Differential Phosphorus Allocation in Leaves and Roots of Yushania shuichengensis Across Soil Phosphorus Gradients: Implications for Ecological Adaptation and Resource Use Efficiency
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling
2.3. Measurement of Functional Traits of Leaves and Roots
2.3.1. Leaf Traits
2.3.2. Root Traits
2.4. Measurement of P Fractions, and Total P
2.5. Calculations of Photosynthetic P Efficiency and P Resorption Efficiency
2.6. Statistical Analysis
3. Results
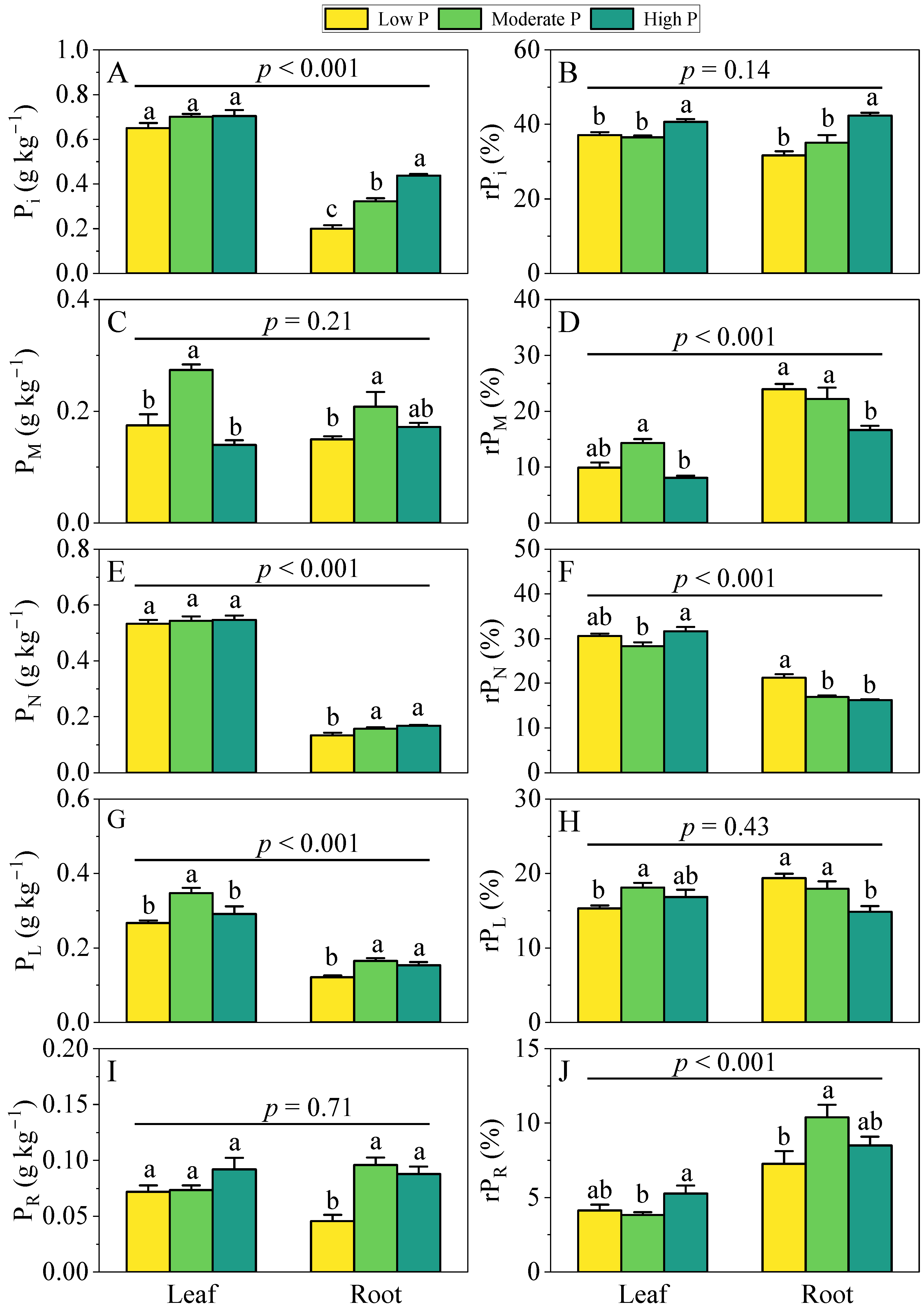
3.1. Variation in P Fractions Among Sites
3.2. Variation in P Fractions Between Leaves and Roots
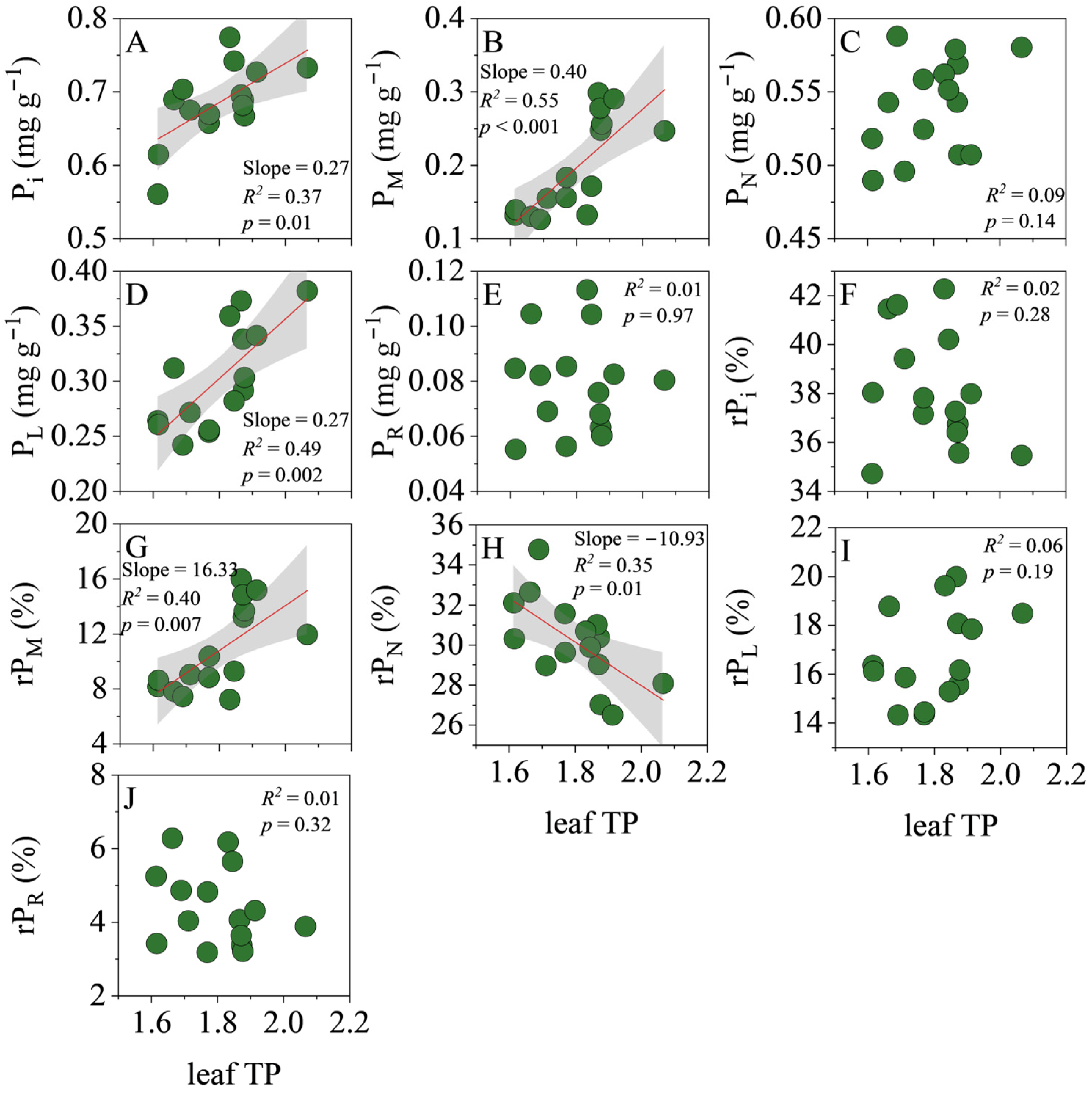
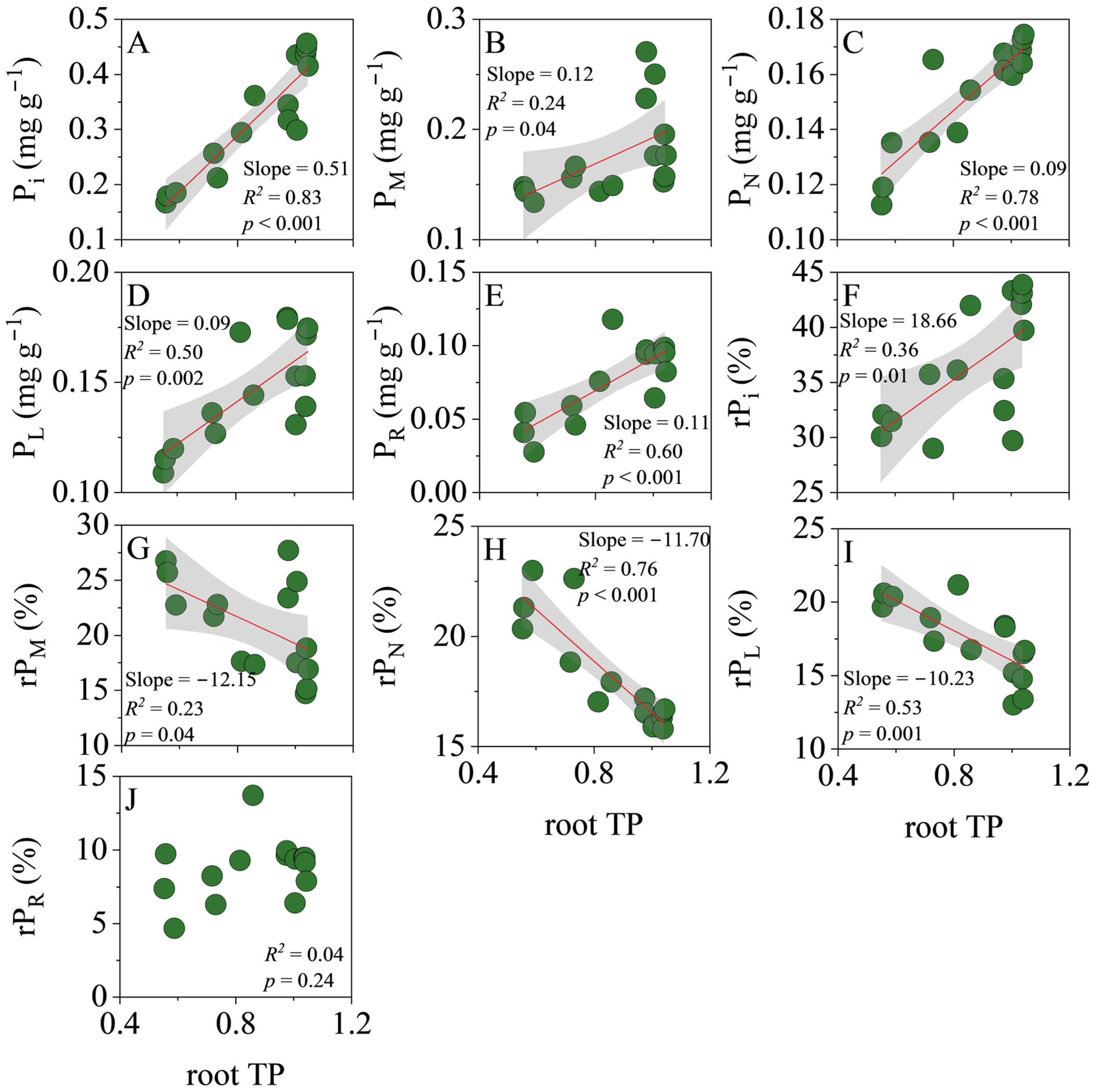
3.3. Correlations of TP with P Fractions in Leaves and Roots
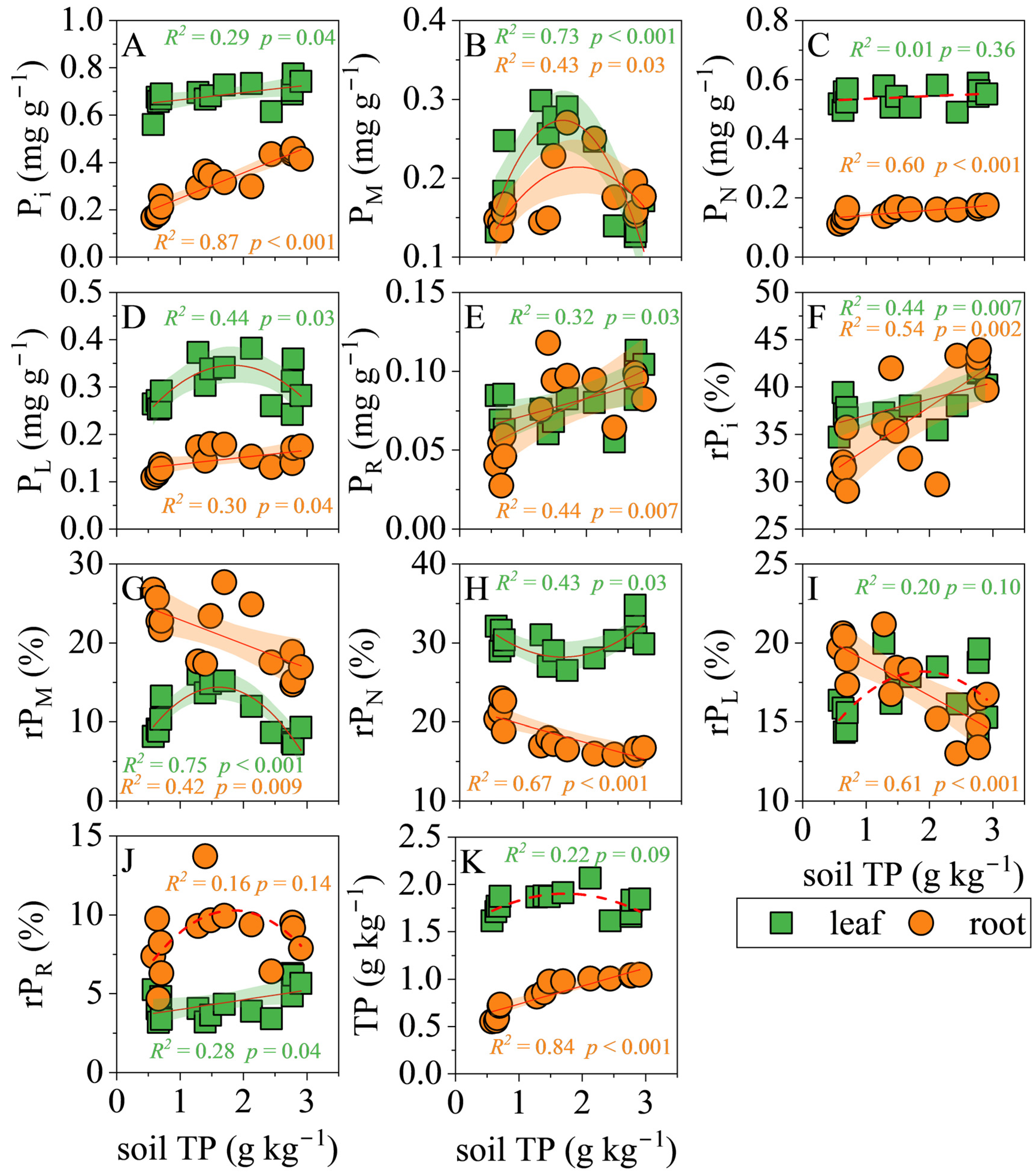
3.4. Correlations of Soil TP with P Fractions
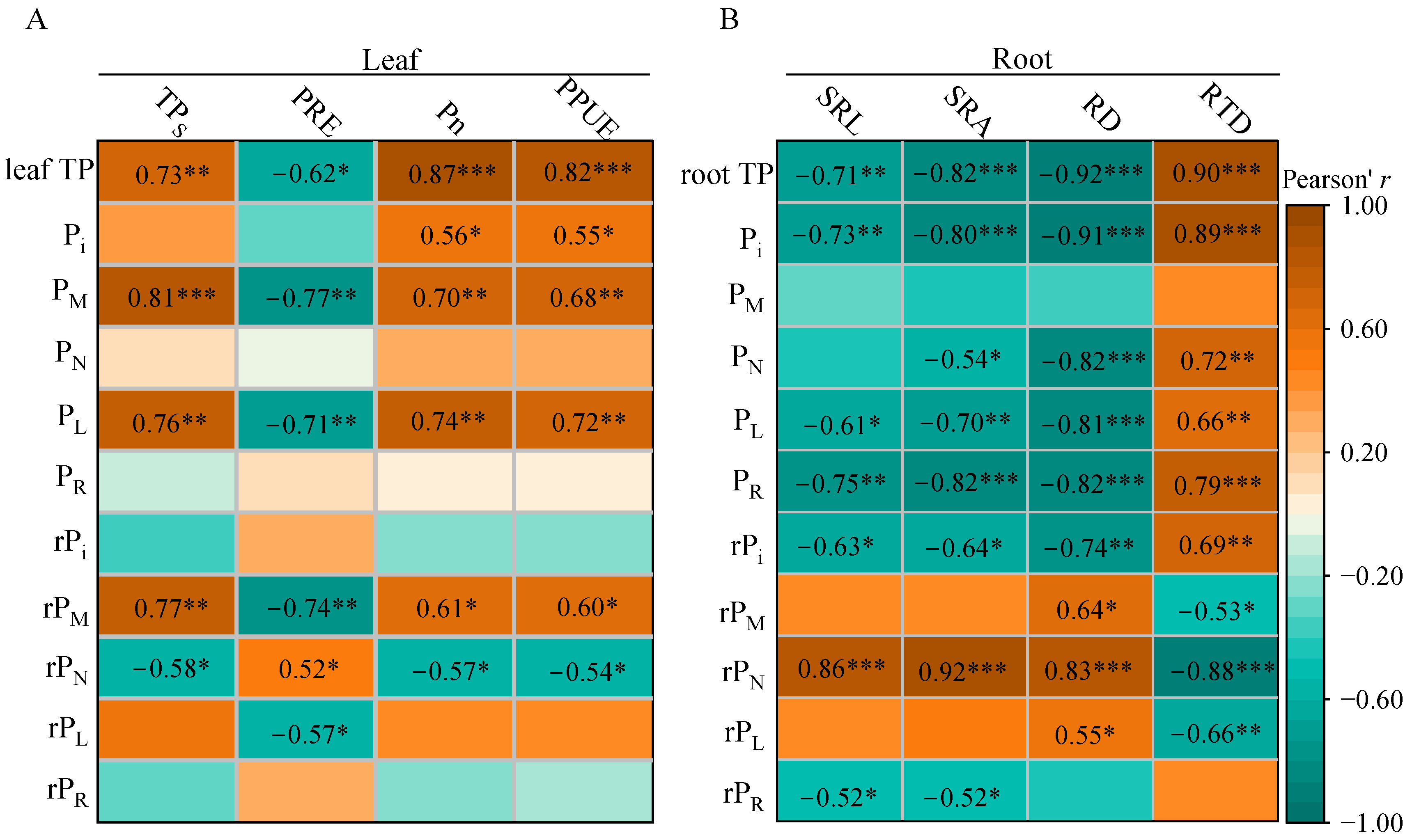
3.5. Correlations of P Fractions with Functional Traits in Leaves and Roots
4. Discussion
4.1. The Response of Leaf and Root P Fractions to Soil P Supply
4.2. Differences in P Fraction Concentrations and Relative Allocations Between Leaves and Roots
4.3. Effects of Plant P Fractions on Functional Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P | Phosphorus |
| TP | Total phosphorus |
| Pi | orthophosphate P |
| PM | low-molecular-weight metabolite P |
| PN | nucleic acid P |
| PL | lipid P |
| PR | residual P |
| rPi | relative allocation of Pi |
| rPM | relative allocation of PM |
| rPN | relative allocation of PN |
| rPL | relative allocation of PL |
| rPR | relative allocation of PR |
| PPM | primary metabolic P |
| TPS | senesced leaf TP |
| PRE | P resorption efficiency |
| Pn | net photosynthetic rate |
| PPUE | photosynthetic P-use efficiency |
| SRL | specific root length |
| SRA | specific root surface area |
| RD | root diameter |
| RTD | root tissue density |
References
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Cunha, H.F.V.; Andersen, K.M.; Lugli, L.F.; Santana, F.D.; Aleixo, I.F.; Moraes, A.M.; Garcia, S.; Di Ponzio, R.; Mendoza, E.O.; Brum, B.; et al. Direct evidence for phosphorus limitation on Amazon forest productivity. Nature 2022, 608, 558–562. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Laliberté, E.; Pearse, S.J.; Ryan, M.H.; Shane, M.W.; Veneklaas, E.J. Phosphorus Nutrition of Proteaceae in Severely Phosphorus-Impoverished Soils: Are There Lessons to Be Learned for Future Crops? Plant Physiol. 2011, 156, 1058–1066. [Google Scholar] [CrossRef]
- Augusto, L.; Achat, D.L.; Jonard, M.; Vidal, D.; Ringeval, B. Soil parent material—A major driver of plant nutrient limitations in terrestrial ecosystems. Glob. Change Biol. 2017, 23, 3808–3824. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Relationship between photosynthetic phosphorus-use efficiency and foliar phosphorus fractions in tropical tree species. Ecol. Evol. 2013, 3, 4872–4880. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, A.; Kitayama, K. Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. J. Ecol. 2009, 97, 984–991. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.H.; Han, Z.M.; Pang, J.Y.; Lambers, H.; Finnegan, P.M. Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol. 2019, 223, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.F.; Li, Z.A.; Sayer, E.J.; Lambers, H.; Li, Y.W.; Zou, B.; Tang, J.W.; Heskel, M.; Ding, Y.Z.; Wang, F.M. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2019, 33, 503–513. [Google Scholar] [CrossRef]
- Yu, Q.S.; Ni, X.F.; Cheng, X.L.; Ma, S.H.; Tian, D.; Zhu, B.A.; Zhu, J.L.; Ji, C.J.; Tang, Z.Y.; Fang, J.Y. Foliar phosphorus allocation and photosynthesis reveal plants’ adaptative strategies to phosphorus limitation in tropical forests at different successional stages. Sci. Total Environ. 2022, 846, 157456. [Google Scholar] [CrossRef]
- Wen, Z.H.; Pang, J.Y.; Wang, X.; Gille, C.E.; De Borda, A.; Hayes, P.E.; Clode, P.L.; Ryan, M.H.; Siddique, K.H.M.; Shen, J.B.; et al. Differences in foliar phosphorus fractions, rather than in cell-specific phosphorus allocation, underlie contrasting photosynthetic phosphorus use efficiency among chickpea genotypes. J. Exp. Bot. 2023, 74, 1974–1989. [Google Scholar] [CrossRef] [PubMed]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Salter, W.T.; Turnbull, T.L.; Okazaki, Y.; Saito, K.; Kreuzwieser, J.; Rennenberg, H.; Adams, M.A. Plant and soil P determine functional attributes of subalpine Australian plants. Arct. Antarct. Alp. Res. 2018, 50, e1420246. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhang, L.L.; Wen, D.Z. Photosynthesis of subtropical forest species from different successional status in relation to foliar nutrients and phosphorus fractions. Sci. Rep. 2018, 8, 10455. [Google Scholar] [CrossRef]
- Tsujii, Y.; Atwell, B.J.; Lambers, H.; Wright, I.J. Leaf phosphorus fractions vary with leaf economic traits among 35 Australian woody species. New Phytol. 2024, 241, 1985–1997. [Google Scholar] [CrossRef]
- Yang, L.; Yi, T. Two New Species of Yushania Keng f. (Poaceae) from Guizhou, China and the Name about Bashania faberi (Rendle) Yi. J. Bamboo Res. 2013, 32, 5–8+17, (In Chinese with English abstract). [Google Scholar]
- Wang, Y.; Wang, S. Emeishan Large Igneous Provinces and Basalt Copper Deposits: An Example From Permian Basalt Areas in Guizhou. Guizhou Geol. 2003, 1, 5–10+14, (In Chinese with English abstract). [Google Scholar]
- Zhan, H.; He, Q.; Zeng, F.; Deng, Y.; Wang, X.; Li, L.; Lou, Y.; Xie, F.; Wang, Y.; Lang, X. Geochemical characteristics and geological implications of mudstones and sandstones at the top of the Upper Permian Xuanwei Formation on the western margin of Sichuan Basin. Acta Petrol. Mineral. 2023, 42, 83–103, (In Chinese with English abstract). [Google Scholar]
- Yang, H. A preliminary study on the geological structure of Hezhang, Weining and Shuicheng counties in Guizhou. J. Nanjing Univ. (Nat. Sci.) 1955, 1, 86–94. (In Chinese) [Google Scholar]
- Zou, S.; Huang, C.M.; Feng, T.; Chen, Y.; Bai, X.L.; Li, W.J.; He, B. Phosphorus Differences in Trunk-Epiphytic and Rock-Epiphytic Habitats Modify Pyrrosia sheareri Root Traits but Not Leaf Photosynthetic Rates in a Karst Forest. Forests 2025, 16, 903. [Google Scholar] [CrossRef]
- Bai, X.; Yang, D.; Sher, J.; Zhang, Y.; Zhang, K.; Liu, Q.; Wen, H.; Zhang, J.; Slot, M. Divergences in stem and leaf traits between lianas and coexisting trees in a subtropical montane forest. J. Plant Ecol. 2024, 17, 1. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.H.; Zhang, X.H.; Song, C.C. Responses of plant nutrient resorption to phosphorus addition in freshwater marsh of Northeast China. Sci. Rep. 2015, 5, 8097. [Google Scholar] [CrossRef]
- Hayes, P.E.; Adem, G.D.; Pariasca-Tanaka, J.; Wissuwa, M. Leaf phosphorus fractionation in rice to understand internal phosphorus-use efficiency. Ann. Bot. 2022, 129, 287–302. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Ryan, M.H.; Gille, C.E.; Dayrell, R.L.C.; Finnegan, P.M.; Ranathunge, K.; Nicol, D.; Lambers, H. Phosphorus fractions in leaves. New Phytol. 2023, 237, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.H.; Xie, M.; Liu, T.; Huang, H.G.; Zhang, X.Z.; Yu, H.Y.; Zheng, Z.C.; Wang, Y.D.; Tang, Y.; Li, T.X. Physiological and molecular responses in phosphorus-hyperaccumulating Polygonum species to high phosphorus exposure. Plant Cell Environ. 2024, 47, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Chan, C.; Liao, Y.Y.; Chiou, T.J. The Impact of Phosphorus on Plant Immunity. Plant Cell Physiol. 2021, 62, 582–589. [Google Scholar] [CrossRef]
- Fort, F. Grounding trait-based root functional ecology. Funct. Ecol. 2023, 37, 2159–2169. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.W.; Ran, Q.X.; Pang, J.Y.; Lambers, H.; He, J. Crop domestication increased photosynthetic phosphorus-use efficiency associated with changes in leaf phosphorus fractions under low soil phosphorus conditions. Plant Soil 2025, 509, 915–928. [Google Scholar] [CrossRef]
- Wang, F.-C.; Fang, X.-M.; Wang, G.G.; Mao, R.; Lin, X.-F.; Wang, H.; Chen, F.-S. Effects of nutrient addition on foliar phosphorus fractions and their resorption in different-aged leaves of Chinese fir in subtropical China. Plant Soil 2019, 443, 41–54. [Google Scholar] [CrossRef]
- Tsujii, Y.; Onoda, Y.; Kitayama, K. Phosphorus and nitrogen resorption from different chemical fractions in senescing leaves of tropical tree species on Mount Kinabalu, Borneo. Oecologia 2017, 185, 171–180. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
| Site Name | Low-P Site | Moderate-P Site | High-P Site |
|---|---|---|---|
| Coordinate | 26°50′40″ N, 104°41′29″ E | 26°50′30″ N, 104°42′10″ E | 27°0′25″ N, 104°43′12″ E |
| Parent rock | Sandstone | Limestone | Basalt |
| Vegetation type | Bamboo shrubland | Bamboo shrubland | Bamboo shrubland |
| MAT (°C) | 8.1 | 8.1 | 8.0 |
| MAP (mm yr−1) | 1094 | 1094 | 1088 |
| Soil TP (g kg−1) | 0.66 | 1.60 | 2.73 |
| Organ | Control Variable | Functional Traits | rPi | rPM | rPN | rPL | rPR |
|---|---|---|---|---|---|---|---|
| Leaf | leaf TP | TPS | −0.18 | 0.56 * | −0.23 | 0.48 | −0.14 |
| PRE | 0.16 | −0.57 * | 0.22 | −0.48 | 0.14 | ||
| Pn | 0.02 | 0.09 | −0.04 | 0.31 | 0.08 | ||
| PPUE | 0.09 | 0.13 | −0.03 | 0.31 | 0.09 | ||
| Root | root TP | SRL | −0.32 | 0.07 | 0.71 ** | −0.26 | −0.44 |
| SRA | −0.26 | 0.06 | 0.72 ** | −0.34 | −0.48 | ||
| RD | −0.51 | 0.45 | 0.09 | −0.53 | −0.37 | ||
| RTD | 0.35 | −0.13 | −0.41 | 0.05 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Huang, C.; Bai, X.; Li, W.; He, B. Differential Phosphorus Allocation in Leaves and Roots of Yushania shuichengensis Across Soil Phosphorus Gradients: Implications for Ecological Adaptation and Resource Use Efficiency. Biology 2025, 14, 1647. https://doi.org/10.3390/biology14121647
Zou S, Huang C, Bai X, Li W, He B. Differential Phosphorus Allocation in Leaves and Roots of Yushania shuichengensis Across Soil Phosphorus Gradients: Implications for Ecological Adaptation and Resource Use Efficiency. Biology. 2025; 14(12):1647. https://doi.org/10.3390/biology14121647
Chicago/Turabian StyleZou, Shun, Chumin Huang, Xiaolong Bai, Wangjun Li, and Bin He. 2025. "Differential Phosphorus Allocation in Leaves and Roots of Yushania shuichengensis Across Soil Phosphorus Gradients: Implications for Ecological Adaptation and Resource Use Efficiency" Biology 14, no. 12: 1647. https://doi.org/10.3390/biology14121647
APA StyleZou, S., Huang, C., Bai, X., Li, W., & He, B. (2025). Differential Phosphorus Allocation in Leaves and Roots of Yushania shuichengensis Across Soil Phosphorus Gradients: Implications for Ecological Adaptation and Resource Use Efficiency. Biology, 14(12), 1647. https://doi.org/10.3390/biology14121647






