Climate-Driven Habitat Shifts of Two Palm Squirrel Species (Sciuridae: Funambulus) and Projected Expansion of Their Range Overlap with Indian Agroecosystems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
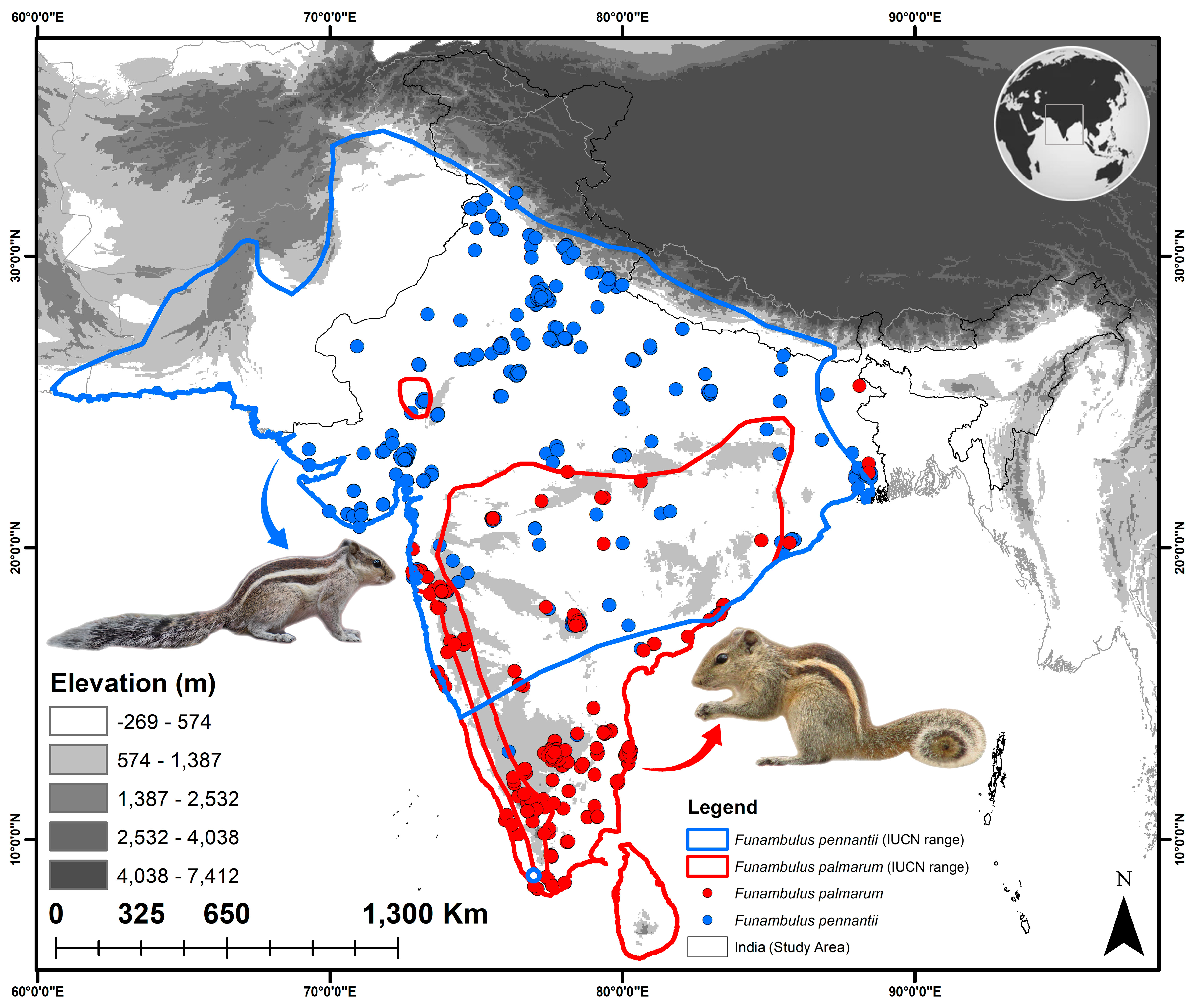
2.1. Study Area and Occurrence Records
2.2. Modeling Predictors
2.3. Ensemble Distribution Model
3. Results
3.1. Model Evaluation
3.2. Predictor Importance and Response
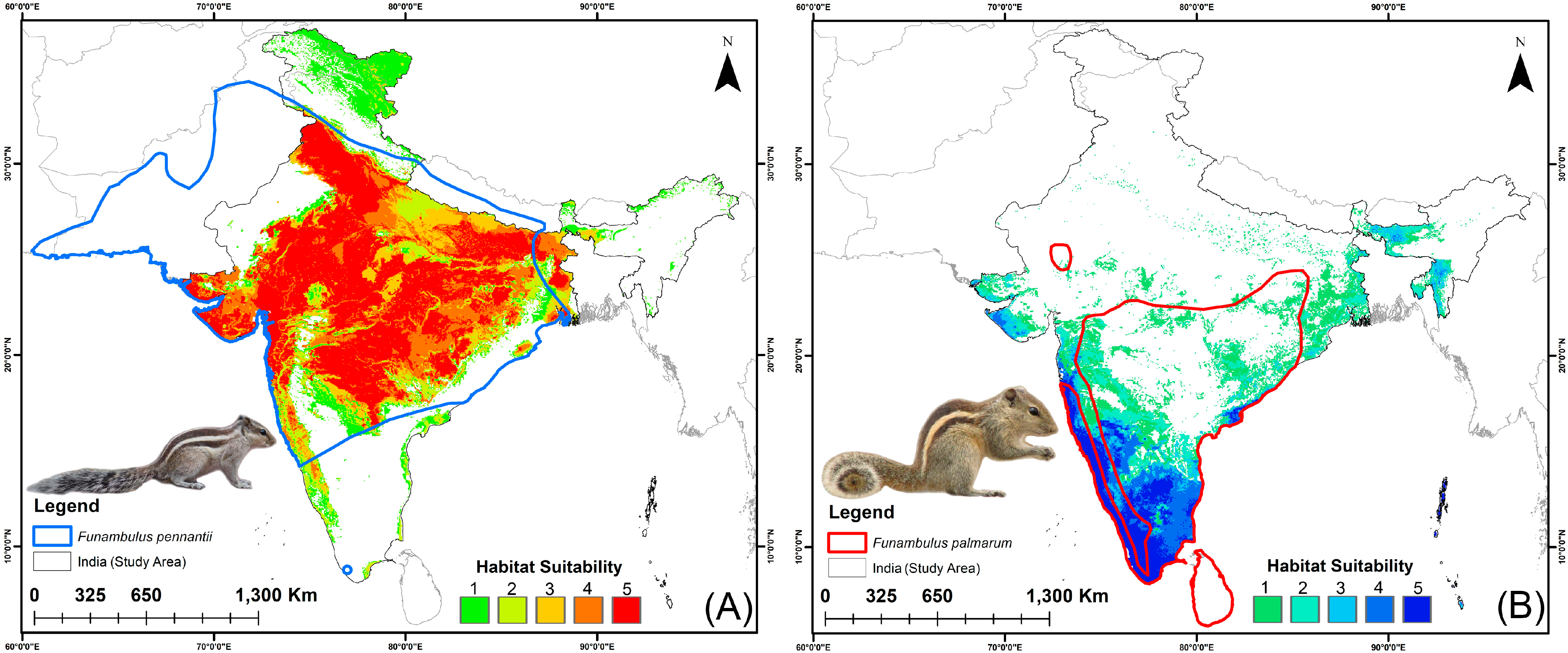
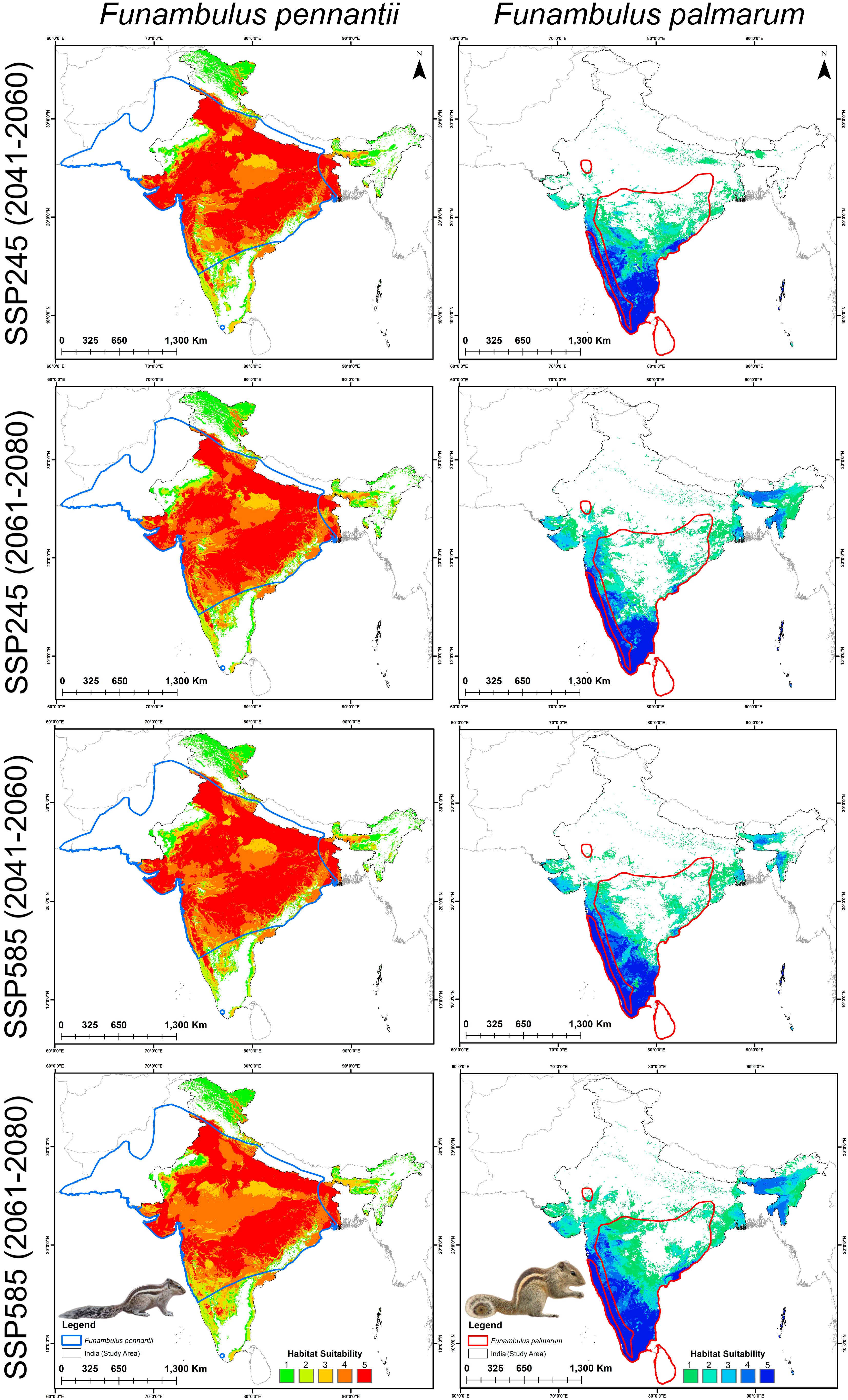
3.3. Habitat Suitability: Present and Future
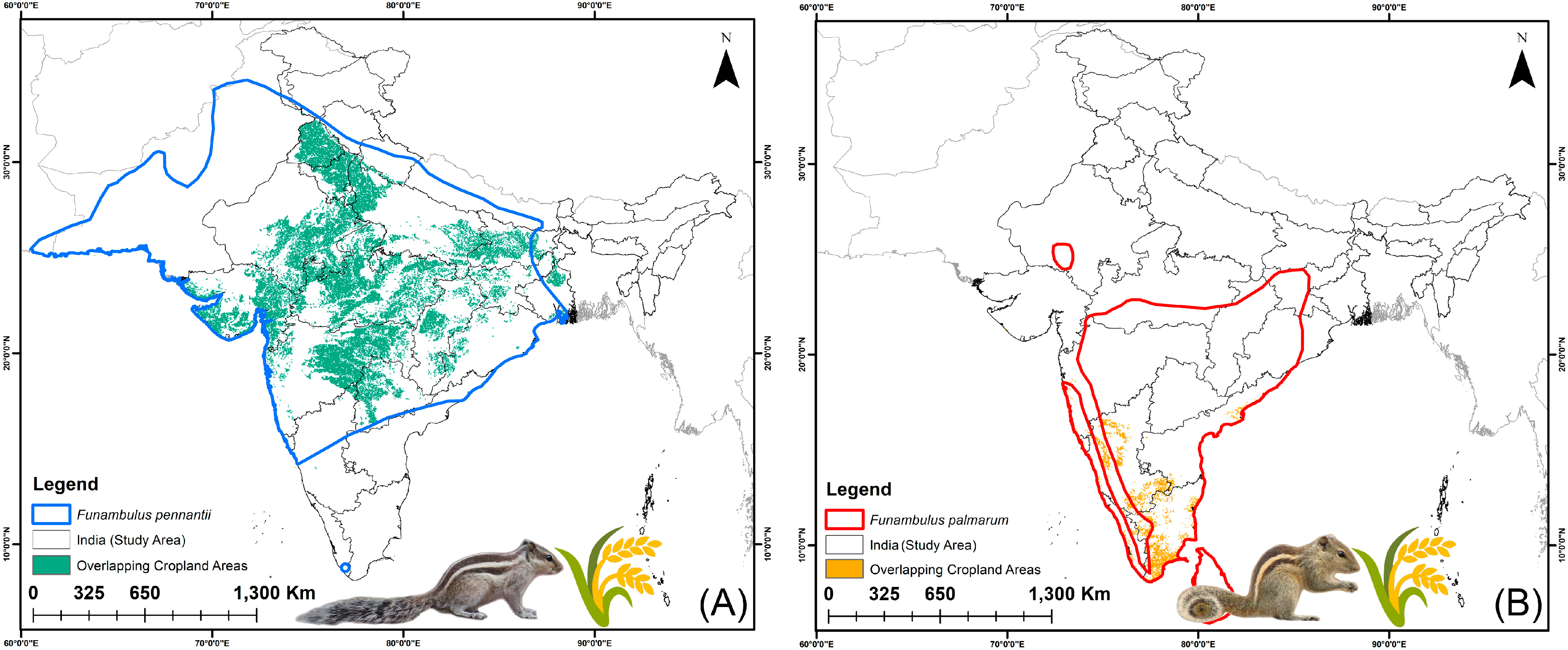
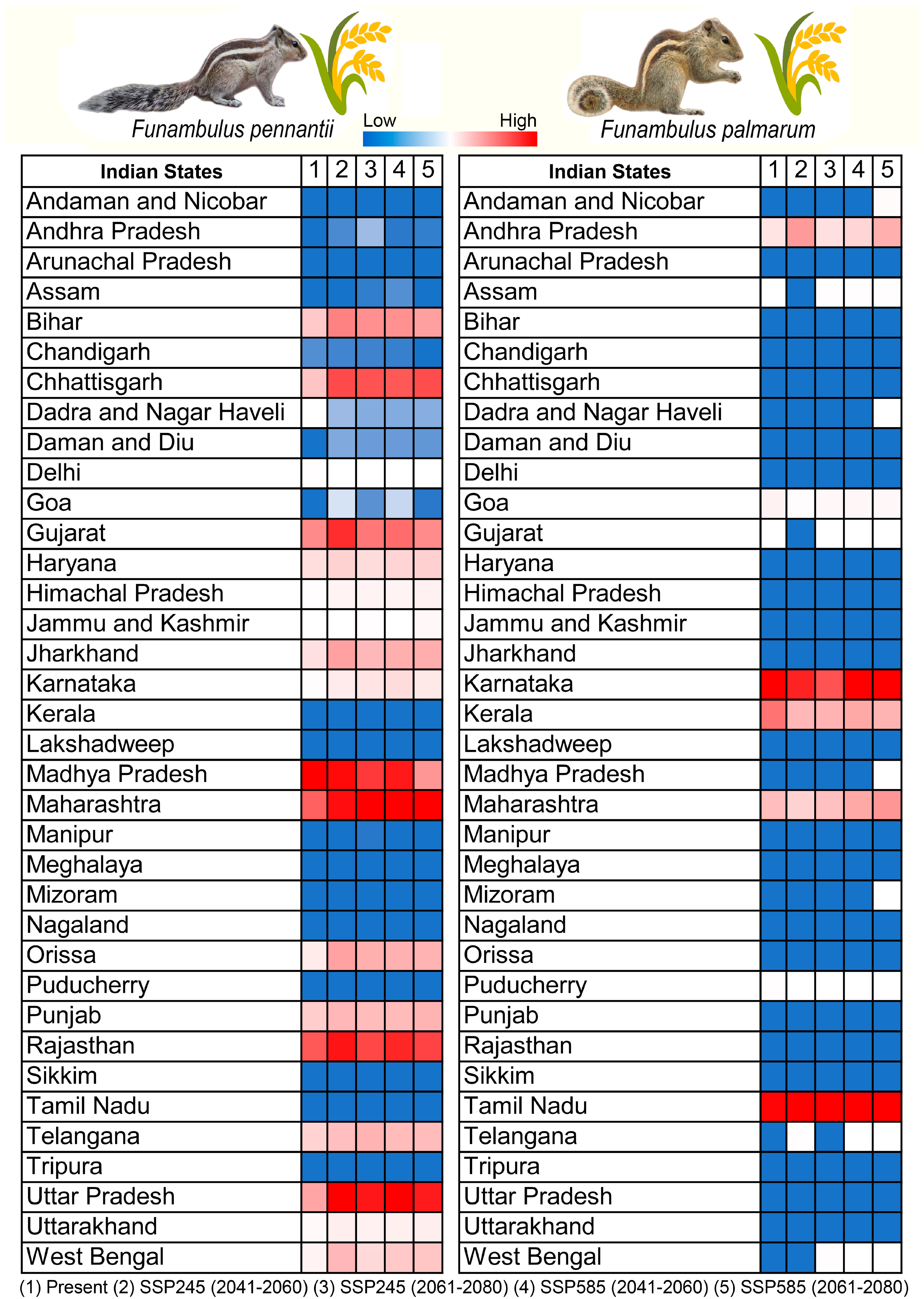
3.4. Agricultural Vulnerability: Present and Future
4. Discussion
5. Recommendations for Management Interventions
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180792. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, X.; Xu, M.; Li, K.; Huan, Y.; Zhou, G.; Lun, F.; Shang, W.; Zhang, R.; Xie, Y. High-resolution global distribution projections of 10 rodent genera under diverse SSP-RCP scenarios, 2021–2100. Sci. Data 2025, 12, 1467. [Google Scholar] [CrossRef]
- Lima, M.; Marquet, P.A.; Jaksic, F.M. El Niño events, precipitation patterns, and rodent outbreaks are statistically associated in semiarid Chile. Ecography 1999, 22, 213–218. [Google Scholar] [CrossRef]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190104. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Yi, X.; Zhou, W.; Liu, R.; Holyoak, M.; Cao, L.; Zhang, M.; Chen, J.; Zhang, Z.; et al. Evolutionary and ecological patterns of scatter- and larder-hoarding behaviours in rodents. Ecol. Lett. 2022, 25, 1202–1214. [Google Scholar] [CrossRef]
- Antão, L.H.; Weigel, B.; Strona, G.; Hällfors, M.; Kaarlejärvi, E.; Dallas, T.; Laine, A.-L. Climate change reshuffles northern species within their niches. Nat. Clim. Change 2022, 12, 587–592. [Google Scholar] [CrossRef]
- Heikonen, S.; Heino, M.; Jalava, M.; Siebert, S.; Viviroli, D.; Kummu, M. Climate change threatens crop diversity at low latitudes. Nat. Food 2025, 6, 331–342. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Aplin, K.P.; Brown, P.R.; Jacob, J.; Krebs, C.J.; Singleton, G.R. Field Methods for Rodent Studies in Asia and the Indo-Pacific; Australian Centre for International Agricultural Research: Melbourne, Australia, 2003. [Google Scholar]
- Stuart, A.M.; Singleton, G.R.; Prescott, C.V. Population ecology of the Asian house rat (Rattus tanezumi) in complex lowland agroecosystems in the Philippines. Wildl. Res. 2015, 42, 165–175. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R.S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- John, A. Rodent outbreaks and rice pre-harvest losses in Southeast Asia. Food Secur. 2014, 6, 249–260. [Google Scholar] [CrossRef]
- Makundi, R.H.; Massawe, A.W. Ecologically based rodent management in Africa: Potential and challenges. Wildl. Res. 2011, 38, 588–595. [Google Scholar] [CrossRef]
- Singleton, G.R.; Belmain, S.; Brown, P.R.; Aplin, K.; Htwe, N.M. Impacts of rodent outbreaks on food security in Asia. Wildl. Res. 2010, 37, 355–359. [Google Scholar] [CrossRef]
- Auffray, J.C.; Renaud, S.; Claude, J. Rodent biodiversity in changing environments. Kasetsart J. Nat. Sci. 2009, 43, 83–93. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Nameer, P.O.; Molur, S. Funambulus pennantii (errata version published in 2017). IUCN Red List Threat. Species 2016, e.T8702A115088099. [Google Scholar] [CrossRef]
- Nameer, P.O.; Molur, S. Funambulus palmarum (errata version published in 2017). IUCN Red List Threat. Species 2016, e.T8701A115087934. [Google Scholar] [CrossRef]
- Ghose, R.K.; Mandal, A.K.; Ghosh, P.S. A contribution to the taxonomy of Indian five-striped squirrel (Funambulus pennanti Wroughton), with description of two new subspecies. Rec. Zool. Surv. India 2004, 102, 89–103. [Google Scholar] [CrossRef]
- Tayade, N.S.; Dabhade, S.D.; Wanjari, V.H. Road casualties of Indian palm squirrel. Int. E-Res. J. 2019, 10, 278–281. [Google Scholar]
- Sharma, S.K. Presence of Indian palm squirrel (Funambulus palmarum Linnaeus) in southern Aravallis. Zoos’ Print J. 2005, 20, 1908–1909. [Google Scholar] [CrossRef]
- Chakravarthy, A.K. Field observations on the squirrel, Funambulus palmarum (Linnaeus) in a cardamom ecosystem in India. Pest Manag. Hortic. Ecosyst. 1996, 2, 89–91. [Google Scholar]
- Chakravarthy, A.K.; Thyagaraj, N.E.; Kumar, L.V.; Girish, A.C. Crop raiding and management of Funambulus palmarum in cardamom (Elettaria cardamomum) plantations of Western Ghats of Karnataka, south India. Curr. Sci. 2008, 94, 907–911. [Google Scholar]
- Chakravarthy, A.; Thyagaraj, N. The palm squirrel in coconut plantations: Ecosystem services by therophily. Mammalia 2012, 76, 193–199. [Google Scholar] [CrossRef]
- Ramesh Singh, Y.; Painkra, G.P.; Kerketta, D.; Kumar, D. First record of leucism in five-striped palm squirrel, Funambulus pennantii (Rodentia: Sciuridae) from North India. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1956–1961. [Google Scholar]
- Ahmed, T.; Nath, S.; Naher, H.; Jowel, H.; Nasrin, S. Abundance and feeding habit of squirrels (Mammalia: Rodentia: Sciuridae) in an urban park, Bangladesh. Taprobanica 2021, 10, 47–53. [Google Scholar] [CrossRef]
- Perodaskalaki, A.; Rammou, D.L.; Thapamagar, T.; Bhandari, S.; Bhusal, D.R.; Youlatos, D. Habitat use and positional behavior of northern palm squirrels (Funambulus pennantii) in an urban forest in central Nepal. Land 2023, 12, 690. [Google Scholar] [CrossRef]
- Puri, K.; Joshi, R. First record of melanism in the northern palm squirrel Funambulus pennantii in Delhi, India. Therya Notes 2023, 4, 249–252. [Google Scholar] [CrossRef]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-De La Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in Southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.E.; Johnston, K.M.; Schmitz, O.J. Global climate change and mammalian species diversity in US national parks. Proc. Natl. Acad. Sci. USA 2003, 100, 11474–11477. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Gholamrezaei, M.; Mohebali, M.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Shirzadi, M.R. Ecological niche modeling of main reservoir hosts of zoonotic cutaneous leishmaniasis in Iran. Acta Trop. 2016, 160, 44–52. [Google Scholar] [CrossRef]
- Jacob, J. In focus: Vertebrate management and risk mitigation. Pest Manag. Sci. 2021, 77, 597–598. [Google Scholar] [CrossRef]
- Biancolini, D.; Pacifici, M.; Falaschi, M.; Bellard, C.; Blackburn, T.M.; Ficetola, G.F.; Rondinini, C. Global distribution of alien mammals under climate change. Glob. Change Biol. 2024, 30, e17560. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Bennett, D.; Richard, F.J. Distribution modelling of the garden dormouse Eliomys quercinus (Linnaeus, 1766) with novel climate change indicators. Mamm. Biol. 2021, 101, 589–599. [Google Scholar] [CrossRef]
- Hamidi, K.; Mohammadi, S.; Eskandarzadeh, N. How will climate change affect the temporal and spatial distributions of a reservoir host, the Indian gerbil (Tatera indica), and the spread of zoonotic diseases that it carries? Evol. Ecol. Res. 2018, 19, 215–226. [Google Scholar]
- Kunwar, B.; Baral, S.; Jeong, Y.-H.; Park, S.-M.; Choi, S.-H.; Oh, H.-S. Predicting the potential distribution of a rodent pest, brown rat (Rattus norvegicus), associated with changes in climate and land cover in South Korea. Ecol. Evol. 2024, 14, e70573. [Google Scholar] [CrossRef]
- Lin, S.; Yao, D.; Jiang, H.; Qin, J.; Feng, Z. Predicting current and future potential distributions of the greater bandicoot rat (Bandicota indica) under climate change conditions. Pest Manag. Sci. 2024, 80, 734–743. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ebrahimi, E.; Moghadam, M.S.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inform. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial Conservation Assessment Tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the concepts right. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gautam, S.; Shany, V.J. Navigating climate change in Southern India: A study on dynamic dry-wet patterns and urgent policy interventions. Geosyst. Geoenviron. 2024, 3, 100263. [Google Scholar] [CrossRef]
- Li, L.; Xie, F.; Yuan, N. On the long-term memory characteristic in land surface air temperatures: How well do CMIP6 models perform? Atmos. Ocean. Sci. Lett. 2023, 16, 100291. [Google Scholar] [CrossRef]
- Karra, K.; Kontgis, C.; Statman-Weil, Z.; Mazzariello, J.C.; Mathis, M.; Brumby, S.P. Global land use/land cover with Sentinel 2 and deep learning. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–16 July 2021; IEEE: Brussels, Belgium, 2021; pp. 4704–4707. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and workflow management for species habitat modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Miller, J. Species distribution modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails; U.S. Geological Survey: Reston, VA, USA, 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 11 January 2025).
- Lavazza, L.; Morasca, S.; Rotoloni, G. On the reliability of the area under the ROC curve in empirical software engineering. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 14–16 June 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 93–100. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination capacity in species distribution models depends on the representativeness of the environmental domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC plots: Calibrating species distribution models with presence-only data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef]
- Liu, Z.; Zhan, W.; Bechtel, B.; Voogt, J.; Lai, J.; Chakraborty, T.; Wang, Z.-H.; Li, M.; Huang, F.; Lee, X. Surface warming in global cities is substantially more rapid than in rural background areas. Commun. Earth Environ. 2022, 3, 219. [Google Scholar] [CrossRef]
- Richardson, J.L.; McCoy, E.P.; Parlavecchio, N.; Szykowny, R.; Beech-Brown, E.; Buijs, J.A.; Buckley, J.; Corrigan, R.M.; Costa, F.; DeLaney, R.; et al. Increasing rat numbers in cities are linked to climate warming, urbanization, and human population. Sci. Adv. 2025, 11, eads6782. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Erauskin-Extramiana, M.; Arrizabalaga, H.; Cabré, A.; Coelho, R.; Rosa, D.; Ibaibarriaga, L.; Chust, G. Are shifts in species distribution triggered by climate change? A swordfish case study. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104666. [Google Scholar] [CrossRef]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Davey, C.M.; Chamberlain, D.E.; Newson, S.E.; Noble, D.G.; Johnston, A. Rise of the generalists: Evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr. 2012, 21, 568–578. [Google Scholar] [CrossRef]
- Rivrud, I.M.; Meisingset, E.L.; Loe, L.E.; Mysterud, A. Future suitability of habitat in a migratory ungulate under climate change. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190442. [Google Scholar] [CrossRef] [PubMed]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet different: Distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mamm. Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Fitak, R.R.; Koprowski, J.L.; Culver, M. Severe reduction in genetic variation in a montane isolate: The endangered Mount Graham red squirrel (Tamiasciurus hudsonicus grahamensis). Conserv. Genet. 2013, 14, 1233–1241. [Google Scholar] [CrossRef]
- Steiner, M.; Huettmann, F. Where do the world’s squirrel hotspots and coldspots of 230+ species go with climate change in 2100? A first BIG DATA minimum estimate from an open access climate niche rapid model assessment. In Sustainable Squirrel Conservation: A Modern Reassessment of Family Sciuridae; Springer International Publishing: Cham, Switzerland, 2023; pp. 317–331. [Google Scholar]
- de Gabriel Hernando, M.; Roa, I.; Fernández-Gil, J.; Juan, J.; Fuertes, B.; Reguera, B.; Revilla, E. Trends in weather conditions favor generalist over specialist species in rear-edge alpine bird communities. Ecosphere 2022, 13, e3953. [Google Scholar] [CrossRef]
- Salunke, P.; Keshri, N.P.; Mishra, S.K.; Dash, S.K. Future projections of seasonal temperature and precipitation for India. Front. Clim. 2023, 5, 1069994. [Google Scholar] [CrossRef]
- Jatav, S.S.; Naik, K.; Nayak, S. Assessing adaptive capacity to climate change of farmers in Gangetic Plains region, India. Discov. Agric. 2024, 2, 97. [Google Scholar] [CrossRef]
- Athira, K.S.; Roxy, M.K.; Dasgupta, P.; Saranya, J.S.; Singh, V.K.; Attada, R. Regional and temporal variability of Indian summer monsoon rainfall in relation to El Niño Southern Oscillation. Sci. Rep. 2023, 13, 12643. [Google Scholar]
- Csurhes, S. Indian Palm Squirrel Risk Assessment; Department of Agriculture and Fisheries, Biosecurity Queensland, Queensland Government: Brisbane, Australia, 2010. [Google Scholar]
- Jatav, S.S.; Naik, K. Measuring the agricultural sustainability of India: An application of Pressure-State-Response (PSR) model. Reg. Sustain. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Lurz, P.W.; Genovesi, P.; Maiorano, L.; Girardello, M.; Bertolino, S. The use of climatic niches in screening procedures for introduced species to evaluate risk of spread: A case with the American eastern grey squirrel. PLoS ONE 2013, 8, e66559. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Martinoli, A.; Russo, D.; Preatoni, D.; Bertolino, S. Modelling the effects of climate change on the risk of invasion by alien squirrels. Hystrix 2016, 27, 1–8. [Google Scholar]
- Habibi, I.; Achour, H.; Bounaceur, F.; Benaradj, A.; Aulagnier, S. Predicting the future distribution of the Barbary ground squirrel (Atlantoxerus getulus) under climate change using niche overlap analysis and species distribution modeling. Environ. Monit. Assess. 2024, 196, 1140. [Google Scholar] [CrossRef]
- Kaur, J.; Singla, N.; Kaur, N. Evaluating non-lethal management strategies against five-striped northern palm squirrel (Funambulus pennantii). Indian J. Entomol. 2025, 87, 67–71. [Google Scholar]
- Malhi, C.S.; Kaur, K. Food preference behaviour of the five-striped squirrel, Funambulus pennanti Wroughton. Behav. Process. 1995, 34, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Chaudhury, V.; Tripathi, R.S. Current Status of Rodent Problem in Indian Agriculture; Technical Bulletin No. 01/2017; All India Network Project on Vertebrate Pest Management (Rodent Control), Indian Council of Agricultural Research (ICAR): New Delhi, India, 2017; 74p. [Google Scholar]
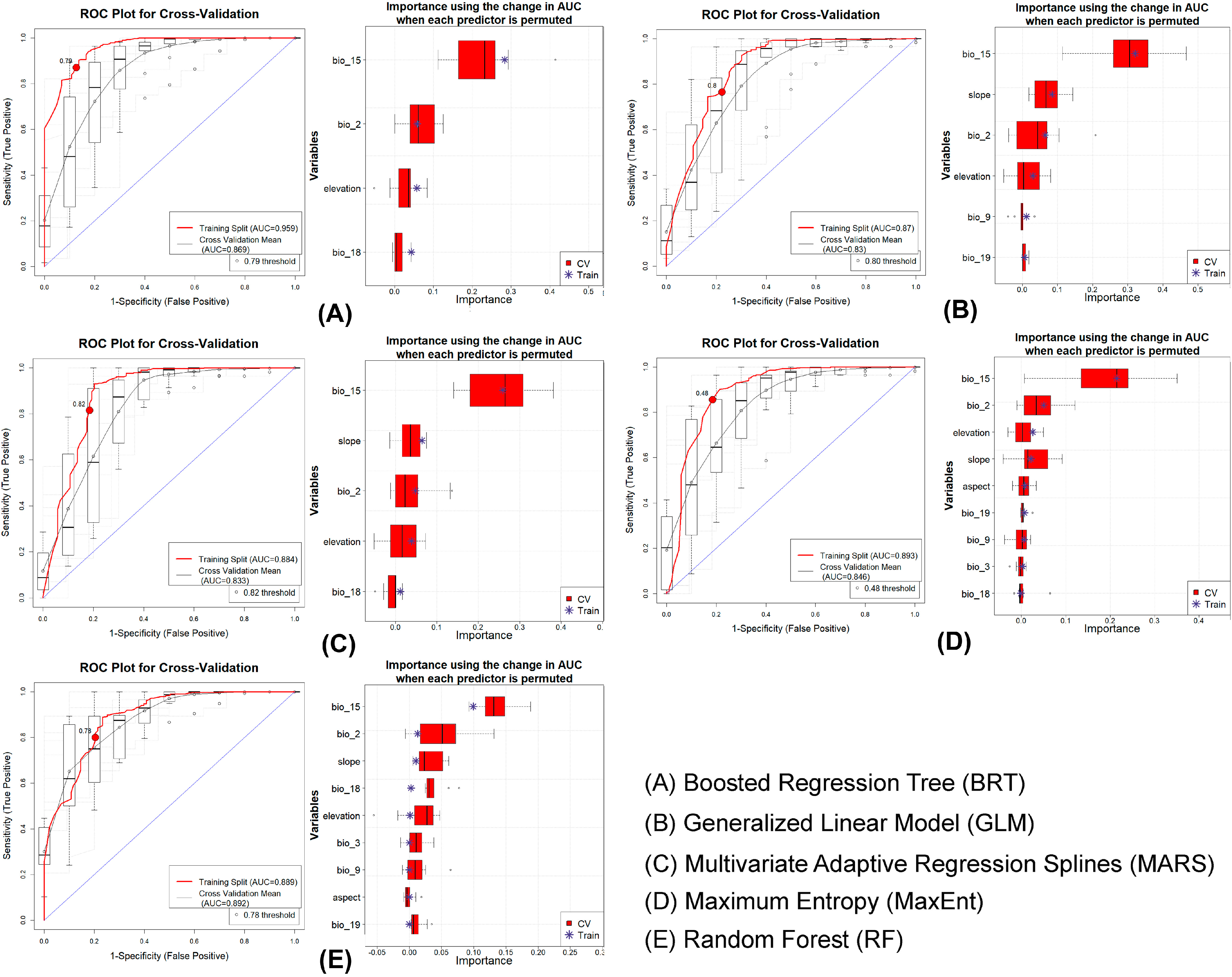
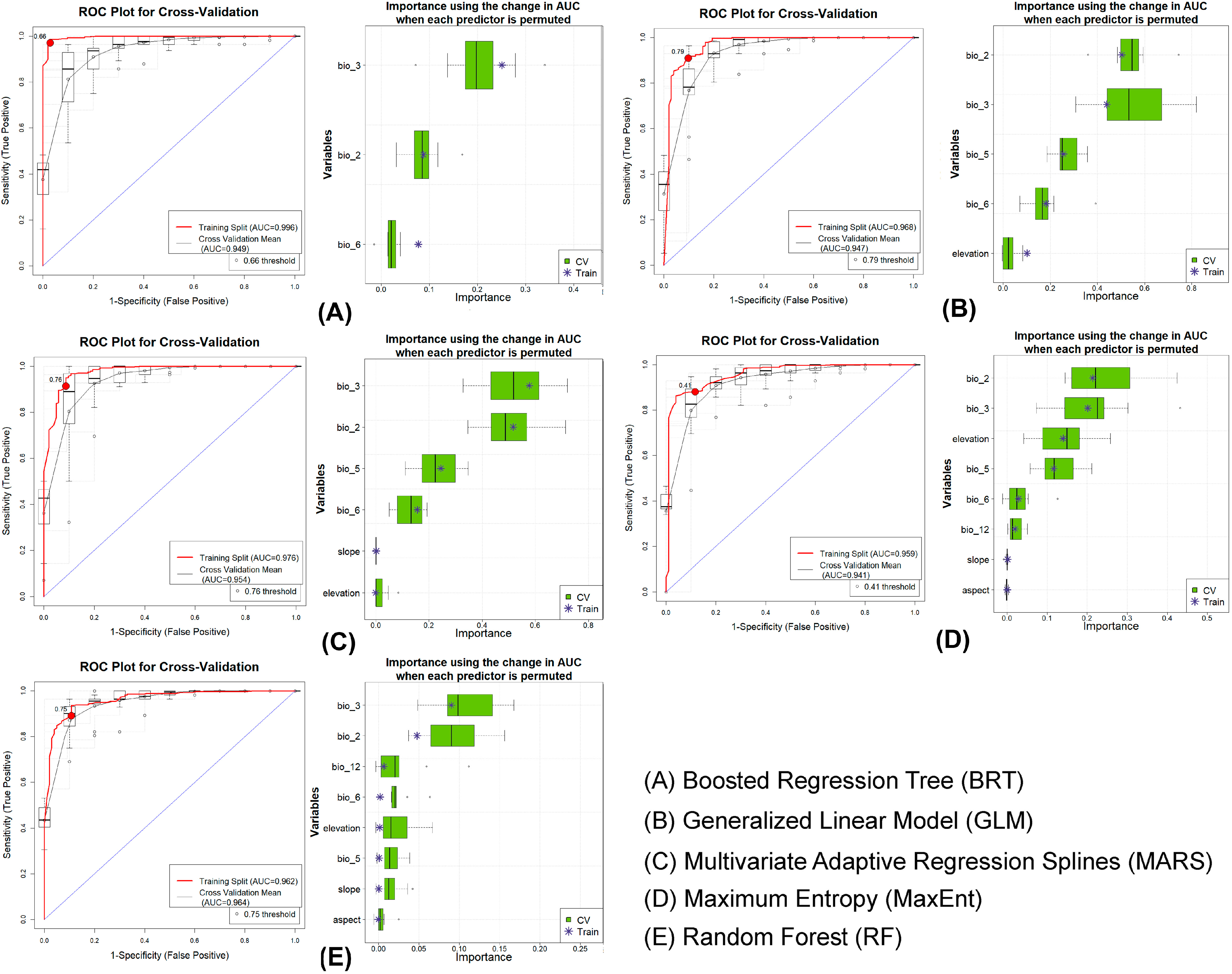

| Species | Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|
| F. pennantii | BRT | Train | 0.959 | 0.090 | 87.100 | 0.744 | 0.693 | 0.874 | 0.871 |
| CV | 0.869 | 81.200 | 0.592 | 0.550 | 0.760 | 0.832 | |||
| GLM | Train | 0.870 | 0.040 | 76.900 | 0.542 | 0.477 | 0.777 | 0.766 | |
| CV | 0.830 | 75.300 | 0.506 | 0.447 | 0.751 | 0.755 | |||
| MARS | Train | 0.884 | 0.051 | 81.500 | 0.630 | 0.570 | 0.816 | 0.815 | |
| CV | 0.833 | 80.500 | 0.595 | 0.549 | 0.780 | 0.815 | |||
| MaxEnt | Train | 0.893 | 0.047 | 84.500 | 0.672 | 0.629 | 0.816 | 0.856 | |
| CV | 0.846 | 79.400 | 0.568 | 0.522 | 0.760 | 0.808 | |||
| RF | Train | 0.889 | 0.003 | 79.900 | 0.597 | 0.537 | 0.796 | 0.801 | |
| CV | 0.892 | 86.100 | 0.596 | 0.622 | 0.663 | 0.934 | |||
| F. palmarum | BRT | Train | 0.996 | 0.047 | 97.100 | 0.942 | 0.928 | 0.971 | 0.971 |
| CV | 0.949 | 87.200 | 0.718 | 0.693 | 0.833 | 0.885 | |||
| GLM | Train | 0.968 | 0.021 | 90.800 | 0.813 | 0.777 | 0.903 | 0.910 | |
| CV | 0.947 | 88.200 | 0.741 | 0.713 | 0.845 | 0.896 | |||
| MARS | Train | 0.976 | 0.022 | 91.300 | 0.826 | 0.790 | 0.913 | 0.914 | |
| CV | 0.954 | 90.800 | 0.806 | 0.774 | 0.892 | 0.914 | |||
| MaxEnt | Train | 0.959 | 0.018 | 88.200 | 0.764 | 0.718 | 0.883 | 0.881 | |
| CV | 0.941 | 87.100 | 0.732 | 0.692 | 0.854 | 0.878 | |||
| RF | Train | 0.962 | 0.002 | 89.200 | 0.785 | 0.742 | 0.893 | 0.892 | |
| CV | 0.964 | 90.300 | 0.712 | 0.737 | 0.755 | 0.957 |
| Species | Variables | BRT | GLM | MARS | MAXENT | RF | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|---|
| F. pennantii | Aspect | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.002 | 0.449 |
| bio_15 | 0.283 | 0.321 | 0.259 | 0.215 | 0.099 | 0.235 | 63.921 | |
| bio_18 | 0.043 | 0.000 | 0.011 | 0.001 | 0.003 | 0.012 | 3.166 | |
| bio_19 | 0.000 | 0.006 | 0.000 | 0.007 | 0.000 | 0.003 | 0.695 | |
| bio_2 | 0.058 | 0.064 | 0.048 | 0.050 | 0.013 | 0.047 | 12.649 | |
| bio_3 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.137 | |
| bio_9 | 0.000 | 0.011 | 0.000 | 0.006 | 0.000 | 0.003 | 0.924 | |
| Elevation | 0.057 | 0.030 | 0.039 | 0.026 | 0.001 | 0.030 | 8.271 | |
| Slope | 0.000 | 0.084 | 0.064 | 0.022 | 0.011 | 0.036 | 9.789 | |
| F. palmarum | Aspect | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| bio_12 | 0.000 | 0.000 | 0.000 | 0.020 | 0.007 | 0.005 | 0.625 | |
| bio_2 | 0.088 | 0.504 | 0.517 | 0.214 | 0.048 | 0.274 | 32.121 | |
| bio_3 | 0.251 | 0.440 | 0.577 | 0.201 | 0.090 | 0.312 | 36.559 | |
| bio_5 | 0.000 | 0.256 | 0.244 | 0.116 | 0.001 | 0.124 | 14.479 | |
| bio_6 | 0.077 | 0.182 | 0.156 | 0.029 | 0.002 | 0.089 | 10.446 | |
| Elevation | 0.000 | 0.102 | 0.000 | 0.140 | 0.001 | 0.049 | 5.702 | |
| Slope | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Chatterjee, P.; Singha, H.; Kim, H.-W.; Kundu, S. Climate-Driven Habitat Shifts of Two Palm Squirrel Species (Sciuridae: Funambulus) and Projected Expansion of Their Range Overlap with Indian Agroecosystems. Biology 2025, 14, 1666. https://doi.org/10.3390/biology14121666
Abedin I, Chatterjee P, Singha H, Kim H-W, Kundu S. Climate-Driven Habitat Shifts of Two Palm Squirrel Species (Sciuridae: Funambulus) and Projected Expansion of Their Range Overlap with Indian Agroecosystems. Biology. 2025; 14(12):1666. https://doi.org/10.3390/biology14121666
Chicago/Turabian StyleAbedin, Imon, Paromit Chatterjee, Hilloljyoti Singha, Hyun-Woo Kim, and Shantanu Kundu. 2025. "Climate-Driven Habitat Shifts of Two Palm Squirrel Species (Sciuridae: Funambulus) and Projected Expansion of Their Range Overlap with Indian Agroecosystems" Biology 14, no. 12: 1666. https://doi.org/10.3390/biology14121666
APA StyleAbedin, I., Chatterjee, P., Singha, H., Kim, H.-W., & Kundu, S. (2025). Climate-Driven Habitat Shifts of Two Palm Squirrel Species (Sciuridae: Funambulus) and Projected Expansion of Their Range Overlap with Indian Agroecosystems. Biology, 14(12), 1666. https://doi.org/10.3390/biology14121666









