Chagas Disease in the 21st Century: Global Spread, Ecological Shifts, and Research Frontiers
Simple Summary
Abstract
1. Introduction
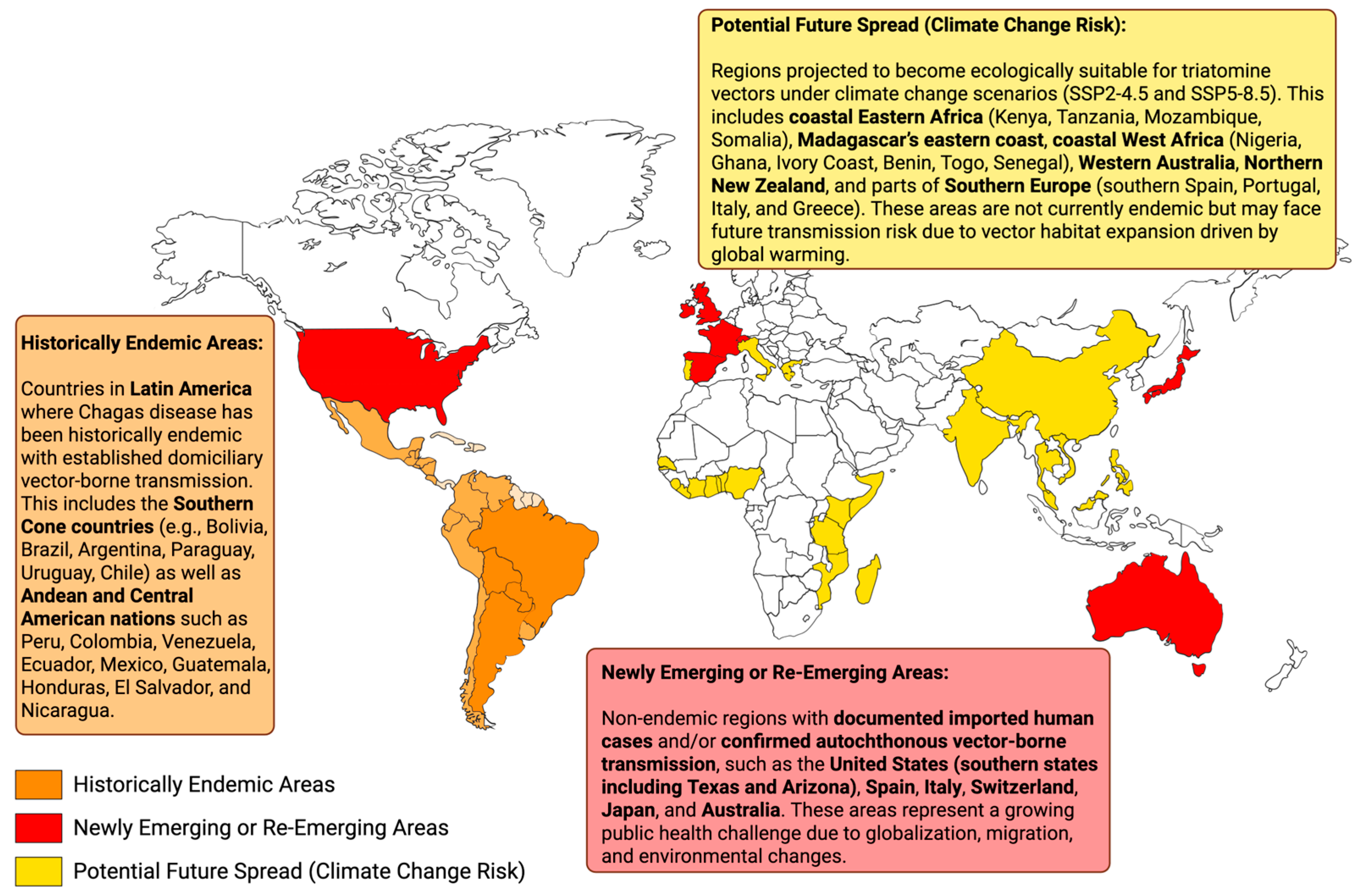
2. Global Spread
2.1. Latin America: The Historic Epicenter
2.2. Emergence in Non-Endemic Regions: A Global Health Challenge
2.3. Oral Transmission: A Growing Driver of Global Outbreaks
2.4. Congenital Transmission: A Silent Global Threat
3. Ecological Shifts
3.1. Environmental and Ecological Factors in Vector Spread
3.2. Insecticide Resistance and Operational Challenges
3.3. Innovations and Strategic Needs
4. Research Frontiers
4.1. Advances in Vector Surveillance and Ecology
- Genomic, Transcriptomic, and Proteomic Profiling of Triatomine Vectors
- Climate-Based Predictive Mapping of Triatomine Vector Distribution
4.2. Digital Surveillance Innovations
4.3. One Health Integration
5. Recommendations
- Global Spread—Strengthen Cross-Border Surveillance and Health System Integration
- Ecological Shifts—Integrate Environmental and Wildlife Surveillance
- Research Frontiers—Foster Innovation in Diagnostics and Digital Epidemiology
- One Health Implementation—Promote Intersectoral Collaboration
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CFR | Case Fatality Rate |
| DNA | Deoxyribonucleic Acid |
| DTU | Discrete Typing Unit |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EU | European Union |
| GIS | Geographic Information System |
| IFA | Immunofluorescence Assay |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LAMP | Loop-Mediated Isothermal Amplification |
| ML | Machine Learning |
| NGS | Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| RDT | Rapid Diagnostic Test |
| RNA | Ribonucleic Acid |
| SSP | Shared Socioeconomic Pathway |
| WHO | World Health Organization |
References
- de Sousa, A.S.; Vermeij, D.; Ramos ANJr Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Montgomery, S.P. Chagas disease. Ann. Intern. Med. 2023, 176, ITC17–ITC32. [Google Scholar] [CrossRef]
- Cucunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramírez, J.-D.; Velásquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.-G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health–Am. 2024, 37, 100881. [Google Scholar] [CrossRef]
- Alarcón de Noya, B.; Jackson, Y. Chagas Disease Epidemiology: From Latin America to the World. In Chagas Disease: A Neglected Tropical Disease; Pinazo Delgado, M.-J., Gascón, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–36. [Google Scholar]
- López-García, A.; Gilabert, J.A. Oral transmission of Chagas disease from a One Health approach: A systematic review. Trop. Med. Int. Health 2023, 28, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Montgomery, S.P. Congenital Chagas disease: Progress toward implementation of pregnancy-based screening. Curr. Opin. Infect. Dis. 2021, 34, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas disease in the United States: A public health approach. Clin. Microbiol. Rev. 2019, 33, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.N.; Bosch-Nicolau, P.; Nascimento, B.R.; Martins-Melo, F.R.; Perel, P.; Geissbühler, Y.; Demacq, C.; Quijano, M.; Mosser, J.F.; Cousin, E.; et al. Prevalence of Chagas disease among Latin American immigrants in non-endemic countries: An updated systematic review and meta-analysis. Lancet Reg. Health–Eur. 2024, 46, 101040. [Google Scholar]
- Rodriguez, F.; Luna, B.S.; Calderon, O.; Manriquez-Roman, C.; Amezcua-Winter, K.; Cedillo, J.; Garcia-Vazquez, R.; Tejeda, I.A.; Romero, A.; Waldrup, K.; et al. Surveillance of Trypanosoma cruzi infection in Triatomine vectors, feral dogs and cats, and wild animals in and around El Paso county, Texas, and New Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009147. [Google Scholar] [CrossRef]
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.; Chiodini, P.L. Diagnosis and clinical management of Chagas disease: An increasing challenge in non-endemic areas. Res. Rep. Trop. Med. 2022, 13, 25–40. [Google Scholar] [CrossRef]
- Ocaña-Mayorga, S.; Lobos, S.E.; Crespo-Pérez, V.; Villacís, A.G.; Pinto, C.M.; Grijalva, M.J. Influence of ecological factors on the presence of a triatomine species associated with the arboreal habitat of a host of Trypanosoma cruzi. Parasites Vectors 2018, 11, 567. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Cecere, M.C. Chagas disease vector control. In Triatominae—The Biology of Chagas Disease Vectors; Springer: Berlin/Heidelberg, Germany, 2021; pp. 491–535. [Google Scholar]
- Vargas-Abasolo, R.; Gutiérrez-Cabrera, A.E.; Cruz-López, L.; Alavez-Rosas, D.; Benelli, G.; Córdoba-Aguilar, A. Chagas disease vector control strategies: Where we are and where we should go from here. Entomol. Gen. 2023, 43, 771–788. [Google Scholar] [CrossRef]
- Chagas, C. Über eine neue Trypanosomiasis des Menschen. Arch. Für Schiffs Und Trop. Hyg. 1909, 13, 351–353. [Google Scholar]
- Swett, M.C.; Rayes, D.L.; Campos, S.V.; Kumar, R.N. Chagas disease: Epidemiology, diagnosis, and treatment. Curr. Cardiol. Rep. 2024, 26, 1105–1112. [Google Scholar] [CrossRef]
- Steverding, D. The history of Chagas disease. Parasites Vectors 2014, 7, 317. [Google Scholar] [CrossRef]
- Goldenberg, S.; Zingales, B.; Colli, W. Basic research on Chagas disease: Fifty years of a successful initiative. Acta Trop. 2025, 265, 107598. [Google Scholar] [CrossRef]
- Schofield, C.J.; Dias, J.C. The southern cone initiative against Chagas disease. Adv. Parasitol. 1999, 42, 1–27. [Google Scholar]
- Jimeno, I.; Mendoza, N.; Zapana, F.; de la Torre, L.; Torrico, F.; Lozano, D.; Billot, C.; Pinazo, M.J.; InSPIRES Consortium. Social determinants in the access to health care for Chagas disease: A qualitative research on family life in the “Valle Alto” of Cochabamba, Bolivia. PLoS ONE 2021, 16, e0255226. [Google Scholar] [CrossRef]
- Pinto, J.; Skjefte, M.; Alonso-Padilla, J.; Lozano Beltran, D.F.; Pinto, L.V.; Casellas, A.; Arteaga Terrazas, M.E.; Toledo Galindo, K.A.; Challapa Quechover, R.; Escobar Caballero, M.; et al. Five-year serological and clinical evolution of chronic Chagas disease patients in Cochabamba, Bolivia. PLoS Negl. Trop. Dis. 2023, 17, e0011498. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Pereira, H.; Scofield, A.; Júnior, P.S.B.; Dos Santos, D.L.; de Sousa Siqueira, J.; Chaves, J.F.; de Jesus Cardoso, R.; dos Anjos Lima, A.H.; Sarmento, N.M.F.P.; Júnior, F.D.; et al. Chagas disease in urban and peri-urban environment in the Amazon: Sentinel hosts, vectors, and the environment. Acta Trop. 2021, 217, 105858. [Google Scholar] [CrossRef]
- Madeira, F.P.; Jesus, A.C.d.; Moraes, M.H.d.S.; Barroso, N.F.; Castro, G.V.d.S.; Ribeiro, M.A.L.; Mendes, J.E.T.; Camargo, L.M.A.; Meneguetti, D.U.d.O.; Bernarde, P.S.; et al. Chagas disease in the western Brazilian Amazon: Epidemiological overview from 2007 to 2018. J. Hum. Growth Dev. 2021, 31, 84–92. [Google Scholar] [CrossRef]
- Paixão, D.d.S.; Portela Madeira, F.; Costa de Jesus, A.; Paixão, H.C.d.S.; Camargo, J.d.S.A.A.; Ribeiro, M.A.L.; José Ramos, L.; de Oliveira, J.; Aristeu da Rosa, J.; Bernarde, P.S.; et al. Mapping the Silent Threat: A Comprehensive Analysis of Chagas Disease Occurrence in Riverside Communities in the Western Amazon. Pathogens 2024, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.R.T.; de Oliveira Guerra, J.A.; Ortiz, J.V.; do Nascimento Couceiro, K.; da Silva e Silva, M.R.H.; Jorge Brandão, A.R.; Guevara, E.; Arcanjo, A.R.L.; de Oliveira Júnior, E.F.; Smith-Doria, S.; et al. Acute Chagas disease associated with ingestion of contaminated food in Brazilian western Amazon. Trop. Med. Int. Health 2023, 28, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Guhl, F.; Ramírez, J.D. Poverty, migration, and Chagas disease. Curr. Trop. Med. Rep. 2021, 8, 52–58. [Google Scholar] [CrossRef]
- Schijman, A.G.; Alonso-Padilla, J.; Britto, C.; Bernal, C.P.H. Retrospect, advances and challenges in Chagas disease diagnosis: A comprehensive review. Lancet Reg. Health–Am. 2024, 36, 100821. [Google Scholar] [CrossRef]
- Urbano, P.; Hernández, C.; Velásquez-Ortiz, N.; Ballesteros, N.; Páez-Triana, L.; Vega, L.; Urrea, V.; Ramírez, A.; Muñoz, M.; Ibarra-Cerdeña, C.N.; et al. Transmission ecology of Trypanosoma cruzi by Rhodnius prolixus (Reduviidae: Triatominae) infesting palm-tree species in the Colombian Orinoco, indicates risks to human populations. PLoS Negl. Trop. Dis. 2024, 18, e0011981. [Google Scholar] [CrossRef]
- Ravazi, A.; Oliveira, J.d.; Madeira, F.F.; Nunes, G.M.; Reis, Y.V.d.; Oliveira, A.B.B.d.; Azevedo, L.M.S.; Galvão, C.; Azeredo-Oliveira, M.T.V.d.; Rosa, J.A.d.; et al. Climate and environmental changes and their potential effects on the dynamics of chagas disease: Hybridization in Rhodniini (Hemiptera, Triatominae). Insects 2023, 14, 378. [Google Scholar] [CrossRef]
- Forsyth, C.; Higuita, N.I.A.; Hamer, S.A.; Ibarra-Cerdeña, C.N.; Valdez-Tah, A.; Granados, P.S.; Hamer, G.L.; Vingiello, M.; Beatty, N.L. Climate change and Trypanosoma cruzi transmission in North and central America. Lancet Microbe 2024, 5, 100946. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.D.; Paz-Soldán, V.A.; Condori Pino, C.E.; Malaga Chavez, F.S.; Levy, M.Z.; Gonçalves, R. Barriers to surveillance and control of re-emergence of the Chagas disease vector Triatoma infestans in Arequipa, Peru. PLoS Negl. Trop. Dis. 2025, 19, e0013373. [Google Scholar] [CrossRef]
- Abras, A.; Ballart, C.; Fernández-Arévalo, A.; Pinazo, M.-J.; Gascón, J.; Muñoz, C.; Gállego, M. Worldwide control and management of Chagas disease in a new era of globalization: A close look at congenital Trypanosoma cruzi infection. Clin. Microbiol. Rev. 2022, 35, e0015221. [Google Scholar] [CrossRef]
- Navarro, M.; Reguero, L.; Subirà, C.; Blázquez-Pérez, A.; Requena-Méndez, A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain. Travel Med. Infect. Dis. 2022, 47, 102284. [Google Scholar] [CrossRef]
- Hotez, P.J.; Dumonteil, E.; Woc-Colburn, L.; Serpa, J.A.; Bezek, S.; Edwards, M.S.; Hallmark, C.J.; Musselwhite, L.W.; Flink, B.J.; Bottazzi, M.E. Chagas disease: “The new HIV/AIDS of the Americas”. PLoS Negl. Trop. Dis. 2012, 6, e1498. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- Basile, L.; Jansà, J.M.; Carlier, Y.; Salamanca, D.D.; Angheben, A.; Bartoloni, A.; Seixas, J.; Van Gool, T.; Cañavate, C.; Flores-Chávez, M.; et al. Chagas disease in European countries: The challenge of a surveillance system. Eurosurveillance 2011, 16, 19968. [Google Scholar] [CrossRef]
- Navarro, M.; Navaza, B.; Guionnet, A.; López-Vélez, R. Chagas disease in Spain: Need for further public health measures. PLoS Negl. Trop. Dis. 2012, 6, e1962. [Google Scholar] [CrossRef]
- Montgomery, S.P.; Starr, M.C.; Cantey, P.T.; Edwards, M.S.; Meymandi, S.K. Neglected parasitic infections in the United States: Chagas disease. Am. J. Trop. Med. Hyg. 2014, 90, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Gómez i Prat, J.; Peremiquel-Trillas, P.; Claveria Guiu, I.; Choque, E.; Oliveira Souto, I.; Serre Delcor, N.; Sulleiro, E.; Espasa, M.; Pastoret, C.; de Los Santos, J.J. A community-based intervention for the detection of Chagas disease in Barcelona, Spain. J. Community Health 2019, 44, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Montgomery, S.P.; Herwaldt, B.L.; Rassi, A.; Marin-Neto, J.A.; Dantas, R.O.; Maguire, J.H.; Acquatella, H.; Morillo, C.; Kirchhoff, L.V. Evaluation and treatment of Chagas disease in the United States: A systematic review. JAMA 2007, 298, 2171–2181. [Google Scholar] [CrossRef]
- Echeverría, L.E.; Marcus, R.; Novick, G.; Sosa-Estani, S.; Ralston, K.; Zaidel, E.J.; Forsyth, C.; Ribeiro, A.L.P.; Mendoza, I.; Falconi, M.L.; et al. WHF IASC roadmap on Chagas disease. Glob. Heart 2020, 15, 26. [Google Scholar] [CrossRef]
- Carlier, Y.; Altcheh, J.; Angheben, A.; Freilij, H.; Luquetti, A.O.; Schijman, A.G.; Segovia, M.; Wagner, N.; Albajar Vinas, P. Congenital Chagas disease: Updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl. Trop. Dis. 2019, 13, e0007694. [Google Scholar] [CrossRef]
- Edwards, M.S.; Stimpert, K.K.; Bialek, S.R.; Montgomery, S.P. Evaluation and management of congenital Chagas disease in the United States. J. Pediatr. Infect. Dis. Soc. 2019, 8, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.M.I.; Miura, S.; Maeda, T.; Imai, K.; Smith, C.; Velasquez, C.V.; Honda, S.; Hirayama, K. Analysis of the Chagas disease situation in Japan: A cross sectional study and cost-effectiveness analysis of a Chagas disease screening program. Lancet Reg. Health–West. Pac. 2023, 31, 100574. [Google Scholar]
- Harada, Y.; Iwashita, H.; Moriyasu, T.; Nagi, S.; Saito, N.; Sugawara-Mikami, M.; Yoshioka, K.; Yotsu, R.; Japan NTD Study Group. The current status of neglected tropical diseases in Japan: A scoping review. PLoS Negl. Trop. Dis. 2024, 18, e0011854. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Liu, H.; Xu, Y.; Lu, H.; Zhao, L.; He, Y.; Zhang, M.; Zhang, J.; Si, G. Clinical and epidemiological investigation of human infection with zoonotic parasite Trypanosoma dionisii in China. J. Infect. 2024, 89, 106290. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y. Introduction: Japan Overseas Cooperation Volunteers as a State-Managed International Voluntary Service. In State-Managed International Voluntary Service: The Case of Japan Overseas Cooperation Volunteers; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–23. [Google Scholar]
- Picanço, M.M.; Guedes, R.N.C.; da Silva, R.S.; Galvão, C.; Souza, P.G.C.; Barreto, A.B.; Sant’Ana, L.C.d.S.; Lopes, P.H.Q.; Picanço, M.C. Unveiling the overlooked: Current and future distribution dynamics of kissing bugs and palm species linked to oral Chagas disease transmission. Acta Trop. 2024, 258, 107367. [Google Scholar] [CrossRef]
- Shaw, J.A. Parasitic diseases. In Historical Diseases from a Modern Perspective: The American Experience; Springer: Berlin/Heidelberg, Germany, 2024; pp. 147–159. [Google Scholar]
- Moreira, C.H.V.; Azevedo, L.; Ferreira, A.M.; Oliveira, A.C.G.; de Souza, A.B.; Haikal, D.S.A.; Oliveira, C.D.L.; Cardoso, C.S.; Spinelli, M.; Bierrenbach, A.L.; et al. Bridging the gap in Chagas disease management: A mixed-methods study using an implementation science approach within the Brazilian primary health care system—‘Implementa-Chagas/SaMi-Trop project’. Lancet Reg. Health–Am. 2025, 47, 101136. [Google Scholar]
- Ramos-Sesma, V.; Navarro, M.; Llenas-García, J.; Gil-Anguita, C.; Torrus-Tendero, D.; Wikman-Jorgensen, P.; García-López, M.; Amador-Prous, C.; Ventero-Martín, M.P.; Guevara-Hernández, P.; et al. Community-based screening of Chagas disease among Latin American migrants in a non-endemic country: An observational study. Infect. Dis. Poverty 2021, 10, 117. [Google Scholar] [CrossRef]
- Salazar, R.; Castillo-Neyra, R.; Tustin, A.W.; Borrini-Mayorí, K.; Náquira, C.; Levy, M.Z. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2015, 92, 331–335. [Google Scholar] [CrossRef]
- Thompson, C.K.; Thompson, R.A. Trypanosomes of Australian mammals: Knowledge gaps regarding transmission and biosecurity. Trends Parasitol. 2015, 31, 553–562. [Google Scholar] [CrossRef]
- Meraj, S.; Phung, P.; Lau, K.; Lowenberger, C.; Gries, G. Common Bed Bugs: Non-Viable Hosts for Trypanosoma rangeli Parasites. Cells 2024, 13, 2042. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Lane, T.R.; Bernatchez, J.A.; Calvet Alvarez, C.M.; Barbosa da Silva, E.; Giardini, M.A.; Ekins, S. Oral Pyronaridine Tetraphosphate Reduces Tissue Presence of Parasites in a Mouse Model of Chagas Disease. ACS Omega 2024, 9, 37288–37298. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Sousa, A.S.d.; Ramos, A.N., Jr. New contributions to the elimination of Chagas disease as a public health problem: Towards the sustainable development goals by 2030. Trop. Med. Infect. Dis. 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.V.; Chuit, R. Editorial: New opportunities for diagnosis and control of Chagas Disease to reach the 2030 goals for elimination. Front. Parasitol. 2023, 2, 1258999. [Google Scholar] [CrossRef]
- Sabaini, F.; Giacomelli, A.; Tassis, B.; Ierardi, M.; Marconi, A.M.; Ronchi, A.; Cetin, I.; Testa, L.; Savasi, V.; Fabbri, E.; et al. Screening for Chagas disease in Latin-American pregnant women and their newborns: A prospective observational, multicenter study in Milan, Italy. Travel Med. Infect. Dis. 2025, 65, 102846. [Google Scholar] [CrossRef]
- Llenas-García, J.; Wikman-Jorgensen, P.; Gil-Anguita, C.; Ramos-Sesma, V.; Torrús-Tendero, D.; Martínez-Goñi, R.; Romero-Nieto, M.; García-Abellán, J.; Esteban-Giner, M.J.; Antelo, K.; et al. Chagas disease screening in pregnant Latin American women: Adherence to a systematic screening protocol in a non-endemic country. PLoS Negl. Trop. Dis. 2021, 15, e0009281. [Google Scholar] [CrossRef] [PubMed]
- Rapp, E. Impact of structural barriers on undocumented migrants at risk of chagas disease in Switzerland: A double burden of neglect. Lat. Am. Perspect. 2025, 52, 234–249. [Google Scholar] [CrossRef]
- Jackson, Y.; Basile, L.; Chappuis, F. Chagas Disease in Europe. In Neglected Tropical Diseases-Europe and Central Asia; Springer: Berlin/Heidelberg, Germany, 2022; pp. 101–122. [Google Scholar]
- Higuita, N.I.A.; Beatty, N.L.; Forsyth, C.; Henao-Martínez, A.F.; Manne-Goehler, J.; Bourque, D.; Bowman, N.M.; Carrion, M.; Coyle, C.; Dauphinais, M.; et al. Chagas disease in the United States: A call for increased investment and collaborative research. Lancet Reg. Health–Am. 2024, 34, 100768. [Google Scholar]
- Monsalve-Lara, J.; Lilioso, M.; Valença-Barbosa, C.; Thyssen, P.J.; Miguel, D.C.; Limeira, C.; Gadelha, F.R.; Fontes, F.V.; Pires-Silva, D.; Dornak, L.L.; et al. The risk of oral transmission in an area of a Chagas disease outbreak in the Brazilian northeast evaluated through entomological, socioeconomic and schooling indicators. Acta Trop. 2021, 215, 105803. [Google Scholar] [CrossRef]
- Beatty, N.L.; Arango-Ferreira, C.; Gual-Gonzalez, L.; Zuluaga, S.; Nolan, M.S.; Cantillo-Barraza, O. Oral Chagas disease in Colombia—Confirmed and suspected routes of transmission. Trop. Med. Infect. Dis. 2024, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Villamil-Gómez, W.E.; Schultz, J.; Henao-Martínez, A.F.; Parra-Henao, G.; Rassi Jr, A.; Rodríguez-Morales, A.J.; Suarez, J.A. A deadly feast: Elucidating the burden of orally acquired acute Chagas disease in Latin America–Public health and travel medicine importance. Travel Med. Infect. Dis. 2020, 36, 101565. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef]
- de Noya, B.A.; Colmenares, C.; Díaz-Bello, Z.; Ruiz-Guevara, R.; Medina, K.; Muñoz-Calderón, A.; Mauriello, L.; Cabrera, E.; Montiel, L.; Losada, S.; et al. Orally-transmitted Chagas disease: Epidemiological, clinical, serological and molecular outcomes of a school microepidemic in Chichiriviche de la Costa, Venezuela. Parasite Epidemiol. Control 2016, 1, 188–198. [Google Scholar] [CrossRef]
- Rincón-Acevedo, C.Y.; Parada-García, A.S.; Olivera, M.J.; Torres-Torres, F.; Zuleta-Dueñas, L.P.; Hernández, C.; Ramírez, J.D. Clinical and epidemiological characterization of acute Chagas disease in Casanare, Eastern Colombia, 2012–2020. Front. Med. 2021, 8, 681635. [Google Scholar] [CrossRef]
- Gutiérrez, S.A.; Jaimes-Dueñez, J.; Cruz-Saavedra, L.; Hernández, C.; Cantillo-Barraza, O.; Álvarez, F.; Blanco, M.; Leal, B.; Martínez, L.; Medina, M.; et al. An outbreak of acute Chagas disease possibly spread through oral transmission involving animal reservoirs in Eastern Colombia. Am. J. Trop. Med. Hyg. 2023, 110, 36–39. [Google Scholar] [CrossRef]
- Brasil, L.S.; Silvério, D.V.; Silva, J.O.A.; Santos, W.S.; de Melo, L.V.; Juen, L.; França, F.M.; Vieira, T.B. Potential geographic displacement of Chagas disease vectors under climate change. Med. Vet. Entomol. 2025, 39, 709–717. [Google Scholar] [CrossRef]
- Silva-dos-Santos, D.; Barreto-de-Albuquerque, J.; Guerra, B.; Moreira, O.C.; Berbert, L.R.; Ramos, M.T.; Mascarenhas, B.A.S.; Britto, C.; Morrot, A.; Serra Villa-Verde, D.M.; et al. Unraveling Chagas disease transmission through the oral route: Gateways to Trypanosoma cruzi infection and target tissues. PLoS Negl. Trop. Dis. 2017, 11, e0005507. [Google Scholar] [CrossRef]
- Antunes, D.; Marins-Dos-Santos, A.; Ramos, M.T.; Mascarenhas, B.A.S.; Moreira, C.J.d.C.; Farias-de-Oliveira, D.A.; Savino, W.; Monteiro, R.Q.; de Meis, J. Oral route driven acute Trypanosoma cruzi infection unravels an IL-6 dependent hemostatic derangement. Front. Immunol. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Xavier, I.G.G.; Vieira, M.C.; Rodrigues Junior, L.F.; Sperandio da Silva, G.M.; da Silva, P.S.; de Holanda, M.T.; Maciel, E.R.; Carneiro, F.M.; Mazzoli-Rocha, F.; Sangenis, L.H.C.; et al. Prevalence of metabolic syndrome and associated factors among patients with chronic Chagas disease. PLoS ONE 2021, 16, e0249116. [Google Scholar] [CrossRef]
- Basso, B. Modulation of immune response in experimental Chagas disease. World J. Exp. Med. 2013, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cristovão-Silva, A.C.; Brelaz-de-Castro, M.C.A.; Hernandes, M.Z.; Pereira, V.R.A. Chagas disease: Immunology of the disease at a glance. Cytokine Growth Factor Rev. 2021, 62, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pacini, M.F.; Bulfoni Balbi, C.; Dinatale, B.; Farré, C.; Cacik, P.; Gonzalez, F.B.; Marcipar, I.; Pérez, A.R. Intranasal trans-sialidase vaccine mitigates acute and chronic pathology in a preclinical oral chagas disease model. Vaccines 2024, 12, 1171. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Bern, C.; Clark, E.H.; Teixeira, A.L.; Molina, I. Clinical features of Chagas disease progression and severity. Lancet Reg. Health–Am. 2024, 37, 100832. [Google Scholar] [CrossRef]
- Bruneto, E.G.; Fernandes-Silva, M.M.; Toledo-Cornell, C.; Martins, S.; Ferreira, J.M.; Corrêa, V.R.; da Costa, J.M.; Pinto, A.Y.d.N.; de Souza, D.d.S.; Pinto, M.C.G.; et al. Case-fatality from orally-transmitted acute Chagas disease: A systematic review and meta-analysis. Clin. Infect. Dis. 2021, 72, 1084–1092. [Google Scholar] [CrossRef]
- Matthews, S.; Tannis, A.; Puchner, K.P.; Bottazzi, M.E.; Cafferata, M.L.; Comandé, D.; Buekens, P. Estimation of the morbidity and mortality of congenital Chagas disease: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010376. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vega, C.; Billot, C.; Torrico, F. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: Results 2004–2009. PLoS Negl. Trop. Dis. 2013, 7, e2304. [Google Scholar] [CrossRef]
- Carlier, Y.; Dumonteil, E.; Herrera, C.; Waleckx, E.; Tibayrenc, M.; Buekens, P.; Truyens, C.; Muraille, E. Coinfection by multiple Trypanosoma cruzi clones: A new perspective on host-parasite relationship with consequences for pathogenesis and management of Chagas disease. Microbiol. Mol. Biol. Rev. 2025, 89, e00242-24. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.J.; Xiong, X.; Carlier, Y.; Sosa-Estani, S.; Buekens, P. Frequency of the congenital transmission of Trypanosoma cruzi: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 22–33. [Google Scholar] [CrossRef]
- Lynn, M.K.; Aquino, M.S.R.; Rivas, C.A.B.; Landrove, A.M.; de Juarez, A.M.P.; Cerón, R.A.; López, D.G.; Nolan, M.S.; Rivas, P.M.C.; González, R.D.; et al. Prenatal Chagas disease screening in Latin America: The current policy landscape and potential utility of an expanded maternal-familial Trypanosoma cruzi testing framework. Lancet Reg. Health–Am. 2025, 47, 101139. [Google Scholar] [CrossRef]
- Chancey, R.J.; Edwards, M.S.; Montgomery, S.P. Congenital Chagas Disease. Pediatr. Rev. 2023, 44, 213–221. [Google Scholar] [CrossRef]
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Proaño, A.; Shah, N.C.; Guarnizo, S.A.G.; Miranda-Schaeubinger, M.; Levy, M.Z.; Gilman, R.H.; Flannery, D.D. Targeted Maternal Chagas Disease Screening Among Individuals Born in a Chagas-Endemic Country. JAMA Netw. Open 2024, 7, e2449120. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Aldasoro, E.; de Lazzari, E.; Sicuri, E.; Brown, M.; Moore, D.A.; Gascon, J.; Muñoz, J. Prevalence of Chagas disease in Latin-American migrants living in Europe: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2015, 9, e0003540. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The impact of deforestation, urbanization, and changing land use patterns on the ecology of mosquito and tick-borne diseases in Central America. Insects 2021, 13, 20. [Google Scholar] [CrossRef]
- Alarcón de Noya, B.; Díaz-Bello, Z.; Ruiz-Guevara, R.; Noya, O. Chagas disease expands its epidemiological frontiers from rural to urban areas. Front. Trop. Dis. 2022, 3, 799009. [Google Scholar] [CrossRef]
- Rengifo-Correa, L.; Abad-Franch, F.; Martínez-Hernández, F.; Salazar-Schettino, P.M.; Téllez-Rendón, J.L.; Villalobos, G.; Morrone, J.J. A biogeographic–ecological approach to disentangle reticulate evolution in the Triatoma phyllosoma species group (Heteroptera: Triatominae), vectors of Chagas disease. J. Zool. Syst. Evol. Res. 2021, 59, 94–110. [Google Scholar] [CrossRef]
- Gómez-Bravo, A.; Cirignoli, S.; Wehrendt, D.; Schijman, A.; León, C.M.; Flores-Chaves, M.; Nieto, J.; Kieran, T.J.; Abril, M.; Guhl, F. Zoonotic cycle of American Trypanosomiasis in an endemic region of the Argentine Chaco, factors that influenced a paradigm shift. Insects 2024, 15, 471. [Google Scholar] [CrossRef]
- Alevi, K.C.C.; de Oliveira, J.; da Silva Rocha, D.; Galvão, C. Trends in taxonomy of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to integrative taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Madigan, R.; Briñez, W.D.; Paniz-Mondolfi, A.; Ramírez, J.D. Characterizing the transmission dynamics of Trypanosoma cruzi in Triatoma sanguisuga collected from dog kennels in southern Texas. Parasites Vectors 2025, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-De-La-Fuente, A.L.; Sánchez-Casaccia, P.; Piccinali, R.V.; Provecho, Y.; Salvá, L.; Meli, S.; Cano, F.; Hernández, R.; Nattero, J. Urban vectors of Chagas disease in the American continent: A systematic review of epidemiological surveys. PLoS Negl. Trop. Dis. 2022, 16, e0011003. [Google Scholar] [CrossRef] [PubMed]
- Busselman, R.E.; Hamer, S.A. Chagas disease ecology in the United States: Recent advances in understanding Trypanosoma cruzi transmission among triatomines, wildlife, and domestic animals and a quantitative synthesis of vector–host interactions. Annu. Rev. Anim. Biosci. 2022, 10, 325–348. [Google Scholar] [CrossRef]
- Jackson, Y.; Pinto, A.; Pett, S. Chagas disease in Australia and New Zealand: Risks and needs for public health interventions. Trop. Med. Int. Health 2014, 19, 212–218. [Google Scholar] [CrossRef]
- Germano, M.D.; Ines Picollo, M. Reproductive and developmental costs of deltamethrin resistance in the Chagas disease vector Triatoma infestans. J. Vector Ecol. 2015, 40, 59–65. [Google Scholar] [CrossRef]
- Santo-Orihuela, P.L.; Vassena, C.V.; Carvajal, G.; Clark, E.; Menacho, S.; Bozo, R.; Gilman, R.H.; Bern, C.; Marcet, P.L. Toxicological, enzymatic, and molecular assessment of the insecticide susceptibility profile of Triatoma infestans (Hemiptera: Reduviidae, Triatominae) populations from rural communities of Santa Cruz, Bolivia. J. Med. Entomol. 2017, 54, 187–195. [Google Scholar] [CrossRef]
- Tian, Y.; Durden, C.; Hamer, G.L. A scoping review of triatomine control for Chagas disease prevention: Current and developing tools in Latin America and the United States. J. Med. Entomol. 2024, 61, 1290–1308. [Google Scholar] [CrossRef]
- Dulbecco, A.B.; Calderón-Fernández, G.M.; Pedrini, N. Cytochrome P450 genes of the CYP4 clan and pyrethroid resistance in chagas disease vectors. Front. Trop. Dis. 2022, 3, 823093. [Google Scholar] [CrossRef]
- Roca-Acevedo, G.; Matamoros, G.; Toloza, A. Pyrethroid-Resistance in Triatoma Infestans (Hemiptera: Reduviidae): A Systematic Review and Meta-Analysis Study. Curr. Trop. Med. Rep. 2025, 12, 5. [Google Scholar] [CrossRef]
- Cecere, M.C.; Gaspe, M.S.; Macchiaverna, N.P.; Enriquez, G.F.; Alvedro, A.; Laiño, M.A.; Alvarado-Otegui, J.A.; Cardinal, M.V.; Gürtler, R.E. Slow recovery rates and spatial aggregation of Triatoma infestans populations in an area with high pyrethroid resistance in the Argentine Chaco. Parasites Vectors 2024, 17, 287. [Google Scholar] [CrossRef]
- Maza, V.A.; Cardinal, M.V.; Nattero, J. Morphofunctional characteristics of flight-related traits in deltamethrin-resistant and susceptible Triatoma infestans (Klug, 1834) of the Argentinean Chaco. Parasites Vectors 2025, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.C.F.; Freitas, M.V.S.d.; de Paulo, G.L.; Barbosa, S.E.; de Souza, J.M.B.; Amaral, B.S.; Diotaiuti, L.G.; Ferreira, R.A. Community-based surveillance of Chagas Disease: Characterization and use of triatomine information posts (TIPs) in a high-risk area for triatomine reinfestation in Latin America. PLoS Negl. Trop. Dis. 2025, 19, e0013153. [Google Scholar] [CrossRef]
- Baldiviezo, L.V.; Nieva, L.B.; Pedrini, N.; Cardozo, R.M. Microencapsulation of a native strain of the entomopathogenic fungus Beauveria bassiana and bioinsecticide activity against pyrethroid-resistant Triatoma infestans to vector control of Chagas disease in the argentine Gran Chaco region. Trop. Med. Infect. Dis. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Valença-Barbosa, C.; Finamore-Araujo, P.; Moreira, O.C.; Alvarez, M.V.N.; Borges-Veloso, A.; Barbosa, S.E.; Diotaiuti, L.; de Souza, R.d.C.M. High parasitic loads quantified in sylvatic Triatoma melanica, a Chagas disease vector. Pathogens 2022, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Fronza, G.; Toloza, A.C.; Mougabure-Cueto, G.A.; Carbajo, A.E. Pyrethroid resistance distribution in Triatoma infestans and environmental association along the Argentine endemic zone. Acta Trop. 2024, 257, 107307. [Google Scholar] [CrossRef]
- Delgado-Noguera, L.A.; Hernández-Pereira, C.E.; Ramírez, J.D.; Hernández, C.; Velasquez-Ortíz, N.; Clavijo, J.; Ayala, J.M.; Forero-Peña, D.; Marquez, M.; Suarez, M.J.; et al. Tele-entomology and tele-parasitology: A citizen science-based approach for surveillance and control of Chagas disease in Venezuela. Parasite Epidemiol. Control. 2022, 19, e00273. [Google Scholar] [CrossRef]
- Musah, A.; Browning, E.; Aldosery, A.; Valerio Graciano Borges, I.; Ambrizzi, T.; Tunali, M.; Başibüyük, S.; Yenigün, O.; Moreno, G.M.M.; De Lima, C.L.; et al. Coalescing disparate data sources for the geospatial prediction of mosquito abundance, using Brazil as a motivating case study. Front. Trop. Dis. 2023, 4, 1039735. [Google Scholar] [CrossRef]
- Aldosery, A.; Musah, A.; Birjovanu, G.; Moreno, G.; Boscor, A.; Dutra, L.; Santos, G.; Nunes, V.; Oliveira, R.; Ambrizzi, T.; et al. MEWAR: Development of a cross-platform Mobile application and web dashboard system for real-time mosquito surveillance in Northeast Brazil. Front. Public Health 2021, 9, 754072. [Google Scholar] [CrossRef]
- de Miranda, V.L.; de Souza, E.P.; Bambil, D.; Khalighifar, A.; Peterson, A.T.; de Oliveira Nascimento, F.A.; Gurgel-Gonçalves, R.; Abad-Franch, F. Cellphone picture-based, genus-level automated identification of Chagas disease vectors: Effects of picture orientation on the performance of five machine-learning algorithms. Ecol. Inform. 2024, 79, 102430. [Google Scholar] [CrossRef]
- Dorn, P.L.; Monroy, M.C.; Stevens, L. Sustainable, integrated control of native vectors: The case of Chagas disease in Central America. Front. Trop. Dis. 2022, 3, 971000. [Google Scholar] [CrossRef]
- Hernandez-Castro, L.E.; Paterno, M.; Villacis, A.G.; Andersson, B.; Costales, J.A.; De Noia, M.; Ocana-Mayorga, S.; Yumiseva, C.A.; Grijalva, M.J.; Llewellyn, M.S. 2b-RAD genotyping for population genomic studies of Chagas disease vectors: Rhodnius ecuadoriensis in Ecuador. PLoS Negl. Trop. Dis. 2017, 11, e0005710. [Google Scholar] [CrossRef] [PubMed]
- Gysin, G.; Urbano, P.; Brandner-Garrod, L.; Begum, S.; Kristan, M.; Walker, T.; Hernández, C.; Ramírez, J.D.; Messenger, L.A. Towards environmental detection of Chagas disease vectors and pathogen. Sci. Rep. 2022, 12, 9849. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Auckland, L.D.; Snowden, K.F.; Hamer, G.L.; Hamer, S.A. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect. Genet. Evol. 2018, 58, 171–180. [Google Scholar] [CrossRef]
- Peterson, J.K.; MacDonald, M.L.; Ellis, V.A. Genome report: First whole genome sequence of Triatoma sanguisuga (Le Conte, 1855), vector of Chagas disease. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Duan, L.; Tian, Y.; Wang, Z.; Yang, L.; Guo, Y.; Li, Y.; Zhou, Z.; Shen, Y.; Zhang, Y.; Liu, Q. Transcriptomic comparison analysis across seven developmental stages of the Triatoma rubrofasciata, a vector of Chagas disease. BMC Genom. 2025, 26, 444. [Google Scholar] [CrossRef]
- Traverso, L.; Latorre Estivalis, J.M.; da Rocha Fernandes, G.; Fronza, G.; Lobbia, P.; Mougabure Cueto, G.; Ons, S. Transcriptomic modulation in response to an intoxication with deltamethrin in a population of Triatoma infestans with low resistance to pyrethroids. PLoS Negl. Trop. Dis. 2022, 16, e0010060. [Google Scholar] [CrossRef]
- Praça, Y.R.; Santiago, P.B.; Charneau, S.; Mandacaru, S.C.; Bastos, I.M.D.; Bentes, K.L.d.S.; Silva, S.M.M.; da Silva, W.M.C.; da Silva, I.G.; de Sousa, M.V.; et al. An Integrative Sialomic Analysis Reveals Molecules From Triatoma sordida (Hemiptera: Reduviidae). Front. Cell. Infect. Microbiol. 2022, 11, 798924. [Google Scholar] [CrossRef]
- Mizushima, D.; Tabbabi, A.; Yamamoto, D.S.; Kien, L.T.; Kato, H. Salivary gland transcriptome of the Asiatic Triatoma rubrofasciata. Acta Trop. 2020, 210, 105473. [Google Scholar] [CrossRef]
- Barbosa, H.J.; Quevedo, Y.S.; Torres, A.M.; Veloza, G.A.G.; Carranza Martínez, J.C.; Urrea-Montes, D.A.; Robello-Porto, C.; Vallejo, G.A. Comparative proteomic analysis of the hemolymph and salivary glands of Rhodnius prolixus and R. colombiensis reveals candidates associated with differential lytic activity against Trypanosoma cruzi Dm28c and T. cruzi Y. PLoS Negl. Trop. Dis. 2024, 18, e0011452. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.D.; Gollob, K.J.; Zingales, B.; Dutra, W.O. Pathogen diversity, immunity, and the fate of infections: Lessons learned from Trypanosoma cruzi human-host interactions. Lancet Microbe 2022, 3, e711–e722. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Fimbres-Macias, J.P.; Juarez, J.G.; Downs, C.H.; Carbajal, E.; Melo, M.; Garza, D.Y.; Killets, K.C.; Wilkerson, G.K.; Carrera-Treviño, R.; et al. Development of an operational trap for collection, killing, and preservation of triatomines (Hemiptera: Reduviidae): The kissing bug kill trap. J. Med. Entomol. 2024, 61, 1322–1332. [Google Scholar] [CrossRef]
- Gurgel-Gonçalves, R.; Komp, E.; Campbell, L.P.; Khalighifar, A.; Mellenbruch, J.; Mendonça, V.J.; Owens, H.L.; de la Cruz Felix, K.; Peterson, A.T.; Ramsey, J.M. Automated identification of insect vectors of Chagas disease in Brazil and Mexico: The Virtual Vector Lab. PeerJ 2017, 5, e3040. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Jr, G.; Abad-Franch, F.; de Sousa, O.M.; Dos Santos, C.G.; Fonseca, E.O.; Dos Santos, R.F.; Cunha, G.M.; de Carvalho, C.M.; Reis, R.B.; Gurgel-Goncalves, R.; et al. TriatoScore: An entomological-risk score for Chagas disease vector control-surveillance. Parasites Vectors 2021, 14, 492. [Google Scholar] [CrossRef]
- Hill, J.; Teal, E.; Cross, C.L.; Sanchez, Z.; Webber, M.M.; Oxborough, R.M.; Messenger, L.A. Using iNaturalist presence data to produce suitability maps for Triatoma protracta, T. rubida and T. recurva in the American Southwest, Texas and northern Mexico, to identify potential transmission zones of Chagas disease. Sci. Rep. 2024, 14, 26879. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Justi, S.A.; Rabinovich, J.E.; Diniz Filho, J.A.F.; Villalobos, F. Phylogenetic structure of geographical co-occurrence among New World Triatominae species, vectors of Chagas disease. J. Biogeogr. 2020, 47, 1218–1231. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Balsalobre, A.; Vicente, M.E.; Curtis-Robles, R.; Hamer, S.A.; Ayala Landa, J.M.; Rabinovich, J.E.; Marti, G.A. American triatomine species occurrences: Updates and novelties in the DataTri database. GigaByte 2022, 2022, gigabyte62. [Google Scholar] [CrossRef]
- Shirey, V.; Rabinovich, J. Climate change-induced degradation of expert range maps drawn for kissing bugs (Hemiptera: Reduviidae) and long-standing current and future sampling gaps across the Americas. Mem. Inst. Oswaldo Cruz 2024, 119, e230100. [Google Scholar] [CrossRef]
- Pacheco, G.J.; Fulton, L.; Betancourt, J.; Shanmugam, R.; Granados, P.S. Geospatial analysis as a tool to identify target areas for Chagas disease education for healthcare providers. BMC Infect. Dis. 2022, 22, 590. [Google Scholar] [CrossRef]
- De Rose Ghilardi, F.; Silva, G.; Vieira, T.M.; Mota, A.; Bierrenbach, A.L.; Damasceno, R.F.; Oliveira, L.C.d.; Dias Porto Chiavegatto Filho, A.; Sabino, E. Machine learning for predicting Chagas disease infection in rural areas of Brazil. PLoS Negl. Trop. Dis. 2024, 18, e0012026. [Google Scholar] [CrossRef] [PubMed]
- Ledien, J.; Cucunubá, Z.M.; Parra-Henao, G.; Rodríguez-Monguí, E.; Dobson, A.P.; Adamo, S.B.; Basáñez, M.-G.; Nouvellet, P. Linear and Machine Learning modelling for spatiotemporal disease predictions: Force-of-Infection of Chagas disease. PLoS Negl. Trop. Dis. 2022, 16, e0010594. [Google Scholar] [CrossRef] [PubMed]
- Cochero, J.; Pattori, L.; Balsalobre, A.; Ceccarelli, S.; Marti, G. A convolutional neural network to recognize Chagas disease vectors using mobile phone images. Ecol. Inform. 2022, 68, 101587. [Google Scholar] [CrossRef]
- Gurgel-Gonçalves, R. Stronger control-surveillance systems for vector-borne Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210130chgsb. [Google Scholar] [CrossRef]
- WHO. Implementing an Information and Surveillance System of Chagas Disease; WHO: Geneva, Switzerland, 2024.
- Paz, M.A.; Meireles, A.C.A.; Galvão, C.; Gil-Santana, H.R.; Julião, G.R. “WhatsBarb” Citizen Surveillance: Survey of Insects Mistaken for Triatomines. Rev. Soc. Bras. Med. Trop. 2025, 58, e004032025. [Google Scholar] [CrossRef]
- Velázquez-Ramírez, D.D.; Pérez de Léon, A.A.; Ochoa-Díaz-López, H. Review of American Trypanosomiasis in Southern Mexico Highlights Opportunity for Surveillance Research to Advance Control Through the One Health Approach. Front. Public Health 2022, 10, 838949. [Google Scholar] [CrossRef]
- Padilla, A.M.; Yao, P.Y.; Landry, T.J.; Cooley, G.M.; Mahaney, S.M.; Ribeiro, I.; VandeBerg, J.L.; Tarleton, R.L. High variation in immune responses and parasite phenotypes in naturally acquired Trypanosoma cruzi infection in a captive non-human primate breeding colony in Texas, USA. PLoS Negl. Trop. Dis. 2021, 15, e0009141. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.N.; Aguilar, D.; Gorchakov, R.; Rossmann, S.N.; Montgomery, S.P.; Rivera, H.; Woc-Colburn, L.; Hotez, P.J.; Murray, K.O. Evidence of autochthonous Chagas disease in southeastern Texas. Am. J. Trop. Med. Hyg. 2015, 92, 325–330. [Google Scholar] [CrossRef]
- Davila, E.; Fernandez-Santos, N.A.; Estrada-Franco, J.G.; Wei, L.; Velázquez-Ramírez, D.D.; García-Miranda, R.; Irecta Nájera, C.; Cruz-Cadena, R.; Guichard-Romero, C.; Rodriguez, C.; et al. Domestic Dog Infection with Trypanosoma cruzi from Northern and Southern Regions of Mexico. Vector-Borne Zoonotic Dis. 2024, 24, 510–519. [Google Scholar] [CrossRef]
- Meyer, M.; Eibner, G.; Heni, A.C.; Wilhelm, K.; Sommer, S. Changes in biodiversity drive trypanosome infections of wildlife in Panama. One Health 2025, 21, 101113. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Ramírez, D.D.; Ochoa-Díaz-López, H.; Garza-Ramos, J.; Ocampo-López-Escalera, J.; Espinoza-Medinilla, E.E.; Irecta-Nájera, C.A.; Navarro-López, R.; Delgado-Enciso, I.; Pérez de León, A.Á.; Debboun, M. One Health research to mitigate health burden of American trypanosomiasis in Mexico. Acta Trop. 2025, 263, 107567. [Google Scholar] [CrossRef] [PubMed]

| Route | Mechanism | Predominant Regions | Key Data | Public Health Impact |
|---|---|---|---|---|
 Vectorial | Feces of infected triatomines contaminate skin/mucosa | Rural Latin America (Bolivia, Gran Chaco, NE Brazil) | Prevalence > 6% in Bolivia; control success in Southern Cone, but insecticide resistance rising | Still a driver of chronic burden; risk of resurgence |
 Oral | Contaminated food/beverages (açaí, sugarcane, guava) | Amazonia (Brazil, Venezuela, Colombia, French Guiana) | >70% of acute cases in Amazonia; outbreaks up to 100+ cases; CFR 10–35% | Aggressive acute disease (fulminant myocarditis, systemic inflammation) |
 Congenital | Mother-to-child (transplacental) | Bolivia, Brazil, Paraguay, Colombia; migrants in Spain, Italy, U.S. | 8000–15,000 cases/year; 1–10% transmission; 5–7% in Bolivian/Brazilian migrants | Nearly 100% curable if detected early; WHO 2030 elimination target |
 Transfusional | Infected blood products | Latin America (historic); risk in U.S./Europe without screening | Screening widespread in endemic countries; patchy in others | Sporadic risk where screening inconsistent |
 Transplantation | Infected organ donors | U.S., Spain, Italy, Switzerland, Japan | Isolated cases; often underrecognized | High mortality in immunosuppressed if undetected |
 Sylvatic/Spillover | Contact with infected vectors or reservoirs | Southern U.S. (Texas, New Mexico), Amazonia Basin, Gran Chaco, and other endemic rural regions of Latin America. | Infected vectors (Triatoma spp.) and dogs/cats documented; human cases rare | Emerging zoonotic concern, climate change may expand risk |
| Reference | Type | Vector Species | Approach | Key Findings |
|---|---|---|---|---|
| Traverso et al., 2022 [117] | Transcriptomics—Insecticide Response | Triatoma infestans | RNA-seq after sub-lethal deltamethrin exposure | Differential expressions of ABC transporters, heat shock proteins, odorant-binding proteins, and cuticle-related genes. Highlights pathways linked to detoxification and insecticide resistance. |
| Praça et al., 2022 [118] | Sialomics/Proteomics—Salivary Glands | Triatoma sordida | Integrated proteomics & transcriptomics | >26,000 salivary transcripts and 132 secreted proteins identified. Lipocalins were dominant (~89% of secretory output), along with apyrases and protease inhibitors, showing mechanisms of anticoagulation and immune evasion. |
| Peterson et al., 2024 [115] | Genomics | Triatoma sanguisuga | Whole-genome sequencing | First high-quality genome assembly (1.16 Gb, ~17,799 predicted genes, 99.1% BUSCO completeness, 61% repetitive DNA). Foundation for studies on blood-feeding, host-seeking, and vector competence. |
| Barbosa et al., 2024 [120] | Comparative Proteomics—Immune Factors | Rhodnius prolixus vs. R. colombiensis | Comparative hemolymph & saliva proteomics | R. prolixus had higher levels of trypanolytic molecules (lysozyme, prolixin, nitrophorins, serpins). Correlated with ability to lyse TcII strains (Y strain). Highlights vector-parasite immune interactions and evasion mechanisms by TcI strains. |
| Duan et al., 2025 [116] | Transcriptomics—Developmental | Triatoma rubrofasciata | RNA-seq across developmental stages | Identified stage-specific gene expression. Venom-like salivary proteins (e.g., histidine phosphatase, serine carboxypeptidase) upregulated in late nymphs. Adult stage showed high CYP425A1 expression, indicating detoxification adaptation. |
| Reference | Geographic Scope | Approach | Key Findings |
|---|---|---|---|
| Ceccarelli et al., 2020 [126] | New World triatomines (multiple countries) | Clustering and regression-tree analyses of occurrence data with remote-sensing variables (elevation, vegetation indices, precipitation) | Defined biogeographic regions and identified environmental factors influencing species distributions, providing an environmental baseline for niche modeling. |
| Hill et al., 2024 [125] | Southwestern U.S. & Northern Mexico (T. protracta, T. rubida, T. recurva) | Citizen science data (iNaturalist) integrated with MaxEnt modeling; >700 geotagged observations with remote-sensing climate variables | Identified high-risk zones in Southern California, Sierra Nevada foothills, Southern Arizona, and Texas/N. Mexico border. Precipitation was the strongest predictor of vector presence. |
| Shirey & Rabinovich, 2024 [128] | Global focus, with emphasis on Latin America | High-resolution climate surfaces combined with Bayesian additive regression trees (BART) | Demonstrated that static expert-drawn maps degrade under climate change as species move beyond known ranges. Highlighted sampling gaps, stressing need for intensified field surveys. |
| Brasil et al., 2025 [69] | Latin America (55 triatomine species) | Ensemble ecological niche modeling using machine learning algorithms (MaxEnt, Random Forest); >11,000 occurrence records; climate scenarios SSP2-4.5 & SSP5-8.5 for 2050 and 2080 | By 2050, distributions remained mostly stable. By 2080, major expansion of suitable habitats, especially in the Brazilian Amazonia and deforestation arc, increasing risk to previously unaffected populations. |
| Study | Geographic Scope | Components Integrated | Key Findings |
|---|---|---|---|
| Rodriguez et al., 2021 [9] | El Paso County, Texas & New Mexico, USA | Triatomine vectors/Feral dogs and cats/Wild animals | Found high T. cruzi prevalence: 66.7% of triatomines, 45.3% of feral dogs, 29.2% of feral cats, and 71.4% of wild animals were infected. Triatoma rubida was the dominant species (98.2%). Bloodmeal analysis identified humans, dogs, cats, and wildlife as feeding sources, suggesting active enzootic and potential zoonotic transmission. |
| Velázquez-Ramírez, Pérez de León & Ochoa-Díaz-López, 2022 [136] | Chiapas & Oaxaca, Mexico | Domestic animals/Wildlife/Humans/Ecology | Wildlife maintain T. cruzi in nature; domestic animals also act as reservoirs; environmental & ecological gaps in surveillance identified; need for molecular diagnostics and electronic data reporting. |
| Busselman & Hamer et al., 2022 [94] | Southern USA | Wildlife reservoirs (opossums, rodents, raccoons, etc.), domestic dogs, triatomines, human dwellings | Quantitative synthesis shows many animal species are infected; triatomines’ bloodmeal analysis implicates human/domestic hosts; highlighting underrecognized transmission risk and diagnostic gaps. |
| Dávila et al., 2024 [139] | Northern & Southern Mexico | Dogs (domestic animals)/Humans (shared spaces)/Vector interface | Dogs found infected in various regions; dogs share habitats with humans; suggests utility of using dogs as sentinel/reservoirs to monitor transmission risk. |
| Velázquez-Ramírez et al., 2025 [141] | Mexico | Human/Domestic animals/Vectors/Environment | Surveys and data show domestic animals and wildlife carry T. cruzi; vector control and surveillance strategies need to integrate all sectors. Emphasis on surveillance in rural and semi-rural regions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.d.S.; Maldonado, R.A.; Farani, P.S.G. Chagas Disease in the 21st Century: Global Spread, Ecological Shifts, and Research Frontiers. Biology 2025, 14, 1631. https://doi.org/10.3390/biology14111631
Ferreira MdS, Maldonado RA, Farani PSG. Chagas Disease in the 21st Century: Global Spread, Ecological Shifts, and Research Frontiers. Biology. 2025; 14(11):1631. https://doi.org/10.3390/biology14111631
Chicago/Turabian StyleFerreira, Marina da Silva, Rosa Amelia Maldonado, and Priscila Silva Grijó Farani. 2025. "Chagas Disease in the 21st Century: Global Spread, Ecological Shifts, and Research Frontiers" Biology 14, no. 11: 1631. https://doi.org/10.3390/biology14111631
APA StyleFerreira, M. d. S., Maldonado, R. A., & Farani, P. S. G. (2025). Chagas Disease in the 21st Century: Global Spread, Ecological Shifts, and Research Frontiers. Biology, 14(11), 1631. https://doi.org/10.3390/biology14111631







