Improving Soil Properties and Microbiomes by Mixed Eucalyptus–Cupressus Afforestation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. Soil Physical and Chemical Properties
2.4. DNA Extraction and High-Throughput Sequencing
2.5. Sequence Processing and Bioinformatic Analysis
2.6. Metabolome Sequencing and Differentially Expressed Metabolites (DEMs) Analysis
3. Results
3.1. Soil Properties in Different Mixed Forests
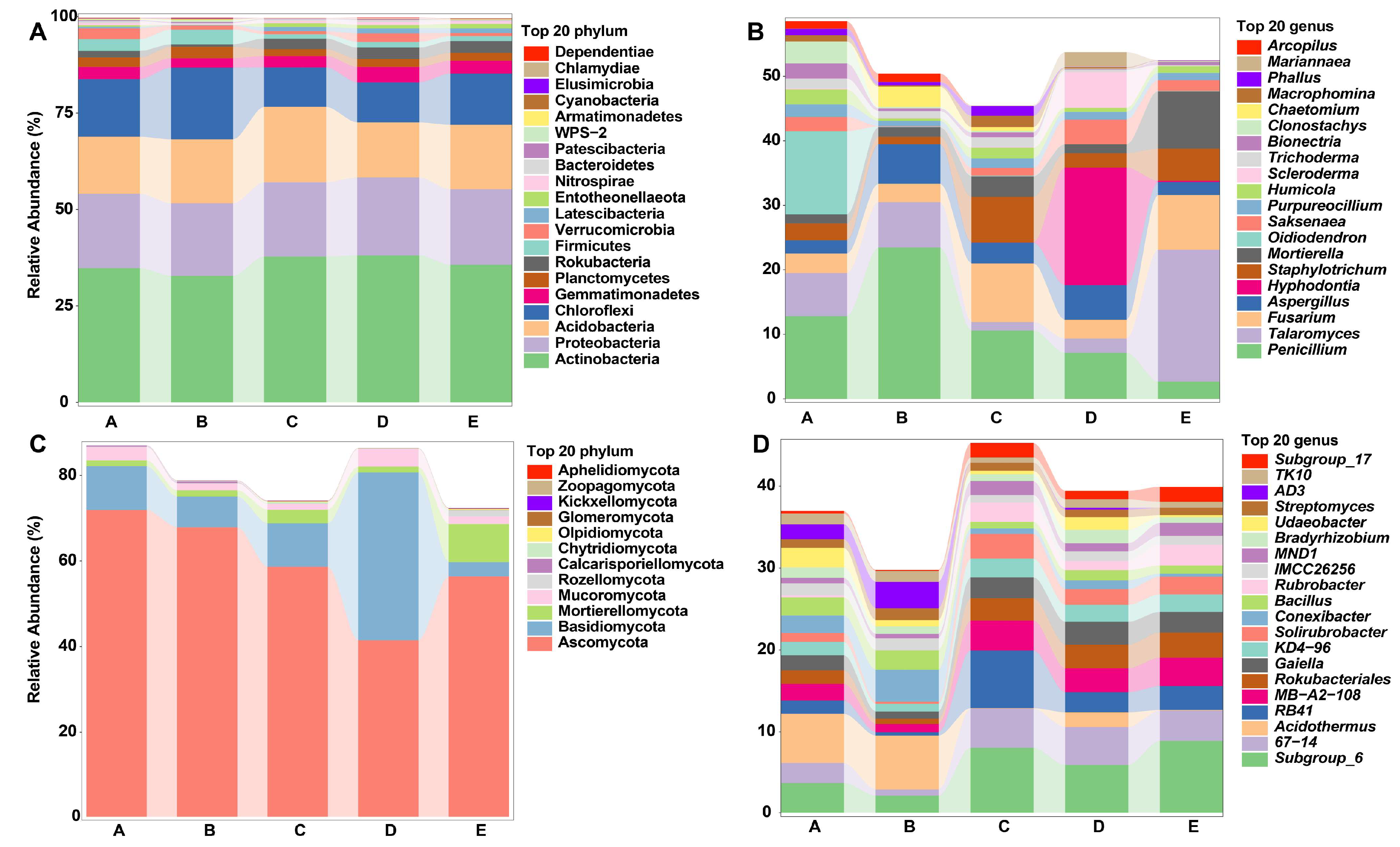
3.2. Microbial Community Composition in Different Mixed Forests
3.3. Soil Microbial Diversity in Different Mixed Forests
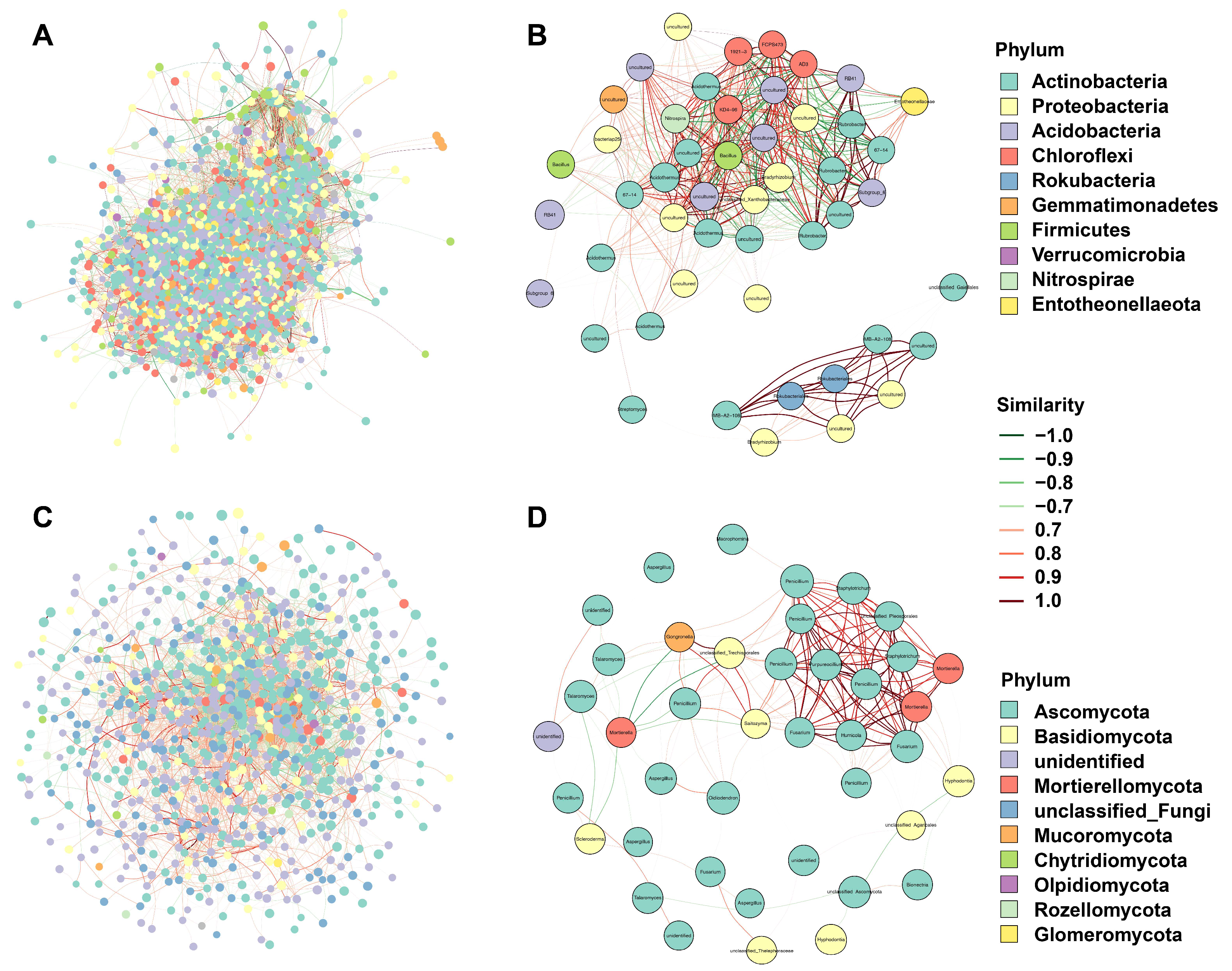
3.4. Co-Occurrence Network Analyses of Bacteria and Fungi
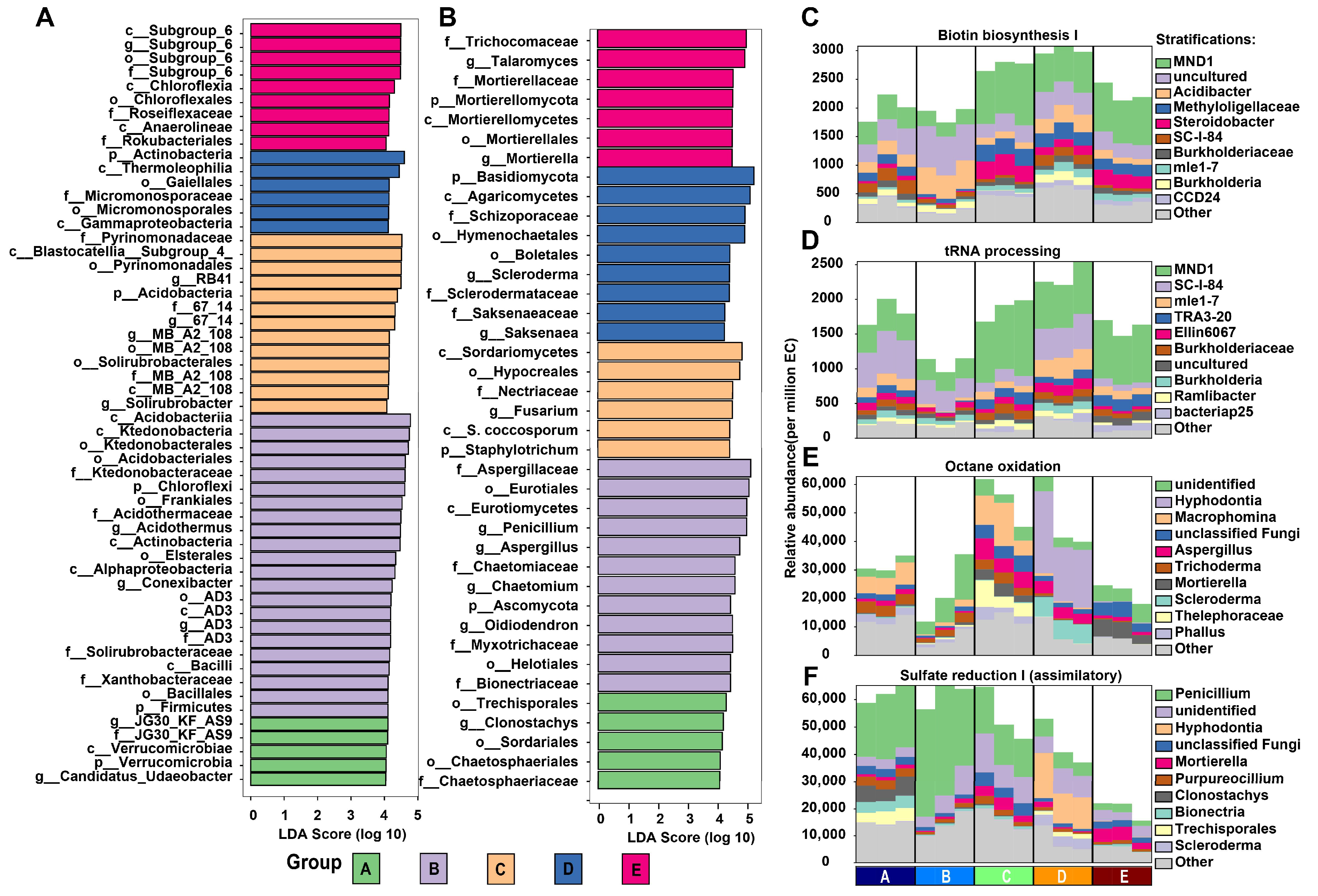
3.5. Microbial Biomarkers and Functional Analysis in Different Mixed Forests
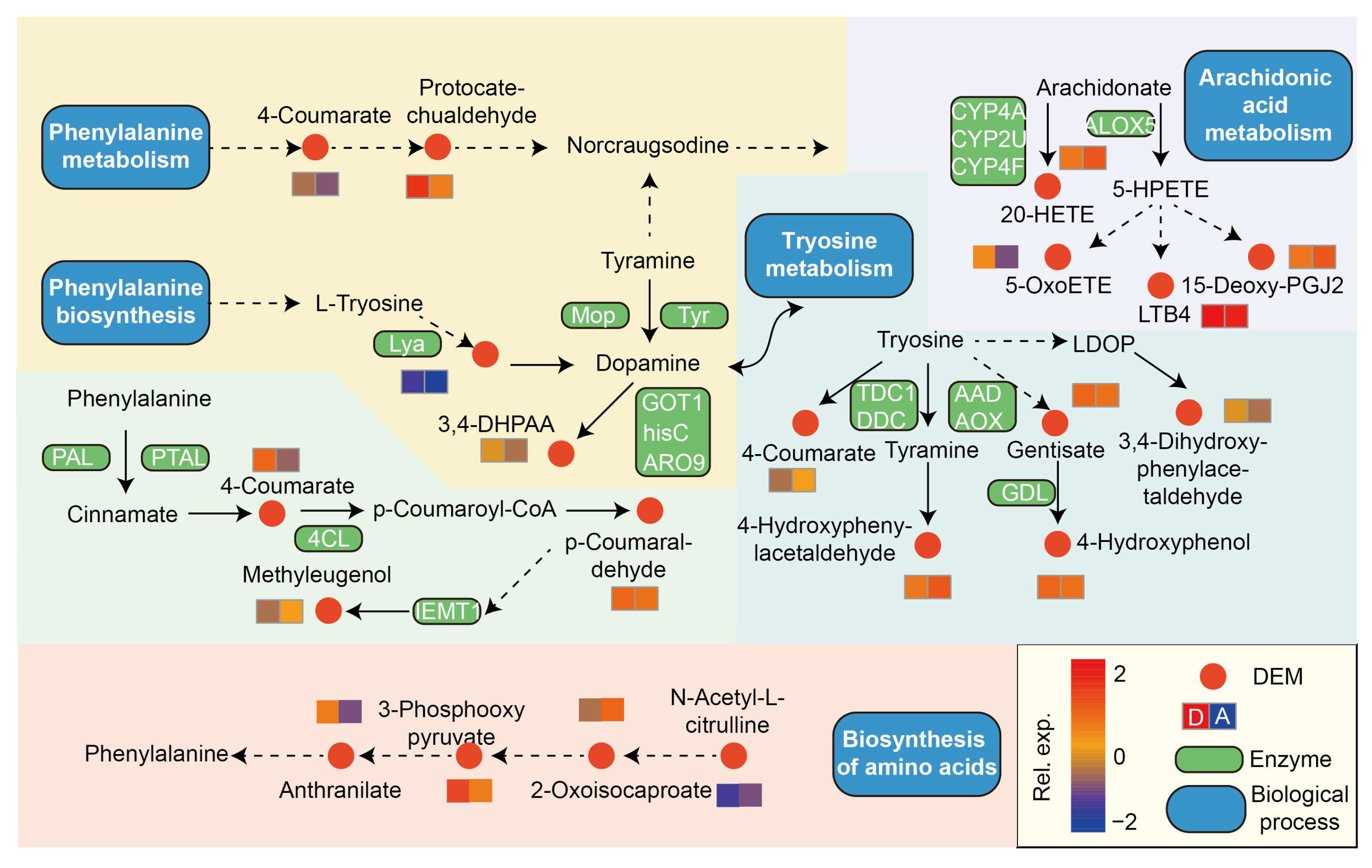
3.6. Identification of Metabolites and Functional Annotation of DEMs
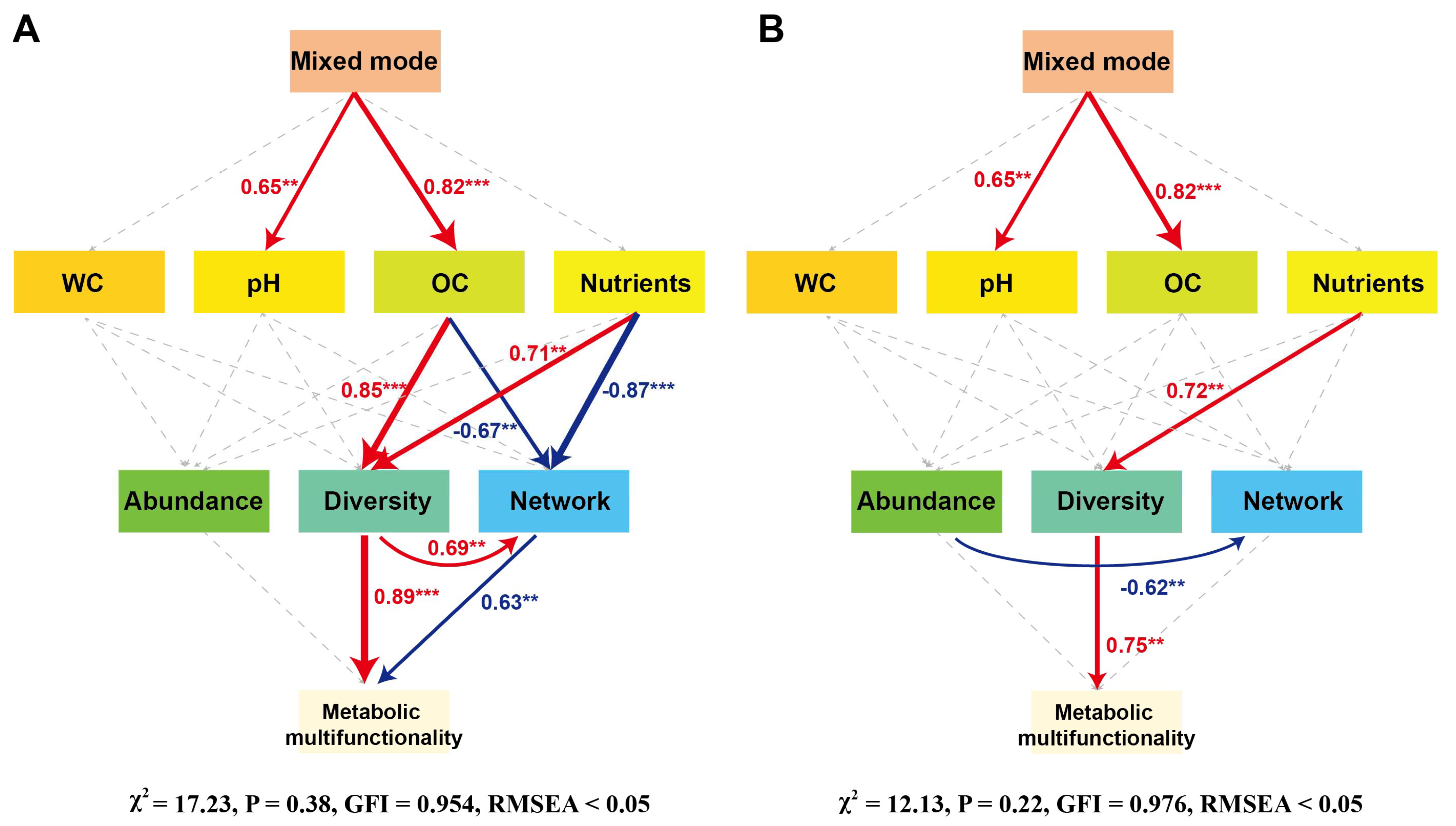
3.7. Interactions Among Soil Properties, Microbial Communities, and Soil Metabolic Multifunctionality
4. Discussion
4.1. Effects of Tree Species Composition on Soil Physicochemical Properties
4.2. Microbial Community Structure and Ecological Implications
4.3. Functional Microbial Adaptations and Ecosystem Processes
4.4. Metabolomic Shifts and Microbe–Metabolite Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, C.; Li, L.; Ouyang, L.; Su, M.; Guo, K. E. urophylla × E. grandis high-quality genome and comparative genomics provide insights on evolution and diversification of eucalyptus. BMC Genom. 2023, 24, 223. [Google Scholar] [CrossRef]
- Kaur, A.; Monga, R. Eucalyptus trees plantation: A review on suitability and their beneficial role. Int. J. Bio-Resour. Stress Manag. 2021, 12, 16–25. [Google Scholar] [CrossRef]
- Molla, G.; Addisie, M.B.; Ayele, G.T. Expansion of eucalyptus plantation on fertile cultivated lands in the North-Western highlands of Ethiopia. Remote Sens. 2023, 15, 661. [Google Scholar] [CrossRef]
- Tomé, M.; Almeida, M.H.; Barreiro, S.; Branco, M.R.; Deus, E.; Pinto, G.; Silva, J.S.; Soares, P.; Rodríguez-Soalleiro, R. Opportunities and challenges of Eucalyptus plantations in Europe: The Iberian Peninsula experience. Eur. J. For. Res. 2021, 140, 489–510. [Google Scholar] [CrossRef]
- Medeiros, P.L.D.; Pimenta, A.S.; Miranda, N.D.O.; Melo, R.R.D.; Amorim, J.D.S.; Azevedo, T.K.B.D. The myth that eucalyptus trees deplete soil water—A review. Forests 2025, 16, 423. [Google Scholar] [CrossRef]
- MRahman, A.; Das, A.K.; Al Riyadh, Z.; Suhag, M.; Rahman, M.M. Eucalyptus in Agriculture: Friend or Foe? Analyzing its impact on crop yields, soil dynamics, and farmers’ perceptions in Bangladesh. Agrofor. Syst. 2024, 98, 3109–3128. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisbing, S.; Grossenbacher, D.L.; Ritter, M.; Yost, J.M. Testing an invasion mechanism for Eucalyptus globulus: Is there evidence of allelopathy. Am. J. Bot. 2021, 108, 607–615. [Google Scholar] [CrossRef]
- Duff, T.; Porteous, M.G.; Goodger, J.Q. Wildfires, Flammable Oils, and Eucalyptus Trees: The Persistence and Volatility of Terpenes in Excised Leaves. SSRN 4120325. 2022. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4120325 (accessed on 6 April 2025).
- Guerrero, F.; Carmona, C.; Hernández, C.; Toledo, M.; Arriagada, A.; Espinoza, L.; Bergmann, J.; Taborga, L.; Yañez, K.; Carrasco, Y. Drivers of flammability of Eucalyptus globulus Labill leaves: Terpenes, essential oils, and moisture content. Forests 2022, 13, 908. [Google Scholar] [CrossRef]
- Williams, R.A. Mitigating biodiversity concerns in Eucalyptus plantations located in South China. J. Biosci. Med. 2015, 3, 1. [Google Scholar] [CrossRef]
- Xie, Y.; Arnold, R.J.; Wu, Z.; Chen, S.; Du, A.; Luo, J. Advances in eucalypt research in China. Front. Agric. Sci. Eng. 2017, 4, 380–390. [Google Scholar] [CrossRef]
- Kindt, R.; Dawson, I.K.; Lillesø, J.-P.B.; Muchugi, A.; Pedercini, F.; Roshetko, J.; van Noordwijk, M.; Graudal, L.; Jamnadass, R. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. World Agrofor. 2021, 312, 1–21. [Google Scholar]
- Nair, P.R.; Kumar, B.M.; Nair, V.D. Multipurpose trees (MPTs) and other agroforestry species. In An Introduction to Agroforestry: Four Decades of Scientific Developments; Springer: Berlin, Germany, 2022; pp. 281–351. [Google Scholar]
- Jiang, X.; Yang, J.; Yang, Y.; Yang, J.; Dong, Q.; Zeng, H.; Zhang, K.; Xu, N.; Yuan, J.; Liu, M. Response of Thinning to C: N: P Stoichiometric Characteristics and Seasonal Dynamics of Leaf-Litter-Soil System in Cupressus funebris Endl. Artificial Forests in Southwest, China. Forests 2024, 15, 1435. [Google Scholar] [CrossRef]
- Abiyu, A.; Lemenih, M.; Gratzer, G.; Aerts, R.; Teketay, D.; Glatzel, G. Status of native woody species diversity and soil characteristics in an exclosure and in plantations of Eucalyptus globulus and Cupressus lusitanica in Northern Ethiopia. Mt. Res. Dev. 2011, 31, 144–152. [Google Scholar] [CrossRef]
- Alem, S.; Pavlis, J.; Urban, J.; Kucera, J. Pure and mixed plantations of Eucalyptus camaldulensis and Cupressus lusitanica: Their growth interactions and effect on diversity and density of undergrowth woody plants in relation to light. Open J. For. 2015, 5, 375. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Pereira, A.P.D.A.; Santana, M.C.; Zagatto, M.R.; Brandani, C.B.; Wang, J.-T.; Verma, J.P.; Singh, B.K.; Cardoso, E.J. Nitrogen-fixing trees in mixed forest systems regulate the ecology of fungal community and phosphorus cycling. Sci. Total Environ. 2021, 758, 143711. [Google Scholar] [CrossRef]
- He, Y.; Han, X.; Wang, X.; Wang, L.; Liang, T. Long-term ecological effects of two artificial forests on soil properties and quality in the eastern Qinghai-Tibet Plateau. Sci. Total Environ. 2021, 796, 148986. [Google Scholar] [CrossRef]
- Qian, J.; Ji, C.; Yang, J.; Zhao, H.; Wang, Y.; Fu, L.; Liu, Q. The advantage of afforestation using native tree species to enhance soil quality in degraded forest ecosystems. Sci. Rep. 2024, 14, 20022. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Liu, Y.; Yao, B.; Yu, M.; Ma, J.; Yang, X.; Xue, J.; Xiang, Y.; Li, Y. Impact of mixed plantations on soil physicochemical properties: Variations and controlling factors in China. For. Ecol. Manag. 2024, 568, 122107. [Google Scholar] [CrossRef]
- Li, D.; Fan, S.; He, A.; Yin, F. Forest resources and environment in China. J. For. Res. 2004, 9, 307–312. [Google Scholar] [CrossRef]
- Yao, L.; Jiao, J.; Wu, C.; Jiang, B.; Fan, L. Effects of thinning on the structure of soil microbial communities in a subtropical secondary evergreen broad-leaved forest. Front. Plant Sci. 2024, 15, 1465237. [Google Scholar] [CrossRef] [PubMed]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Yu, A.; Tang, Z.; Yin, H.; Wu, S.; Xiang, Y.; Yang, J.; Chen, G.; Hou, G.; Fan, C.; Zhao, K. Thinning and replanting enhance soil environmental stability in Cupressus funebris plantations: Insights from soil nematodes. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Zhan, C. Microbial decomposition and soil health: Mechanisms and ecological implications. Mol. Soil Biol. 2024, 15. [Google Scholar] [CrossRef]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; von Wettberg, E.J.; Sachs, J.L.; Martinez-Romero, E. Microbially mediated plant functional traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. The Aspergilli and their mycotoxins: Metabolic interactions with plants and the soil biota. Front. Microbiol. 2020, 10, 2921. [Google Scholar] [CrossRef]
- Vassileva, M.; Mendes, G.D.O.; Deriu, M.A.; Benedetto, G.D.; Flor-Peregrin, E.; Mocali, S.; Martos, V.; Vassilev, N. Fungi, P-solubilization, and plant nutrition. Microorganisms 2022, 10, 1716. [Google Scholar] [CrossRef]
- Yilmaz, N.; López-Quintero, C.A.; Vasco-Palacios, A.M.; Frisvad, J.C.; Theelen, B.; Boekhout, T.; Samson, R.A.; Houbraken, J. Four novel Talaromyces species isolated from leaf litter from Colombian Amazon rain forests. Mycol. Prog. 2016, 15, 1041–1056. [Google Scholar] [CrossRef]
- Binyamin, R.; Nadeem, S.M.; Akhtar, S.; Khan, M.Y.; Anjum, R. Beneficial and pathogenic plant-microbe interactions: A review. Soil Environ. 2019, 38, 127–150. [Google Scholar] [CrossRef]
- Rice, K.C.; Turner, M.E.; Carney, O.N.V.; Gu, T.; Ahn, S.-J. Modification of the Streptococcus mutans transcriptome by LrgAB and environmental stressors. Microb. Genom. 2017, 3, e000104. [Google Scholar] [CrossRef]
- Nowicka, B.; Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef] [PubMed]
- Raza, A. Metabolomics: A systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2020, 41, 741–763. [Google Scholar] [CrossRef]
- PDivekar, A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]


| Indexes | WC | pH | OC | AN | AP | AK | TN | TP | TK | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16S | α-diversity | Chao1 | 0.428 | 0.508 | 0.769 | 0.849 | 0.216 | 0.775 | 0.856 | −0.285 | 0.142 |
| Shannon | 0.23 | 0.583 | 0.865 | 0.886 * | 0.243 | 0.904 * | 0.938 * | −0.178 | 0.223 | ||
| Pielou | 0.063 | 0.549 | 0.917 * | 0.885 * | 0.18 | 0.945 * | 0.948 * | −0.058 | 0.308 | ||
| Phylum | Firmicutes | −0.104 | −0.765 | −0.741 | −0.777 | −0.551 | −0.872 | −0.899 * | 0.039 | −0.293 | |
| Gemmatimonadetes | 0.173 | 0.278 | 0.902 * | 0.868 | −0.124 | 0.815 | 0.843 | −0.112 | 0.267 | ||
| Acidobacteria | 0.057 | 0.402 | −0.273 | −0.162 | 0.648 | −0.086 | −0.052 | 0.022 | −0.028 | ||
| Verrucomicrobia | 0.398 | −0.47 | 0.005 | −0.006 | −0.748 | −0.125 | −0.148 | −0.62 | −0.532 | ||
| Rokubacteria | 0.046 | 0.762 | 0.811 | 0.812 | 0.477 | 0.946 * | 0.944 * | −0.064 | 0.272 | ||
| Chloroflexi | −0.476 | −0.48 | −0.734 | −0.825 | −0.192 | −0.737 | −0.822 | 0.335 | −0.093 | ||
| Actinobacteria | 0.527 | 0.398 | 0.757 | 0.856 | 0.112 | 0.707 | 0.815 | −0.316 | 0.132 | ||
| ITS | α-diversity | Chao1 | 0.348 | −0.444 | −0.604 | −0.457 | −0.098 | −0.739 | −0.606 | −0.006 | −0.124 |
| Shannon | 0.35 | 0.612 | 0.098 | 0.217 | 0.549 | 0.34 | 0.346 | −0.479 | −0.307 | ||
| Pielou | 0.17 | 0.788 | 0.315 | 0.368 | 0.598 | 0.619 | 0.564 | −0.45 | −0.252 | ||
| Phylum | Ascomycota | −0.192 | −0.229 | −0.967 ** | −0.985 ** | 0.006 | −0.755 | −0.892 * | −0.264 | −0.68 | |
| Basidiomycota | 0.568 | −0.349 | 0.714 | 0.769 | −0.629 | 0.329 | 0.502 | −0.11 | 0.298 | ||
| Calcarisporiellomycota | −0.151 | −0.643 | −0.834 | −0.877 | −0.436 | −0.861 | −0.935 * | −0.075 | −0.45 | ||
| Chytridiomycota | 0.106 | 0.352 | −0.28 | −0.159 | 0.602 | −0.118 | −0.07 | 0.006 | −0.034 | ||
| Mortierellomycota | −0.76 | 0.916 * | 0.274 | 0.149 | 0.918 | 0.646 | 0.489 | 0.458 | 0.368 | ||
| Olpidiomycota | −0.463 | 0.963 ** | 0.338 | 0.293 | 0.959 | 0.683 | 0.592 | 0.288 | 0.316 | ||
| Rozellomycota | −0.852 | 0.826 | 0.313 | 0.149 | 0.8 | 0.647 | 0.475 | 0.535 | 0.424 | ||
| Zoopagomycota | 0.331 | −0.466 | −0.361 | −0.221 | −0.14 | −0.598 | −0.417 | 0.208 | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.-W.; Liu, Y.-Y.; Jiang, Y.-X.; Li, W.-Q.; Peng, Y.; Zhou, S.-M.; You, S.-Q.; Liu, S.-Q.; Deng, H.-P. Improving Soil Properties and Microbiomes by Mixed Eucalyptus–Cupressus Afforestation. Biology 2025, 14, 1667. https://doi.org/10.3390/biology14121667
Zuo Y-W, Liu Y-Y, Jiang Y-X, Li W-Q, Peng Y, Zhou S-M, You S-Q, Liu S-Q, Deng H-P. Improving Soil Properties and Microbiomes by Mixed Eucalyptus–Cupressus Afforestation. Biology. 2025; 14(12):1667. https://doi.org/10.3390/biology14121667
Chicago/Turabian StyleZuo, You-Wei, Yu-Ying Liu, Ya-Xin Jiang, Wen-Qiao Li, Yang Peng, Sheng-Mao Zhou, Shi-Qi You, Sheng-Qiao Liu, and Hong-Ping Deng. 2025. "Improving Soil Properties and Microbiomes by Mixed Eucalyptus–Cupressus Afforestation" Biology 14, no. 12: 1667. https://doi.org/10.3390/biology14121667
APA StyleZuo, Y.-W., Liu, Y.-Y., Jiang, Y.-X., Li, W.-Q., Peng, Y., Zhou, S.-M., You, S.-Q., Liu, S.-Q., & Deng, H.-P. (2025). Improving Soil Properties and Microbiomes by Mixed Eucalyptus–Cupressus Afforestation. Biology, 14(12), 1667. https://doi.org/10.3390/biology14121667








