Comparative Profiling of Antibiotic Resistance Genes and Microbial Communities in Pig and Cow Dung from Rural China: Insights into Environmental Dissemination and Public Health Risks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Extraction of Bacterial Genomic DNA
2.3. PCR Array Analysis
2.4. 16S rDNA Sequencing Analysis
2.5. Process of Sequencing Data
2.6. Statistical Analysis
3. Results
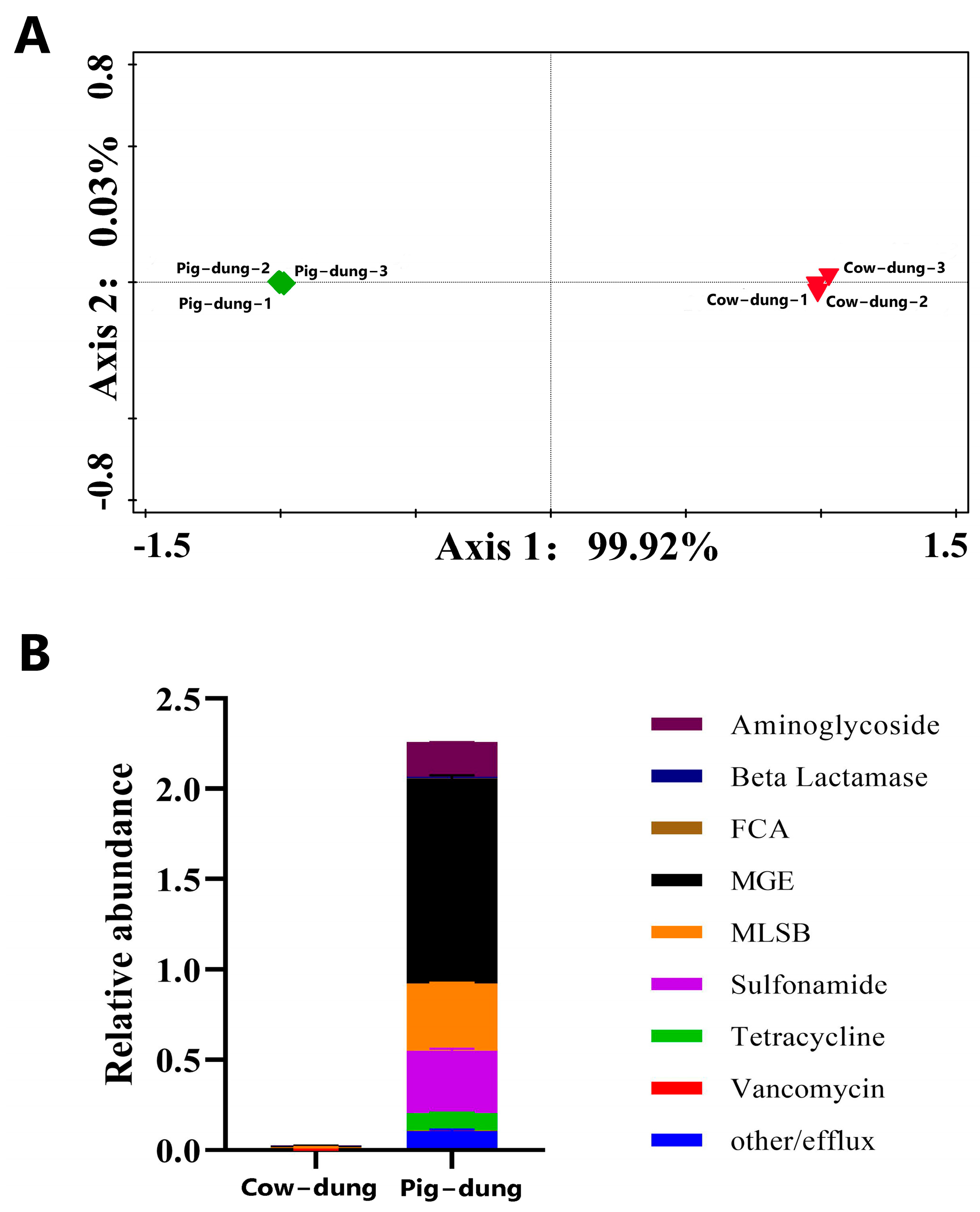
3.1. Analysis of Microbial ARGs in Cow and Pig Dung Samples
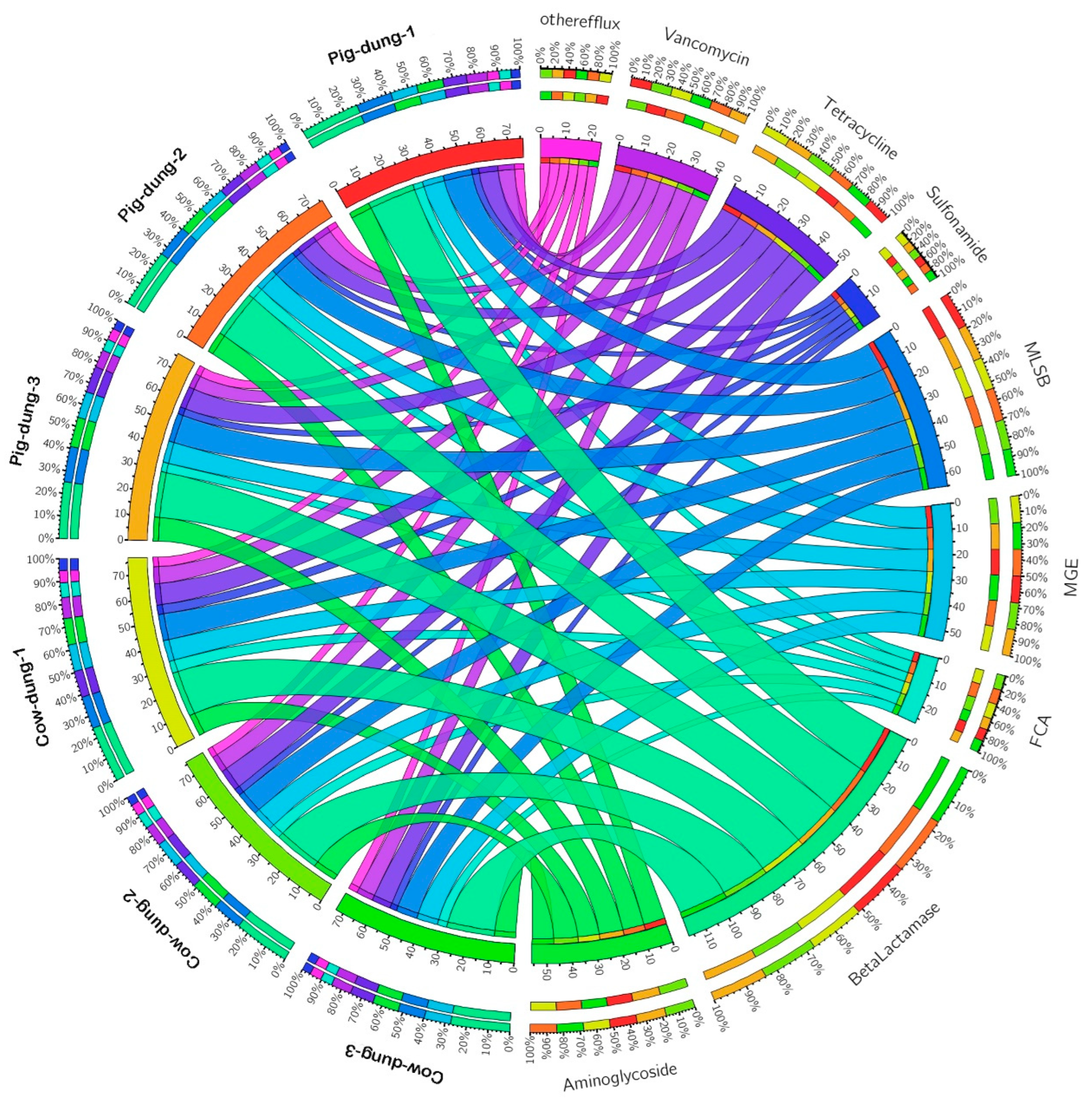
3.2. Differentially Expressed ARGs Between Cow and Pig Dung Samples
3.3. Analysis of 16S rDNA Sequencing Data
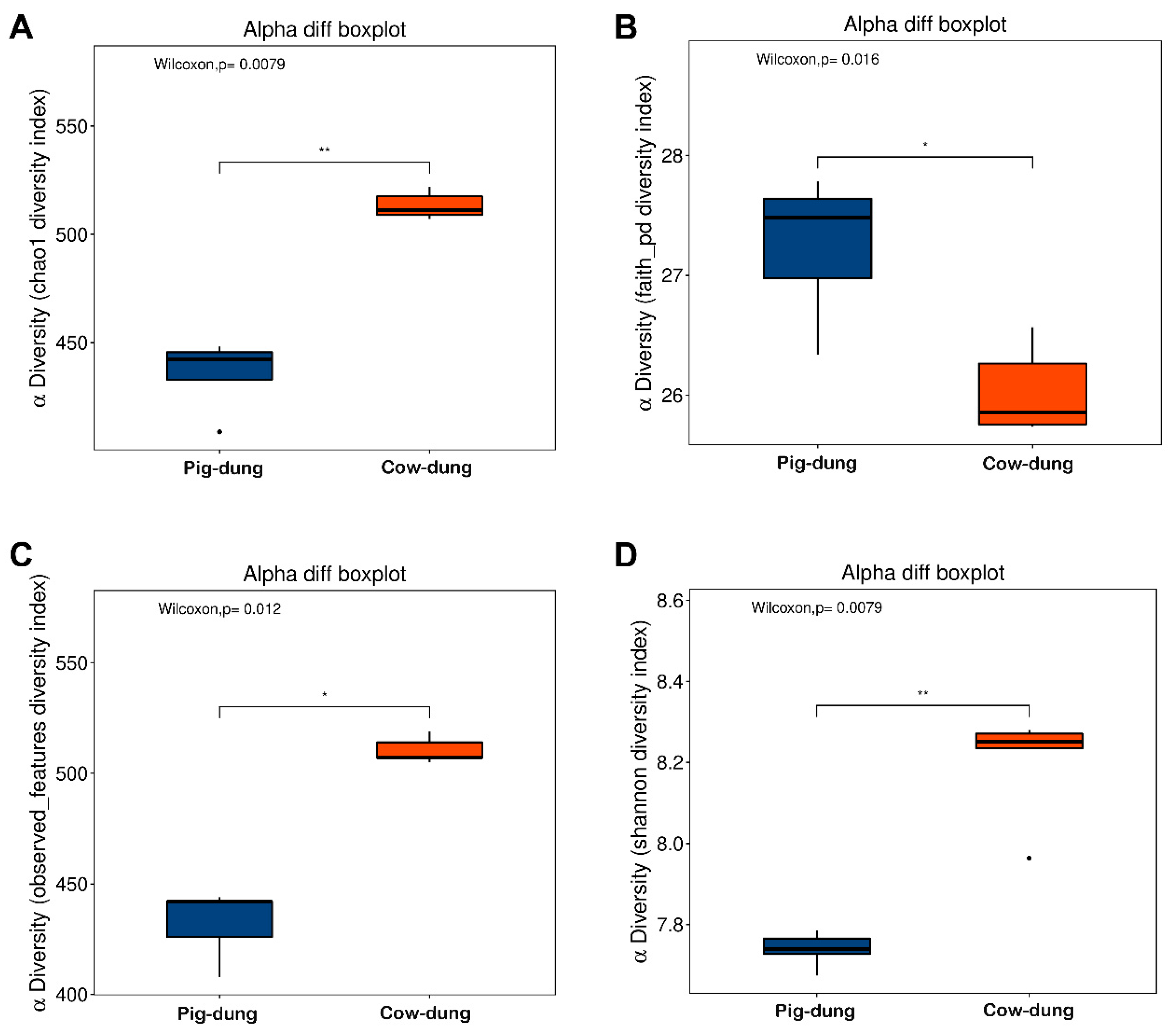
3.4. Comparative Evaluation of Dung Microbiota Variations Between Two Groups
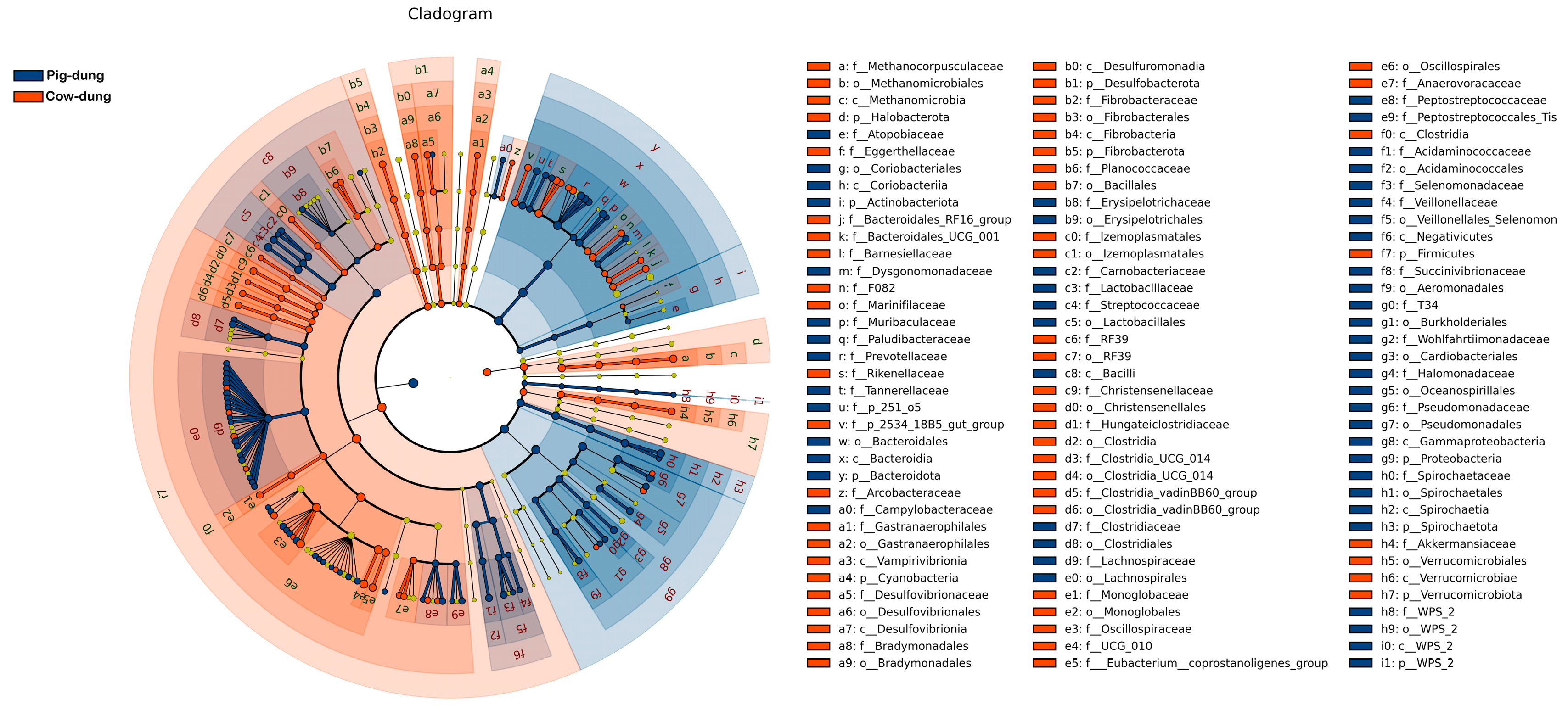
3.5. Important Bacteria in Cow and Pig Dung Samples
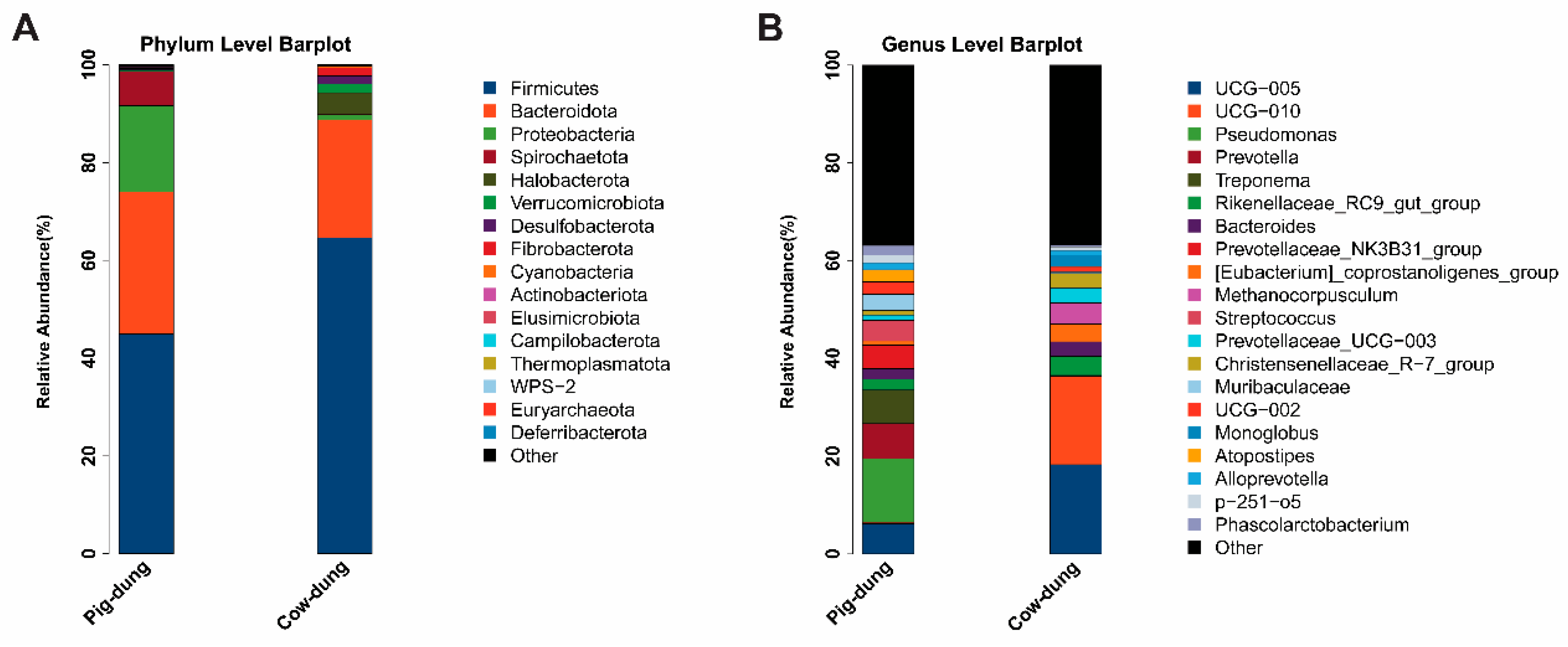
3.6. Microbial Community Composition at Phylum and Genus Levels in Pig and Cow Dung Samples
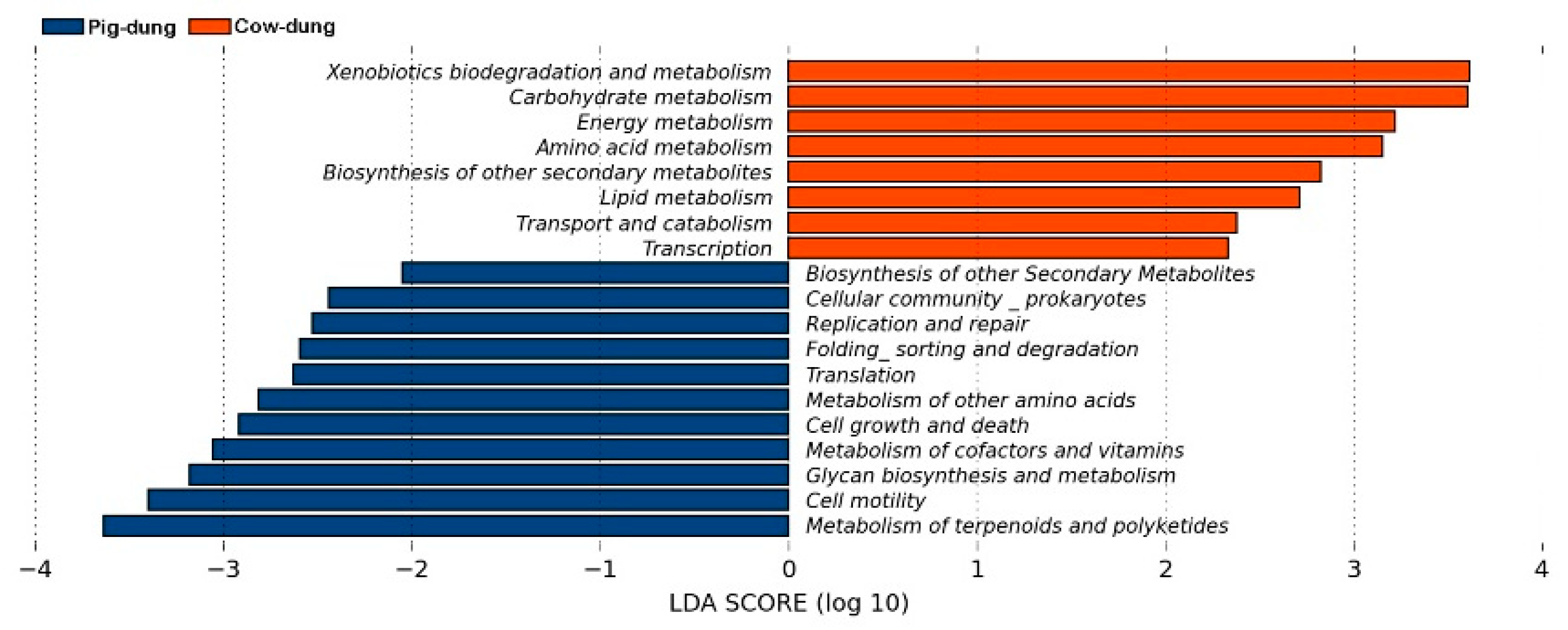
3.7. Analysis of Metabolic Pathways Between Cow and Pig Dung Samples
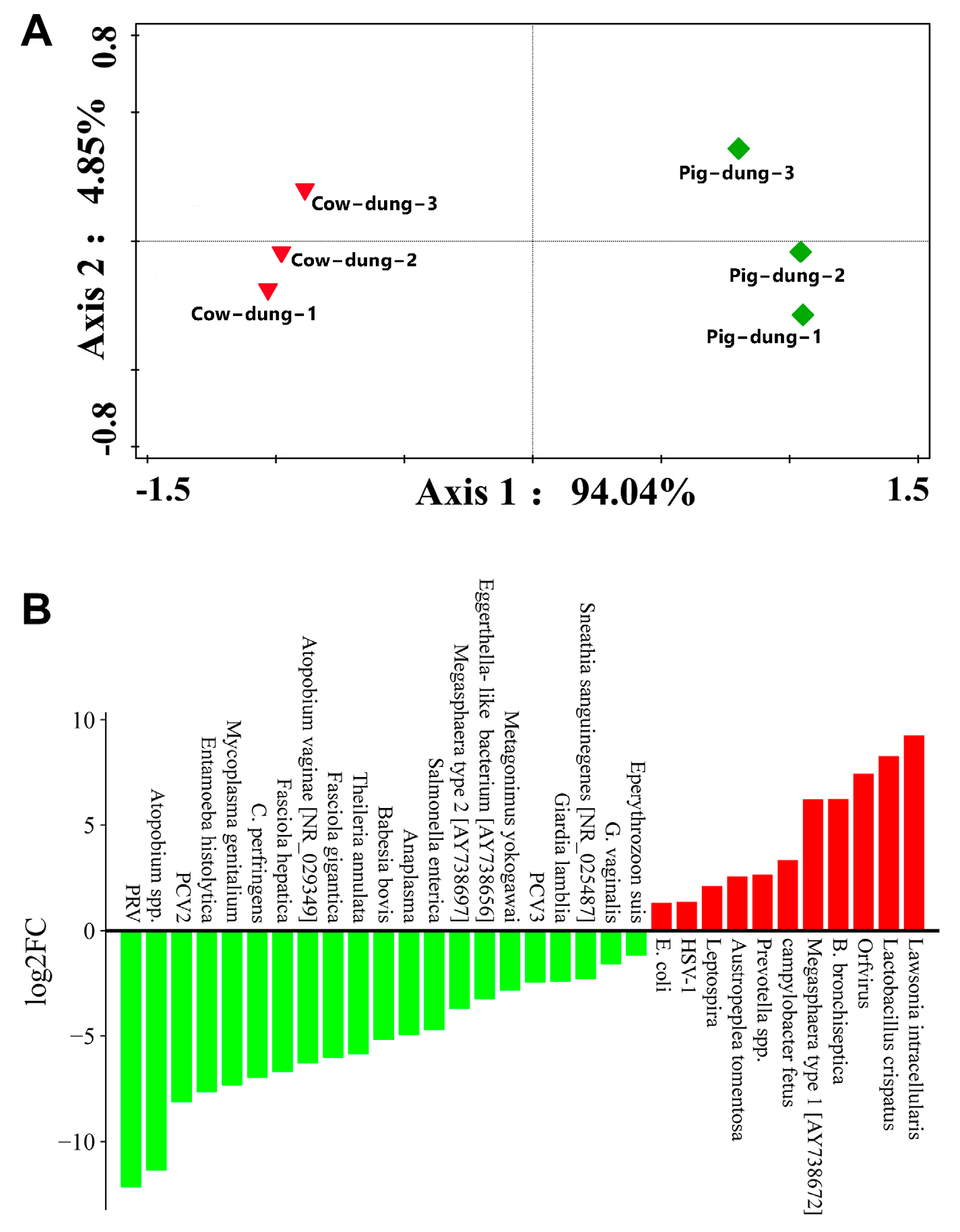
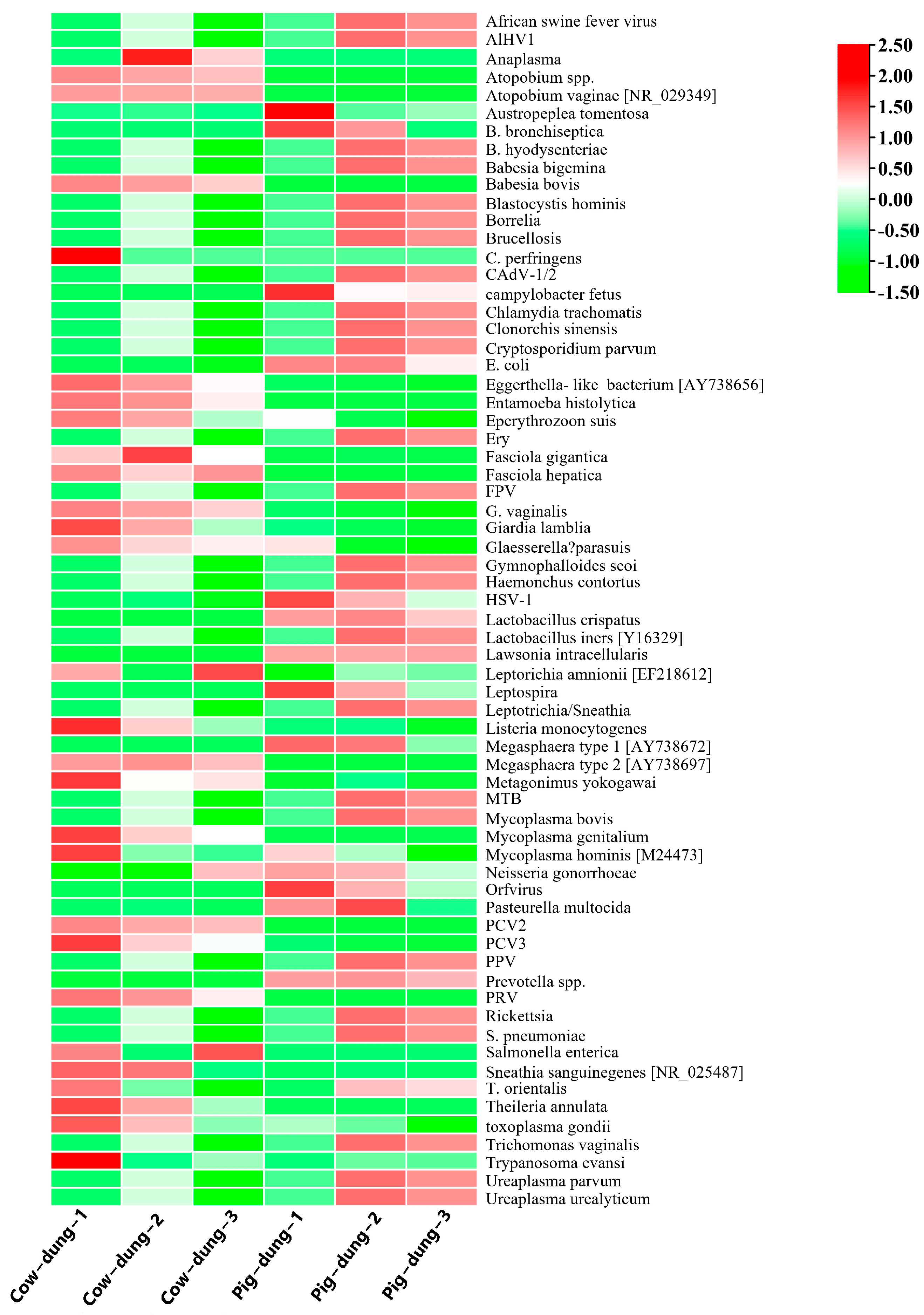
3.8. Analysis of Specific Microorganisms Between Cow and Pig Dung Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, B.K.; Behera, B.K.; Chakraborty, H.J.; Paria, P.; Gangopadhyay, A.; Rout, A.K.; Nayak, K.K.; Parida, P.K.; Rai, A. Metagenomic study focusing on antibiotic resistance genes from the sediments of River Yamuna. GENE 2020, 758, 144951. [Google Scholar] [CrossRef] [PubMed]
- Tello, A.; Austin, B.; Telfer, T.C. Selective Pressure of Antibiotic Pollution on Bacteria of Importance to Public Health. Environ. Health Perspect. 2012, 120, 1100–1106. [Google Scholar] [CrossRef]
- Rout, A.K.; Tripathy, P.S.; Dixit, S.; Behera, D.U.; Behera, B.; Das, B.K.; Behera, B.K. Unveiling the Microbiome Landscape: A Metagenomic Study of Bacterial Diversity, Antibiotic Resistance, and Virulence Factors in the Sediments of the River Ganga, India. Antibiotics 2023, 12, 1735. [Google Scholar] [CrossRef]
- Luo, C.Y.; Zhang, T.; Mustafa, M.F.; Li, M.Y.; Xu, S. Removal efficiency of ARGs in different wastewater treatment plants and their potential risks in effluent. NPJ Clean Water 2025, 8, 45. [Google Scholar] [CrossRef]
- Nava, A.R.; Daneshian, L.; Sarma, H. Antibiotic resistant genes in the environment-exploring surveillance methods and sustainable remediation strategies of antibiotics and ARGs. Environ. Res. 2022, 215, 114212. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, J.Z.; Luo, Y.J.; Muhammad, I.; Dong, H.F.; Ma, H.X.; Kong, L.C. Acidifiers promoted antibiotic resistance gene transfer via plasmid conjugation. J. Environ. Chem. Eng. 2025, 13, 117203. [Google Scholar] [CrossRef]
- Li, R.C.; Pei, X.; Zhang, M.; Deng, X.H.; Tao, C.Y.; Wang, J.B.; Chen, X.L.; Clarke, N.; Sas-Paszt, L.; Shen, Z.Z.; et al. Cessation of manure application diminishes the dissemination potential of antibiotic resistance genes by altering bacterial interaction patterns in soil-lettuce systems. Appl. Soil Ecol. 2025, 211, 106100. [Google Scholar] [CrossRef]
- Wang, G.Y.; Gao, X.; Cai, Y.; Li, G.X.; Ma, R.A.; Yuan, J. Dynamics of antibiotic resistance genes during manure composting: Reduction in herbivores manure and accumulation in carnivores. Environ. Int. 2024, 190, 108900. [Google Scholar] [CrossRef]
- Feng, W.Y.; Ye, Y.H.; Xiang, Y.P.; Peng, S.D.; He, S.Y.; Peng, H.H.; Zhang, Z.F.; Yang, Z.H.; Xiong, W.P. Unraveling the dual role in enhancing methane production and mitigating antibiotic resistance gene spread in anaerobic co-digestion of microalgae and waste activated sludge. J. Hazard. Mater. 2025, 494, 138606. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Kannimuthu, D. Comparative resistome analysis of Aeromonas species in aquaculture reveals antibiotic resistance patterns and phylogeographic distribution. Environ. Res. 2023, 239, 117273. [Google Scholar] [CrossRef]
- Rao, J.; Wei, X.C.; Li, H.; Zhang, Z.W.; Liu, J.H.; Lian, M.J.; Cao, W.W.; Yuan, L.; Dou, B.B.; Tian, Y.H.; et al. Novel Multiplex PCR Assay and Its Application in Detecting Prevalence and Antibiotic Susceptibility of Porcine Respiratory Bacterial Pathogens in Guangxi, China. Microbiol. Spectr. 2023, 11, e0397122. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Qiu, J.Y.; Zheng, Y.L.; Chang, L.J.; Li, H.H.; Xu, F.E.; Zhang, W.; Yin, L.Z.; Fu, H.L.; Yan, Q.G.; et al. Association between Phenotypes of Antimicrobial Resistance, ESBL Resistance Genes, and Virulence Genes of Salmonella Isolated from Chickens in Sichuan, China. Animals 2023, 13, 2770. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Z.; He, L.Y.; Bai, H.; He, L.X.; Zhang, M.; Chen, Z.Y.; Liu, Y.S.; Ying, G.G. Airborne bacterial community and antibiotic resistome in the swine farming environment: Metagenomic insights into livestock relevance, pathogen hosts and public risks. Environ. Int. 2023, 172, 107751. [Google Scholar] [CrossRef]
- Khairunisa, B.H.; Heryakusuma, C.; Ike, K.; Mukhopadhyay, B.; Susanti, D. Evolving understanding of rumen methanogen ecophysiology. Front. Microbiol. 2023, 14, 1296008. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.P.; Zhang, N.N.; Zhao, Z.Q.; Bai, Z.H.; Ma, L. Nitrogen budgets of contrasting crop-livestock systems in China. Environ. Pollut. 2021, 288, 117633. [Google Scholar] [CrossRef]
- Gao, F.Z.; He, L.Y.; He, L.X.; Bai, H.; Zhang, M.; Chen, Z.Y.; Qiao, L.K.; Liu, Y.S.; Ying, G.G. Swine farming shifted the gut antibiotic resistome of local people. J. Hazard. Mater. 2024, 465, 133082. [Google Scholar] [CrossRef]
- Xin, H.B.; Qiu, T.L.; Guo, Y.J.; Gao, H.Z.; Zhang, L.Q.; Gao, M. Aerosolization behavior of antimicrobial resistance in animal farms: A field study from feces to fine particulate matter. Front. Microbiol. 2023, 14, 1175265. [Google Scholar] [CrossRef]
- Zaidi, S.E.Z.; Zaheer, R.; Poulin-Laprade, D.; Scott, A.; Rehman, M.A.; Diarra, M.; Topp, E.; Domselaar, G.V.; Zovoilis, A.; McAllister, T.A. Comparative Genomic Analysis of Enterococci across Sectors of the One Health Continuum. Microorganisms 2023, 11, 727. [Google Scholar] [CrossRef]
- Blazejewska, A.; Zalewska, M.; Grudniak, A.; Popowska, M. A Comprehensive Study of the Microbiome, Resistome, and Physical and Chemical Characteristics of Chicken Waste from Intensive Farms. Biomolecules 2022, 12, 1132. [Google Scholar] [CrossRef]
- Sardar, P.; Elhottová, D.; Pérez-Valera, E. Soil-specific responses in the antibiotic resistome of culturable Acinetobacter spp. and other non-fermentative Gram-negative bacteria following experimental manure application. FEMS Microbiol. Ecol. 2023, 99, fiad148. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Z.; Liu, P.; Xiong, W.G.; Zhou, Q.; Wangxiao, J.Y.; Zeng, Z.L.; Sun, Y.X. Fate of potential indicator antimicrobial resistance genes (ARGs) and bacterial community diversity in simulated manure-soil microcosms. Ecotoxicol. Environ. Saf. 2018, 147, 817–823. [Google Scholar] [CrossRef]
- Bloois, L.V.; Duim, B.; Looft, T.; Veldman, K.T.; Zomer, A.L.; Wagenaar, J.A. Antimicrobial resistance in Campylobacter fetus: Emergence and genomic evolution. Microb. Genom. 2023, 9, 000934. [Google Scholar] [CrossRef]
- Khan, I.; Yasir, M.; Farman, M.; Kumosani, T.; AlBasri, S.F.; Bajouh, O.S.; Azhar, E.I. Evaluation of gut bacterial community composition and antimicrobial resistome in pregnant and non-pregnant women from Saudi population. Infect. DRUG Resist. 2019, 12, 1749–1761. [Google Scholar] [CrossRef]
- Pais, S.; Costa, M.; Barata, A.R.; Rodrigues, L.; Afonso, I.M.; Almeida, G. Evaluation of Antimicrobial Resistance of Different Phylogroups of Escherichia coli Isolates from Feces of Breeding and Laying Hens. Antibiotics 2023, 12, 20. [Google Scholar] [CrossRef]
- Bonvegna, M.; Tomassone, L.; Christensen, H.; Olsen, J.E. Whole Genome Sequencing (WGS) Analysis of Virulence and AMR Genes in Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli from Animal and Environmental Samples in Four Italian Swine Farms. Antibiotics 2022, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Fayad, E.; El-Bazzal, B.; Daoud, Z.; Rolain, J.M. Prevalence of Extended-Spectrum Beta-Lactamase-Producing Gram-Negative Bacilli and Emergence of mcr-1 Colistin Resistance Gene in Lebanese Swine Farms. Microb. DRUG Resist. 2019, 25, 233–240. [Google Scholar] [CrossRef]
- Verstegen, M.W.A.; Williams, B.A. Alternatives to the use of antibiotics as growth promoters for monogastric animals. Anim. Biotechnol. 2002, 13, 113–127. [Google Scholar] [CrossRef]
- Saettone, V.; Biasato, I.; Radice, E.; Schiavone, A.; Bergero, D.; Meineri, G. State-of-the-Art of the Nutritional Alternatives to the Use of Antibiotics in Humans and Monogastric Animals. Animals 2020, 10, 2199. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecien, M.; Jachimowicz, K.; Muszynski, S.; Tomaszewska, E. Bioactive compounds, antibiotics and heavy metals: Effects on the intestinal structure and microbiome of monogastric animals—A non-systematic review. Ann. Anim. Sci. 2023, 23, 289–313. [Google Scholar] [CrossRef]
- Kerek, A.; Németh, V.; Szabó, A.; Papp, M.; Bányai, K.; Kardos, G.; Kaszab, E.; Bali, K.; Nagy, Z.; Süth, M.; et al. Monitoring Changes in the Antimicrobial-Resistance Gene Set (ARG) of Raw Milk and Dairy Products in a Cattle Farm, from Production to Consumption. Vet. Sci. 2024, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.B.; Zhai, Y.F.; Mao, B.Y.; Hu, N. Antibiotic resistance genes and intI1 prevalence in a swine wastewater treatment plant and correlation with metal resistance, bacterial community and wasteWater parameters. Ecotoxicol. Environ. Saf. 2018, 161, 251–259. [Google Scholar] [CrossRef]
- Klinsoda, J.; Boonsoongnern, A.; Thanantong, N.; Kaminsonsakul, T.; Treesuwan, K.; Trevanich, S.; Metzler-Zebeli, B.U. The Microbiome Characterization of Edible Visceral Organs and Fresh Meat During Production in a Pig Processing Facility in Thailand. Pathogens 2025, 14, 475. [Google Scholar] [CrossRef]
- Fan, Z.Z.; Du, Y.W.; Zhang, J.L.; Yang, X.P. Pathogenic bacteria and high-risk ARGs removal in subsurface flow constructed wetlands treating swine tailwater: Performance and influencing factors. Chem. Eng. J. 2025, 507, 160582. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, H.H.; Yu, S.B.; Li, D.H.; Gillings, M.R.; Ren, H.Q.; Mao, D.Q.; Guo, J.H.; Luo, Y. Inter-plasmid transfer of antibiotic resistance genes accelerates antibiotic resistance in bacterial pathogens. ISME J. 2024, 18, wrad032. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Ban-Tokuda, T.; Matsui, H. Diversity of Subgroup of Fibrobacter succinogenes in the Rumen and Feces of Cattle and Sheep. Anim. Sci. J. 2025, 96, e70043. [Google Scholar] [CrossRef]
- Ahvenjärvi, S.; Bayat, A.R.; Toivanen, M.; Mäntysaari, P.; Tapio, I. The effects of residual energy intake on nutrient use, methane emissions and microbial composition in dairy cows. Sci. Rep. 2024, 14, 613. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Ali, M.; Zhu, Y.; Chen, X.Y.; Lu, D.Y.; Liu, Y.; Li, K.; Zhang, C.F. Comparative analysis of gut microbiota in free range and house fed yaks from Linzhou County. Sci. Rep. 2025, 15, 14317. [Google Scholar] [CrossRef]
- Xi, L.; Wen, X.H.; Jia, T.; Han, J.C.; Qin, X.X.; Zhang, Y.Z.; Wang, Z.H. Comparative study of the gut microbiota in three captive Rhinopithecus species. BMC Genom. 2023, 24, 398. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Yang, F.; Liu, R.; Gao, Q.; Cheng, B.; Lin, X.; Huang, L.; Chen, C.; Xiang, J.; et al. Signaling metabolite succinylacetone activates HIF-1α and promotes angiogenesis in GSTZ1-deficient hepatocellular carcinoma. JCI Insight 2023, 8, e164968. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Lee, G.Y.; Hossain, M.A.; Awji, E.G.; Park, S.C. Gut microbiome perturbation and its correlation with tylosin pharmacokinetics in healthy and infected pigs. Sci. Rep. 2024, 14, 18670. [Google Scholar] [CrossRef]
- Han, R.; Liu, L.; Meng, Y.; Han, H.R.; Xiong, R.B.; Li, Y.; Chen, L.S. Archaeal and bacterial community structures of rural household biogas digesters with different raw materials in Qinghai Plateau. Biotechnol. Lett. 2021, 43, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.Y.; Zhang, X.X.; Miao, Y.; Zhao, Y.T.; Ye, L.; Li, B.; Zhang, T. Fate of antibiotic resistance genes and their associations with bacterial community in livestock breeding wastewater and its receiving river water. WATER Res. 2017, 124, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.; Weisbjerg, M.R.; Abdelhafiz, Y.; Bauer, S.L.; Kiron, V.; Novoa-Garrido, M. Feed characteristics and potential effects on ruminal bacteria of ensiled sugar kelp and winged kelp for Holstein dairy cows. Animal 2024, 18, 101274. [Google Scholar] [CrossRef]
- Yegorov, B.; Yegorova, A.; Yeryganov, K. Microbiomes of human, livestock animal gastrointestinal tracts and of food products and compound feeds: Connections and impacts. Part 1. J. Food Sci. Technol.-Ukr. 2023, 17, 15–26. [Google Scholar] [CrossRef]
- Jost, B.H.; Post, K.W.; Songer, J.G.; Billington, S.J. Isolation of Arcanobacterium pyogenes from the porcine gastric mucosa. Vet. Res. Commun. 2002, 26, 419–425. [Google Scholar] [CrossRef]
- Li, Y.P.; Fan, H.X.; Li, B.Q.; Liu, X.B. Environmental Impact of Xenobiotic Aromatic Compounds and Their Biodegradation Potential in Comamonas testosteroni. Int. J. Mol. Sci. 2024, 25, 13317. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Zheng, J.W.; Lv, X.R.; Han, Y.Q.; Zhang, H.M.; Ren, Y.; Yin, G.F.; Ren, L.Z. Infectivity and Potential Zoonotic Characteristics of Porcine Pseudorabies Virus in Human Cells. Transbound. Emerg. Dis. 2024, 2024, 5929976. [Google Scholar] [CrossRef]
- Xu, T.; Chen, X.M.; Fu, Y.; Ai, Y.; Wang, D.M.; Wei, Z.Y.; Li, X.S.; Zheng, L.L.; Chen, H.Y. Cross-species transmission of an emerging porcine circovirus (PCV4): First molecular detection and retrospective investigation in dairy cows. Vet. Microbiol. 2022, 273, 109528. [Google Scholar] [CrossRef]
- Ortiz-Chura, A.; Gere, J.; Marcoppido, G.; Depetris, G.; Cravero, S.; Faverín, C.; Pinares-Patiño, C.; Cataldi, A.; Cerón-Cuhhi, M.E. Dynamics of the ruminal microbial ecosystem, and inhibition of methanogenesis and propiogenesis in response to nitrate feeding to Holstein calves. Anim. Nutr. 2021, 7, 1205–1218. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.L.; Li, Y.; Wang, Q.; Shao, J.R.; Chen, Y.; Xin, J.Q. Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1β production through the NF-κB pathway via toll-like receptor 2 and MyD88. Dev. Comp. Immunol. 2016, 55, 111–118. [Google Scholar] [CrossRef]
- Sato, Y.; Shioya, H.; Uda, Y.; Asano, H.; Nagao, Y.; Kuno, H.; Yoshizawa, F. Effects of two types of Coccomyxa sp. KJ on in vitro ruminal fermentation, methane production, and the rumen microbiota. PLoS ONE 2024, 19, e0308646. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhu, S.L.; Tang, Y.F.; Gu, F.F.; Valencak, T.G.; Liu, J.X.; Sun, H.Z. Age- and Microbiota-Dependent Cell Stemness Plasticity Revealed by Cattle Cell Landscape. Research 2023, 6, 0025. [Google Scholar] [CrossRef]
- Sardar, S.K.; Das, K.; Maruf, M.; Haldar, T.; Saito-Nakano, Y.; Kobayashi, S.; Dutta, S.; Ganguly, S. Molecular evidence suggests the occurrence of Entamoeba moshkovskii in pigs with zoonotic potential from eastern India. FOLIA Parasitol. 2022, 69, 012. [Google Scholar] [CrossRef]
- Sharma, D.; Gupta, S.; Sethi, K.; Kumar, S.; Kumar, R. Polymerase Spiral Reaction (PSR) as a novel rapid colorimetric isothermal point of care assay for detection of Trypanosoma evansi genomic DNA. Vet. Parasitol. 2022, 302, 109644. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Nonaka, N.; Nakao, R. Tick-borne diseases in Egypt: A one health perspective. ONE Health 2022, 15, 100443. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Z.; Kang, C.; Wei, J.Q.; Ma, H.; Liu, G.; Zhao, J.P.; Zhang, H.S.; Yang, X.B.; Wang, X.Y.; Yang, L.H.; et al. Meta-Analysis of the Prevalence of Giardia duodenalis in Cattle in China. Foodborne Pathog. Dis. 2023, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Nambuya, S.; Kalinda, C.; Vudriko, P.; Adriko, M.; Phiri, M.; Mindu, T.; Wagaba, D.; Mugisha, L. Meta-analysis and systematic review of the prevalence and risk factors of animal fascioliasis in Eastern and Southern Africa between 2000 and 2023. Prev. Vet. Med. 2025, 239, 106490. [Google Scholar] [CrossRef]
- Zhan, X.D.; Li, C.P.; Yang, B.H.; Zhu, Y.X.; Tian, Y.; Shen, J.; Zhao, J.H. Investigation on the zoonotic trematode species and their natural infection status in Huainan areas of China. Nutr. Hosp. 2017, 34, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Cardin, R.D.; Smith, G.A.; Pickard, G.E.; Sollars, P.J.; Dixon, D.A.; Pasula, R.; Bravo, F.J. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model. NPJ Vaccines 2020, 5, 104. [Google Scholar] [CrossRef]
- Fu, S.L.; Yuan, Y.Z.; Tian, X.Y.; Zhou, L.L.; Guo, L.; Zhang, D.; He, J.; Peng, C.; Qiu, Y.S.; Ye, C.; et al. Detection of Colistin Sulfate on Piglet Gastrointestinal Tract Microbiome Alterations. Vet. Sci. 2022, 9, 666. [Google Scholar] [CrossRef]
- Choi, Y.; Hosseindoust, A.; Ha, S.H.; Kim, J.; Min, Y.J.; Jeong, Y.; Mun, J.; Sa, S.; Kim, J. Effects of dietary supplementation of bacteriophage cocktail on health status of weanling pigs in a non-sanitary environment. J. Anim. Sci. Biotechnol. 2023, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhu, X.Y.; Sun, L.L.; Xie, C.L.; Wang, X.K.; Ma, L.B.; Yan, X.H. Akkermansia muciniphila Promotes SIgA Production and Alters the Reactivity Toward Commensal Bacteria in Early-Weaned Piglets. J. Nutr. 2025, 155, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Meng, M.L.; Qi, L.; Li, Y.Y.; Yao, H.Y. Environmental risks in swine biogas slurry-irrigated soils: A comprehensive analysis of antibiotic residues, resistome, and bacterial pathogens. Environ. Int. 2024, 191, 108954. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Raguideau, S.; Sirén, K.; Asnicar, F.; Cumbo, F.; Hildebrand, F.; Segata, N.; Cha, C.J.; Quince, C. Population-level impacts of antibiotic usage on the human gut microbiome. Nat. Commun. 2023, 14, 1191. [Google Scholar] [CrossRef]
- Swarthout, J.M.; Fuhrmeister, E.R.; Hamzah, L.; Harris, A.R.; Ahmed, M.A.; Gurley, E.S.; Satter, S.M.; Boehm, A.B.; Pickering, A.J. Differential Overlap in Human and Animal Fecal Microbiomes and Resistomes in Rural versus Urban Bangladesh. Appl. Environ. Microbiol. 2022, 88, e0075922. [Google Scholar] [CrossRef]
- Liu, Y.H.; Dyall-Smith, M.; Marenda, M.; Hu, H.W.; Browning, G.; Billman-Jacobe, H. Antibiotic Resistance Genes in Antibiotic-Free Chicken Farms. Antibiotics 2020, 9, 120. [Google Scholar] [CrossRef]
- Gehlot, P.; Hariprasad, P. Unveiling the ecological landscape of bacterial β-lactam resistance in Delhi-national capital region, India: An emerging health concern. J. Environ. Manag. 2024, 363, 121288. [Google Scholar] [CrossRef]
- Yuan, K.; Yu, K.; Yang, R.Q.; Zhang, Q.H.; Yang, Y.; Chen, E.Z.; Lin, L.; Luan, T.G.; Chen, W.; Chen, B.W. Metagenomic characterization of antibiotic resistance genes in Antarctic soils. Ecotoxicol. Environ. Saf. 2019, 176, 300–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Wang, M.Y.; Luo, M.; Peng, Y.; Li, Z.P.; Xu, J.L.; Ou, M.L.; Kan, B.; Li, X.; et al. The prevalence and distribution of aminoglycoside resistance genes. Biosaf. Health 2023, 5, 14–20. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, S.; An, Y.W.; Wang, Y.Q.; Lei, Y.; Song, L.Y. Antibiotics and antibiotic resistance genes in landfills: A review. Sci. TOTAL Environ. 2022, 806, 150647. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, T.; Fang, H. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, J.H.; Liu, X.W.; Zhan, X.M.; Chen, Q.C. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Res. 2014, 58, 248–257. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, J.; Chen, X. Comparative Profiling of Antibiotic Resistance Genes and Microbial Communities in Pig and Cow Dung from Rural China: Insights into Environmental Dissemination and Public Health Risks. Biology 2025, 14, 1623. https://doi.org/10.3390/biology14111623
Wang H, Guo J, Chen X. Comparative Profiling of Antibiotic Resistance Genes and Microbial Communities in Pig and Cow Dung from Rural China: Insights into Environmental Dissemination and Public Health Risks. Biology. 2025; 14(11):1623. https://doi.org/10.3390/biology14111623
Chicago/Turabian StyleWang, Haifeng, Juan Guo, and Xing Chen. 2025. "Comparative Profiling of Antibiotic Resistance Genes and Microbial Communities in Pig and Cow Dung from Rural China: Insights into Environmental Dissemination and Public Health Risks" Biology 14, no. 11: 1623. https://doi.org/10.3390/biology14111623
APA StyleWang, H., Guo, J., & Chen, X. (2025). Comparative Profiling of Antibiotic Resistance Genes and Microbial Communities in Pig and Cow Dung from Rural China: Insights into Environmental Dissemination and Public Health Risks. Biology, 14(11), 1623. https://doi.org/10.3390/biology14111623




