Circadian Light Hygiene Is Associated with Anemia Markers in Young Adults
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Actigraphy
2.3. Complete Blood Count
2.4. Morningness–Eveningness Questionnaire (MEQ)
2.5. Data Analysis
3. Results
3.1. Study Participants
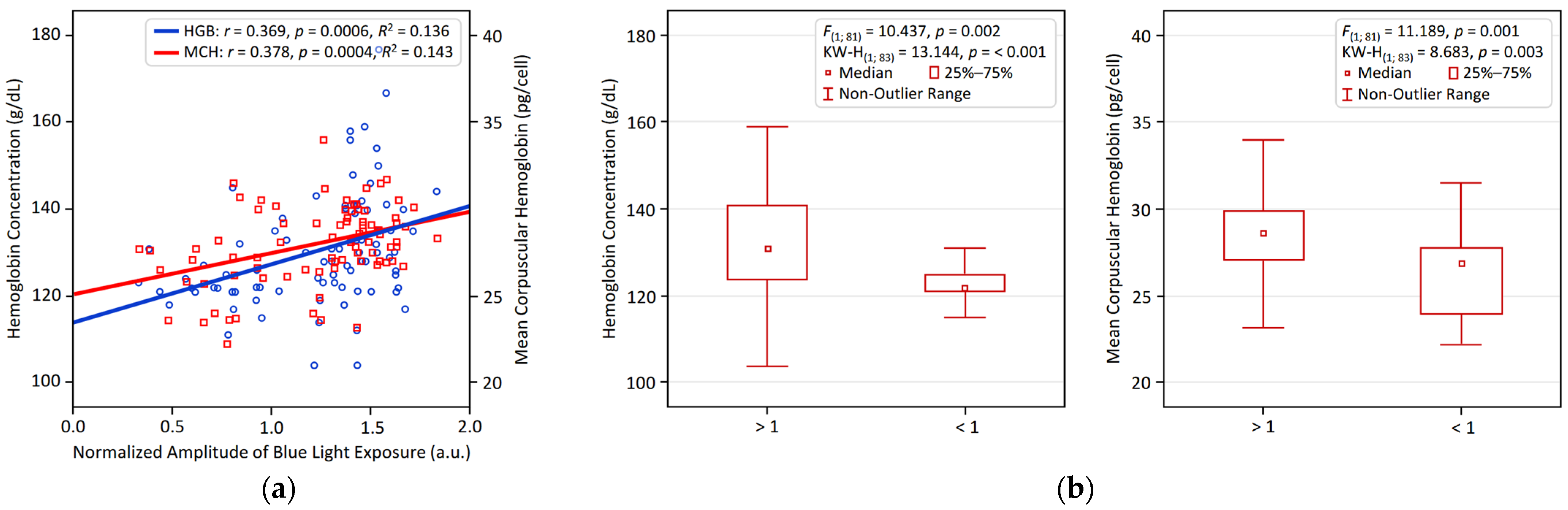
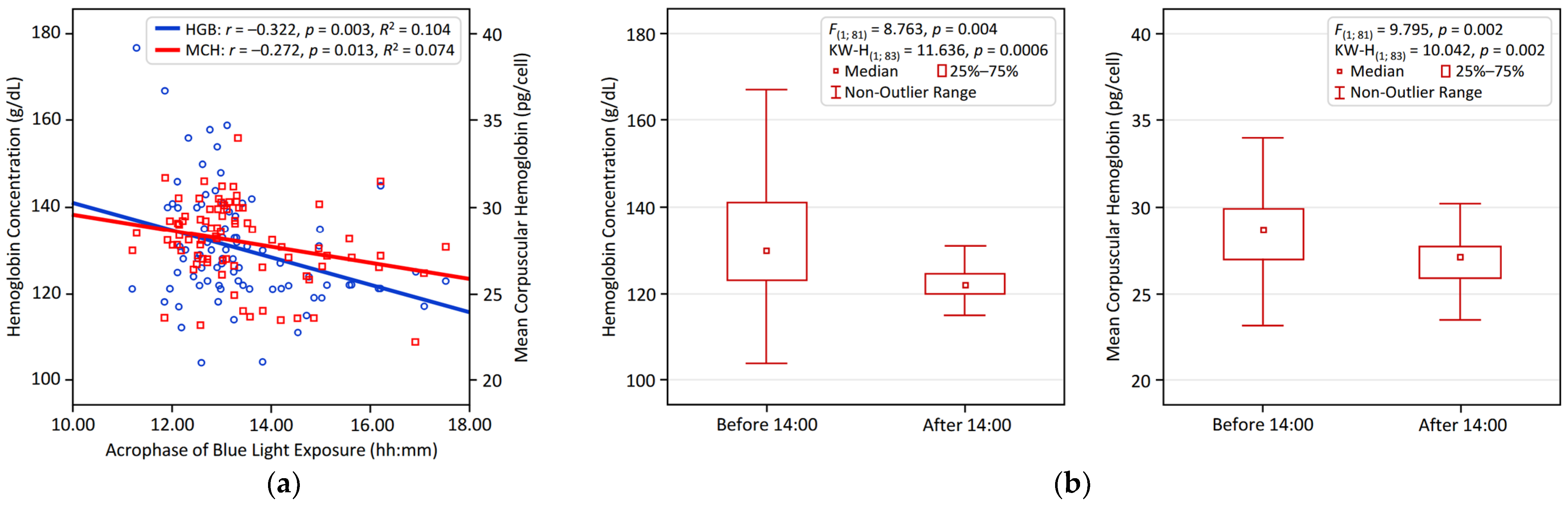
3.2. Univariate Associations Between Circadian Parameters and Hematological Variables
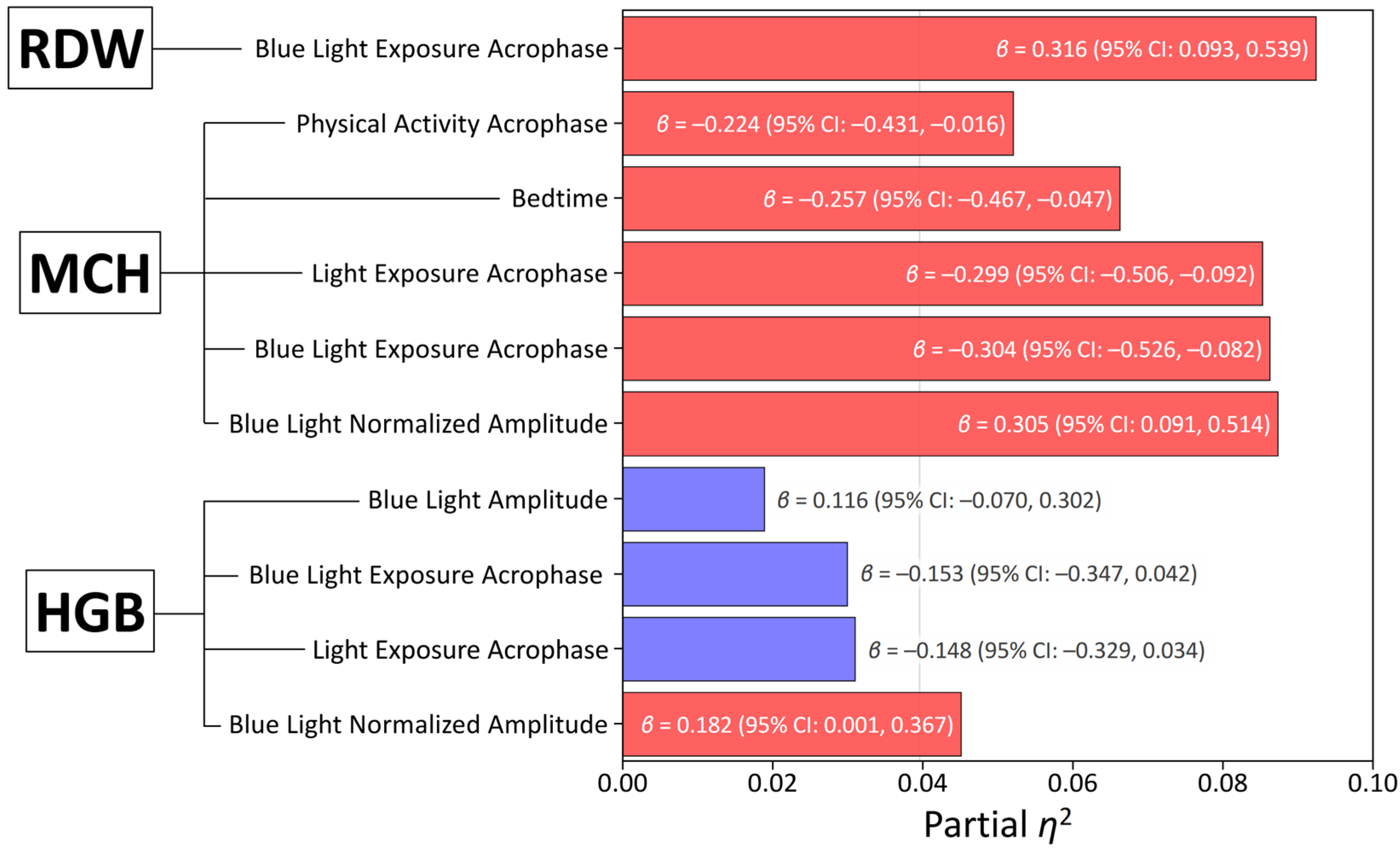
3.3. Multivariate Associations Between Circadian Parameters and Hematological Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| List of abbreviations | |
| BLE | Blue Light Exposure (irradiance in the short-wavelength range, measured by the Blue channel of the Condor AcTrust2 device). |
| BLE NA | Normalized Amplitude of Blue Light Exposure (ratio of the 24 h amplitude of the fitted cosine curve for BLE data to its MESOR, used to standardize the dynamic range of blue light exposure relative to its average value). |
| BMI | Body Mass Index. |
| CBC | Complete Blood Count. |
| CIE | Commission Internationale de l’Éclairage (International Commission on Illumination, referenced in relation to melanopic irradiance standards). |
| FDR | False Discovery Rate (a statistical method used to correct for multiple comparisons, with a critical value of 0.1 in the study). |
| GBD | Global Burden of Disease (a comprehensive assessment of health loss due to diseases, injuries, and risk factors). |
| HCT | Hematocrit (percentage of blood volume made up of red blood cells). |
| HGB | Hemoglobin (amount of oxygen-carrying protein in blood). |
| HSCs | Hematopoietic Stem Cells |
| IS | Inter-Daily Stability (a non-parametric index measuring consistency of activity or exposure across days). |
| IV | Intra-Daily Variability (a non-parametric index measuring within-day fragmentation of activity or exposure). |
| JBI | Joanna Briggs Institute (an organization that provides tools for evidence-based healthcare, including critical appraisal checklists). |
| L5 | Least Active/Exposed 5 h Period (the 5 h of lowest values for physical activity or light exposure). |
| LE | Light Exposure (general light exposure, measured via actigraphy in lux). |
| M10 | Most Active/Exposed 10 h Period (the 10 h of highest values for physical activity or light exposure). |
| MCH | Mean Corpuscular Hemoglobin (average amount of hemoglobin per red blood cell). |
| MCHC | Mean Corpuscular Hemoglobin Concentration (average concentration of hemoglobin in red blood cells). |
| MCV | Mean Corpuscular Volume (average size of red blood cells). |
| MEQ | Morningness–Eveningness Questionnaire (a 19-item self-report tool to assess chronotype, with scores ranging from 16 to 86; higher scores indicate morning type). |
| MESOR | Midline-Estimating Statistic Of Rhythm (a rhythm-adjusted mean). |
| NR1D1 | Nuclear Receptor Subfamily 1 Group D Member 1 (also known as REV-ERB). |
| PA | Physical Activity. |
| PIM | Proportional Integrative Mode (a method for estimating motor activity in actigraphy). |
| RBCs | Red Blood Cells (erythrocytes; also referred to as red blood cell count in 1012/L). |
| RDW-CV | Red Cell Distribution Width—Coefficient of Variation (variation in red blood cell size, expressed as a percentage). |
| VIFs | Variance Inflation Factors (used to evaluate potential multi-collinearity among variables in regression models). |
| WASO | Wake After Sleep Onset (time spent awake after initially falling asleep). |
| List of terms | |
| Acrophase | Timing of the peak in a circadian rhythm. |
| Amplitude | Magnitude of variation in a circadian rhythm, peak-to-trough difference (e.g., daily light fluctuation). |
| Chronotype | Individual’s natural preference for morning or evening activity. |
| Actigraphy | Non-invasive wrist-worn device monitoring movement, sleep, and light exposure for objective data. |
References
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Borisenkov, M.F.; Kolomeichuk, S.N.; Markov, A.A.; Weinert, D.; Cornelissen, G.; Stefani, O. Evaluating circadian light hygiene: Methodology and health implications. Russ. Open Med. J. 2024, 13, e0415. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in chronomedicine and interpretation of circadian health. Diagnostics 2025, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.; Stefani, O.; Cornelissen, G. Light hygiene for circadian health: A molecular perspective. Front. Biosci. 2025, 30, 39097. [Google Scholar] [CrossRef]
- Meng, J.J.; Shen, J.W.; Li, G.; Ouyang, C.J.; Hu, J.X.; Li, Z.S.; Zhao, H.; Shi, Y.M.; Zhang, M.; Liu, R.; et al. Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis. Cell 2023, 186, 398–412.e17. [Google Scholar] [CrossRef]
- Rao, F.; Xue, T. Circadian-independent light regulation of mammalian metabolism. Nat. Metab. 2024, 6, 1000–1007. [Google Scholar] [CrossRef]
- Li, A.; Wei, X.; Xie, Y.; Ren, Y.; Zhu, X.; Liu, M.; Liu, S. Light exposure and its applications in human health. J. Biophotonics 2024, 17, e202400023. [Google Scholar] [CrossRef]
- Boyce, P. Light, lighting and human health. Light. Res. Technol. 2021, 54, 101–144. [Google Scholar] [CrossRef]
- GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: Findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e713–e734, Erratum in Lancet Haematol. 2023, 10, e796. https://doi.org/10.1016/S2352-3026(23)00283-1. Erratum in Lancet Haematol. 2024, 11, e10. https://doi.org/10.1016/S2352-3026(23)00373-3. [Google Scholar] [CrossRef]
- Williams, A.M.; Ansai, N.; Ahluwalia, N.; Nguyen, D.T. Anemia Prevalence: United States, August 2021–August 2023. In NCHS Data Briefs; National Center for Health Statistics: Hyattsville, MD, USA, 2024; p. 519. Available online: https://www.ncbi.nlm.nih.gov/books/NBK612586/ (accessed on 21 September 2025). [CrossRef]
- Bikbov, M.M.; Kazakbaeva, G.M.; Zainullin, R.M.; Salavatova, V.F.; Gilmanshin, T.R.; Yakupova, D.F.; Uzianbaeva, Y.V.; Arslangareeva, I.I.; Panda-Jonas, S.; Mukhamadieva, S.R.; et al. Prevalence and associated factors of anemia in a Russian population: The Ural eye and medical study. BMC Public Health 2019, 19, 762. [Google Scholar] [CrossRef]
- De la Cruz-Góngora, V.; Villalpando, S.; Shamah-Levy, T. Overview of trends in anemia and iron deficiency in the Mexican population from 1999 to 2018-19. Food Nutr. Bull. 2024, 45, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Laerum, O.D. The haematopoietic system. In Chronobiology and Chronomedicine: From Molecular and Cellular Mechanisms to Whole Body Interdigitating Networks; Cornelissen, G., Hirota, T., Eds.; Royal Society of Chemistry: London, UK, 2024; pp. 304–322. [Google Scholar]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.D.; Labeed, F.H.; Kitcatt, S.J.; O’Neill, J.S. Detecting circadian rhythms in human red blood cells by dielectrophoresis. Methods Mol. Biol. 2022, 2482, 255–264. [Google Scholar] [CrossRef]
- Beale, A.D.; Hayter, E.A.; Crosby, P.; Valekunja, U.K.; Edgar, R.S.; Chesham, J.E.; Maywood, E.S.; Labeed, F.H.; Reddy, A.B.; Wright, K.P., Jr.; et al. Mechanisms and physiological function of daily haemoglobin oxidation rhythms in red blood cells. EMBO J. 2023, 42, e114164. [Google Scholar] [CrossRef]
- Chun, M.Y.; Kim, J.H.; Kang, J.S. Relationship between Self-Reported Sleep Duration and Risk of Anemia: Data from the Korea National Health and Nutrition Examination Survey 2016–2017. Int. J. Environ. Res. Public Health 2021, 18, 4721. [Google Scholar] [CrossRef]
- Nam, H.K.; Park, J.; Cho, S.I. Association between depression, anemia and physical activity using isotemporal substitution analysis. BMC Public Health 2023, 23, 2236. [Google Scholar] [CrossRef]
- Blackwell, T.; Redline, S.; Ancoli-Israel, S.; Schneider, J.L.; Surovec, S.; Johnson, N.L.; Cauley, J.A.; Stone, K.L.; Study of Osteoporotic Fractures Research Group. Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF Study. Sleep 2008, 31, 283–291. [Google Scholar] [CrossRef]
- Price, L.L.; Lyachev, A.; Khazova, M. Optical performance characterization of light-logging actigraphy dosimeters. J. Opt. Soc. Am. A 2017, 34, 545–557. [Google Scholar] [CrossRef]
- CIE S 026:2018; CIE System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light. CIE Central Bureau: Vienna, Austria, 2018.
- Gubin, D.; Boldyreva, J.; Stefani, O.; Kolomeichuk, S.; Danilova, L.; Shigabaeva, A.; Cornelissen, G.; Weinert, D. Higher vulnerability to poor circadian light hygiene in individuals with a history of COVID-19. Chronobiol. Int. 2025, 42, 133–146. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Putilov, A.A.; Putilov, D.A. Sleepless in Siberia and Alaska: Cross-validation of factor structure of the individual adaptability of the sleep-wake cycle. Ergon. Int. J. Ergon. Hum. Factors 2005, 27, 207–226. [Google Scholar]
- Sato, M.; Roszak, M.; Hashimoto, T.; Kołodziejczak, B.; Gubin, D.; Boudville, N.; Kawka, E.; Bręborowicz, A.; Witowski, J.; Kanikowska, D. Comparison of chronotype and learning motivation in medical university students. BMC Med. Educ. 2024, 24, 1160. [Google Scholar] [CrossRef] [PubMed]
- Busza, A.; Sharma, V.; Ferguson, K.; Fawcett, A.; Knoll, J.; Iwanaszko, M.; Zee, P.; Fishbein, A. Systematic review: Differences in complete blood count component rhythms. Sleep Adv. 2024, 5, zpae086. [Google Scholar] [CrossRef]
- Gubin, D.; Danilenko, K.; Stefani, O.; Kolomeichuk, S.; Markov, A.; Petrov, I.; Voronin, K.; Mezhakova, M.; Borisenkov, M.; Shigabaeva, A.; et al. Light environment of Arctic solstices is coupled with melatonin phase-amplitude changes and decline of metabolic health. J. Pineal Res. 2025, 77, e70023. [Google Scholar] [CrossRef]
- Gubin, D.; Kolomeichuk, S.; Danilenko, K.; Stefani, O.; Markov, A.; Petrov, I.; Voronin, K.; Mezhakova, M.; Borisenkov, M.; Shigabaeva, A.; et al. Timing and amplitude of light exposure, not photoperiod, predict blood lipids in Arctic residents: A circadian light hypothesis. Biology 2025, 14, 799. [Google Scholar] [CrossRef]
- Gubin, D.; Kolomeichuk, S.; Danilenko, K.; Stefani, O.; Markov, A.; Petrov, I.; Voronin, K.; Mezhakova, M.; Borisenkov, M.; Shigabaeva, A.; et al. Light exposure, physical activity, and indigeneity modulate seasonal variation in NR1D1 (REV-ERBα) expression. Biology 2025, 14, 231. [Google Scholar] [CrossRef]
- Sennels, H.P.; Jørgensen, H.L.; Hansen, A.L.; Goetze, J.P.; Fahrenkrug, J. Diurnal variation of hematology parameters in healthy young males: The Bispebjerg study of diurnal variations. Scand. J. Clin. Lab. Investig. 2011, 71, 532–541. [Google Scholar] [CrossRef]
- Hilderink, J.M.; Klinkenberg, L.J.J.; Aakre, K.M.; de Wit, N.C.J.; Henskens, Y.M.C.; van der Linden, N.; Bekers, O.; Rennenberg, R.J.M.W.; Koopmans, R.P.; Meex, S.J.R. Within-day biological variation and hour-to-hour reference change values for hematological parameters. Clin. Chem. Lab. Med. 2017, 55, 1013–1024. [Google Scholar] [CrossRef]
- Ikeda, R.; Tsuchiya, Y.; Koike, N.; Umemura, Y.; Inokawa, H.; Ono, R.; Inoue, M.; Sasawaki, Y.; Grieten, T.; Okubo, N.; et al. REV-ERBα and REV-ERBβ function as key factors regulating mammalian circadian output. Sci. Rep. 2019, 9, 10171. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Lazar, M.A. Nuclear receptor Rev-erbalpha: A heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal. 2010, 8, e001. [Google Scholar] [CrossRef]
- Simcox, J.A.; Mitchell, T.C.; Gao, Y.; Just, S.F.; Cooksey, R.; Cox, J.; Ajioka, R.; Jones, D.; Lee, S.H.; King, D.; et al. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes 2015, 64, 1108–1119. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, Q.; Wang, X.; Bai, H.; Tian, D.; Gao, G.; Wang, F.; Yu, P.; Chang, Y.Z. Cellular iron depletion enhances behavioral rhythm by limiting brain Per1 expression in mice. CNS Neurosci. Ther. 2024, 30, e14592. [Google Scholar] [CrossRef]
- Alves, S.; Silva, F.; Esteves, F.; Costa, S.; Slezakova, K.; Alves, M.; Pereira, M.; Teixeira, J.; Morais, S.; Fernandes, A.; et al. The impact of sleep on haematological parameters in firefighters. Clocks Sleep 2024, 6, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, Q.; Wang, M.; Qian, H.; Song, Q.; Liu, B. Sleep behaviors modify the association between hemoglobin concentration and respiratory infection: A prospective cohort analysis. Front. Physiol. 2025, 16, 1638819. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lin, W. Effects of exercise training on red blood cell production: Implications for anemia. Acta Haematol. 2012, 127, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Caimi, G.; Carlisi, M.; Presti, R.L. Red blood cell distribution width, erythrocyte indices, and elongation index at baseline in a group of trained subjects. J. Clin. Med. 2023, 13, 151. [Google Scholar] [CrossRef]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; van Assen, S. Exercise-induced anaemia: A forgotten cause of iron deficiency anaemia in young adults. Br. J. Gen. Pract. 2015, 65, 268–269. [Google Scholar] [CrossRef]
- Cichoń-Woźniak, J.; Dziewiecka, H.; Ostapiuk-Karolczuk, J.; Kasperska, A.; Gruszka, W.; Basta, P.; Skarpańska-Stejnborn, A. Effect of baseline ferritin levels on post-exercise iron metabolism in male elite youth rowers. Sci. Rep. 2025, 15, 23440. [Google Scholar] [CrossRef]
| Variable | Mean ± Standard Deviation |
|---|---|
| Age (years) | 19.30 ± 1.51 |
| Body mass index (BMI) | 21.76 ± 3.72 |
| Sex (male/female) | 23/62 |
| Red blood cells (RBCs) (1012/L) | 4.68 ± 0.42 |
| Hemoglobin concentration (HGB) (g/dL) | 130.3 ± 12.9 |
| Hematocrit (HCT) (%) | 40.15 ± 3.52 |
| Mean corpuscular hemoglobin (MCH) (pg/cell) | 27.99 ± 2.22 |
| Mean corpuscular volume (MCV) (fL) | 85.01 ± 9.87 |
| Mean corpuscular hemoglobin concentration (MCHC) (g/D) | 321.9 ± 33.7 |
| Red cell distribution width—CV (RDW-CV) (%) | 13.12 ± 1.21 |
| RBC | HGB | HCT | MCV | MCH | MCHC | RDW-CV | |
|---|---|---|---|---|---|---|---|
| Physical Activity | |||||||
| MESOR | 0.098 | −0.024 | 0.024 | −0.081 | −0.137 | −0.023 | 0.088 |
| 24 h Amplitude | 0.190 | 0.176 | 0.146 | −0.079 | 0.010 | −0.007 | 0.051 |
| 24 h Acrophase | 0.088 | −0.153 | −0.086 | −0.069 | −0.274 * | −0.024 | 0.226 |
| IV | 0.042 | 0.012 | 0.039 | 0.096 | −0.063 | 0.043 | 0.012 |
| IS | 0.111 | 0.116 | 0.120 | −0.021 | 0.013 | −0.135 | −0.104 |
| M10 | 0.132 | 0.052 | 0.055 | −0.142 | −0.094 | 0.023 | 0.076 |
| M10 Onset | 0.151 | −0.029 | 0.022 | −0.112 | −0.190 | −0.052 | 0.243 |
| L5 | −0.044 | −0.197 | −0.150 | −0.014 | −0.180 | −0.087 | 0.048 |
| L5 Onset | 0.069 | 0.005 | −0.011 | −0.093 | −0.058 | 0.039 | −0.051 |
| Relative Amplitude | 0.064 | 0.183 | 0.138 | −0.033 | 0.129 | 0.098 | −0.024 |
| Light Exposure (LE)/Blue Light Exposure (BLE) | |||||||
| LE MESOR | 0.161 | 0.189 | 0.199 | 0.118 | 0.030 | 0.084 | −0.049 |
| LE Amplitude | 0.128 | 0.248 | 0.234 | 0.157 | 0.137 | 0.084 | −0.084 |
| LE Acrophase | −0.071 | −0.312 * | −0.223 | −0.128 | −0.280 * | −0.038 | 0.212 |
| BLE MESOR | 0.163 | 0.223 | 0.216 | 0.121 | 0.070 | 0.094 | −0.078 |
| BLE Amplitude | 0.155 | 0.279 * | 0.253 | 0.143 | 0.148 | 0.095 | −0.114 |
| BLE Acrophase | −0.093 | −0.322 * | −0.255 | −0.126 | −0.272 * | −0.029 | 0.291 * |
| BLE M10 | 0.155 | 0.244 | 0.223 | 0.122 | 0.103 | 0.105 | −0.089 |
| BLE M10 Onset | 0.029 | −0.162 | −0.111 | −0.229 | −0.202 | −0.001 | 0.077 |
| BLE L5 | 0.202 | 0.069 | 0.089 | −0.041 | −0.153 | −0.010 | 0.035 |
| BLE L5 Onset | 0.006 | −0.202 | −0.126 | −0.020 | −0.229 | −0.121 | 0.112 |
| BLE NA | 0.047 | 0.369 * | 0.259 | 0.175 | 0.378 * | 0.037 | −0.172 |
| Sleep | |||||||
| Bedtime | 0.045 | −0.225 | −0.154 | −0.133 | −0.314 * | −0.063 | 0.184 |
| Wake Time | 0.107 | −0.078 | 0.011 | −0.029 | −0.212 | −0.030 | 0.214 |
| Time in Bed | 0.058 | 0.091 | 0.105 | 0.043 | 0.037 | 0.014 | 0.056 |
| Total Sleep | 0.072 | 0.108 | 0.108 | 0.037 | 0.044 | 0.009 | 0.030 |
| Sleep Efficacy | 0.055 | 0.056 | 0.004 | −0.004 | 0.017 | −0.046 | −0.095 |
| WASO | −0.056 | −0.056 | 0.008 | 0.030 | −0.024 | 0.028 | 0.138 |
| Chronotype Morningness–Eveningness Questionnaire (MEQ) Score | |||||||
| MEQ Score | −0.020 | 0.051 | 0.010 | 0.074 | 0.100 | −0.032 | −0.047 |
| Dependent Variable | Predictor | β (95% CI) | p-Value | Partial η2 |
|---|---|---|---|---|
| Hemoglobin | Sex | −0.491 (−0.683, −0.299) | <0.001 | 0.247 |
| BLE NA | 0.206 (0.012, 0.400) | 0.037 | 0.054 | |
| Age | 0.028 (−0.154, 0.209) | 0.763 | 0.001 | |
| Mean Corpuscular Hemoglobin | BLE NA | 0.377 (0.159, 0.595) | <0.001 | 0.130 |
| Age | −0.174 (−0.378, 0.032) | 0.096 | 0.035 | |
| Sex | 0.060 (−0.156, 0.277) | 0.580 | 0.004 | |
| Red Cell Distribution Width—CV | BLE Acrophase | 0.316 (0.093, 0.539) | 0.006 | 0.092 |
| Age | 0.205 (−0.005, 0.416) | 0.055 | 0.046 | |
| Sex | 0.023 (−0.245, 0.199) | 0.839 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubin, D.; Boldyreva, J.; Kolomeichuk, S.; Stefani, O.; Shigabaeva, A.; Alkhimova, L.; Tchaikovkaya, M.; Weinert, D.; Cornelissen, G. Circadian Light Hygiene Is Associated with Anemia Markers in Young Adults. Biology 2025, 14, 1649. https://doi.org/10.3390/biology14121649
Gubin D, Boldyreva J, Kolomeichuk S, Stefani O, Shigabaeva A, Alkhimova L, Tchaikovkaya M, Weinert D, Cornelissen G. Circadian Light Hygiene Is Associated with Anemia Markers in Young Adults. Biology. 2025; 14(12):1649. https://doi.org/10.3390/biology14121649
Chicago/Turabian StyleGubin, Denis, Julia Boldyreva, Sergey Kolomeichuk, Oliver Stefani, Aislu Shigabaeva, Larisa Alkhimova, Marina Tchaikovkaya, Dietmar Weinert, and Germaine Cornelissen. 2025. "Circadian Light Hygiene Is Associated with Anemia Markers in Young Adults" Biology 14, no. 12: 1649. https://doi.org/10.3390/biology14121649
APA StyleGubin, D., Boldyreva, J., Kolomeichuk, S., Stefani, O., Shigabaeva, A., Alkhimova, L., Tchaikovkaya, M., Weinert, D., & Cornelissen, G. (2025). Circadian Light Hygiene Is Associated with Anemia Markers in Young Adults. Biology, 14(12), 1649. https://doi.org/10.3390/biology14121649








