Invasion Mechanisms of the Alien Plant Datura stramonium in Xizang: Insights from Genetic Differentiation, Allelopathy, and Ecological Niche Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Methods for Assessing the Allelopathic Effects of D. stramonium
2.2. Molecular Materials and Experimental Methods for D. stramonium
2.3. Experimental Methods for Analyzing D. stramonium Communities
2.4. Data Analysis
3. Results
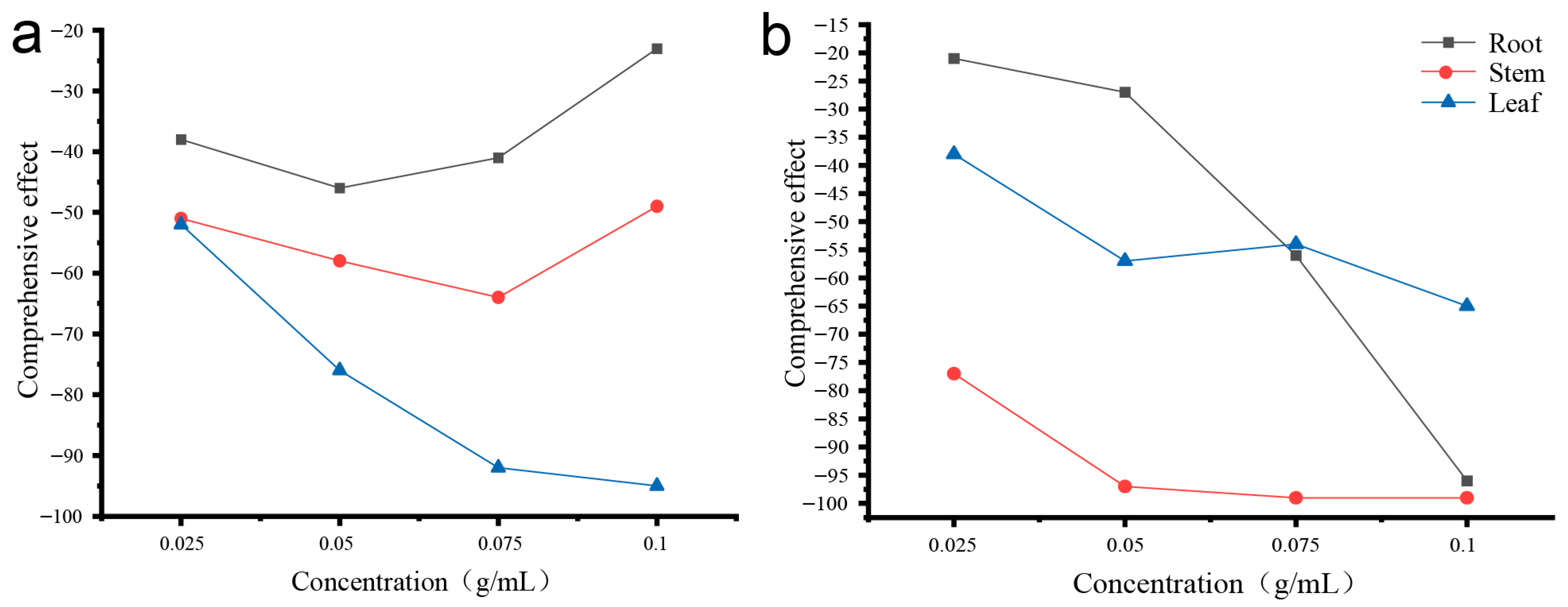
3.1. Allelopathic Effects of D. stramonium
3.2. Population Differentiation, Demographic History, and Haplotype Distribution of D. stramonium in Tibet
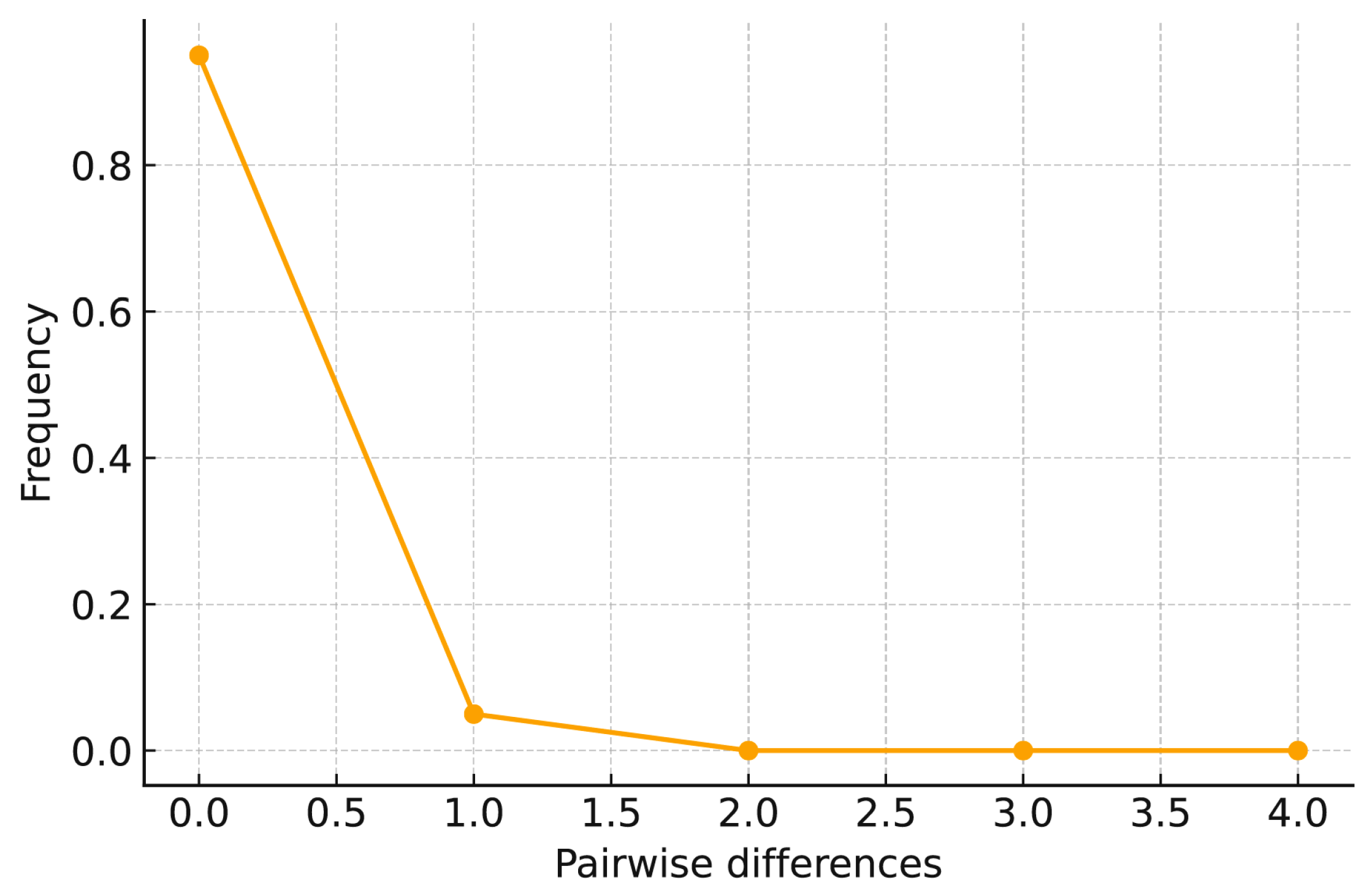
3.2.1. Genetic Differentiation and Historical Demographic Patterns of D. stramonium
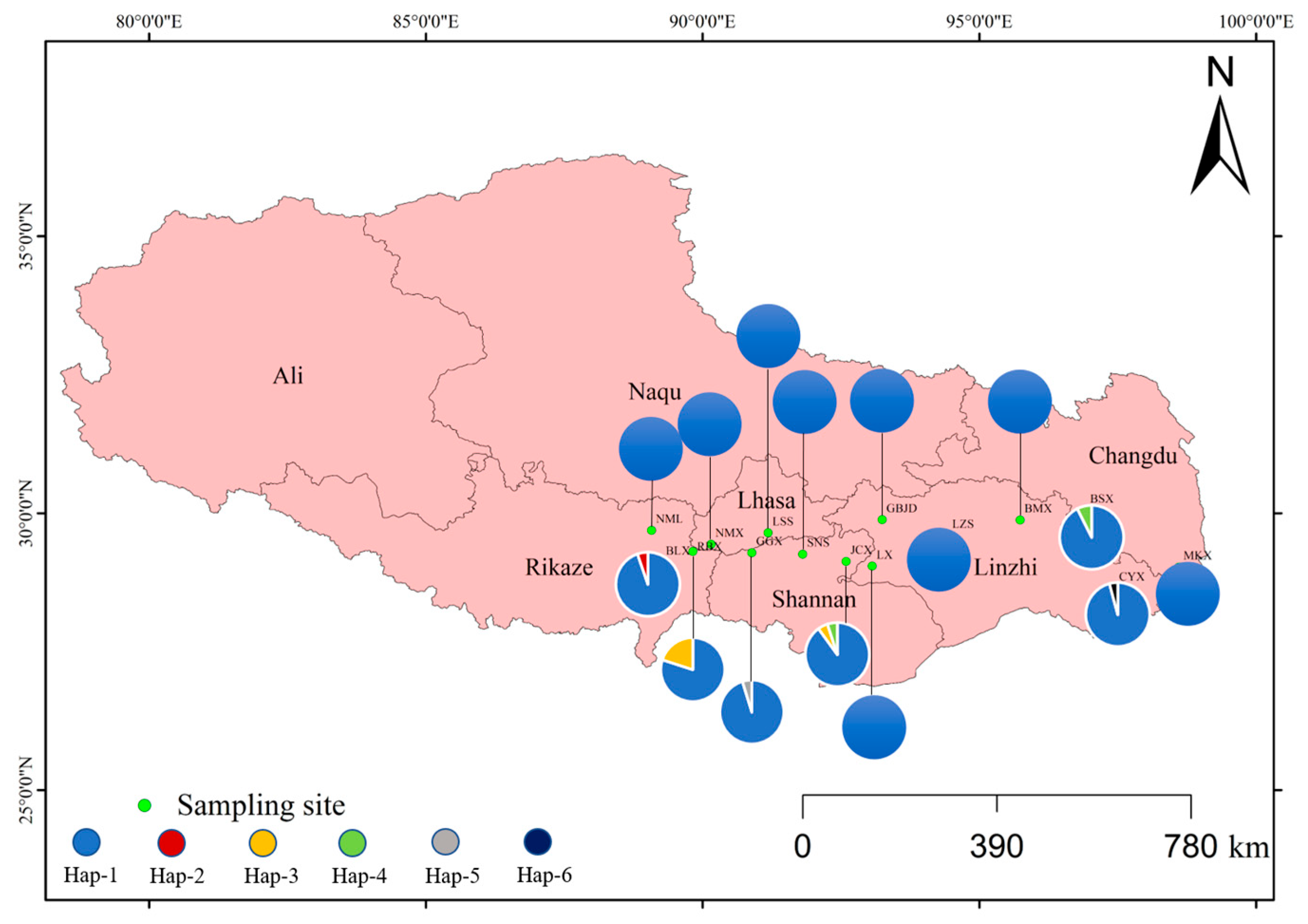
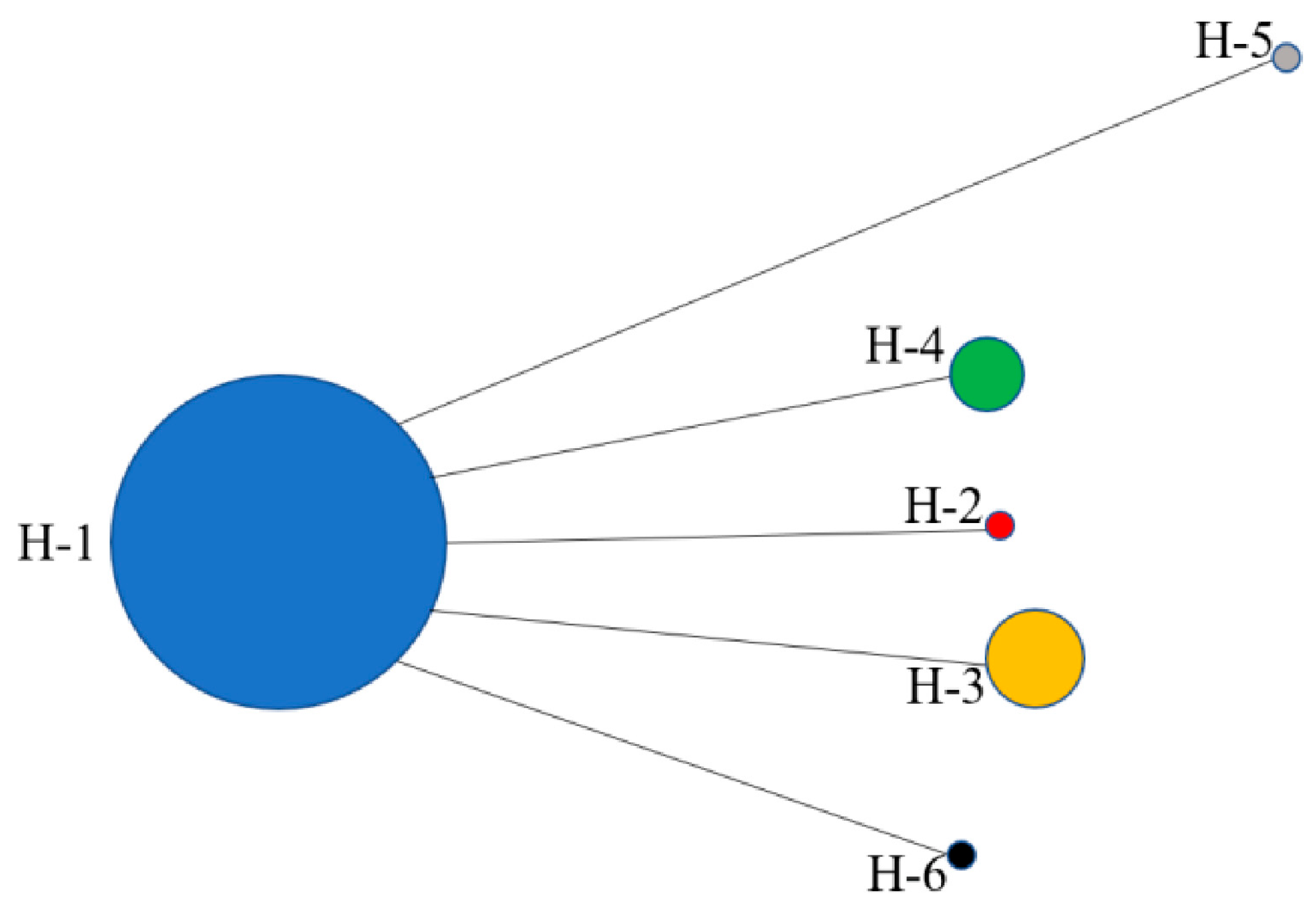
3.2.2. Haplotype Distribution in D. stramonium Populations
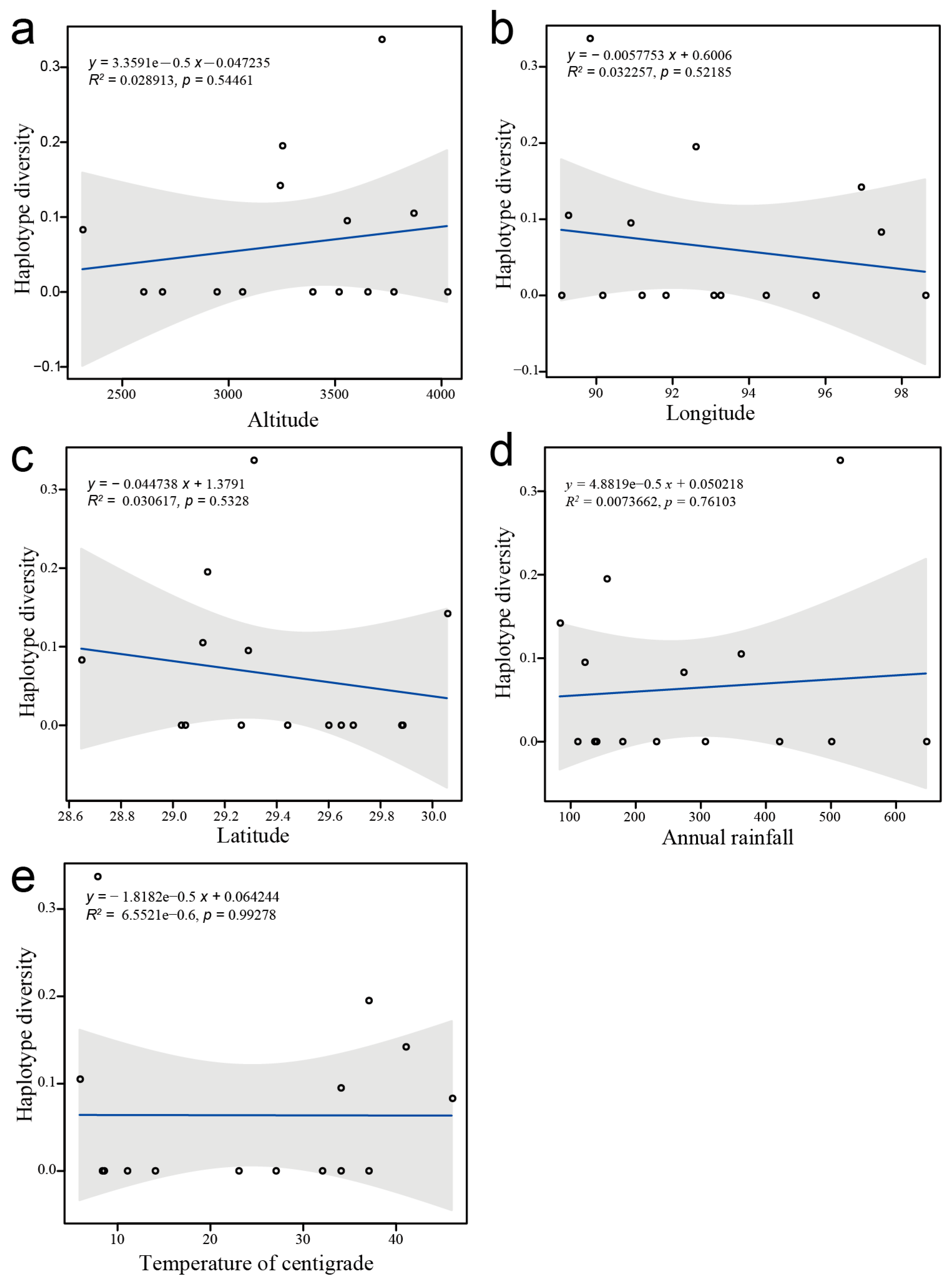
3.2.3. Correlation Analysis of Haplotype Diversity and Environmental Factors
3.3. Niche Characteristics of D. stramonium
3.3.1. Community-Dominant Species Importance Values and Niche Breadth
3.3.2. Niche Overlap Among Community-Dominant Species
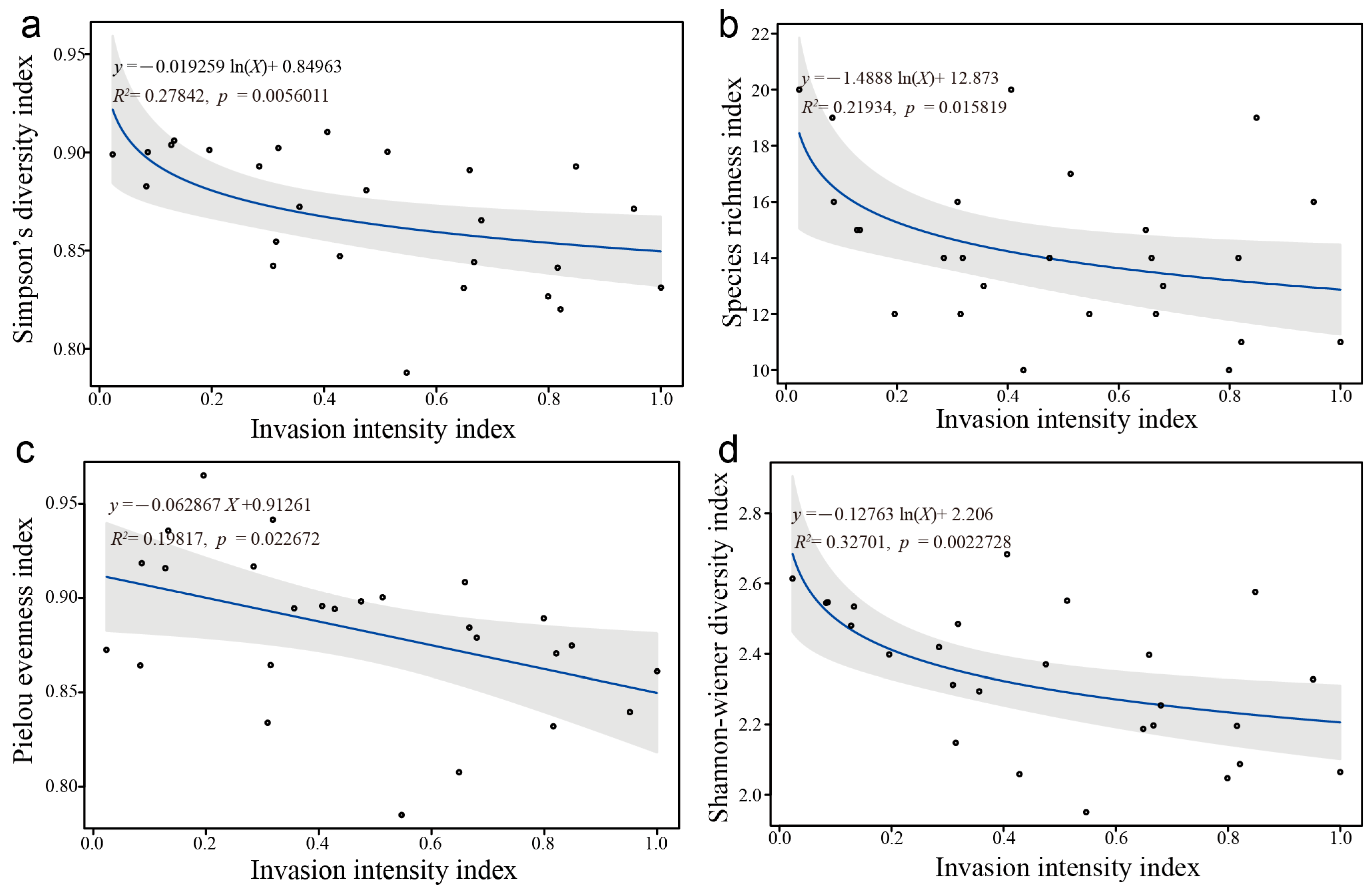
3.3.3. Correlation Analysis Between Community Species Diversity Index and Invasion Intensity Index
4. Discussion
4.1. Mechanistic Interpretation of D. stramonium Allelopathy
4.2. Population Differentiation, Dynamics, and Haplotype Distribution in D. stramonium
4.2.1. Genetic Differentiation in D. stramonium Populations
4.2.2. Historical Population Dynamics of D. stramonium
4.2.3. Interpretation of Haplotype Patterns in D. stramonium
4.3. Niche Characteristics of Dominant Species in D. stramonium Communities
4.3.1. Dominant Species Importance Values and Niche Breadth
4.3.2. Niche Overlap Among Dominant Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Family | Genus | Species |
|---|---|---|
| Amaranthaceae | Amaranthus | Amaranthus hybridus |
| Oxybasis | Oxybasis glauca | |
| Araceae | Arisaema | Arisaema flavum |
| Asteraceae | Galinsoga | Galinsoga parviflora |
| Sonchus | Sonchus oleraceus | |
| Artemisia | Artemisia sieversiana | |
| Cosmos | Cosmos bipinnatus | |
| Senecio | Senecio vulgaris | |
| Cirsium | Cirsium arvense var. alpestre | |
| Cirsium | Cirsium arvense | |
| Gnaphalium | Pseudognaphalium affine | |
| Taraxacum | Taraxacum mongolicum | |
| Artemisia | Artemisia demissa | |
| Artemisia | Artemisia wellbyi | |
| Boraginaceae | Cynoglossum | Cynoglossum amabile |
| Chenopodiaceae | Chenopodium | Dysphania schraderiana |
| Salsola | Kali collinum | |
| Chenopodium | Chenopodium album | |
| Convolvulaceae | Pharbitis | Ipomoea purpurea |
| Convolvulus | Convolvulus arvensis | |
| Cruciferae | Brassica | Brassica napus |
| Rorippa | Rorippa palustris | |
| Lepidium | Lepidium apetalum | |
| Capsella | Capsella bursa-pastoris | |
| Descurainia | Descurainia sophia | |
| Brassica | Brassica rapa | |
| Geraniaceae | Erodium | Erodium cicutarium |
| Gramineae | Echinochloa | Echinochloa crus-galli |
| Pennisetum | Pennisetum flaccidum | |
| Setaria | Setaria viridis | |
| Poa | Poa annua | |
| Chloris | Chloris virgata | |
| Eragrostis | Eragrostis nigra | |
| Digitaria | Digitaria cruciata | |
| Bothriochloa | Bothriochloa ischaemum | |
| Avena | Avena fatua | |
| Elymus | Elymus tangutorum | |
| Labiatae | Elsholtzia | Elsholtzia densa |
| Leguminosae | Sophora | Sophora moorcroftiana |
| Medicago | Medicago edgeworthii | |
| Medicago | Medicago lupulina | |
| Melilotus | Melilotus suaveolens | |
| Astragalus | Astragalus strictus | |
| Loganiaceae | Buddleja | Buddleja alternifolia |
| Malvaceae | Malva | Malva pusilla |
| Malva | Malva verticillata | |
| Plantaginaceae | Plantago | Plantago depressa |
| Polygonaceae | Fagopyrum | Fagopyrum tataricum |
| Persicaria | Persicaria nepalensis | |
| Rumex | Rumex nepalensis | |
| Persicaria | Polygonum aviculare | |
| Persicaria | Persicaria hydropiper | |
| Persicaria | Persicaria lapathifolia | |
| Fagopyrum | Fagopyrum esculentum | |
| Portulacaceae | Portulaca | Portulaca oleracea |
| Rubiaceae | Galium | Galium spurium |
| Solanaceae | Datura | D. stramonium |
| Nicandra | Nicandra physalodes | |
| Datura | Datura metel | |
| Tamaricaceae | Myricaria | Myricaria wardii |
| Zygophyllaceae | Tribulus | Tribulus terrestris |
| Species | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1 | |||||||||||||||
| S2 | 0.845 | 1 | ||||||||||||||
| S3 | 0.849 | 0.867 | 1 | |||||||||||||
| S4 | 0.621 | 0.696 | 0.741 | 1 | ||||||||||||
| S5 | 0.698 | 0.701 | 0.622 | 0.504 | 1 | |||||||||||
| S6 | 0.779 | 0.69 | 0.694 | 0.59 | 0.562 | 1 | ||||||||||
| S7 | 0.722 | 0.672 | 0.695 | 0.411 | 0.593 | 0.489 | 1 | |||||||||
| S8 | 0.824 | 0.707 | 0.694 | 0.456 | 0.78 | 0.503 | 0.778 | 1 | ||||||||
| S9 | 0.582 | 0.685 | 0.502 | 0.379 | 0.536 | 0.487 | 0.439 | 0.573 | 1 | |||||||
| S10 | 0.586 | 0.67 | 0.724 | 0.754 | 0.551 | 0.532 | 0.436 | 0.475 | 0.538 | 1 | ||||||
| S11 | 0.591 | 0.667 | 0.679 | 0.647 | 0.424 | 0.554 | 0.626 | 0.578 | 0.512 | 0.478 | 1 | |||||
| S12 | 0.694 | 0.645 | 0.695 | 0.369 | 0.432 | 0.421 | 0.698 | 0.598 | 0.553 | 0.438 | 0.498 | 1 | ||||
| S13 | 0.529 | 0.483 | 0.445 | 0.285 | 0.691 | 0.292 | 0.534 | 0.699 | 0.391 | 0.242 | 0.36 | 0.463 | 1 | |||
| S14 | 0.64 | 0.553 | 0.574 | 0.662 | 0.49 | 0.594 | 0.399 | 0.344 | 0.232 | 0.573 | 0.255 | 0.32 | 0.177 | 1 | ||
| S15 | 0.658 | 0.553 | 0.738 | 0.546 | 0.45 | 0.625 | 0.363 | 0.461 | 0.329 | 0.471 | 0.41 | 0.461 | 0.244 | 0.389 | 1 | |
| S16 | 0.51 | 0.511 | 0.557 | 0.744 | 0.429 | 0.627 | 0.11 | 0.327 | 0.08 | 0.506 | 0.443 | 0.081 | 0.108 | 0.627 | 0.533 | 1 |
References
- Weaver, S.E.; Warwick, S.I. The biology of Canadian weeds. 64. Datura stramonium L. Can. J. Plant Sci. 1984, 64, 979–991. [Google Scholar] [CrossRef]
- Delecti Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae. Flora Reipublicae Popularis Sinica; Science Press: Beijing, China, 1978; Volume 67, p. 144. [Google Scholar]
- Ma, J.S. The Checklist of the Chinese Invasive Plants; Higher Education Press: Beijing, China, 2013. [Google Scholar]
- Ramona, S.; Alin, C.; Maria, V.A.; Levente, M.; Dan, M.; Ioana, G. Allelopathic effect of aqueous extracts from Datura stramonium on germination and plant growth of maize plants. J. Biotechnol. 2016, 231, S89. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yin, J.; Wang, X.; Chen, Y.; Mao, Z.K.; Lin, F.; Gong, Z.Q.; Wang, X.G. Habitat suitability evaluation of invasive plant species Datura stramonium in Liaoning Province: Based on Biomod 2 combination model. J. Appl. Ecol. 2023, 34, 5. [Google Scholar]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Inderjit; Simberloff, D.; Kaur, H.; Kalisz, S.; Bezemer, T.M. Novel chemicals engender myriad invasion mechanisms. New Phytol. 2021, 232, 1184–1200. [Google Scholar] [CrossRef]
- Li, Y.P.; Feng, Y.L.; Kang, Z.L.; Zheng, Y.L.; Zhang, J.L.; Chen, Y.J. Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. J. Appl. Ecol. 2017, 54, 1281–1290. [Google Scholar] [CrossRef]
- Macdougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T. Accelerating invasion rate in a highly invaded estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef]
- Sax, D.F.; Brown, J.H. The paradox of invasion. Glob. Ecol. Biogeogr. 2000, 9, 363–371. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. J. Ecol. 2005, 93, 5–15. [Google Scholar] [CrossRef]
- Sax, D.F.; Gaines, S.D.; Brown, J.H. Species invasions exceed extinctions on islands worldwide: A comparative study of plants and birds. Am. Nat. 2002, 160, 766–783. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.G.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Pielou; Evelyn, C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Whittaker, R.H. Evolution and measurement of species diversity. TAXON 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Wang, C.; Wei, M.; Wang, S.; Wu, B.; Cheng, H. Erigeron annuus (L.) Pers. and Solidago canadensis L. antagonistically affect community stability and community invasibility under the co-invasion condition. Sci. Total Environ. 2020, 716, 137128. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1998. [Google Scholar]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 3, 53–74. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoung, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic Invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Mukesh, K.; Chandra, S.G. Allelopathy effects of invasive alien Ageratina adenophora on native shrub species of chir pine forest in the central Himalaya, India. J. For. Res. 2022, 27, 53–62. [Google Scholar]
- Bibi, S.; Bibi, A.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems. Agronomy 2023, 13, 590. [Google Scholar] [CrossRef]
- Yorulmaz, M.; Özkan, R.Y. Allelopathic Effect of Jimson Weed (Datura stramonium L.) on Seed Germination. Türk Tarım Doğa Bilim. Derg. 2020, 7, 793–797. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Jones, A.D. Alkaloids of the genus datura: Review of a rich resource for natural product discovery. Molecules 2021, 26, 2629. [Google Scholar] [CrossRef]
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Revolinski, S.R.; Eigenbrode, S.D.; Burke, I.C. Genetic diversity and population structure of a global invader Mayweed chamomile (Anthemis cotula): Management implications. AoB Plants 2021, 13, plab049. [Google Scholar] [CrossRef]
- Cibrián-Jaramillo, A.; Bacon, C.D.; Garwood, N.C.; Bateman, R.M.; Thomas, M.M.; Russell, S.; Bailey, C.D.; Hahn, W.J.; Bridgewater, S.G.; DeSalle, R. Population genetics of the understory fishtail palm Chamaedorea ernesti-augusti in Belize: High genetic connectivity with local differentiation. BMC Genet. 2009, 10, 65. [Google Scholar] [CrossRef]
- Cosacov, A.; Johnson, L.A.; Paiaro, V.; Cocucci, A.A.; Córdoba, F.E.; Sérsic, A.N. Precipitation rather than temperature influenced the phylogeography of the endemic shrub Anarthrophyllum desideratum in the Patagonian steppe. J. Biogeogr. 2013, 40, 168–182. [Google Scholar] [CrossRef]
- Liao, J.C.; Jing, D.D.; Luo, G.J.; Wang, Y.; Zhao, L.M.; Liu, N.F. Comparative phylogeography of Meriones meridianus, Dipus sagitta, and Allactaga sibirica: Potential indicators of the impact of the Qinghai-Tibetan Plateau uplift. Mamm. Biol. 2016, 81, 31–39. [Google Scholar] [CrossRef]
- Pons, O.; Petit, R.J. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 1996, 144, 1237–1245. [Google Scholar] [CrossRef]
- Müller, R.; Nowicki, C.; Barthlott, W.; Ibisch, P.L. Biodiversity and endemism mapping as a tool for regional conservation planning—Case study of the Pleurothallidinae (Orchidaceae) of the Andean rain forests in Bolivia. Biodivers. Conserv. 2003, 12, 2005–2024. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Ingram, T.; Costa-Pereira, R.; Araújo, M.S. The dimensionality of individual niche variation. Ecology 2018, 99, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.G.; Wang, F.K.; Yin, Q.L.; Ten, B.Q. Niche dynamics of species in succession process in the Wetland of Yangtze Rivers Lower Reach, China. Plant Ecol. Evol. 2015, 148, 43–51. [Google Scholar] [CrossRef]
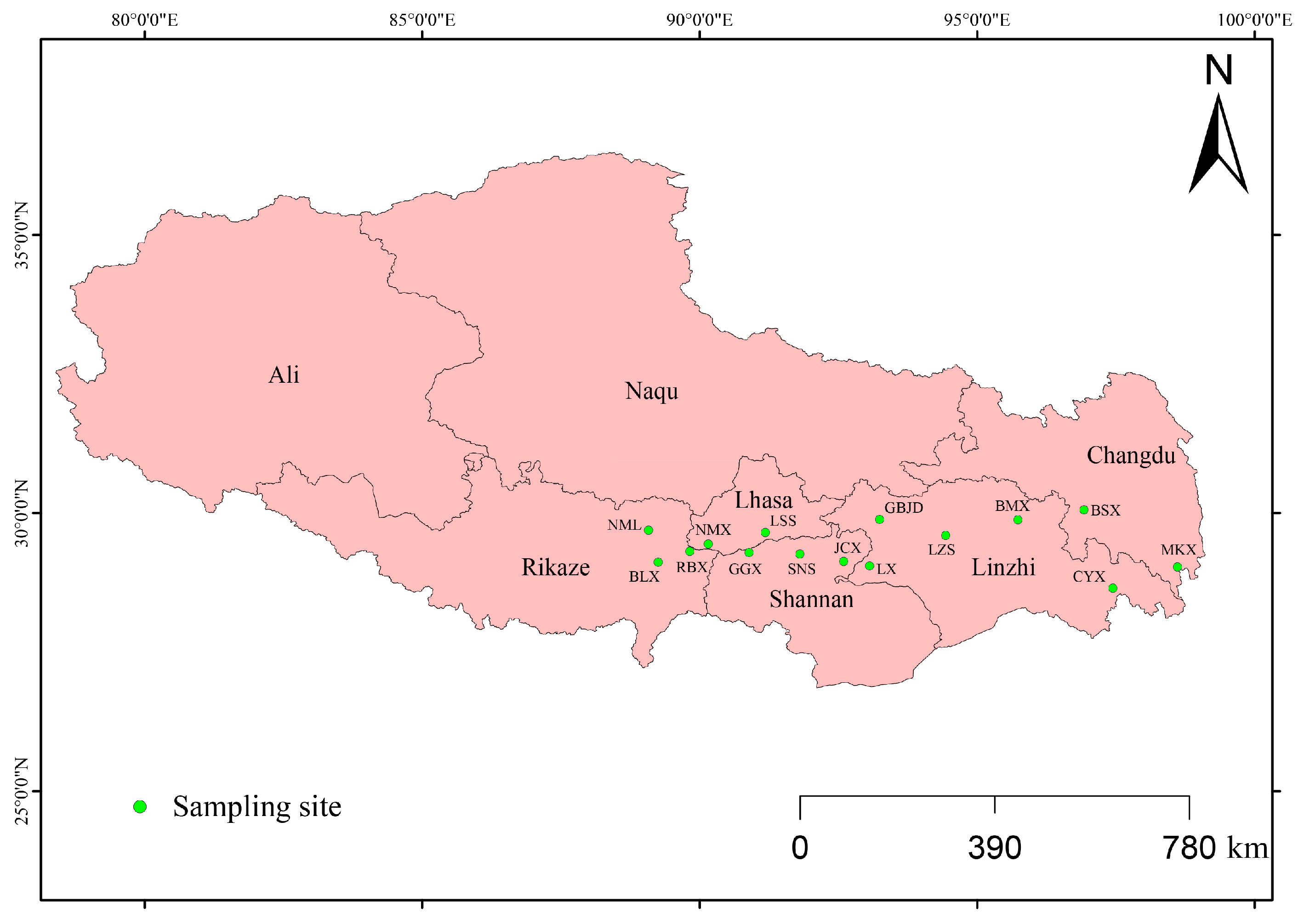
| Location | Code | Longitude (°E) | Latitude (°N) | Individuals Collected | Voucher Number |
|---|---|---|---|---|---|
| Lhasa City, Tibet | LSS | 91.18103 | 29.64584 | 26 | Wjw20230701 |
| Gongga County, Tibet | GGX | 90.88770 | 29.28778 | 21 | Wjw20230702 |
| Nimu County, Tibet | NMX | 90.15169 | 29.43933 | 23 | Wjw20230703 |
| Renbu County, Tibet | RBX | 89.82179 | 29.30988 | 20 | Wjw20230704 |
| Bailang County, Tibet | BLX | 89.25534 | 29.11256 | 19 | Wjw20230705 |
| Nanmulin County, Tibet | NML | 89.07829 | 29.69206 | 23 | Wjw20230706 |
| Shannan City, Tibet | SNS | 91.80690 | 29.26065 | 24 | Wjw20230707 |
| Jiacha County, Tibet | JCX | 92.59348 | 29.13084 | 20 | Wjw20230708 |
| Lang County, Tibet | LX | 93.06258 | 29.04554 | 22 | Wjw20230709 |
| Gongbujiangda County, Tibet | GBJD | 93.24185 | 29.88319 | 23 | Wjw20230710 |
| Linzhi City, Tibet | LZS | 94.43109 | 29.59796 | 20 | Wjw20230711 |
| Bomi County, Tibet | BMX | 95.73593 | 29.87905 | 24 | Wjw20230712 |
| Basu County, Tibet | BSX | 96.92151 | 30.05609 | 27 | Wjw20230713 |
| Mangkang County, Tibet | MKX | 98.60788 | 29.03024 | 20 | Wjw20230714 |
| Chayu County, Tibet | CYX | 97.44478 | 28.64630 | 24 | Wjw20230715 |
| Species | Importance Value | Niche Breadth | |
|---|---|---|---|
| Shannon-Wiener | Levins | ||
| Datura stramonium | 21.92 | 3.198 | 23.326 |
| Dysphania schraderiana | 8.59 | 3.163 | 22.329 |
| Chenopodium album | 8.19 | 3.164 | 22.292 |
| Galinsoga parviflora | 4.94 | 2.856 | 15.419 |
| Tribulus terrestris | 4.36 | 2.78 | 15.238 |
| Amaranthus hybridus | 4.02 | 2.842 | 16.143 |
| Chloris virgata | 3.73 | 2.179 | 14.331 |
| Eragrostis nigra | 3.61 | 2.868 | 17.226 |
| Salsola collina | 3.21 | 2.476 | 10.784 |
| Artemisia sieversiana | 2.69 | 2.673 | 13.843 |
| Pennisetum flaccidum | 2.65 | 2.609 | 13.165 |
| Malva verticillata | 2.61 | 2.549 | 12.595 |
| Lepidium apetalum | 2.41 | 2.221 | 8.501 |
| Echinochloa crus-galli | 2.25 | 2.462 | 11.422 |
| Medicago lupulina | 2.19 | 2.473 | 11.708 |
| Malva pusilla | 2.16 | 2.262 | 9.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zeng, Z.; La, Q.; Wang, J. Invasion Mechanisms of the Alien Plant Datura stramonium in Xizang: Insights from Genetic Differentiation, Allelopathy, and Ecological Niche Analysis. Biology 2025, 14, 1629. https://doi.org/10.3390/biology14111629
Chen Y, Zeng Z, La Q, Wang J. Invasion Mechanisms of the Alien Plant Datura stramonium in Xizang: Insights from Genetic Differentiation, Allelopathy, and Ecological Niche Analysis. Biology. 2025; 14(11):1629. https://doi.org/10.3390/biology14111629
Chicago/Turabian StyleChen, Yonghao, Zhefei Zeng, Qiong La, and Junwei Wang. 2025. "Invasion Mechanisms of the Alien Plant Datura stramonium in Xizang: Insights from Genetic Differentiation, Allelopathy, and Ecological Niche Analysis" Biology 14, no. 11: 1629. https://doi.org/10.3390/biology14111629
APA StyleChen, Y., Zeng, Z., La, Q., & Wang, J. (2025). Invasion Mechanisms of the Alien Plant Datura stramonium in Xizang: Insights from Genetic Differentiation, Allelopathy, and Ecological Niche Analysis. Biology, 14(11), 1629. https://doi.org/10.3390/biology14111629






