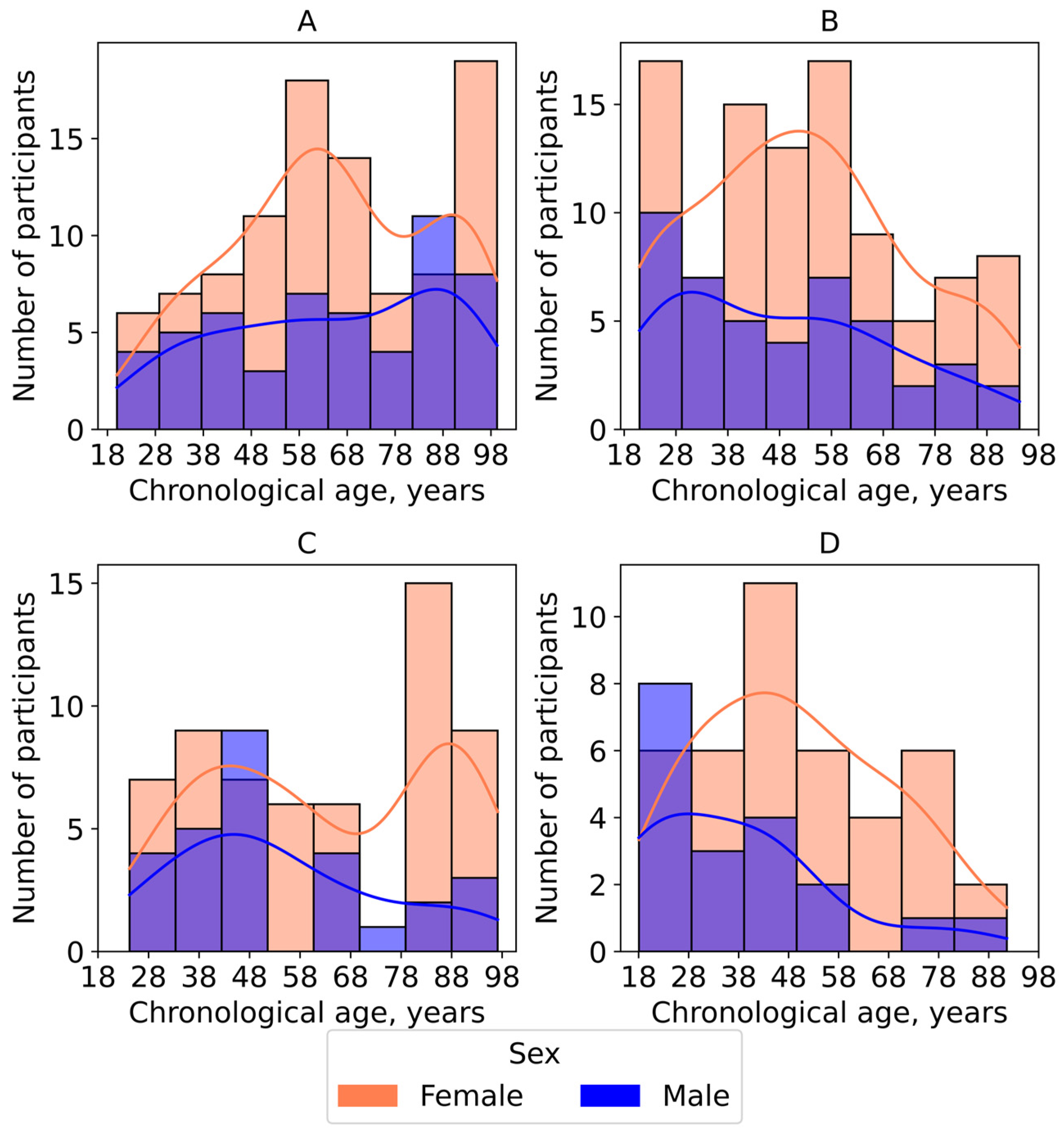
2.1. Data Collection and Cohort Characterization
- (1)
- Yakutia—individuals residing in the extreme climatic conditions of the Republic of Sakha (Yakutia), in small settlements with a moderate level of anthropogenic impact (n = 153);
- (2)
- Nizhny Novgorod—volunteers living in the large metropolis of Nizhny Novgorod in Central Russia, which has a temperate continental climate featuring higher average temperatures and shorter winter period (n = 144);
- (3)
- Dzerzhinsk—residents of the industrial city of Dzerzhinsk in the Nizhny Novgorod region, characterized by elevated levels of air, water, and soil pollution (n = 60);
- (4)
- Small towns—volunteers living in the small towns Semyonov and Pavlovo in the Nizhny Novgorod region, with populations of up to 50,000 (n = 88).
2.2. Sample Handling
2.3. Analytical Methods
2.4. Biological Age Calculation
2.5. Data Processing
2.6. Machine Learning
3. Results
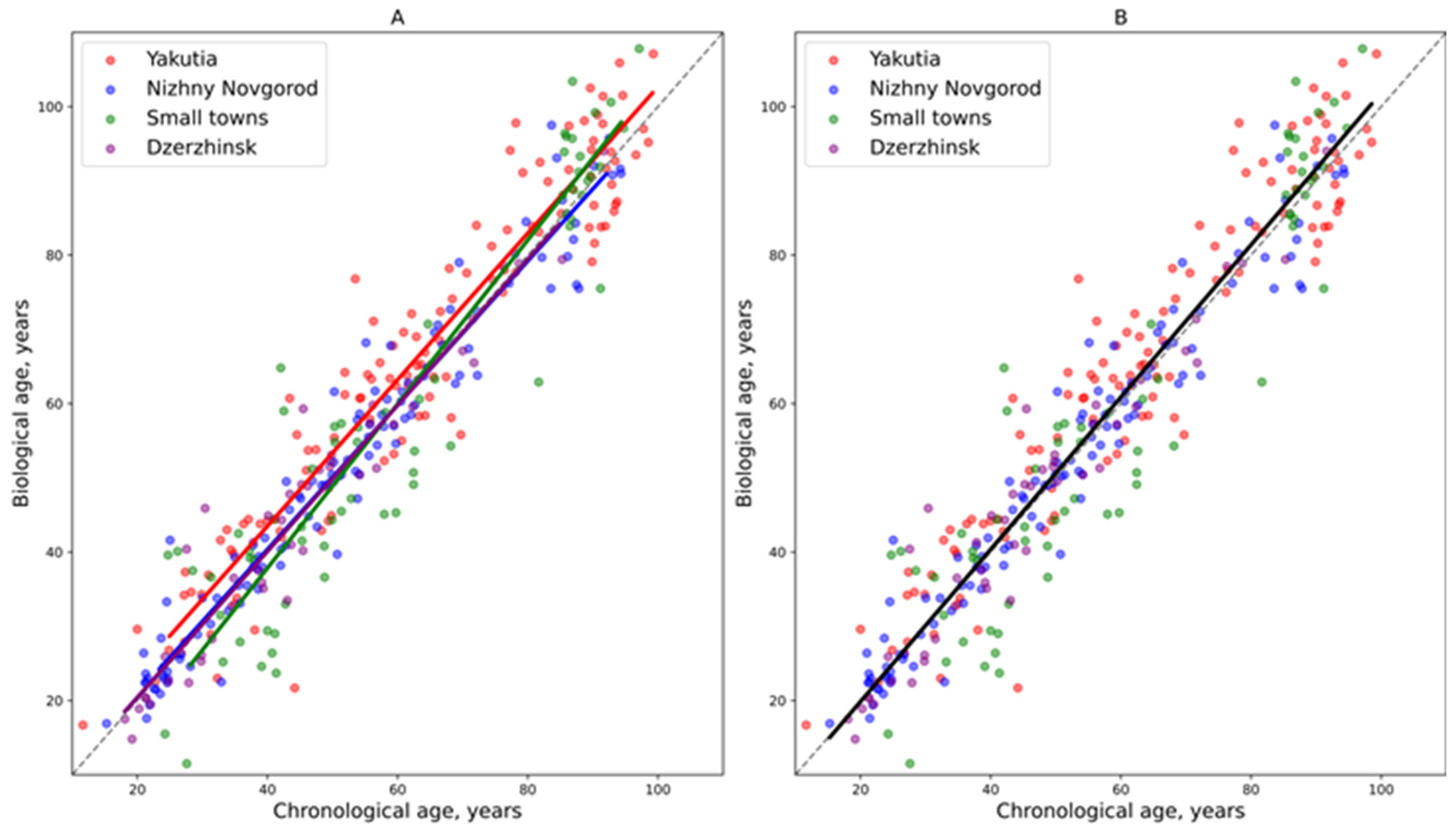
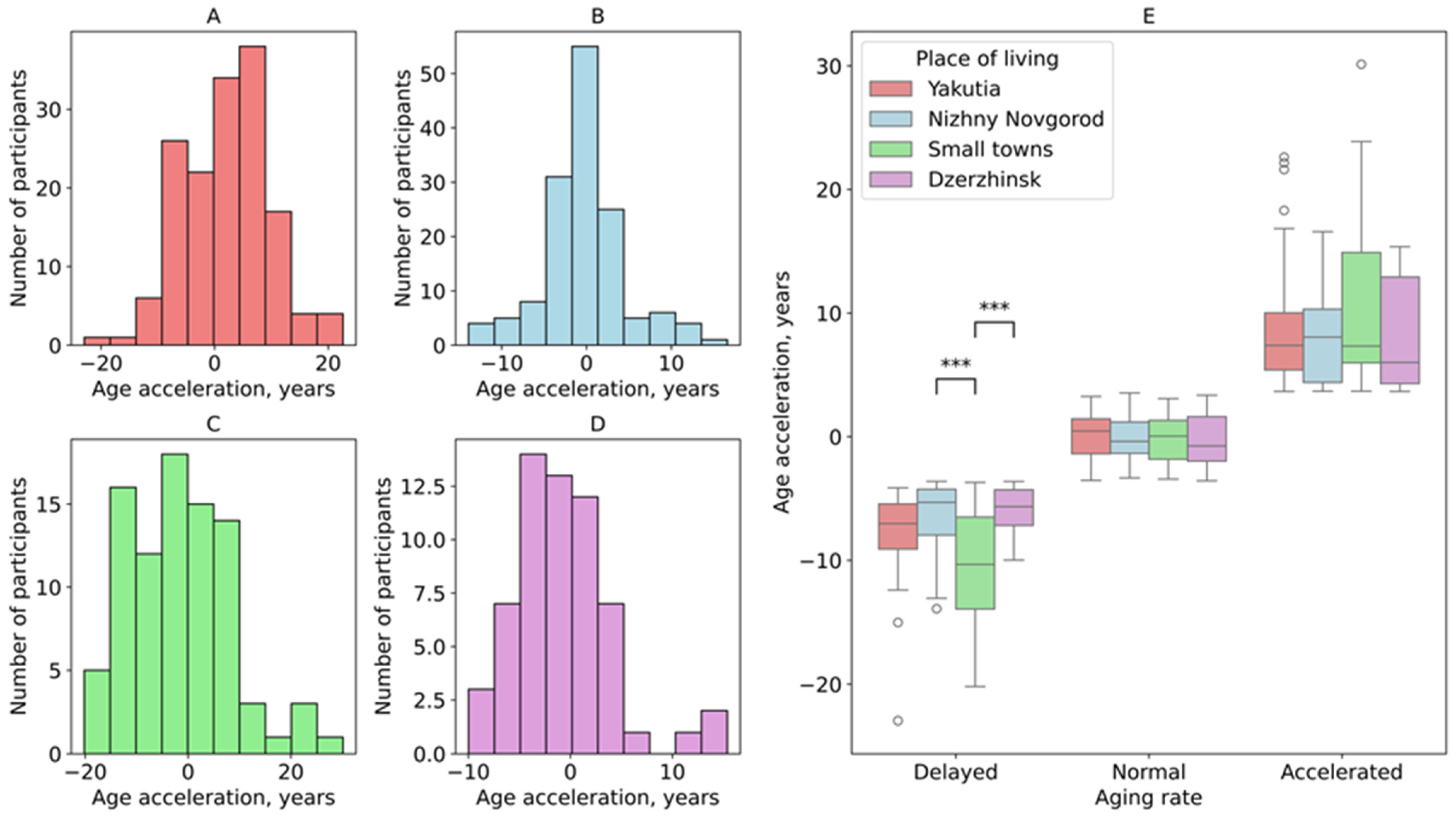
3.1. Age Acceleration Rate Across the Studied Groups
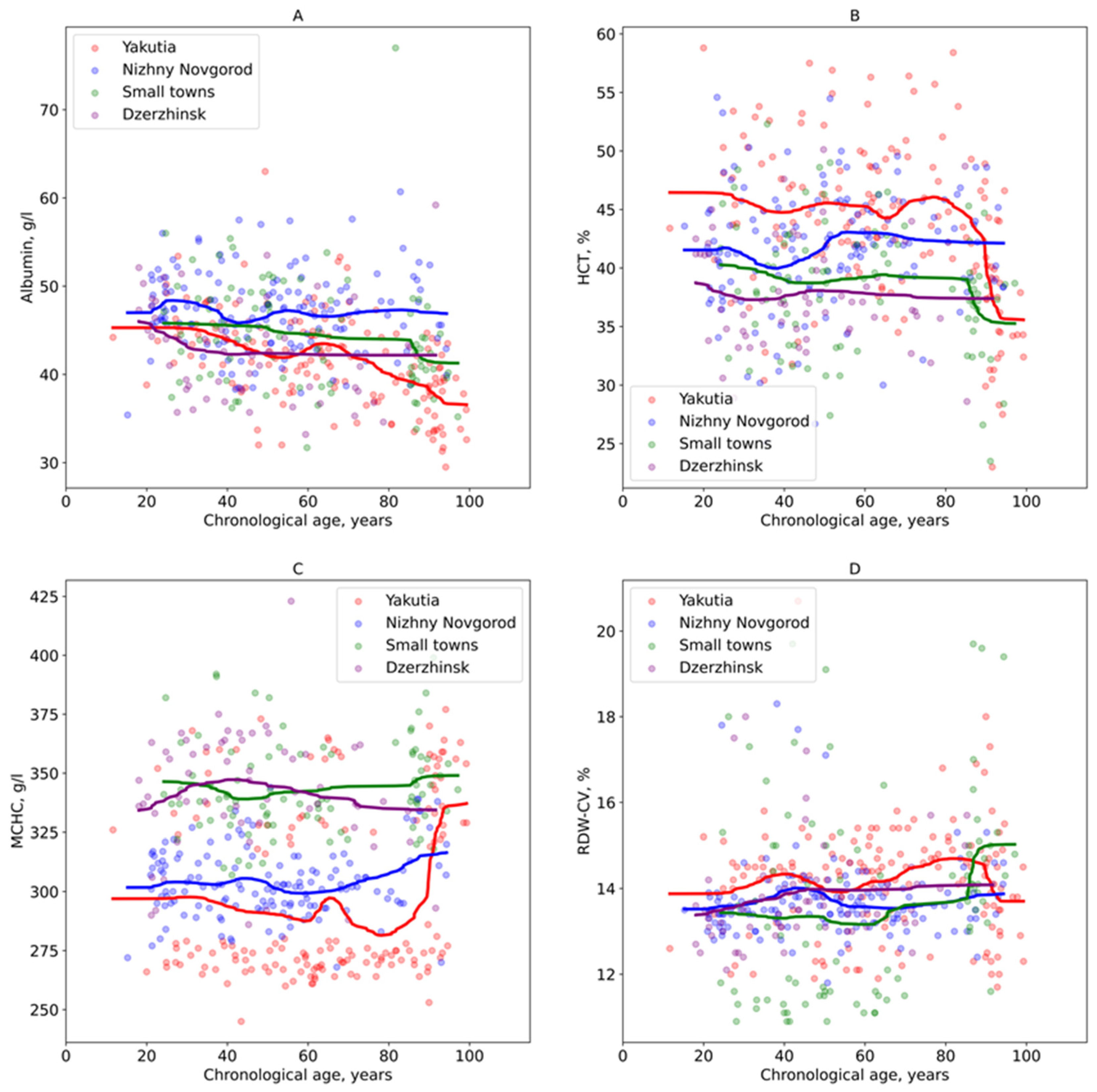
3.2. Analysis of Regional Age-Related Variations in Blood Parameters
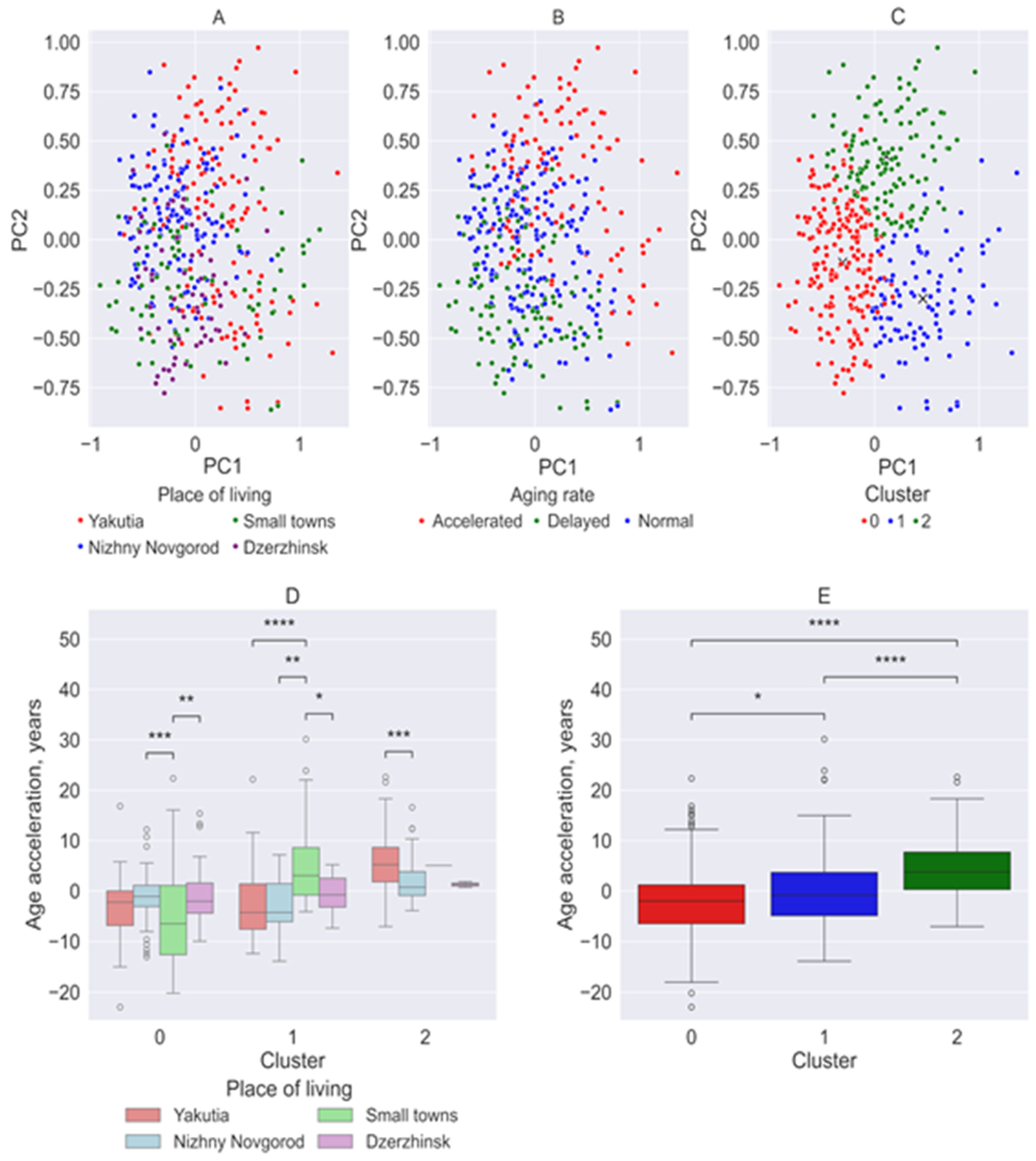
3.3. Principal Component Analysis and Clustering
3.4. Variations in General and Biochemical Blood Parameters in Relation to the Rate of Aging and Environmental Factors
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| CRP | C-reactive protein |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| LYM | Lymphocytes |
| MCH | Mean corpuscular hemoglobin content |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Red blood cell volume |
| ML | Machine learning |
| PCA | Principal component analysis |
| PLT | Platelets |
| RBC | Red blood cells |
| RDW-CV | Red blood cell distribution width by volume |
| WBC | White blood cells |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health. 2022. Available online: https://who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 August 2025).
- Ding, E.; Wang, Y.; Liu, J.; Tang, S.; Shi, X. A review on the application of the exposome paradigm to unveil the environmental determinants of age-related diseases. Hum. Genomics 2022, 16, 54. [Google Scholar] [CrossRef]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sørensen, M.; Hahad, O.; Nieuwenhuijsen, M.; Daiber, A. The contribution of the exposome to the burden of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2021, 42, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, B.; Khraishah, H.; Royé, D.; Vicedo-Cabrera, A.M.; Guo, Y.; Papatheodorou, S.I.; Achilleos, S.; Acquaotta, F.; Armstrong, B.; Bell, M.L.; et al. Associations Between Extreme Temperatures and Cardiovascular Cause-Specific Mortality: Results from 27 Countries. Circulation 2023, 147, 35–46. [Google Scholar] [CrossRef]
- Cardona, A.; Pagani, L.; Antao, T.; Lawson, D.J.; Eichstaedt, C.A.; Yngvadottir, B.; Shwe, M.T.T.; Wee, J.; Romero, I.G.; Raj, S.; et al. Genome-wide analysis of cold adaptation in indigenous Siberian populations. PLoS ONE 2014, 9, e98076. [Google Scholar] [CrossRef]
- Leonard, W.R.; Snodgrass, J.J.; Sorensen, M.V. Metabolic adaptation in indigenous Siberian populations. Annu. Rev. Anthr. 2005, 34, 451–471. [Google Scholar] [CrossRef]
- Bjerregaard, P.; Dewailly, E.; Young, T.K.; Blanchet, C.; Hegele, R.A.; Ebbesson, S.E.; Risica, P.M.; Mulvad, G. Blood pressure among the Inuit (Eskimo) populations in the Arctic. Scand. J. Public. Health 2003, 31, 92–99. [Google Scholar] [CrossRef]
- Snodgrass, J.J.; Leonard, W.R.; Sorensen, M.V.; Tarskaia, L.A.; Mosher, M.J. The influence of basal metabolic rate on blood pressure among indigenous Siberians. Am. J. Phys. Anthropol. 2008, 137, 145–155. [Google Scholar] [CrossRef]
- Cardenas, A.; Fadadu, R.; Bunyavanich, S. Climate change and epigenetic biomarkers in allergic and airway diseases. J. Allergy Clin. Immunol. 2023, 152, 1060–1072. [Google Scholar] [CrossRef]
- Skinner, M.K. Environmental epigenetics and climate change. Environ. Epigenetics 2023, 9, dvac028. [Google Scholar] [CrossRef]
- Kalyakulina, A.; Yusipov, I.; Kondakova, E.; Bacalini, M.G.; Giuliani, C.; Sivtseva, T.; Semenov, S.; Ksenofontov, A.; Nikolaeva, M.; Khusnutdinova, E.; et al. Epigenetics of the far northern Yakutian population. Clin. Epigenetics 2023, 15, 189. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Chervova, O.; Chernysheva, E.; Panteleeva, K.; Widayati, T.A.; Hrbkova, N.; Schneider, J.; Maximov, V.; Ryabikov, A.; Tillmann, T.; Pikhart, H.; et al. Evaluation of Epigenetic Age Acceleration Scores and Their Associations with CVD-Related Phenotypes in a Population Cohort. Biology 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Vandervieren, E. An adjusted boxplot for skewed distributions. Comput. Stat. Data Anal. 2008, 52, 5186–5201. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Sagers, L.; Melas-Kyriazi, L.; Patel, C.J.; Manrai, A.K. Prediction of chronological and biological age from laboratory data. Aging 2020, 12, 7626–7638. [Google Scholar] [CrossRef]
- Erema, V.V.; Yakovchik, A.Y.; Kashtanova, D.A.; Bochkaeva, Z.V.; Ivanov, M.V.; Sosin, D.V.; Matkava, L.R.; Yudin, V.S.; Makarov, V.V.; Keskinov, A.A.; et al. Biological Age Predictors: The Status Quo and Future Trends. Int. J. Mol. Sci. 2022, 23, 15103. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Levine, M.E. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef]
- Misra, B.B. The Chemical Exposome of Human Aging. Front. Genet. 2020, 11, 574936. [Google Scholar] [CrossRef]
- Robine, J.M.; Cubaynes, S. Worldwide demography of centenarians. Mech. Ageing Dev. 2017, 165, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Centenarians by Country 2025. World Population Review. Available online: https://worldpopulationreview.com/country-rankings/centenarians-by-country (accessed on 1 November 2025).
- Roberts, J.D.; Vittinghoff, E.; Lu, A.T.; Alonso, A.; Wang, B.; Sitlani, C.M.; Mohammadi-Shemirani, P.; Fornage, M.; Kornej, J.; Brody, J.A.; et al. Epigenetic Age and the Risk of Incident Atrial Fibrillation. Circulation 2021, 144, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Faul, J.D.; Kim, J.K.; Levine, M.E.; Thyagarajan, B.; Weir, D.R.; Crimmins, E.M. Epigenetic-based age acceleration in a representative sample of older Americans: Associations with aging-related morbidity and mortality. Proc. Natl. Acad. Sci. USA 2023, 120, e2215840120. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V. Epigenetic clocks provide clues to the mystery of uterine ageing. Hum. Reprod. Update 2023, 29, 259–271. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Yusipov, I.; Kondakova, E.; Kalyakulina, A.; Krivonosov, M.; Lobanova, N.; Bacalini, M.G.; Franceschi, C.; Vedunova, M.; Ivanchenko, M. Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease. Geroscience 2022, 44, 817–834. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Walter, H.; Schöbel, B. Climate associated variations in the human serum albumin level. Humangenetik 1975, 30, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Romanova, L.; Balter, V.; Simon, L.; Gerard, P.; Pokatilova, N.; Crubezy, E. Diet of autochthonous populations in Yakutia using isotopic, ethnographic, historical and archaeological data. J. Archaeol. Sci. Rep. 2019, 28, 102022. [Google Scholar] [CrossRef]
- Stepanov, K. The Role of the Yakut Horse in the Traditional Diet of the Peoples of the North. J. Bioinform. Genom. 2024. [Google Scholar] [CrossRef]
- Hsieh, P.; Hallmark, B.; Watkins, J.; Karafet, T.M.; Osipova, L.P.; Gutenkunst, R.N.; Hammer, M.F. Exome Sequencing Provides Evidence of Polygenic Adaptation to a Fat-Rich Animal Diet in Indigenous Siberian Populations. Mol. Biol. Evol. 2017, 34, 2913–2926. [Google Scholar] [CrossRef]
- Butti, S.; Garda, E.; Gattuso, M.; Morganti, F. Aging and Urbanization: Investigating Different “Aging in Place” Models in the City as an Opportunity to Empower Individuals’ Healthy Longevity. In Proceedings of the AESOP Annual Conference 2024, Paris, France, 8–12 July 2024; p. 960. [Google Scholar]
- Kivimäki, M.; Pentti, J.; Frank, P.; Liu, F.; Blake, A.; Nyberg, S.T.; Vahtera, J.; Singh-Manoux, A.; Wyss-Coray, T.; Walker, K.A.; et al. Social disadvantage accelerates aging. Nat. Med. 2025, 31, 1635–1643. [Google Scholar] [CrossRef]
- Arkew, M.; Gemechu, K.; Haile, K.; Asmerom, H. Red Blood Cell Distribution Width as Novel Biomarker in Cardiovascular Diseases: A Literature Review. J. Blood Med. 2022, 13, 413–424. [Google Scholar] [CrossRef]
- Pilling, L.C.; Atkins, J.L.; Duff, M.O.; Beaumont, R.N.; Jones, S.E.; Tyrrell, J.; Kuo, C.L.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; et al. Red blood cell distribution width: Genetic evidence for aging pathways in 116,666 volunteers. PLoS ONE 2017, 12, e0185083. [Google Scholar] [CrossRef]
- Ananthaseshan, S.; Bojakowski, K.; Sacharczuk, M.; Poznanski, P.; Skiba, D.S.; Prahl Wittberg, L.; McKenzie, J.; Szkulmowska, A.; Berg, N.; Andziak, P.; et al. Red blood cell distribution width is associated with increased interactions of blood cells with vascular wall. Sci. Rep. 2022, 12, 13676. [Google Scholar] [CrossRef]
- Hoffmann, J.J.; Nabbe, K.C.; van den Broek, N.M. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin. Chem. Lab. Med. 2015, 53, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lui, L.Y.; Browner, W.S.; Cauley, J.A.; Ensrud, K.E.; Kado, D.M.; Orwoll, E.S.; Schousboe, J.T.; Cummings, S.R. Association Between Variation in Red Cell Size and Multiple Aging-Related Outcomes. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, N.; Tsyrlin, V.; Pliss, M. Seasonal dynamics of red blood parameters in healthy people in regions with different types of climate: A meta-analysis. Izv. Atmos. Ocean. Phys. 2021, 57, 1271–1292. [Google Scholar] [CrossRef]
- Resolution No. 165 Dated December 22, 2017 on the Approval of Hygienic Standards gn 2.1.6.3492-17 “Maximum Permissible Concentrations (MPC) of Pollutants in the Atmospheric Air of Urban and Rural Settlements”. Available online: https://docs.cntd.ru/document/556185926 (accessed on 26 September 2025).
- Chemezov, E.; Sosina, S. State of the atmosphere of the Republic of Sakha (Yakutia). Symb. Sci. 2017, 1. Available online: https://cyberleninka.ru/article/n/sostoyanie-atmosfery-respubliki-saha-yakutiya (accessed on 3 November 2023).
- Nikolaeva, N. Geoecological Approach to Justification of Priority Directions for Reducing Negative Ecological Impacts during Implementation on the South Yakutia of Large Energy Projects in the Republic of Sakha (Yakutia). Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 720, 012016. [Google Scholar] [CrossRef]
- Burtseva, T.; Uvarova, T.; Savvina, M.; Shadrin, V.; Avrusin, S.; Chasnyk, V. Health status of Native people living in the Republic of Sakha (Yakutia). Int. J. Circumpolar Health 2013, 72, 21166. [Google Scholar] [CrossRef]
- Savvinov, G.N.; Velichenko, V.V. Fuel and energy complex of Yakutia: Environmental aspects. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 808, 012062. [Google Scholar] [CrossRef]
- Bolshakova, A.D.; Zaznobina, N.I.; Kovaleva, T.A. The role of green spaces in the improvement of the urban population health quality (on the example of Nizhny Novgorod). Samara J. Sci. 2023, 12, 27–33. [Google Scholar] [CrossRef]
- Aladyshkina, A.; Lakshina, V.; Leonova, L.; Maksimov, A. Ecological and economic modeling of environmental influence on disease incidence in children examplified by Nizhny Novgorod region. Sotsial’nye Aspekty Zdorov’ya Naseleniya 2018, 2, 8. [Google Scholar] [CrossRef]
- State Report “On the Sanitary and Epidemiological Well-Being of the Population in the Nizhny Novgorod Region in 2024”. Available online: https://52.rospotrebnadzor.ru (accessed on 26 September 2025).
- Sotkina, S.A.; Badina, O.N.; Shevchenko, I.A.; Bikmaeva, A.V. The ecological condition of the city of Dzerzhinsk on the degree of soil pollution with heavy metals. Adv. Curr. Nat. Sci. 2017, 6, 96–101. (In Russian) [Google Scholar]
- Georgiadi, A.; Sharapova, E.; Danilenko, A. Urban impact on water quality in the rivers of Central Russia. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 834, 012030. [Google Scholar] [CrossRef]

| Blood Tests | Reference Ranges | Recorded Values of the Studied Cohort (Mean ± SEM [Min–Max]) |
|---|---|---|
| Hematological parameters | ||
| Total number of red blood cells per liter (RBC), 1012/L | 3.8–5.3 | 4.60 ± 0.03 [2.62–6.38] |
| Total number of white blood cells per liter (WBC), 109/L | 4.1–11.1 | 6.14 ± 0.09 [1.82–20.60] |
| Hemoglobin concentration (HGB), g/L | 117–155 | 128.83 ± 0.77 [74.00–174.00] |
| Red blood cell volume (MCV), fL | 83–100 | 89.28 ± 0.40 [79.30–115.60] |
| Hematocrit (HCT), % | 35–45 | 41.17 ± 0.29 [23.00–58.80] |
| Mean corpuscular hemoglobin content (MCH), pg | 32–37 | 28.13 ± 0.14 [16.20–39.60] |
| Mean corpuscular hemoglobin concentration (MCHC), g/L | 318–342 | 315.22 ± 1.57 [245.00–423.00] |
| Percentage of lymphocytes (LYM), % | 16–46 | 32.48 ± 0.39 [2.90–57.00] |
| Red blood cell distribution width by volume (RDW-CV), % | 12.2–14.6 | 13.88 ± 0.07 [10.90–20.70] |
| Platelet count (PLT), 109/L | 150–400 | 265.05 ± 3.41 [81.00–654.00] |
| Biochemical parameters | ||
| Albumin, g/L | 35–50 | 44.08 ± 0.27 [29.50–77.00] |
| Glucose, mmol/L | 3.3–5.6 | 4.66 ± 0.05 [2.59–11.62] |
| Creatinine, umol/L | 44–80 | 90.03 ± 0.96 [38.50–213.00] |
| Alkaline phosphatase (ALP), U/L | 35–130 | 169.25 ± 3.35 [0.00–776.00] |
| C-reactive protein (CRP), mg/L | 0–5 | 6.24 ± 0.34 [0.00–8.70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallio, A.E.; Davydova, E.A.; Mishchenko, T.A.; Sivtseva, T.M.; Nikolaeva, M.M.; Burmistrov, D.E.; Bolshakova, A.D.; Tsybusov, S.N.; Zakharova, R.N.; Vedunova, M.V. Age-Related Human Adaptation to Extreme Climatic Factors and Environmental Conditions. Biology 2025, 14, 1668. https://doi.org/10.3390/biology14121668
Kallio AE, Davydova EA, Mishchenko TA, Sivtseva TM, Nikolaeva MM, Burmistrov DE, Bolshakova AD, Tsybusov SN, Zakharova RN, Vedunova MV. Age-Related Human Adaptation to Extreme Climatic Factors and Environmental Conditions. Biology. 2025; 14(12):1668. https://doi.org/10.3390/biology14121668
Chicago/Turabian StyleKallio, Anna E., Ekaterina A. Davydova, Tatiana A. Mishchenko, Tatiana M. Sivtseva, Maria M. Nikolaeva, Dmitriy E. Burmistrov, Anzhela D. Bolshakova, Sergey N. Tsybusov, Raisa N. Zakharova, and Maria V. Vedunova. 2025. "Age-Related Human Adaptation to Extreme Climatic Factors and Environmental Conditions" Biology 14, no. 12: 1668. https://doi.org/10.3390/biology14121668
APA StyleKallio, A. E., Davydova, E. A., Mishchenko, T. A., Sivtseva, T. M., Nikolaeva, M. M., Burmistrov, D. E., Bolshakova, A. D., Tsybusov, S. N., Zakharova, R. N., & Vedunova, M. V. (2025). Age-Related Human Adaptation to Extreme Climatic Factors and Environmental Conditions. Biology, 14(12), 1668. https://doi.org/10.3390/biology14121668








