Dietary Guanidinoacetic Acid Supplementation Improves Growth Performance of Plateau Yaks Through Plasma Metabolome Modulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Animals and Experimental Design
2.3. Growth Performance
2.4. Blood Sample Collection and Processing
2.5. Serum Biochemical Parameters
2.6. Serum Antioxidant Parameters
2.7. Metabolome Sequencing Data Processing and Analysis
2.8. Data Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Parameters
3.3. Serum Antioxidant Parameters
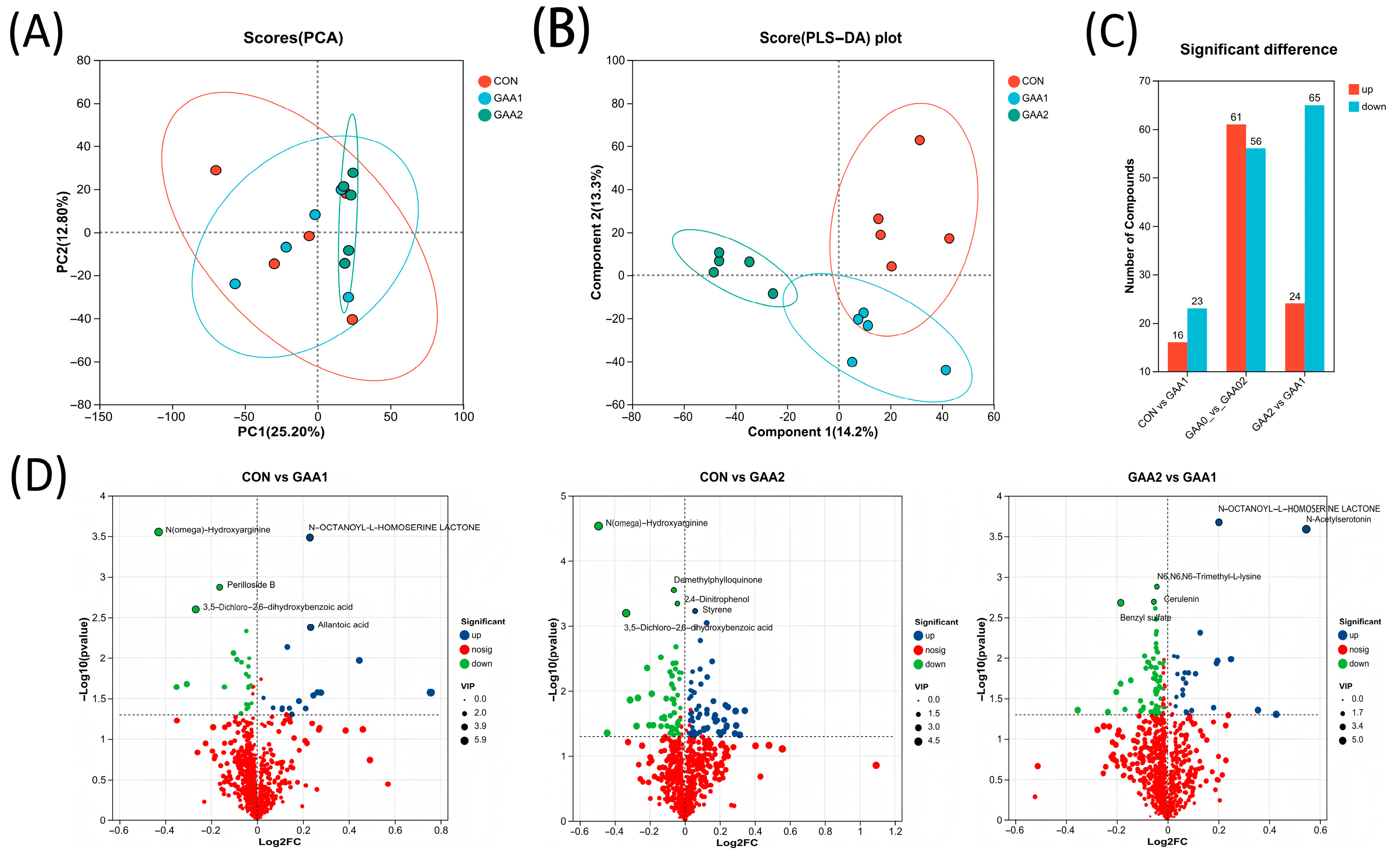
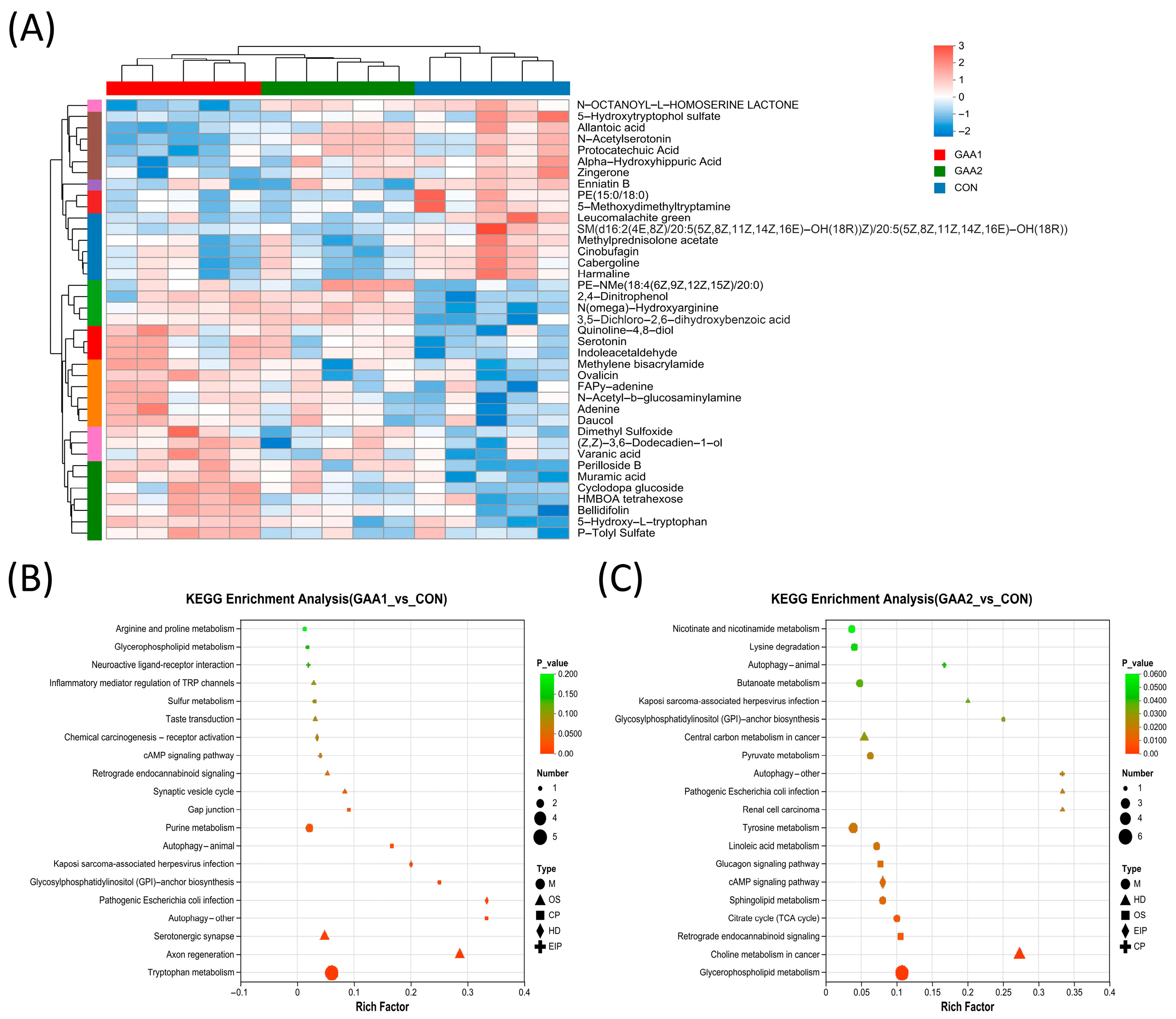
3.4. Plasma Metabolic Profile
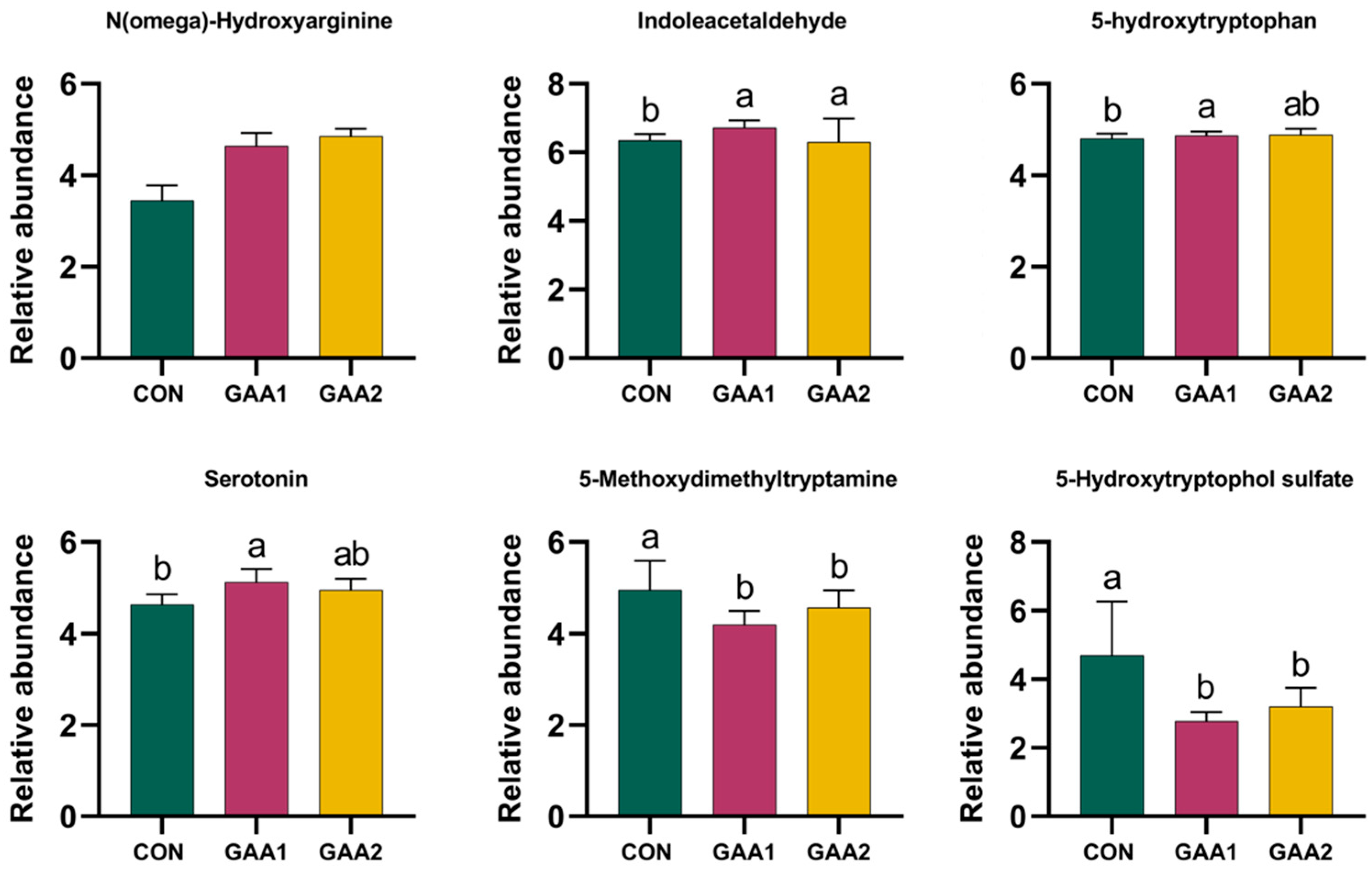
3.5. Critical Plasma Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jing, X.; Ding, L.; Zhou, J.; Huang, X.; Degen, A.; Long, R. The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. 2022, 9, 249–258. [Google Scholar] [CrossRef]
- Kang, K.; Ma, J.; Wang, H.; Wang, Z.; Peng, Q.; Hu, R.; Zou, H.; Bao, S.; Zhang, W.; Sun, B. High-energy diet improves growth performance, meat quality and gene expression related to intramuscular fat deposition in finishing yaks raised by barn feeding. Vet. Med. Sci. 2020, 6, 755–765. [Google Scholar] [CrossRef]
- Ding, X.Z.; Guo, X.; Yan, P.; Liang, C.N.; Bao, P.J.; Chu, M. Seasonal and nutrients intake regulation of lipoprotein lipase (LPL) activity in grazing yak (Bos grunniens) in the Alpine Regions around Qinghai Lake. Livest. Sci. 2012, 143, 29–34. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Portocarero, N.; Braun, U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021, 100, 101203. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Wang, X.F.; Zhu, X.D.; Gao, F.; Zhou, G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019, 98, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zou, T.; Wang, Z.; Yang, J.; Li, L.; Guo, X.; He, Q.; Chen, L.; You, J. Dietary guanidinoacetic acid improves the growth performance and skeletal muscle development of finishing pigs through changing myogenic gene expression and myofibre characteristics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1875–1883. [Google Scholar] [CrossRef]
- Ardalan, M.; Miesner, M.D.; Reinhardt, C.D.; Thomson, D.U.; Armendariz, C.K.; Smith, J.S.; Titgemeyer, E.C. Effects of guanidinoacetic acid supplementation on nitrogen retention and methionine flux in cattle. J. Anim. Sci. 2021, 99, skab172. [Google Scholar] [CrossRef]
- Jayaraman, B.; La, K.V.; La, H.; Doan, V.; Carpena, E.M.; Rademacher, M.; Channarayapatna, G. Supplementation of guanidinoacetic acid to pig diets: Effects on performance, carcass characteristics, and meat quality. J. Anim. Sci. 2018, 96, 2332–2341. [Google Scholar] [CrossRef]
- Córdova-Noboa, H.A.; Oviedo-Rondón, E.O.; Sarsour, A.H.; Barnes, J.; Ferzola, P.; Rademacher-Heilshorn, M.; Braun, U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poult. Sci. 2018, 97, 2479–2493. [Google Scholar] [CrossRef]
- Zhou, J.; Yue, S.; Peng, Q.; Wang, L.; Wang, Z.; Xue, B. Metabonomic Responses of Grazing Yak to Different Concentrate Supplementations in Cold Season. Animals 2020, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.C.; Zhang, J.X.; Wang, Z.S.; Wang, L.Z.; Peng, Q.H.; Da, L.C.; Bao, S.K.; Kong, X.Y.; Xue, B. Metabolism response of grazing yak to dietary concentrate supplementation in warm season. Animal 2021, 15, 100175. [Google Scholar] [CrossRef]
- Yi, S.; Ye, B.; Wang, J.; Yi, X.; Wang, Y.; Abudukelimu, A.; Wu, H.; Meng, Q.; Zhou, Z. Investigation of guanidino acetic acid and rumen-protected methionine induced improvements in longissimus lumborum muscle quality in beef cattle. Meat Sci. 2024, 217, 109624. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yimamu, M.; Zhang, S.; Shah, A.M.; Yang, H.; Cai, W.; Li, C.; Lu, X.; Li, F.; Yang, K. Effects of guanidino acetic acid and betaine supplementation on growth, dietary nutrient digestion and intestinal creatine metabolism in sheep. Vet. Med. Sci. 2024, 10, e1470. [Google Scholar] [CrossRef]
- Yi, S.; Wang, J.; Ye, B.; Yi, X.; Abudukelimu, A.; Wu, H.; Meng, Q.; Zhou, Z. Guanidinoacetic Acid and Methionine Supplementation Improve the Growth Performance of Beef Cattle via Regulating the Antioxidant Levels and Protein and Lipid Metabolisms in Serum and Liver. Antioxidants 2025, 14, 559. [Google Scholar] [CrossRef]
- Yan, Z.; Yan, Z.; Liu, S.; Yin, Y.; Yang, T.; Chen, Q. Regulative Mechanism of Guanidinoacetic Acid on Skeletal Muscle Development and Its Application Prospects in Animal Husbandry: A Review. Front. Nutr. 2021, 8, 714567. [Google Scholar] [CrossRef] [PubMed]
- Majdeddin, M.; Golian, A.; Kermanshahi, H.; de Smet, S.; Michiels, J. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Br. Poult. Sci. 2018, 59, 443–451. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, C.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Zhang, Y.L.; Pei, C.X.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef]
- Potischman, N.; Freudenheim, J.L. Biomarkers of nutritional exposure and nutritional status: An overview. J. Nutr. 2003, 133, 873S–874S. [Google Scholar] [CrossRef]
- Giraldi, G.C.; Wolschick, G.J.; Signor, M.H.; Lago, R.V.P.; de Souza Muniz, A.L.; Draszevski, T.M.R.; Balzan, M.M.; Wagner, R.; da Silva, A.S. Effects of Dietary Guanidinoacetic Acid on the Performance, Rumen Fermentation, Metabolism, and Meat of Confined Steers. Animals 2024, 14, 2617. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Jin, H.; Du, Z.; Fan, X.; Qin, L.; Liu, W.; Zhang, Y.; Ren, J.; Ye, C.; Liu, Q. Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep. Animals 2024, 14, 2052. [Google Scholar] [CrossRef]
- Zheng, J.; Du, M.; Zhang, J.; Liang, Z.; Ahmad, A.A.; Shen, J.; Salekdeh, G.H.; Ding, X. Transcriptomic and Metabolomic Analyses Reveal Inhibition of Hepatic Adipogenesis and Fat Catabolism in Yak for Adaptation to Forage Shortage During Cold Season. Front. Cell Dev. Biol. 2021, 9, 759521. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Jorga, J. Guanidinoacetic acid in human nutrition: Beyond creatine synthesis. Food Sci. Nutr. 2023, 11, 1606–1611. [Google Scholar] [CrossRef]
- Litty, F.-A.; Gudd, J.; Girreser, U.; Clement, B.; Schade, D. Design, Synthesis, and Bioactivation of O-Glycosylated Prodrugs of the Natural Nitric Oxide Precursor N(ω)-Hydroxy-l-arginine. J. Med. Chem. 2016, 59, 8030–8041. [Google Scholar] [CrossRef]
- Tong, B.C.; Barbul, A. Cellular and physiological effects of arginine. Mini Rev. Med. Chem. 2004, 4, 823–832. [Google Scholar] [CrossRef]
- Švob Štrac, D.; Pivac, N.; Mück-Šeler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Shang, H.; Huang, C.; Xiao, Z.; Yang, P.; Zhang, S.; Hou, X.; Zhang, L. Gut microbiota-derived tryptophan metabolites alleviate liver injury via AhR/Nrf2 activation in pyrrolizidine alkaloids-induced sinusoidal obstruction syndrome. Cell Biosci. 2023, 13, 127. [Google Scholar] [CrossRef]
- Beck, O.; Helander, A. 5-hydroxytryptophol as a marker for recent alcohol intake. Addiction 2003, 98 (Suppl. 2), 63–72. [Google Scholar] [CrossRef]
- Valente, E.E.L.; Klotz, J.L.; Markmann, R.C.; Edwards, J.L.; Harmon, D.L. 5-hydroxytryphophan mitigates ergot alkaloid-induced suppression of serotonin and feed intake in cattle. J. Anim. Sci. 2024, 102, skae083. [Google Scholar] [CrossRef]
| Diet Ingredients | Contents, % |
|---|---|
| Corn | 40.15 |
| Wheat bran | 2.75 |
| Soybean | 8.80 |
| Urea | 0.22 |
| NaCl | 0.44 |
| Premix 1 | 2.64 |
| Whole maize silage | 45.00 |
| Total | 100.00 |
| Nutrient component 2 | |
| NEmf, MJ/kg | 6.35 |
| Crude protein, % | 12.88 |
| Neutral detergent fiber, % | 24.98 |
| Acid detergent fiber, % | 8.61 |
| Crude ash, % | 5.31 |
| Total calcium, % | 0.46 |
| Phosphorus, % | 0.40 |
| Item 2 | Treatments 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | GAA1 | GAA2 | |||
| IBW (kg) | 250.44 | 248.13 | 249.56 | 2.28 | 0.924 |
| FBW (kg) | 310.31 | 314.63 | 324.13 | 2.93 | 0.144 |
| ADG (kg/d) | 0.67 | 0.74 | 0.83 | 0.03 | 0.072 |
| F/G | 10.10 | 8.42 | 7.41 | 0.35 | 0.235 |
| DMI (kg/d) | 6.29 | 6.69 | 6.84 | 0.17 | 0.179 |
| Item 2 | Treatments 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | GAA1 | GAA2 | |||
| TP (g/L) | 88.80 | 92.46 | 90.26 | 1.32 | 0.557 |
| ALB (g/L) | 39.63 | 41.24 | 40.76 | 0.91 | 0.787 |
| GLB (g/L) | 49.16 | 51.22 | 49.50 | 1.07 | 0.734 |
| ALP (U/L) | 239.20 | 203.60 | 263.60 | 15.25 | 0.290 |
| GLU (mmol/L) | 5.94 | 5.10 | 4.93 | 0.30 | 0.364 |
| TC (mmol/L) | 1.48 | 2.25 | 2.22 | 0.21 | 0.246 |
| TG (mmol/L) | 0.35 | 0.38 | 0.32 | 0.03 | 0.778 |
| BUN (mmol/L) | 3.23 | 4.03 | 3.95 | 0.21 | 0.234 |
| Item 2 | Treatments 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | GAA1 | GAA2 | |||
| T-AOC (mM) | 0.21 | 0.18 | 0.20 | 0.02 | 0.765 |
| GSH-Px (μmol/L) | 211.83 | 124.70 | 126.26 | 41.25 | 0.087 |
| T-SOD (U/mL) | 239.10 | 253.57 | 238.47 | 14.96 | 0.562 |
| MDA (nmol/mL) | 3.07 | 3.17 | 3.20 | 0.37 | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Zhang, L.; Fu, L.; Dong, X.; Peng, Z.; Zeng, Y.; Wang, G.; Chen, J.; Gao, Y.; Zhou, J. Dietary Guanidinoacetic Acid Supplementation Improves Growth Performance of Plateau Yaks Through Plasma Metabolome Modulation. Biology 2025, 14, 1600. https://doi.org/10.3390/biology14111600
You Y, Zhang L, Fu L, Dong X, Peng Z, Zeng Y, Wang G, Chen J, Gao Y, Zhou J. Dietary Guanidinoacetic Acid Supplementation Improves Growth Performance of Plateau Yaks Through Plasma Metabolome Modulation. Biology. 2025; 14(11):1600. https://doi.org/10.3390/biology14111600
Chicago/Turabian StyleYou, Yinjie, Li Zhang, Lin Fu, Xianwen Dong, Zhongli Peng, Yu Zeng, Gaofu Wang, Juncai Chen, Yanhua Gao, and Jia Zhou. 2025. "Dietary Guanidinoacetic Acid Supplementation Improves Growth Performance of Plateau Yaks Through Plasma Metabolome Modulation" Biology 14, no. 11: 1600. https://doi.org/10.3390/biology14111600
APA StyleYou, Y., Zhang, L., Fu, L., Dong, X., Peng, Z., Zeng, Y., Wang, G., Chen, J., Gao, Y., & Zhou, J. (2025). Dietary Guanidinoacetic Acid Supplementation Improves Growth Performance of Plateau Yaks Through Plasma Metabolome Modulation. Biology, 14(11), 1600. https://doi.org/10.3390/biology14111600






