3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Optical Microscopy
2.3. Immunofluorescence and Histological Analysis of Spheroids Formed by HER2+ BCa Cell Lines
2.4. TEM
2.5. Western Blot
2.6. Statistical Analysis
3. Results
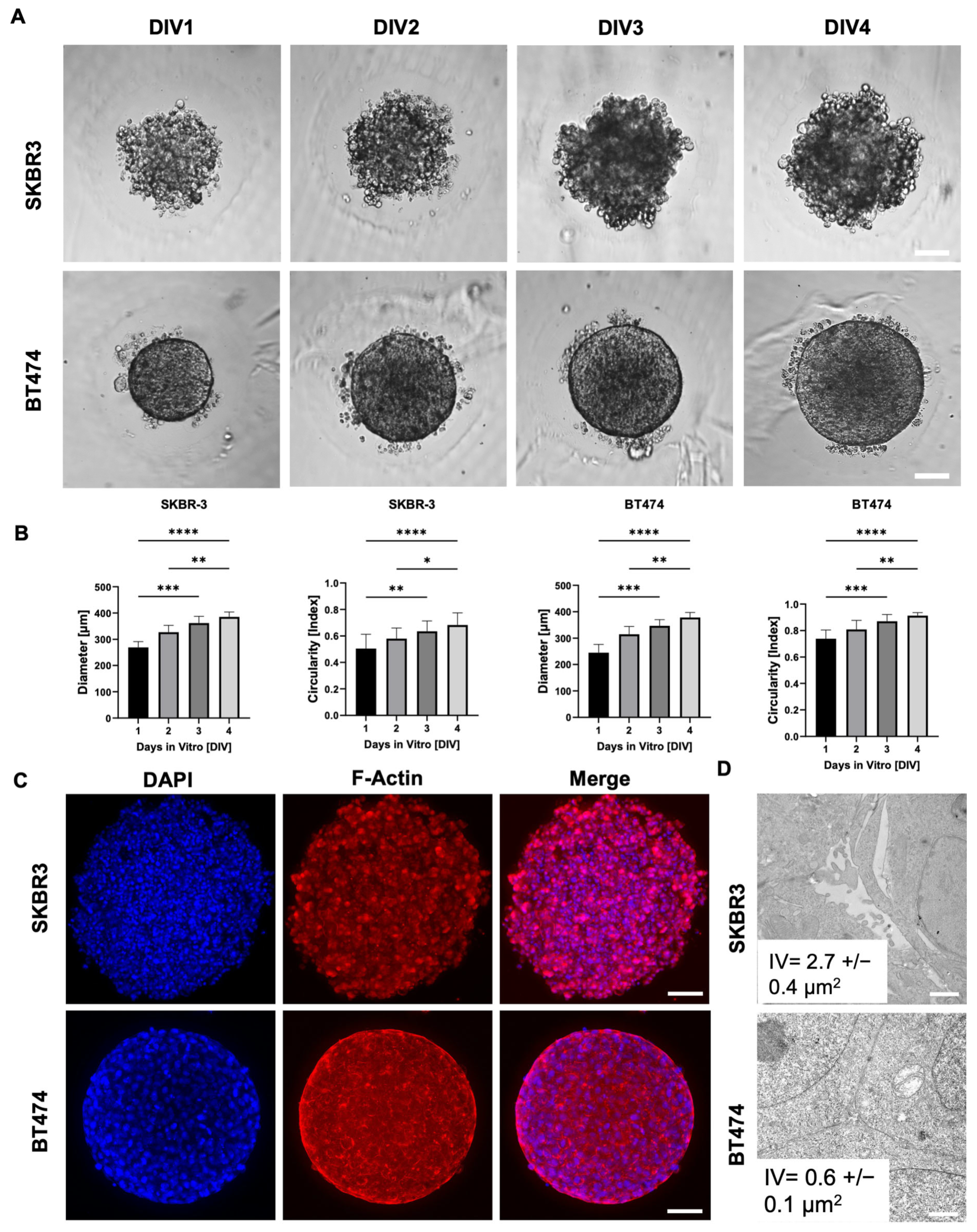
3.1. Distinct Morphologies and 3D Organization of HER2+ Breast Cancer Spheroids
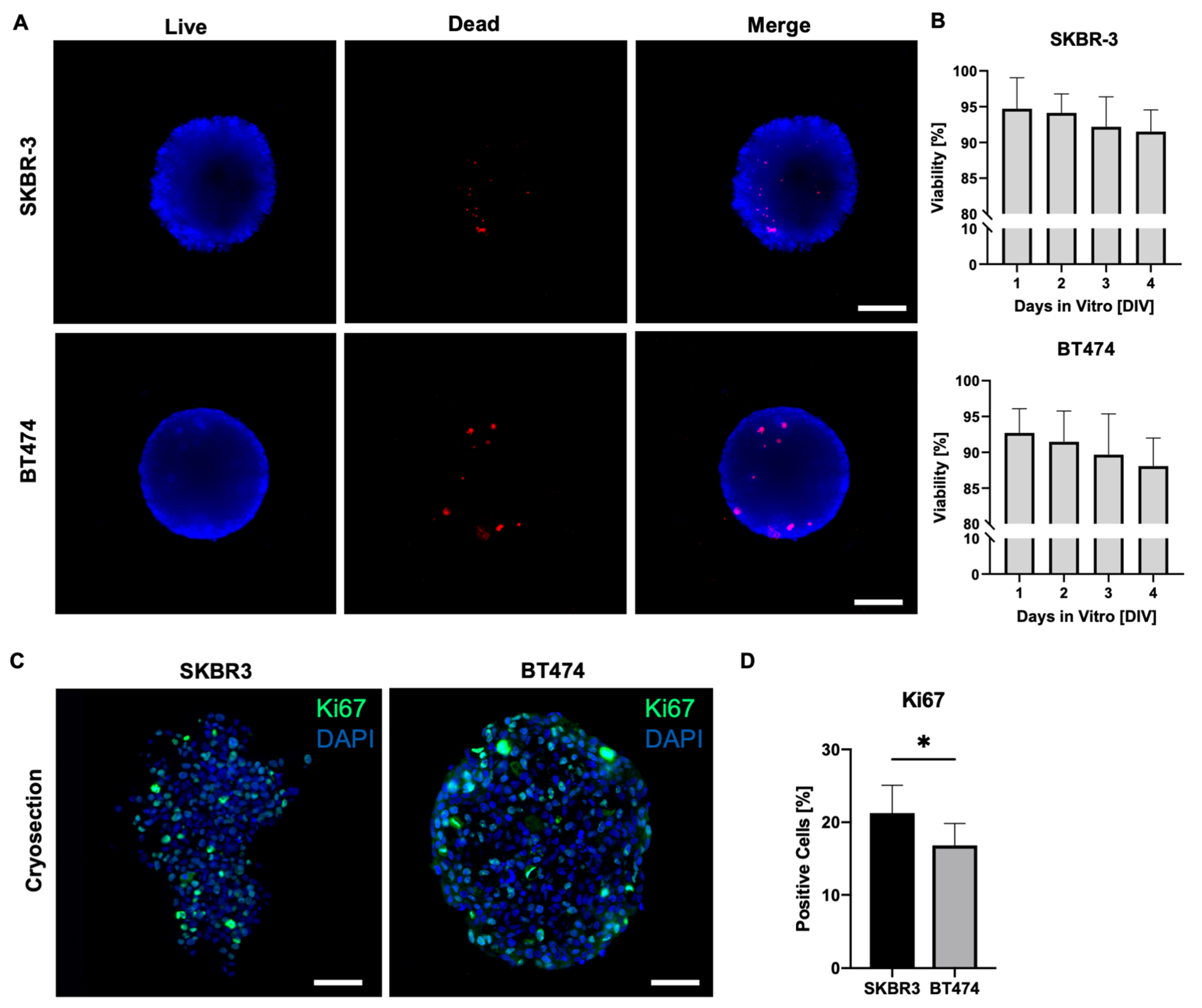
3.2. Assessment of Viability and Proliferation in HER2+ BCa Spheroids
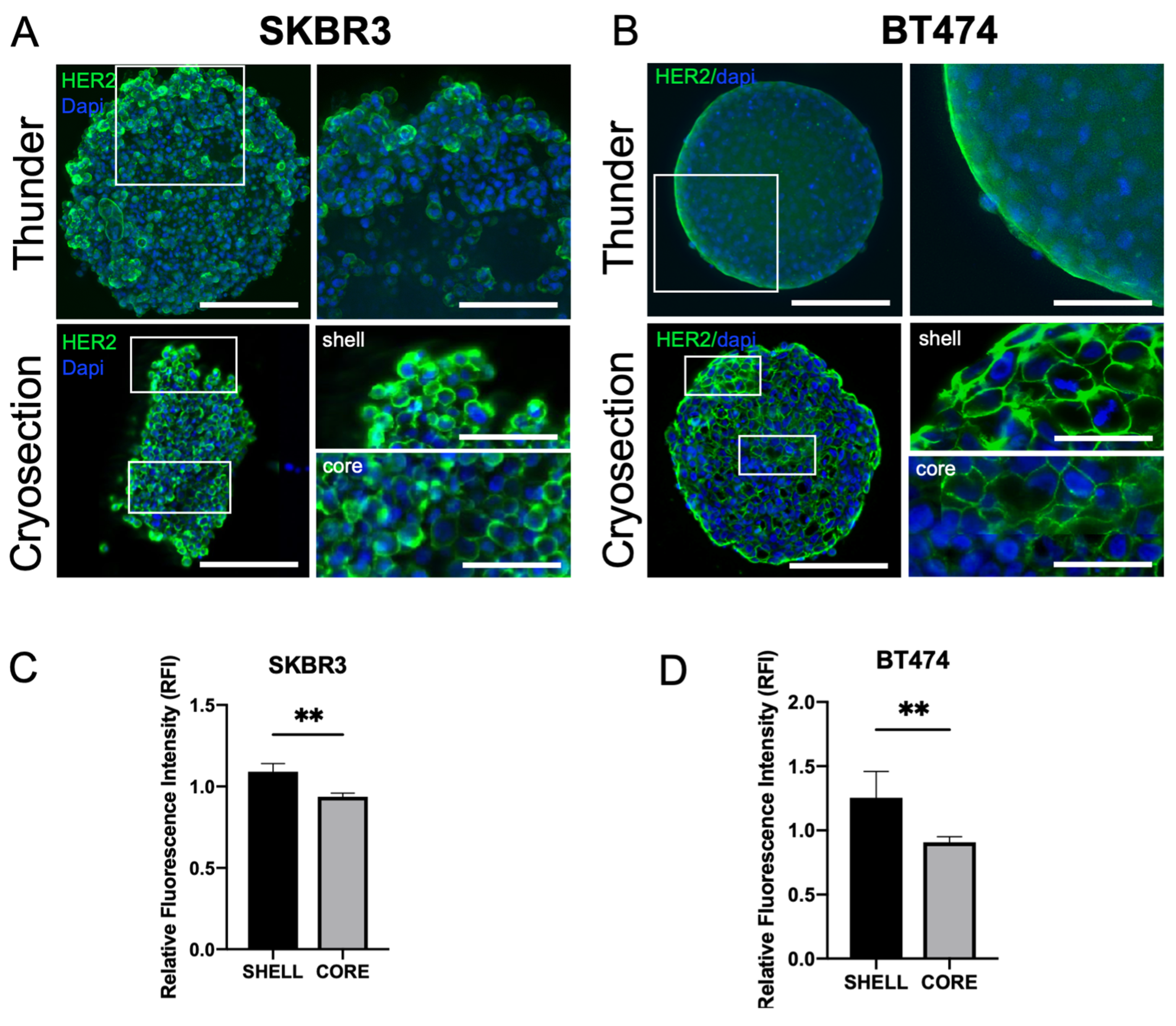
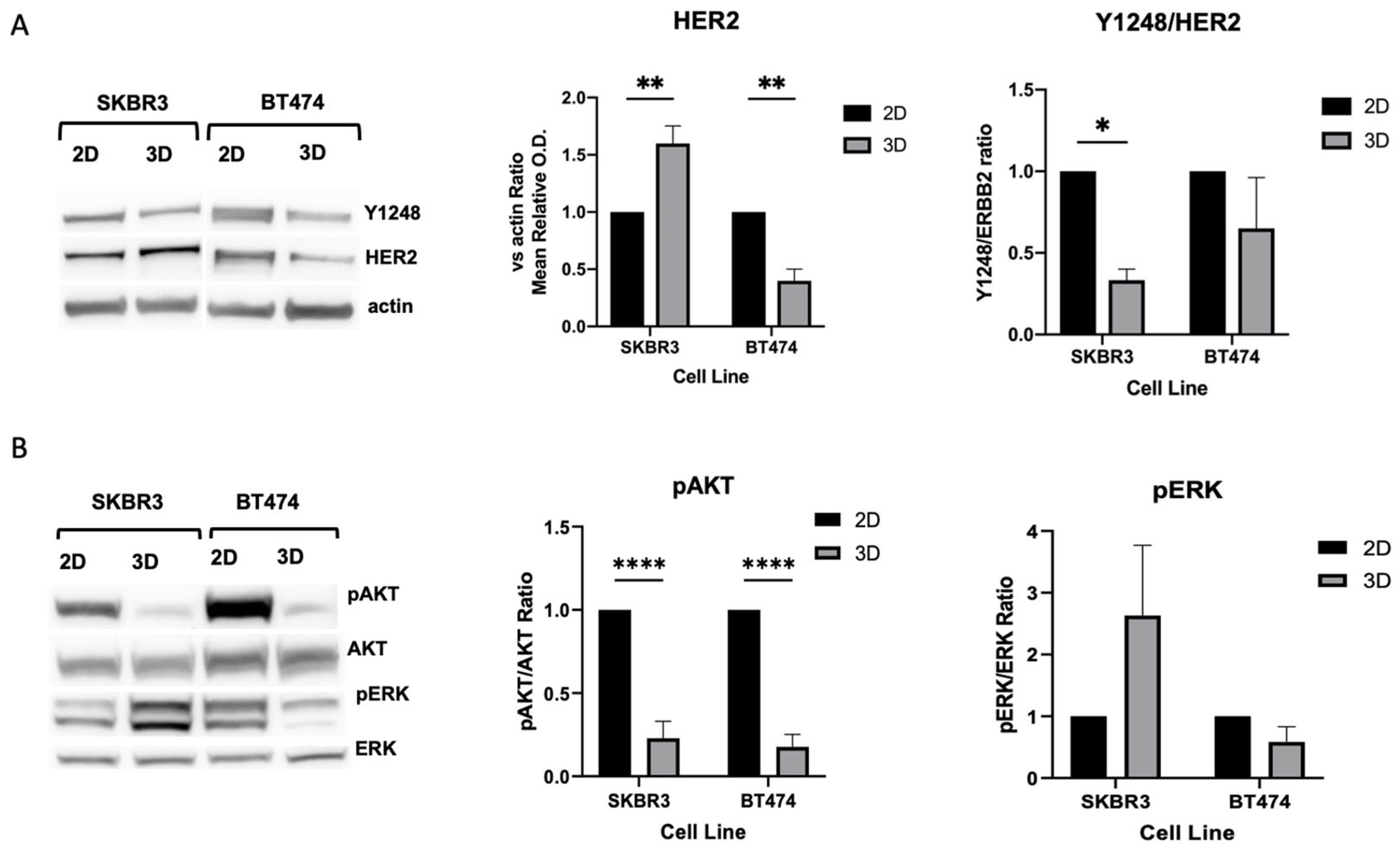
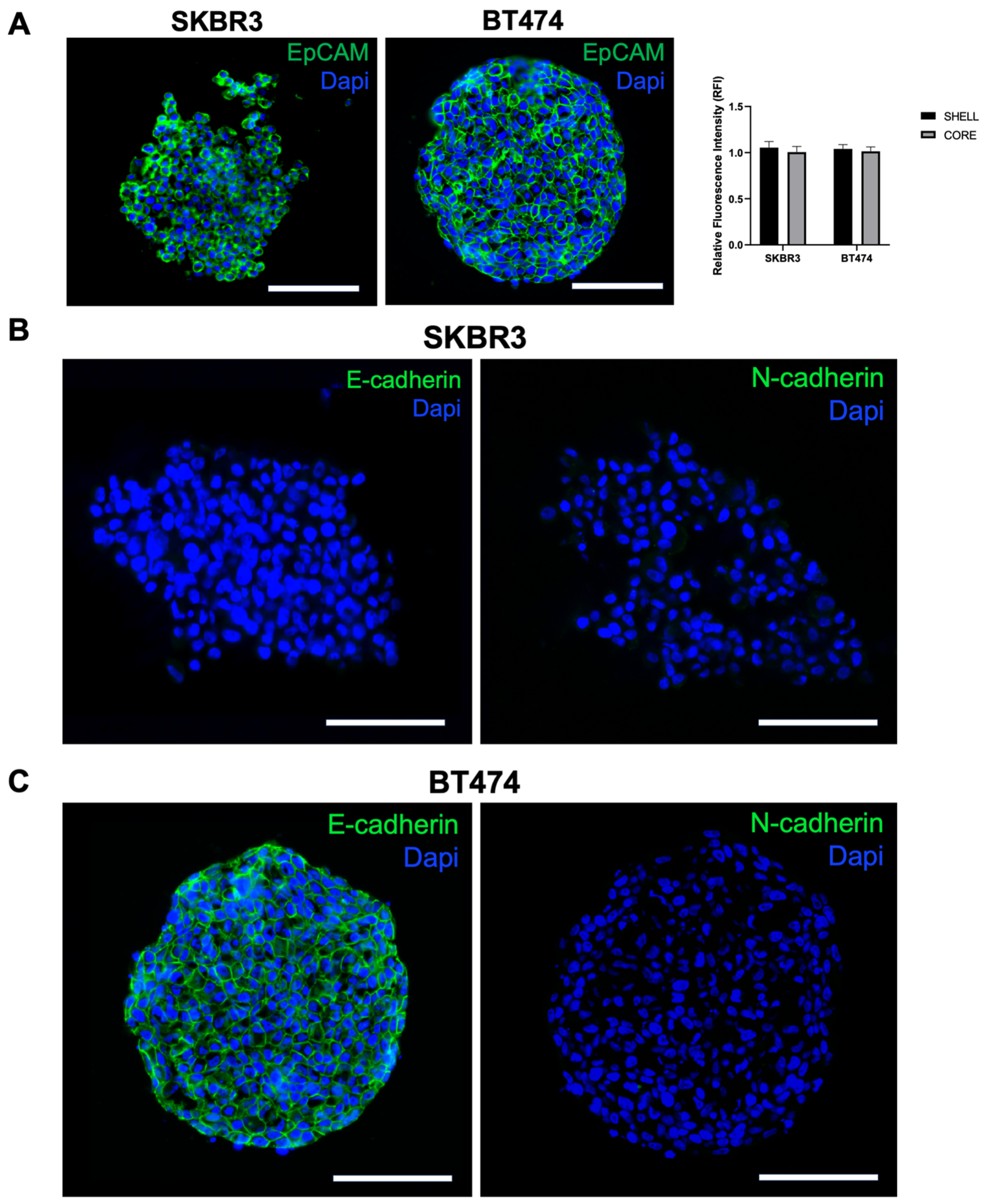
3.3. HER2 Distribution, Signaling, and Epithelial/EMT Markers in 3D Spheroids
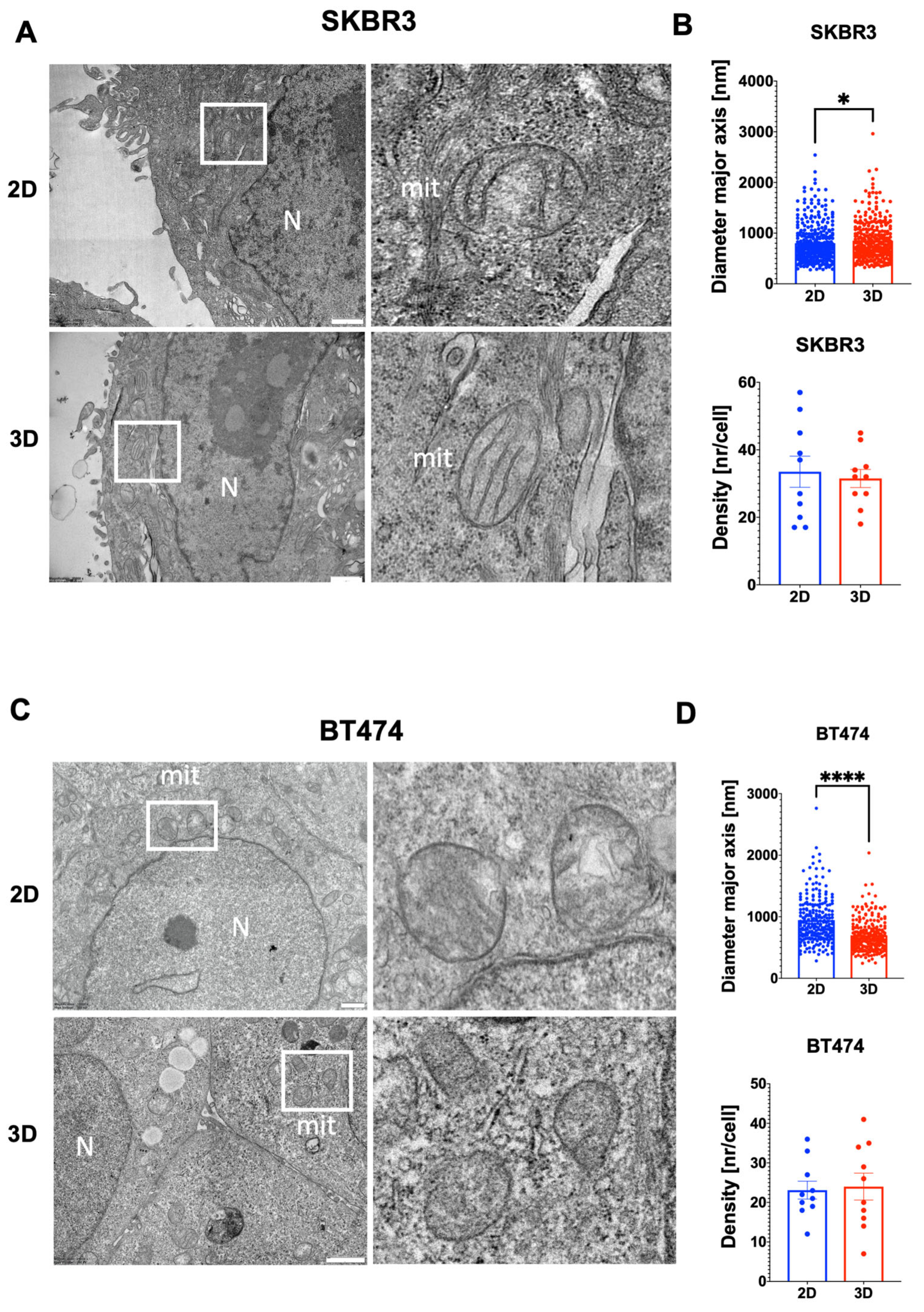
3.4. Ultrastructural Organization and Mitochondrial Remodeling in HER2+ BCa 2D/3D Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ADC | Antibody–drug conjugate |
| AKT | Protein kinase B |
| BCa | Breast cancer |
| BT474 | Human HER2+ breast cancer cell line BT-474 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DIV | Days in vitro |
| ECL | Enhanced chemiluminescence |
| E-cadherin | Epithelial cadherin |
| EMT | Epithelial–mesenchymal transition |
| EpCAM | Epithelial cell adhesion molecule |
| ERBB2/HER2 | Human epidermal growth factor receptor 2 |
| ERK | Extracellular signal-regulated kinase |
| F-actin | Filamentous actin |
| FBS | Fetal bovine serum |
| FCS | Fetal calf serum |
| IVs | Intercellular voids |
| Ki67 | Marker of proliferation Ki-67 |
| NH4Cl | Ammonium chloride |
| O.C.T. | Optimal cutting temperature compound |
| ON | Overnight |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PVDF | Polyvinylidene difluoride |
| RT | Room temperature |
| SKBR3 | Human HER2+ breast cancer cell line SK-BR-3 |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| TEM | Transmission electron microscopy |
| T-DXd | Trastuzumab–deruxtecan |
| UV | Ultraviolet |
| w/v | Weight/volume |
References
- Dessie, Z.G.; Zewotir, T. Global Determinants of Breast Cancer Mortality: A Comprehensive Meta-Analysis of Clinical, Demographic, and Lifestyle Risk Factors. BMC Public Health 2025, 25, 2640. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. HER2-Positive Breast Cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 5, 393. [Google Scholar] [CrossRef]
- Bellese, G.; Tagliatti, E.; Gagliani, M.C.; Santamaria, S.; Arnaldi, P.; Falletta, P.; Rusmini, P.; Matteoli, M.; Castagnola, P.; Cortese, K. Neratinib Is a TFEB and TFE3 Activator That Potentiates Autophagy and Unbalances Energy Metabolism in ERBB2+ Breast Cancer Cells. Biochem. Pharmacol. 2023, 213, 115633. [Google Scholar] [CrossRef]
- Cordeiro, S.; Oliveira, B.B.; Valente, R.; Ferreira, D.; Luz, A.; Baptista, P.V.; Fernandes, A.R. Breaking the Mold: 3D Cell Cultures Reshaping the Future of Cancer Research. Front. Cell Dev. Biol. 2024, 12, 1507388. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The Relevance of Using 3D Cell Cultures, in Addition to 2D Monolayer Cultures, When Evaluating Breast Cancer Drug Sensitivity and Resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef]
- Fröhlich, E. The Variety of 3D Breast Cancer Models for the Study of Tumor Physiology and Drug Screening. Int. J. Mol. Sci. 2023, 24, 7116. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Pickl, M.; Ries, C.H. Comparison of 3D and 2D Tumor Models Reveals Enhanced HER2 Activation in 3D Associated with an Increased Response to Trastuzumab. Oncogene 2009, 28, 461–468. [Google Scholar] [CrossRef]
- Froehlich, K.; Haeger, J.D.; Heger, J.; Pastuschek, J.; Photini, S.M.; Yan, Y.; Lupp, A.; Pfarrer, C.; Mrowka, R.; Schleußner, E.; et al. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J. Mammary Gland. Biol. Neoplasia 2016, 21, 89–98. [Google Scholar] [CrossRef]
- Hongisto, V.; Jernström, S.; Fey, V.; Mpindi, J.-P.; Kleivi Sahlberg, K.; Kallioniemi, O.; Perälä, M. High-Throughput 3D Screening Reveals Differences in Drug Sensitivities between Culture Models of JIMT1 Breast Cancer Cells. PLoS ONE 2013, 8, e77232. [Google Scholar] [CrossRef]
- Arnaldi, P.; Casarotto, E.; Relucenti, M.; Bellese, G.; Gagliani, M.C.; Crippa, V.; Castagnola, P.; Cortese, K. A NSC-34 Cell Line-Derived Spheroid Model: Potential and Challenges for in Vitro Evaluation of Neurodegeneration. Microsc. Res. Tech. 2024, 87, 2785–2800. [Google Scholar] [CrossRef]
- Thongrom, B.; Tang, P.; Arora, S.; Haag, R. Polyglycerol-Based Hydrogel as Versatile Support Matrix for 3D Multicellular Tumor Spheroid Formation. Gels 2023, 9, 938. [Google Scholar] [CrossRef]
- Santamaria, S.; Gagliani, M.C.; Bellese, G.; Marconi, S.; Lechiara, A.; Dameri, M.; Aiello, C.; Tagliatti, E.; Castagnola, P.; Cortese, K. Imaging of Endocytic Trafficking and Extracellular Vesicles Released Under Neratinib Treatment in ERBB2+ Breast Cancer Cells. J. Histochem. Cytochem. 2021, 69, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agashe, A.; Hill, J.C.; Ganguly, K.; Sharma, P.; Richards, T.D.; Huang, W.; Kaczorowski, D.J.; Sanchez, P.G.; Kapania, R.; et al. Mechanical Cues Guide the Formation and Patterning of 3D Spheroids in Fibrous Environments. PNAS Nexus 2025, 4, pgaf263. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; Rogowska-Wrzesinska, A.; Kanlaya, R.; Borkowski, K.; Schwämmle, V.; Dai, J.; Joensen, K.E.; Wojdyla, K.; Carvalho, V.B.; Fey, S.J. The Cultural Divide: Exponential Growth in Classical 2D and Metabolic Equilibrium in 3D Environments. PLoS ONE 2014, 9, e106973. [Google Scholar] [CrossRef] [PubMed]
- Jaros, J.; Petrov, M.; Tesarova, M.; Hampl, A. Revealing 3D Ultrastructure and Morphology of Stem Cell Spheroids by Electron Microscopy. In 3D Cell Culture: Methods and Protocols; Koledova, Z., Ed.; Springer: New York, NY, USA, 2017; pp. 417–431. ISBN 978-1-4939-7021-6. [Google Scholar]
- Smart, C.E.; Morrison, B.J.; Saunus, J.M.; Vargas, A.C.; Keith, P.; Reid, L.; Wockner, L.; Amiri, M.A.; Sarkar, D.; Simpson, P.T.; et al. In Vitro Analysis of Breast Cancer Cell Line Tumourspheres and Primary Human Breast Epithelia Mammospheres Demonstrates Inter- and Intrasphere Heterogeneity. PLoS ONE 2013, 8, e64388. [Google Scholar] [CrossRef]
- Ham, S.L.; Joshi, R.; Luker, G.D.; Tavana, H. Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors. Adv. Healthc. Mater. 2016, 5, 2788–2798. [Google Scholar] [CrossRef]
- Uxa, S.; Castillo-Binder, P.; Kohler, R.; Stangner, K.; Müller, G.A.; Engeland, K. Ki-67 Gene Expression. Cell Death Differ. 2021, 28, 3357–3370. [Google Scholar] [CrossRef]
- Azimi, T.; Loizidou, M.; Dwek, M.V. Cancer Cells Grown in 3D under Fluid Flow Exhibit an Aggressive Phenotype and Reduced Responsiveness to the Anti-Cancer Treatment Doxorubicin. Sci. Rep. 2020, 10, 12020. [Google Scholar] [CrossRef]
- Daverey, A.; Mytty, A.; Kidambi, S. Topography Mediated Regulation of HER-2 Expression in Breast Cancer Cells. Nano Life 2012, 2, 1241009. [Google Scholar] [CrossRef]
- Gangadhara, S.; Smith, C.; Barrett-Lee, P.; Hiscox, S. 3D Culture of Her2+ Breast Cancer Cells Promotes AKT to MAPK Switching and a Loss of Therapeutic Response. BMC Cancer 2016, 16, 345. [Google Scholar] [CrossRef] [PubMed]
- Soysal, S.D.; Muenst, S.; Barbie, T.; Fleming, T.; Gao, F.; Spizzo, G.; Oertli, D.; Viehl, C.T.; Obermann, E.C.; Gillanders, W.E. EpCAM Expression Varies Significantly and Is Differentially Associated with Prognosis in the Luminal B HER2+, Basal-like, and HER2 Intrinsic Subtypes of Breast Cancer. Br. J. Cancer 2013, 108, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Ivascu, A.; Kubbies, M. Diversity of Cell-Mediated Adhesions in Breast Cancer Spheroids. Int. J. Oncol. 2007, 31, 1403–1413. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and n-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Mittal, A.; Desai, N.; Khatri, D.K.; Gugulothu, D.; Lather, V.; Pandita, D.; Vora, L.K. Spheroids in Cancer Research: Recent Advances and Opportunities. J. Drug Deliv. Sci. Technol. 2024, 100, 106033. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Shahrivari, S.; Aminoroaya, N.; Ghods, R.; Latifi, H.; Afjei, S.A.; Saraygord-Afshari, N.; Bagheri, Z. Toxicity of Trastuzumab for Breast Cancer Spheroids: Application of a Novel on-a-Chip Concentration Gradient Generator. Biochem. Eng. J. 2022, 187, 108590. [Google Scholar] [CrossRef]
- Muñoz-Galindo, L.; Melendez-Zajgla, J.; Pacheco-Fernández, T.; Rodriguez-Sosa, M.; Mandujano-Tinoco, E.A.; Vazquez-Santillan, K.; Castro-Oropeza, R.; Lizarraga, F.; Sanchez-Lopez, J.M.; Maldonado, V. Changes in the Transcriptome Profile of Breast Cancer Cells Grown as Spheroids. Biochem. Biophys. Res. Commun. 2019, 516, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Manuel Iglesias, J.; Beloqui, I.; Garcia-Garcia, F.; Leis, O.; Vazquez-Martin, A.; Eguiara, A.; Cufi, S.; Pavon, A.; Menendez, J.A.; Dopazo, J.; et al. Mammosphere Formation in Breast Carcinoma Cell Lines Depends upon Expression of E-Cadherin. PLoS ONE 2013, 8, e77281. [Google Scholar] [CrossRef]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 Activates Wnt/β-Catenin Pathway and Promotes EMT-like Phenotype in Trastuzumab-Resistant HER2-Overexpressing Breast Cancer Cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef]
- Park, A.; Choi, S.; Do, J.; Kim, Y.; Kim, K.S.; Koh, E.; Park, K.S. ZO-1 Regulates the Migration of Mesenchymal Stem Cells in Cooperation with α-Catenin in Response to Breast Tumor Cells. Cell Death Discov. 2024, 10, 19. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. EMT Features in Claudin-Low versus Claudin-Non-Suppressed Breast Cancers and the Role of Epigenetic Modifications. Curr. Issues Mol. Biol. 2023, 45, 6040–6054. [Google Scholar] [CrossRef]
- Zheng Boyer, J.; Lewis Phillips, G.D.; Nitta, H.; Garsha, K.; Admire, B.; Kraft, R.; Dennis, E.; Vela, E.; Towne, P. Activity of Trastuzumab Emtansine (T-DM1) in 3D Cell Culture. Breast Cancer Res. Treat. 2021, 188, 65–75. [Google Scholar] [CrossRef]
- Tapia, I.J.; Perico, D.; Wolos, V.J.; Villaverde, M.S.; Abrigo, M.; Di Silvestre, D.; Mauri, P.; De Palma, A.; Fiszman, G.L. Proteomic Characterization of a 3D HER2+ Breast Cancer Model Reveals the Role of Mitochondrial Complex I in Acquired Resistance to Trastuzumab. Int. J. Mol. Sci. 2024, 25, 7397. [Google Scholar] [CrossRef]
- Li, X.; Huang, T. Advances in the Intrinsic Signaling Pathway Interactions and Clinical Translation of HR+/HER2+ Breast Cancer. Breast Cancer 2025, 32, 1216–1243. [Google Scholar] [CrossRef]
- Relucenti, M.; Francescangeli, F.; De Angelis, M.L.; D’Andrea, V.; Miglietta, S.; Pilozzi, E.; Li, X.; Boe, A.; Chen, R.; Zeuner, A.; et al. The Ultrastructural Analysis of Human Colorectal Cancer Stem Cell-Derived Spheroids and Their Mouse Xenograft Shows That the Same Cells Types Have Different Ratios. Biology 2021, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. Biology of HER2 and Its Importance in Breast Cancer. Oncology 2001, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Falletta, P.; Tagliatti, E.; Cortese, K. Seeing Structure, Losing Sight: The Case for Morphological Thinking in the Age of Integration. Anat. Rec. 2025, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Cortese, K.; Daga, A.; Monticone, M.; Tavella, S.; Stefanelli, A.; Aiello, C.; Bisio, A.; Bellese, G.; Castagnola, P. Carnosic acid induces proteasomal degradation of Cyclin B1, RB and SOX2 along with cell growth arrest and apoptosis in GBM cells. Phytomedicine 2016, 23, 679–685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnaldi, P.; Zotti, V.D.; Bellese, G.; Gagliani, M.C.; Orecchia, P.; Castagnola, P.; Cortese, K. 3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling. Biology 2025, 14, 1654. https://doi.org/10.3390/biology14121654
Arnaldi P, Zotti VD, Bellese G, Gagliani MC, Orecchia P, Castagnola P, Cortese K. 3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling. Biology. 2025; 14(12):1654. https://doi.org/10.3390/biology14121654
Chicago/Turabian StyleArnaldi, Pietro, Valentina Delli Zotti, Grazia Bellese, Maria Cristina Gagliani, Paola Orecchia, Patrizio Castagnola, and Katia Cortese. 2025. "3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling" Biology 14, no. 12: 1654. https://doi.org/10.3390/biology14121654
APA StyleArnaldi, P., Zotti, V. D., Bellese, G., Gagliani, M. C., Orecchia, P., Castagnola, P., & Cortese, K. (2025). 3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling. Biology, 14(12), 1654. https://doi.org/10.3390/biology14121654






