Synthesis of Iron Nanoparticles from Spartina alterniflora for Cadmium Immobilization in Coastal Wetland Sediments
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sediments and Materials
2.2. Synthesis and Characterization of Sa-FeNPs
2.3. Experimental Design
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
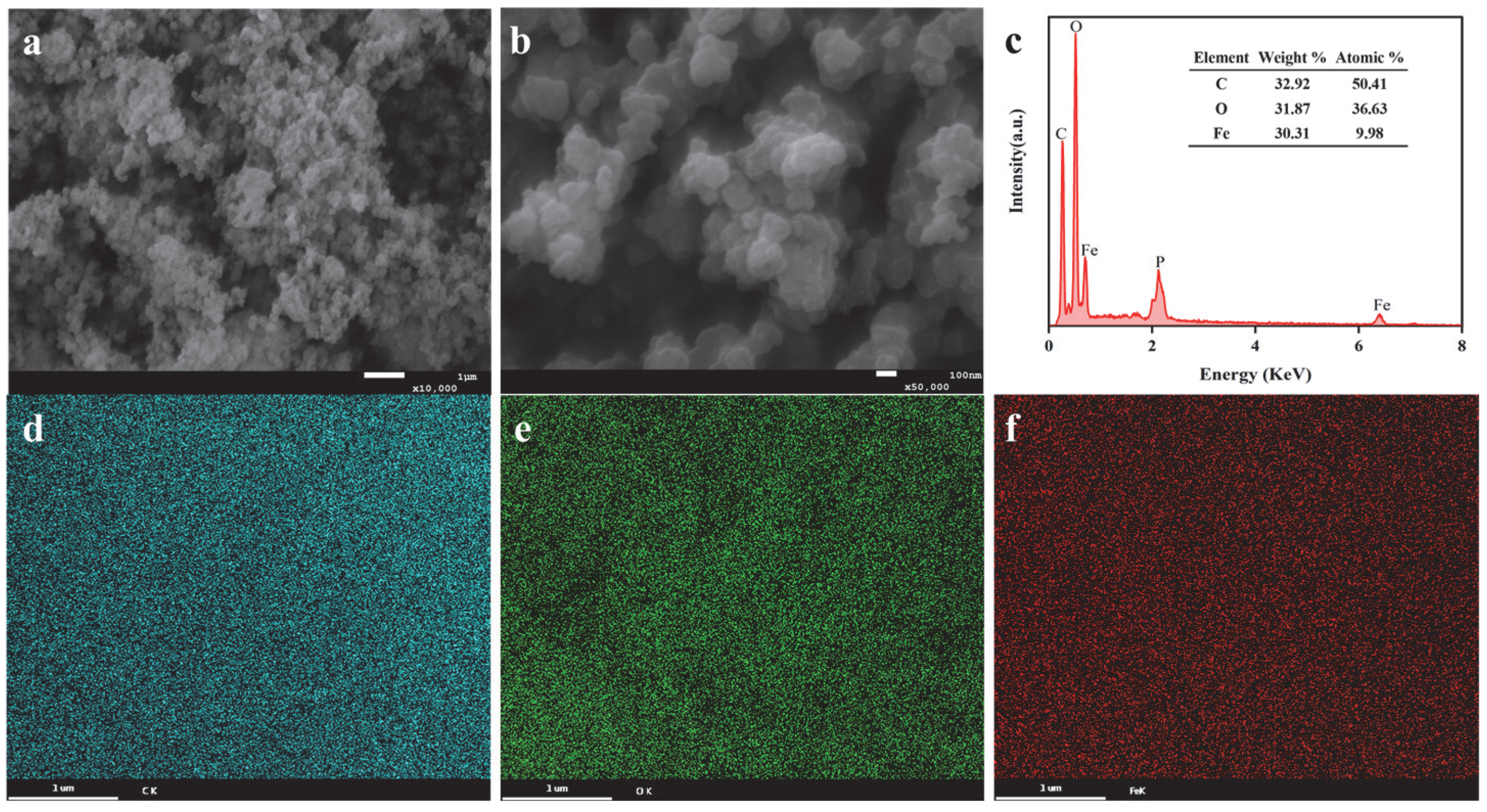
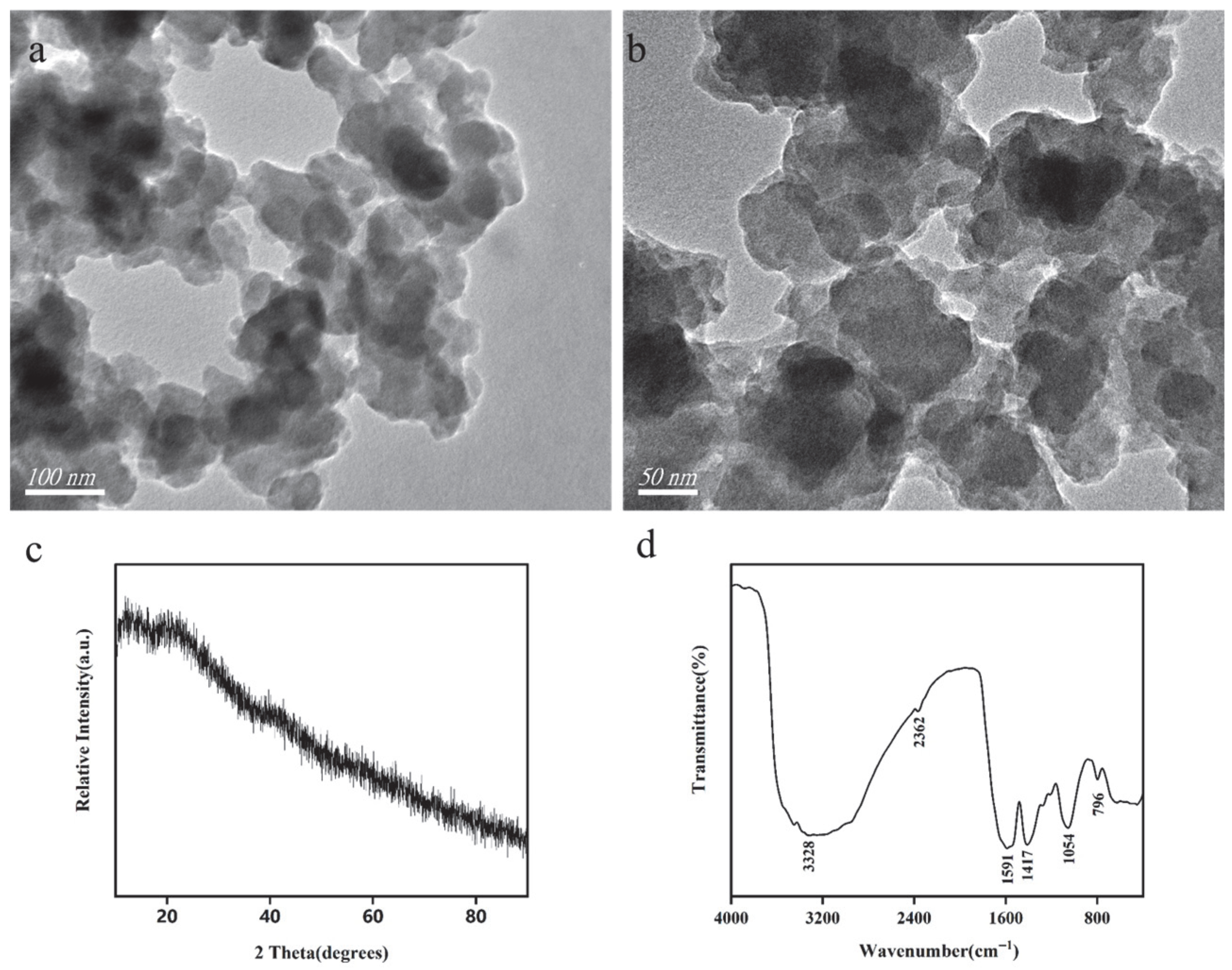
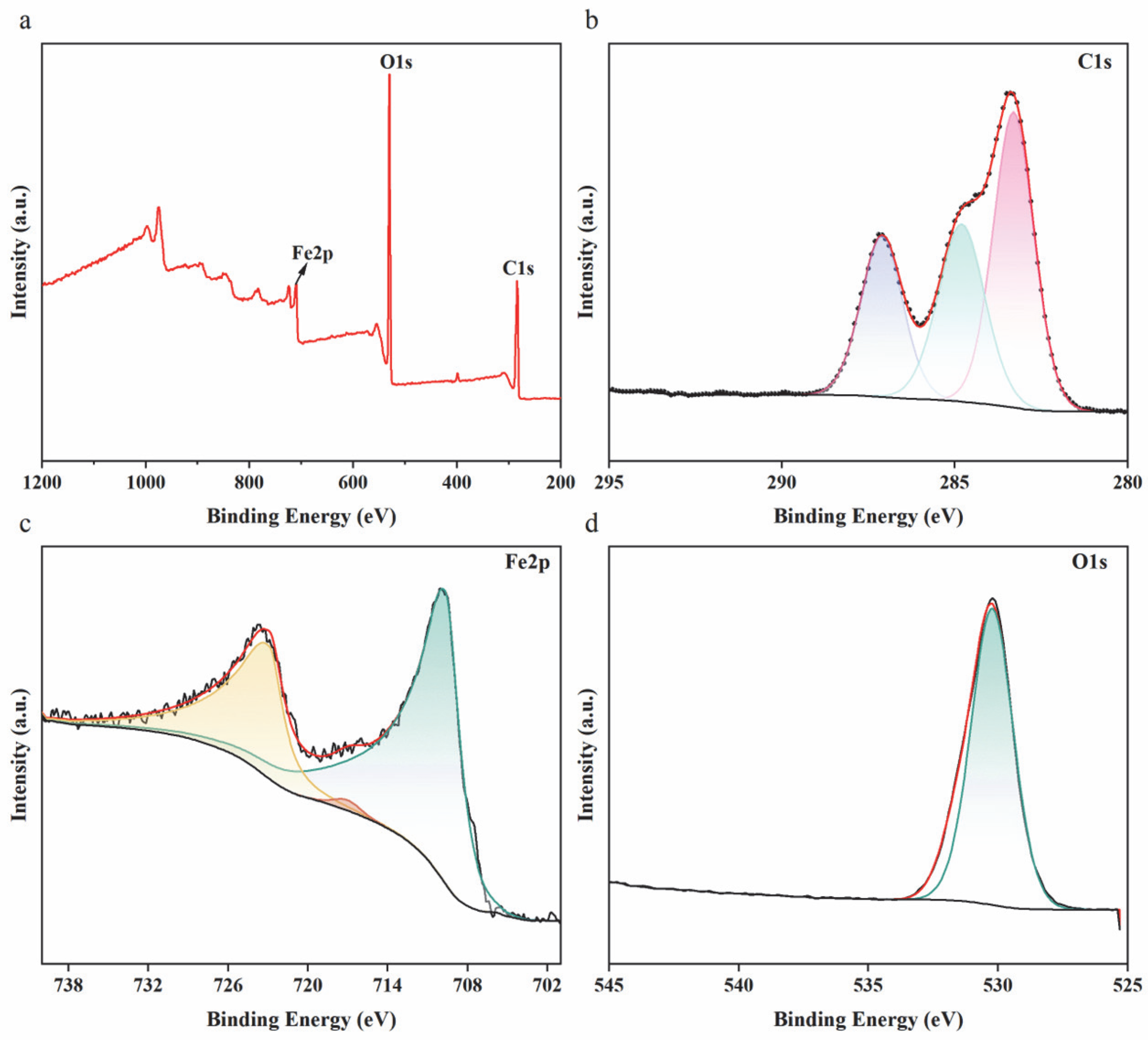
3.1. Characterization of Sa-FeNPs
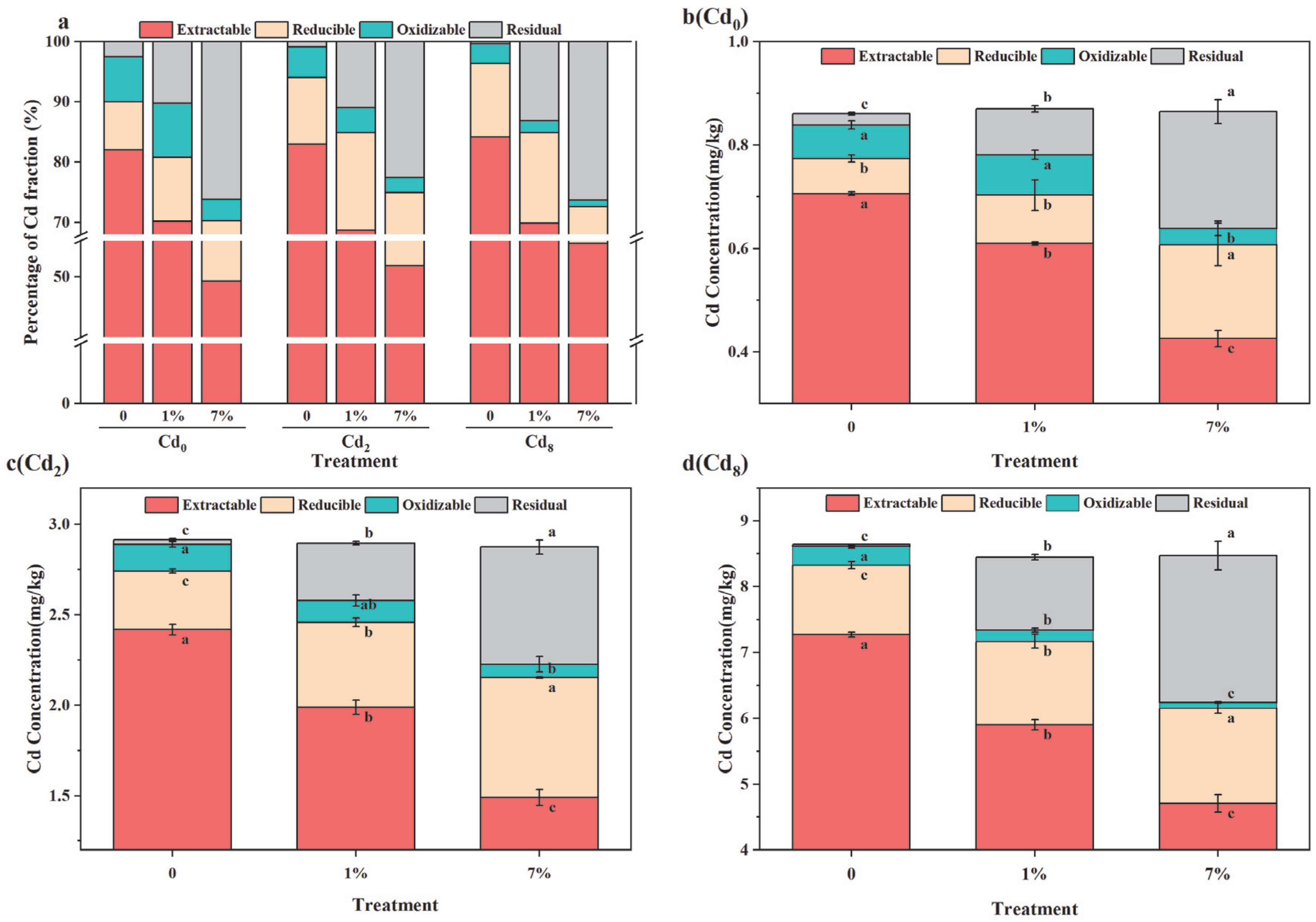
3.2. Effect of Sa-FeNPs on Cd Fractions
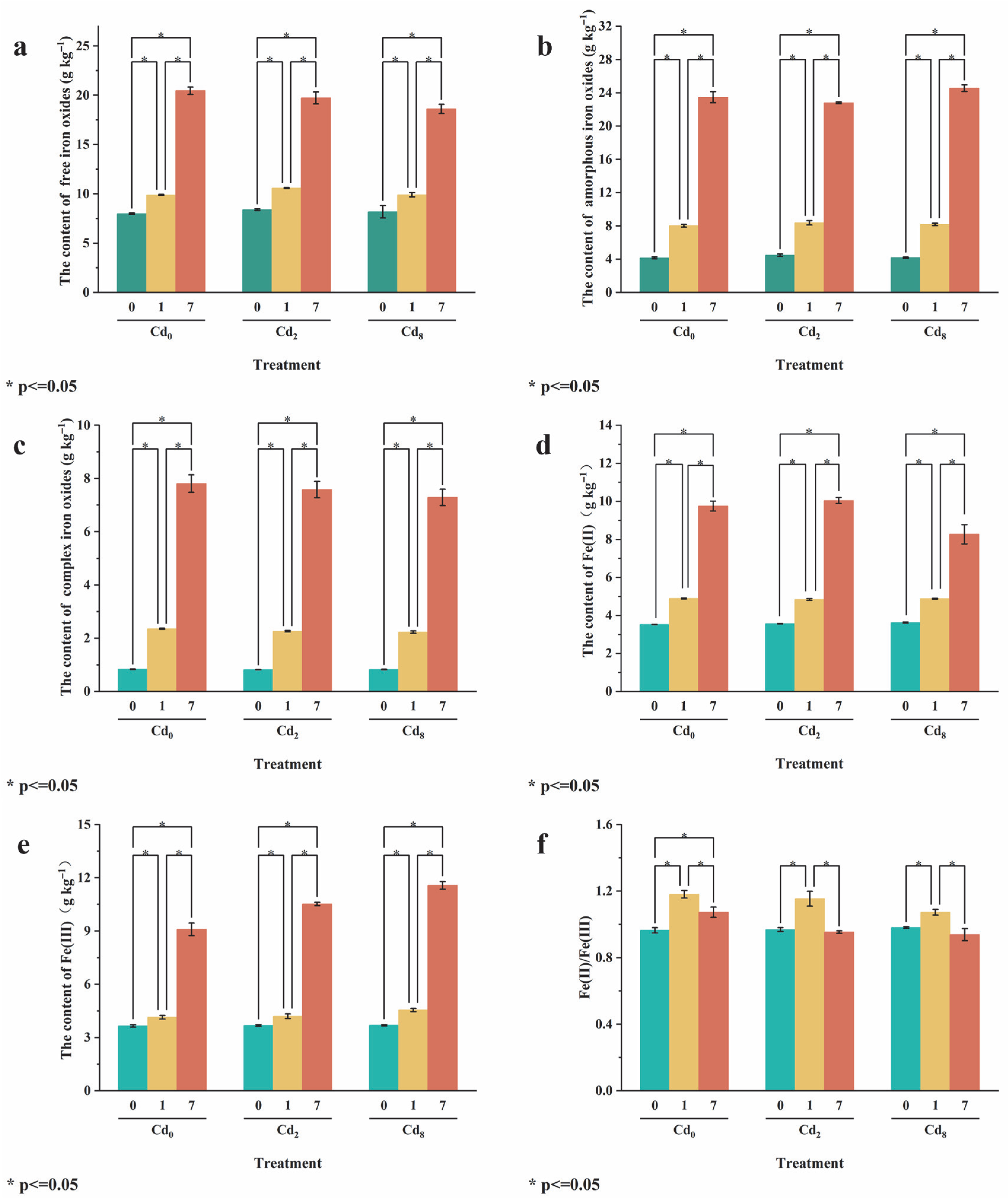
3.3. Effect of Sa-FeNPs on Fe Transformation
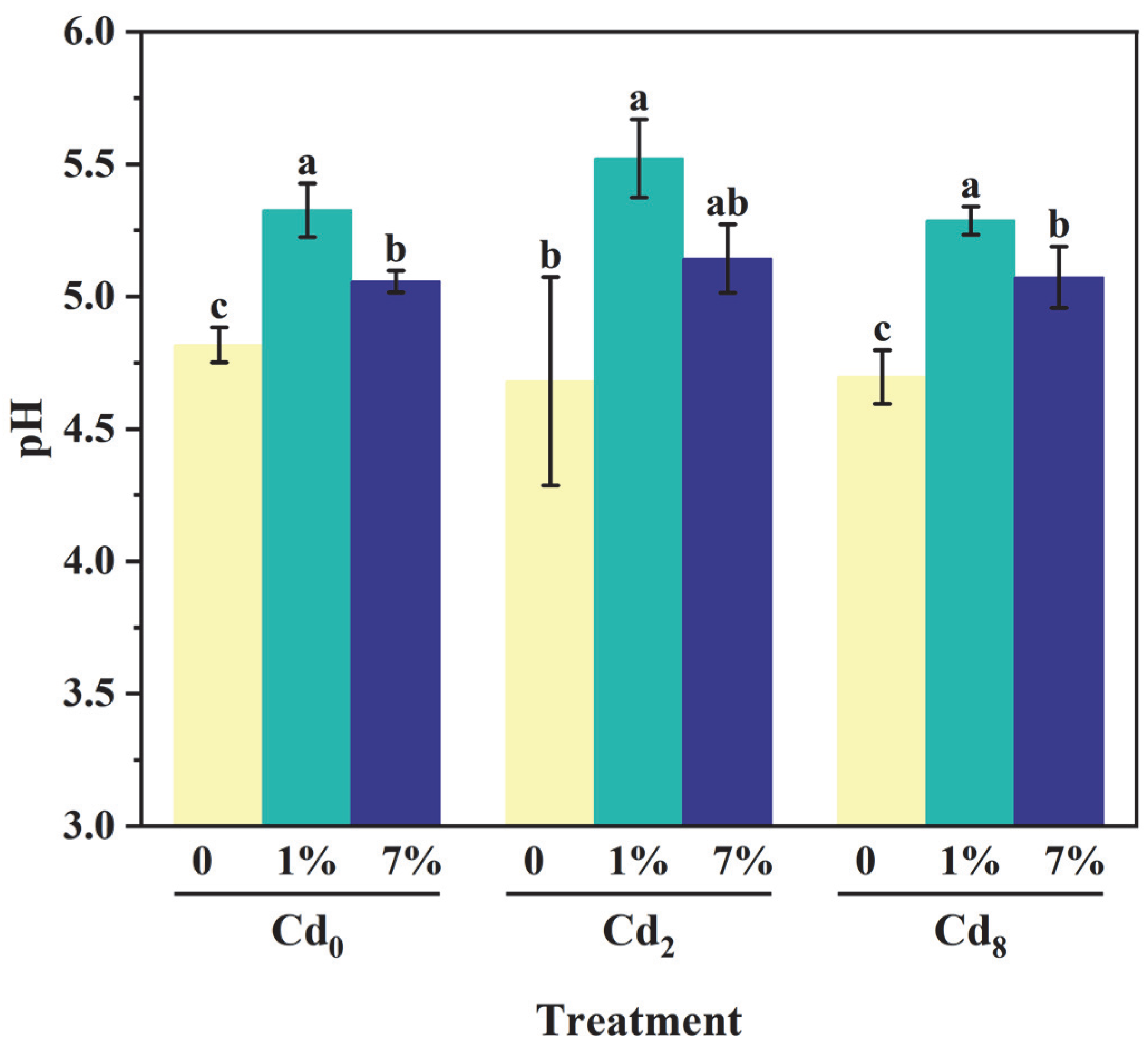
3.4. Effect of Sa-FeNPs on pH of Sediments
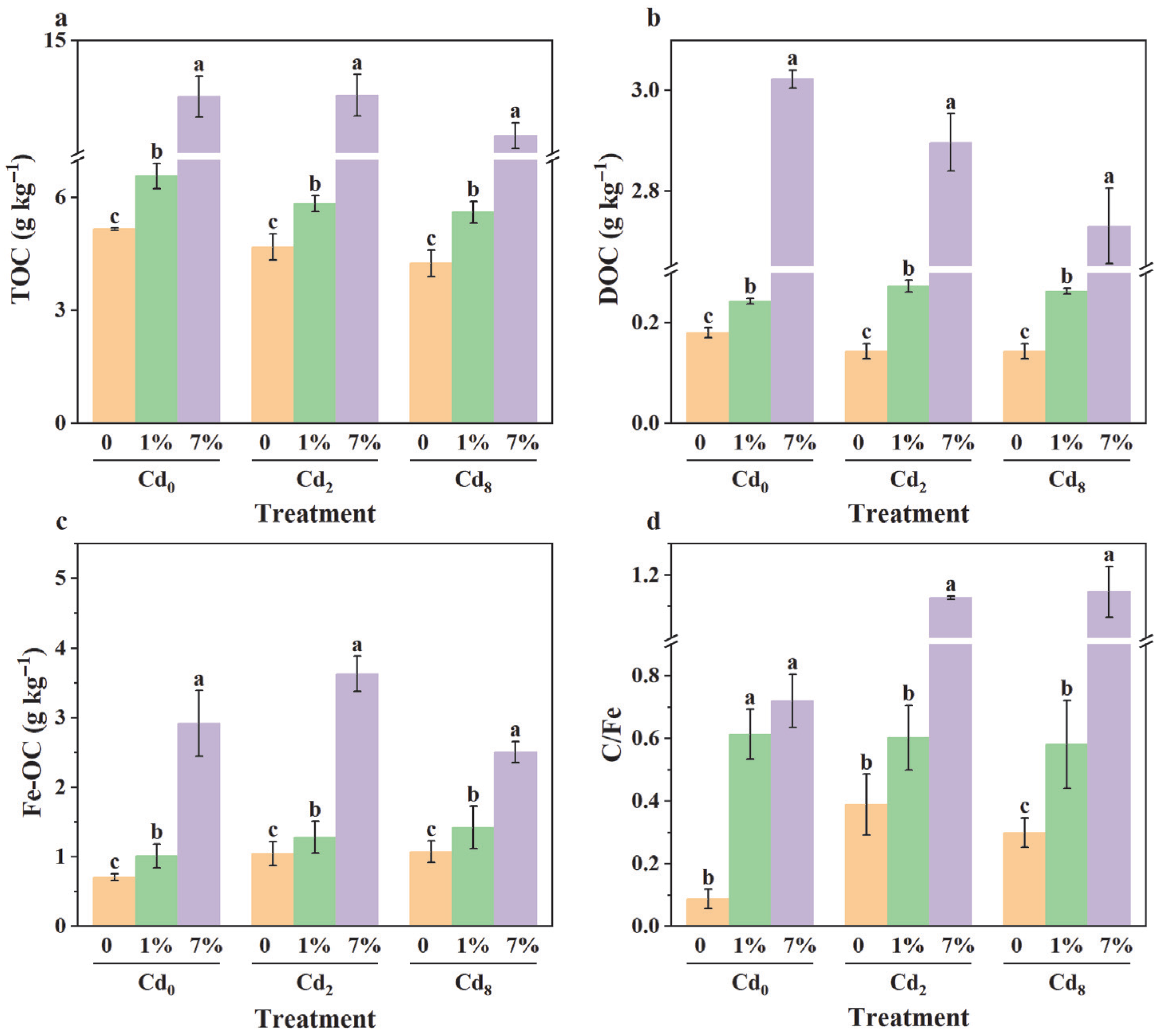
3.5. Effect of Sa-FeNPs on Organic Carbon
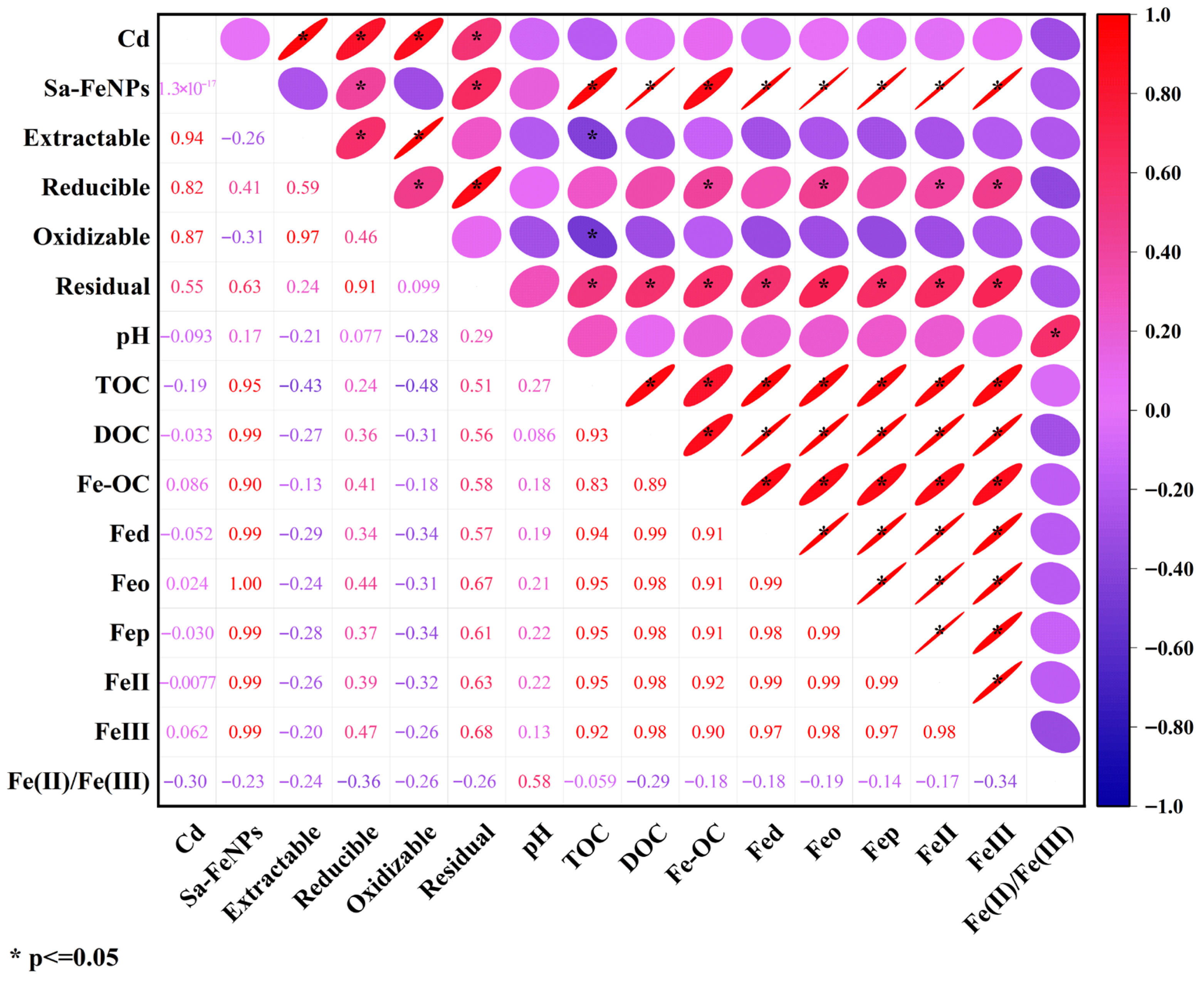
3.6. Correlation Analysis
4. Discussion
4.1. The Immobilization Efficacy of Sa-FeNPs on Cd
4.2. The Mechanism of Sa-FeNPs Immobilizing Cd in Sediments
4.3. Carbon Sequestration Potential and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Fed | Free iron oxides |
| Feo | Amorphous iron oxides |
| Fep | Complex iron oxides |
| TOC | Total organic carbon |
| OM | Organic matter |
| DOC | Dissolved organic carbon |
| Fe-OC | Iron-bound organic carbon |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectrometry |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier transform infrared |
References
- Yuan, R.; Li, Y.; Li, J.; Ji, S.; Wang, S.; Kong, F. The Allelopathic Effects of Aqueous Extracts from Spartina Alterniflora on Controlling the Microcystis Aeruginosa Blooms. Sci. Total Environ. 2020, 712, 136332. [Google Scholar] [CrossRef]
- Qin, F.; Tang, B.; Zhang, H.; Shi, C.; Zhou, W.; Ding, L.; Qin, P. Potential Use of Spartina Alterniflora as Forage for Dairy Cattle. Ecol. Eng. 2016, 92, 173–180. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Zhang, X.; Wang, W.; Wang, X.; Kong, F.; Xi, M. The Amelioration and Improvement Effects of Modified Biochar Derived from Spartina Alterniflora on Coastal Wetland Soil and Suaeda Salsa Growth. Environ. Res. 2024, 240, 117426. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A. The Emerging Roles of Arthropods and Their Metabolites in the Green Synthesis of Metallic Nanoparticles. Nanotechnol. Rev. 2016, 5, 601–622. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Sher, F.; Afshin, S.; Hamzezadeh, A.; Ahmadi, S.; Azhar, O.; Rastegar, A.; Ghosh, S.; Poureshgh, Y. Green Synthesis of Zero-Valent Iron Nanoparticles and Loading Effect on Activated Carbon for Furfural Adsorption. Chemosphere 2022, 287, 132114. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Chapter 6—Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 199–322. [Google Scholar]
- Liu, Y.; Jin, X.; Chen, Z. The Formation of Iron Nanoparticles by Eucalyptus Leaf Extract and Used to Remove Cr(Vi). Sci. Total Environ. 2018, 627, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of Green Zero-Valent Iron Nanoparticles Produced with Tree Leaf Extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef]
- Machado, S.; Stawiński, W.; Slonina, P.; Pinto, A.R.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Application of Green Zero-Valent Iron Nanoparticles to the Remediation of Soils Contaminated with Ibuprofen. Sci. Total Environ. 2013, 461–462, 323–329. [Google Scholar] [CrossRef]
- Leng, Z.; Wu, Y.; Li, J.; Nie, Z.; Jia, H.; Yan, C.; Hong, H.; Wang, X.; Du, D. Phenolic Root Exudates Enhance Avicennia Marina Tolerance to Cadmium under the Mediation of Functional Bacteria in Mangrove Sediments. Mar. Pollut. Bull. 2022, 185, 114227. [Google Scholar] [CrossRef]
- Wu, Y.; Leng, Z.; Li, J.; Yan, C.; Wang, X.; Jia, H.; Chen, L.; Zhang, S.; Zheng, X.; Du, D. Sulfur Mediated Heavy Metal Biogeochemical Cycles in Coastal Wetlands: From Sediments, Rhizosphere to Vegetation. Front. Environ. Sci. Eng. 2021, 16, 102. [Google Scholar] [CrossRef]
- Lin, J.; Sun, M.; Su, B.; Owens, G.; Chen, Z. Immobilization of Cadmium in Polluted Soils by Phytogenic Iron Oxide Nanoparticles. Sci. Total Environ. 2019, 659, 491–498. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Lin, Z.; Khan, N.I.; Owens, G.; Chen, Z. Removal of as(V) by Iron-Based Nanoparticles Synthesized Via the Complexation of Biomolecules in Green Tea Extracts and an Iron Salt. Sci. Total Environ. 2021, 764, 142883. [Google Scholar] [CrossRef]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic Effects of Heavy Metals on Crustaceans and Associated Health Risks in Humans: A Review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Choudhuri, S. Metallothionein: An Intracellular Protein to Protect Against Cadmium Toxicity. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 267–294. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in Plants: Uptake, Toxicity, and Its Interactions with Selenium Fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Roberto, V.P.; Surget, G.; Le Lann, K.; Mira, S.; Tarasco, M.; Guérard, F.; Poupart, N.; Laizé, V.; Stiger-Pouvreau, V.; Cancela, M.L. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina Alterniflora and Salicornia Fragilis Extracts Rich in Polyphenols. Front. Nutr. 2021, 8, 719438. [Google Scholar] [CrossRef]
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The Stabilizing Mechanism of Cadmium in Contaminated Soil Using Green Synthesized Iron Oxide Nanoparticles Under Long-Term Incubation. J. Hazard. Mater. 2019, 379, 120832. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR Three Step Sequential Extraction Procedure Prior to the Certification of New Sediment and Soil Reference Materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Lu, H.; Jia, H.; Yu, J.; Hong, H.; Yan, C. Influence of the Phenols on the Biogeochemical Behavior of Cadmium in the Mangrove Sediment. Chemosphere 2016, 144, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.-Y.; Liu, C.; Li, F.; Xu, X.; Wang, Q. Arsenic Availability in Rice from a Mining Area: Is Amorphous Iron Oxide-bound Arsenic a Source or Sink? Environ. Pollut. 2015, 199, 95–101. [Google Scholar] [CrossRef]
- Phillips, E.J.P.; Lovley, D.R. Determination of Fe(Iii) and Fe(Ii) in Oxalate Extracts of Sediment. Soil Sci. Soc. Am. J. 1987, 51, 938–941. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- García, F.E.; Senn, A.M.; Meichtry, J.M.; Scott, T.B.; Pullin, H.; Leyva, A.G.; Halac, E.B.; Ramos, C.P.; Sacanell, J.; Mizrahi, M.; et al. Iron-Based Nanoparticles Prepared from Yerba Mate Extract. Synthesis, Characterization and Use on Chromium Removal. J. Environ. Manag. 2019, 235, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Fe Nanoparticles Using Eucalyptus Leaf Extracts for Treatment of Eutrophic Wastewater. Sci. Total Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesized Iron Nanoparticles by Green Tea and Eucalyptus Leaves Extracts Used for Removal of Nitrate in Aqueous Solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Pan, Z.; Lin, Y.; Sarkar, B.; Owens, G.; Chen, Z. Green Synthesis of Iron Nanoparticles Using Red Peanut Skin Extract: Synthesis Mechanism, Characterization and Effect of Conditions on Chromium Removal. J. Colloid. Interface Sci. 2020, 558, 106–114. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Simultaneous Removal of Trichloroethylene and Hexavalent Chromium by Green Synthesized Agarose-Fe Nanoparticles Hydrogel. Chem. Eng. J. 2016, 294, 290–297. [Google Scholar] [CrossRef]
- Slijepčević, N.; Tomašević Pilipović, D.; Rađenović, D.; Svirčev, E.; Kulić Mandić, A.; Kerkez, Đ.; Leovac Maćerak, A. The Application of Iron Nanoparticles Biosynthesized Using Citrus Peel Extracts for Immobilization of Metal-Contaminated River Sediment. Int. J. Environ. Sci. Technol. 2024, 21, 3999–4012. [Google Scholar] [CrossRef]
- Keyla, T.S.-H.; Carlos, R.C. Nanoscale Zero Valent Iron for Environmental Cadmium Metal Treatment. In Green Chemistry; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 7. [Google Scholar]
- Li, X.-C.; Yang, Z.-Z.; Zhang, C.; Wei, J.-J.; Zhang, H.-Q.; Li, Z.-H.; Ma, C.; Wang, M.-S.; Chen, J.-Q.; Hu, J.-W. Effects of Different Crystalline Iron Oxides on Immobilization and Bioavailability of Cd in Contaminated Sediment. Chem. Eng. J. 2019, 373, 307–317. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Cheng, M.; Zhang, C.; Hu, C.; Li, J. Performance and Toxicity Assessment of Nanoscale Zero Valent Iron Particles in the Remediation of Contaminated Soil: A Review. Chemosphere 2018, 210, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Aeppli, M.; Vranic, S.; Kaegi, R.; Kretzschmar, R.; Brown, A.R.; Voegelin, A.; Hofstetter, T.B.; Sander, M. Decreases in Iron Oxide Reducibility During Microbial Reductive Dissolution and Transformation of Ferrihydrite. Environ. Sci. Technol. 2019, 53, 8736–8746. [Google Scholar] [CrossRef]
- Robinson, T.C.; Latta, D.E.; Leddy, J.; Scherer, M.M. Redox Potentials of Magnetite Suspensions Under Reducing Conditions. Environ. Sci. Technol. 2022, 56, 17454–17461. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.; Li, W.; Peacock, C.L.; Ma, J.; Wen, H.; Liu, F.; Zhou, Z.; Zhu, C.; Yin, H. Cadmium Isotope Fractionation during Adsorption and Substitution with Iron (Oxyhydr)Oxides. Environ. Sci. Technol. 2021, 55, 11601–11611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Williams, P.N.; Fang, W.; Ji, R.; Han, C.; Ren, J.; Li, H.; Yin, D.; Fan, J.; Xu, H.; et al. Enhanced Mobilization of Cd from Commercial Pigments in the Rhizosphere of Flooded Lowland Rice. Sci. Total Environ. 2022, 807, 151032. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, B.; Antelo, J.; Iglesias, A.; López, R.; Fiol, S.; Arce, F. Analysis of the Variable Charge of Two Organic Soils by Means of the NICA-Donnan Model. Eur. J. Soil Sci. 2007, 58, 1358–1363. [Google Scholar] [CrossRef]
- Polubesova, T.; Chefetz, B. DOM-Affected Transformation of Contaminants on Mineral Surfaces: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 223–254. [Google Scholar] [CrossRef]
- Barekzai, A.; Mengel, K. Effect of Microbial Decomposition of Mature Leaves on Soil pH. Z. Pflanzenernährung Bodenkd. 1993, 156, 93–94. [Google Scholar] [CrossRef]
- Nierop, K.G.J.J.; Jansen, B.; Verstraten, J.M. Dissolved Organic Matter, Aluminium and Iron Interactions: Precipitation Induced by Metal/Carbon Ratio, pH and Competition. Sci. Total Environ. 2002, 300, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of Organic Matter in Sediments Promoted by Iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Wang, H.; Liu, C. Iron-Organic Carbon Interactions in Wetlands: Implications for Wetland Carbon Preservation Under Global Changes. Glob. Change Biol. 2025, 31, e70300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zang, X.; Leng, Z.; Li, Y.; Xu, S.; Wei, N. Synthesis of Iron Nanoparticles from Spartina alterniflora for Cadmium Immobilization in Coastal Wetland Sediments. Biology 2025, 14, 1626. https://doi.org/10.3390/biology14111626
Li J, Zang X, Leng Z, Li Y, Xu S, Wei N. Synthesis of Iron Nanoparticles from Spartina alterniflora for Cadmium Immobilization in Coastal Wetland Sediments. Biology. 2025; 14(11):1626. https://doi.org/10.3390/biology14111626
Chicago/Turabian StyleLi, Jian, Xuejing Zang, Zhanrui Leng, Yan Li, Shiyan Xu, and Na Wei. 2025. "Synthesis of Iron Nanoparticles from Spartina alterniflora for Cadmium Immobilization in Coastal Wetland Sediments" Biology 14, no. 11: 1626. https://doi.org/10.3390/biology14111626
APA StyleLi, J., Zang, X., Leng, Z., Li, Y., Xu, S., & Wei, N. (2025). Synthesis of Iron Nanoparticles from Spartina alterniflora for Cadmium Immobilization in Coastal Wetland Sediments. Biology, 14(11), 1626. https://doi.org/10.3390/biology14111626





