Cardiac–Metabolic Coupling Revealed by Lipid and Energy Metabolomics Determines 80 km Endurance Performance in Yili Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
2.2. Study Design and Blood Sample Collection
2.3. Sample Preparation
2.4. Lipidomics Chromatography and Mass Spectrometry Analysis
- (1)
- Column: Thermo Accucore™ C30 column (2.6 μm, 2.1 mm × 100 mm i.d.);
- (2)
- Mobile phases: Phase A: acetonitrile/water (60:40, v/v) containing 0.1% formic acid and 10 mM ammonium formate; Phase B: acetonitrile/isopropanol (10:90, v/v) containing 0.1% formic acid and 10 mM ammonium formate;
- (3)
- Gradient elution program: 0 min, A/B = 80:20 (v/v); 2 min, 70:30; 4 min, 40:60; 9 min, 15:85; 14 min, 10:90; 15.5 min, 5:95; 17.3 min, 5:95; 17.5 min, 80:20; 20 min, 80:20 (v/v);
- (4)
- Flow rate: 0.35 mL/min; column temperature: 45 °C; injection volume: 2 μL.
2.5. Plasma Energy Metabolites
2.5.1. Sample Preparation
2.5.2. Chromatography and Mass Spectrometry Acquisition Conditions
2.6. Data Analysis
3. Results and Analysis
3.1. Differences in Athletic Performance and Cardiac Structure and Function
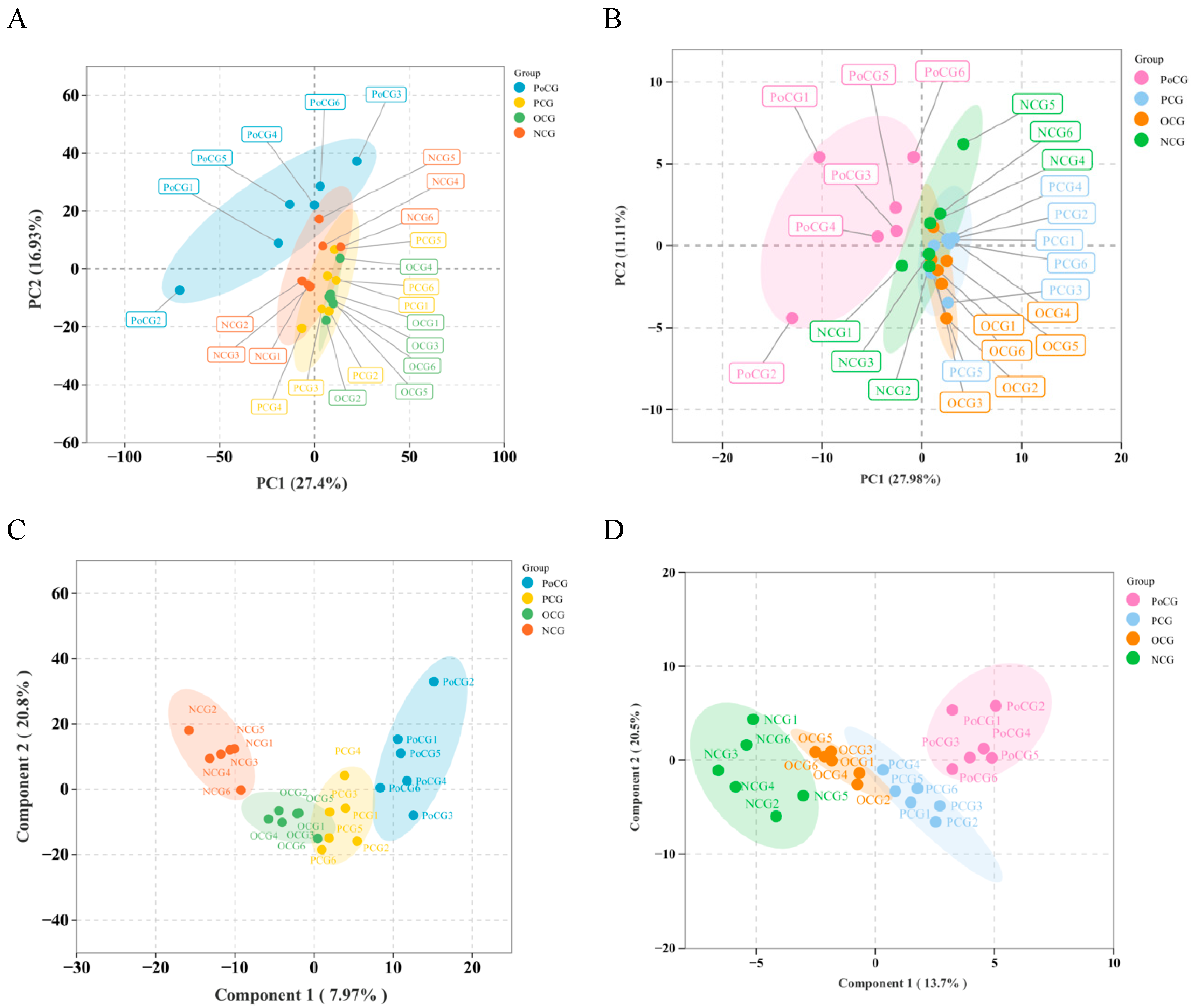
3.2. PCA and OPLS Analysis
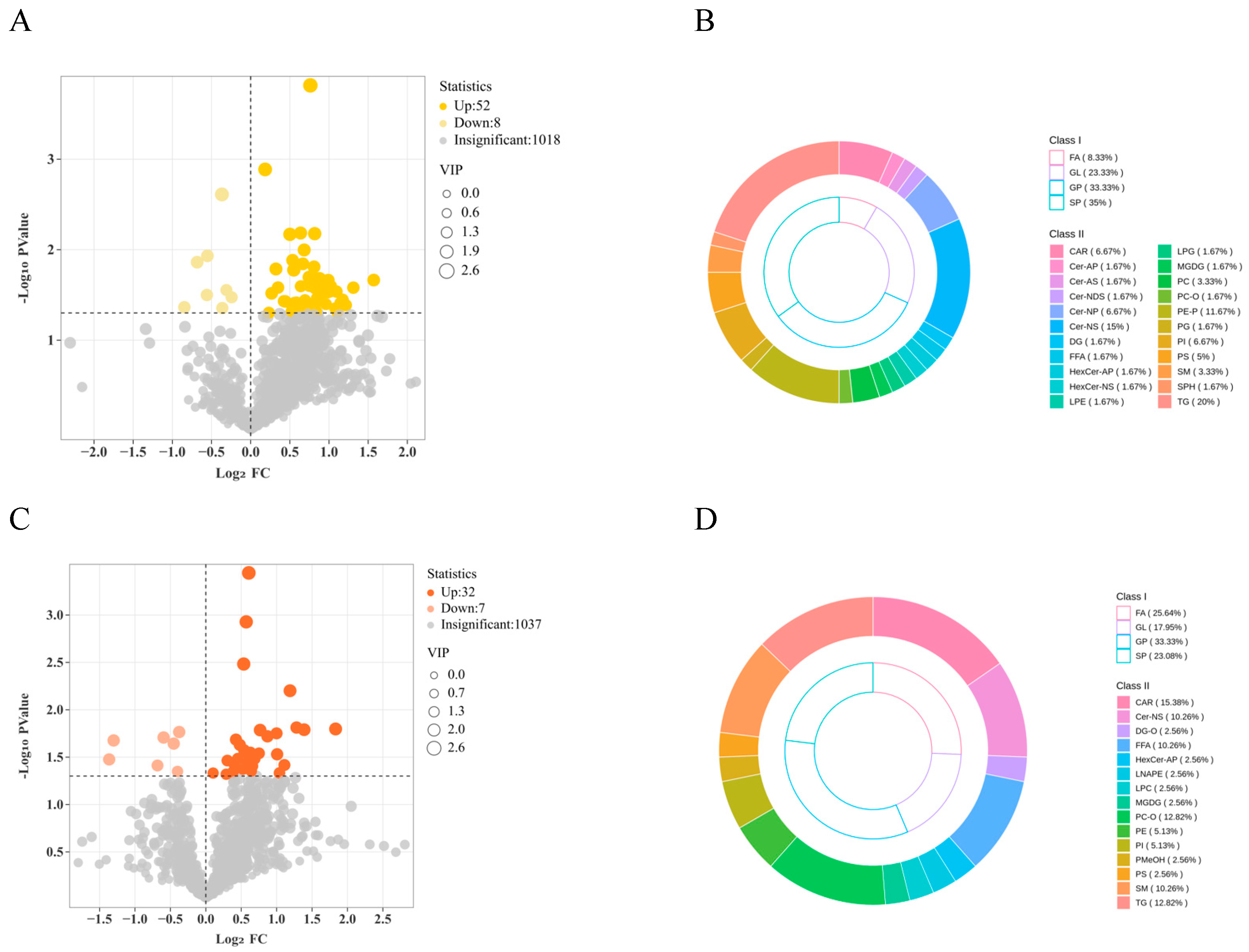
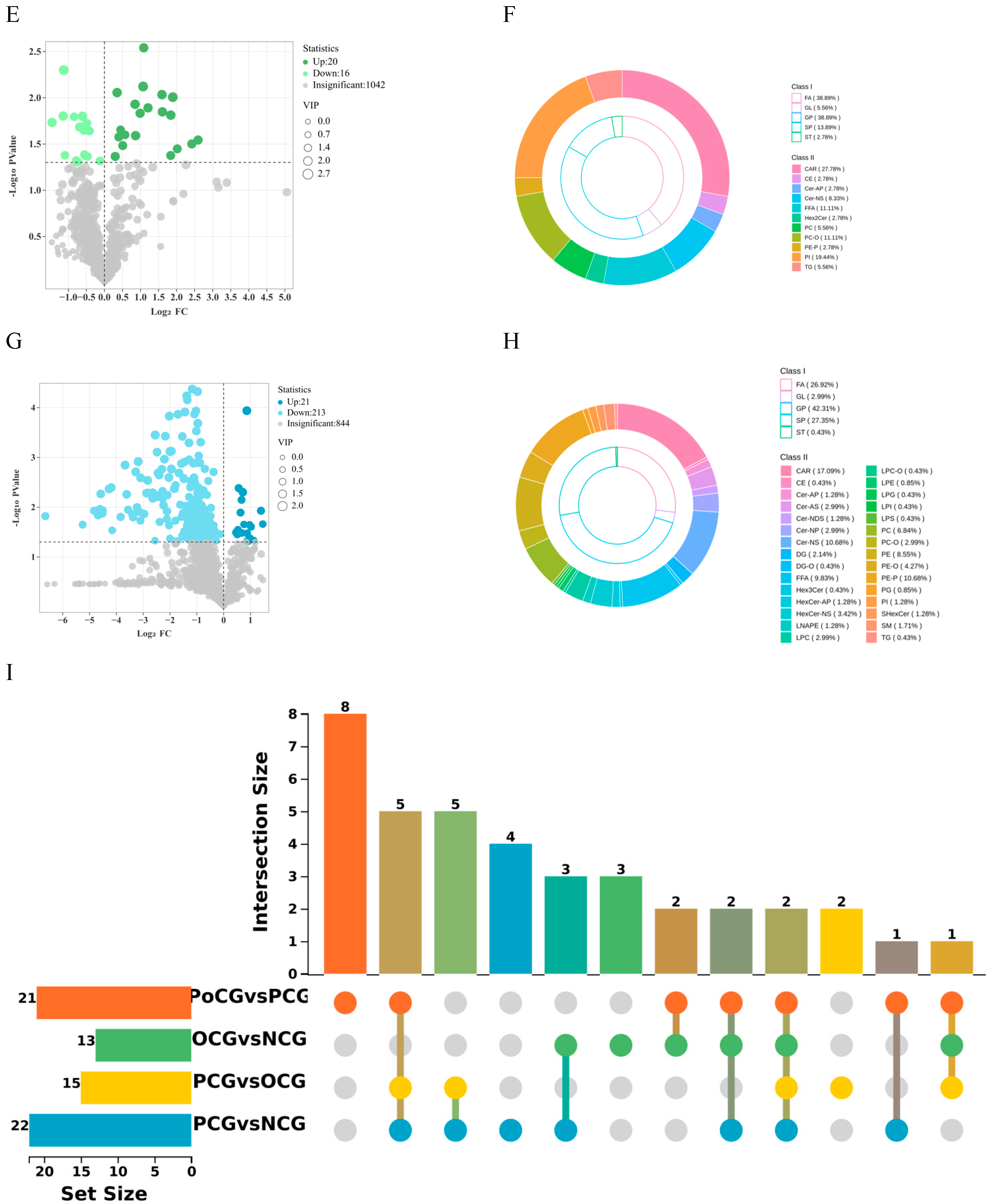
3.3. Volcano Plots of Differential Lipid Metabolites
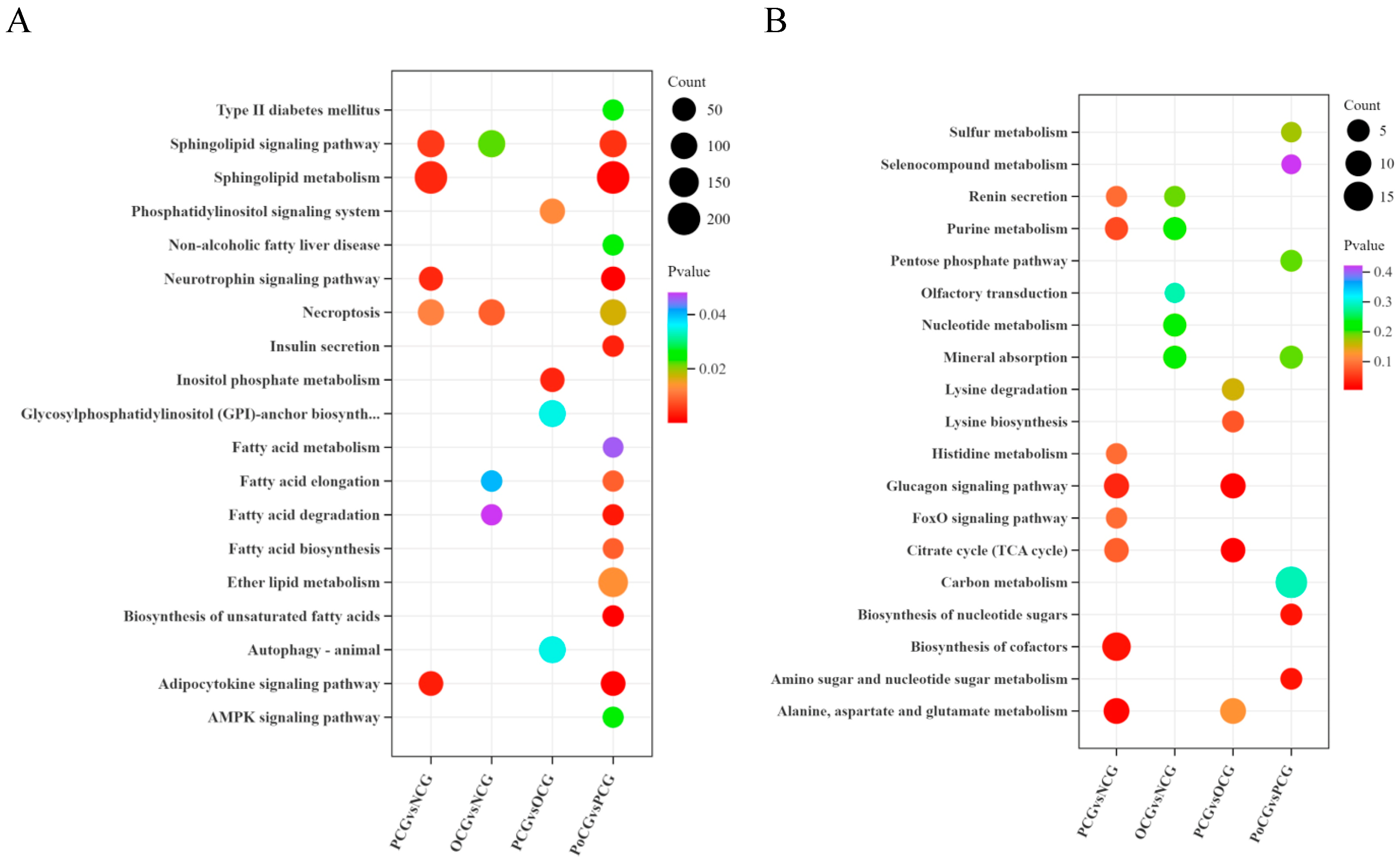
3.4. Enrichment Analysis of Differential Lipid and Energy Metabolites
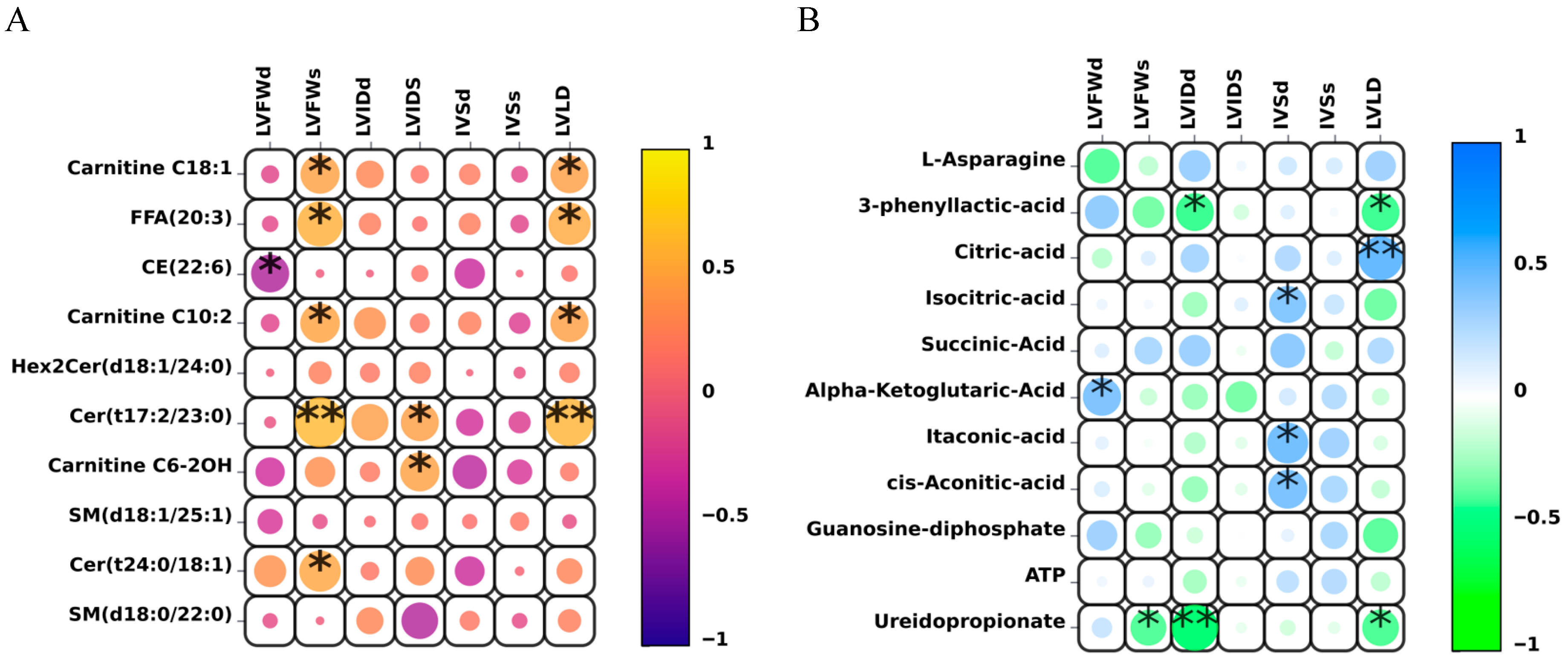
3.5. Correlation Analysis Between Core Quantitative Differential Lipid and Energy Metabolites and Key Left Ventricular Cardiac Indicators
4. Discussion
4.1. Influence of Cardiac Function on Endurance Performance
4.2. Lipid Metabolites
4.2.1. Analysis of Differential Glycerides (GLs)
4.2.2. Analysis of Differential Fatty Acyls (FAs)
4.2.3. Analysis of Differential Sphingolipids (SPs)
4.2.4. Analysis of Energy Metabolites
4.3. Effects of Plasma Metabolic Mechanisms in Yili Horses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, J.M.; Douglas, J.; Davies, E.; Bloom, E. Performance demands in the endurance rider. Comp. Exerc. Physiol. 2021, 17, 199–218. [Google Scholar] [CrossRef]
- Wang, T.; Meng, J.; Peng, X.; Huang, J.; Huang, Y.; Yuan, X.; Li, X.; Yang, X.; Chang, X.; Zeng, Y.; et al. Metabolomics analysis and mRNA/miRNA profiling reveal potential cardiac regulatory mechanisms in Yili racehorses under different training regimens. PLoS ONE 2025, 20, e0322468. [Google Scholar] [CrossRef]
- Rodriguez-López, A.M.; Javier, G.; Carmen, P.; Esteban, P.; Luisa, G.C.; Tomas, F.; Josefa, H.M.; Luis, F. Athlete heart in children and young athletes. Echocardiographic Findings in 331 Cases. Pediatr. Cardiol. 2022, 43, 407–412. [Google Scholar] [CrossRef]
- Young, L.; Rogers, K.; Wood, J. Leftventricular size and systolic function in Thoroughbredracehorses and their relationships to race performance. J. Appl. Physiol. 2005, 99, 1278–1285. [Google Scholar] [CrossRef]
- Williams, J.M.; Douglas, J.; Davies, E.; Chen, F.; Ni, C.; Wang, X.; Cheng, R.; Pan, C.; Wang, Y.; Liang, J.; et al. S1P defects cause a new entity of cataract, alopecia, oral mucosal disorder, and psoriasis-like syndrome. EMBO Mol. Med. 2022, 14, e14904. [Google Scholar]
- Wong, A.; Sun, Q.; Latif, I.I.; Karwi, Q.G. Macrophage energy metabolism in cardiometabolic disease. Mol. Cell. Biochem. 2025, 480, 1763–1783. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Waśkiewicz, Z.; Nikolaidis, P.T.; Mikołajczyk, R.; Kawecki, D.; Rosemann, T.; Knechtle, B. Acute responses of novel cardiac biomarkers to a 24-h ultra-marathon. J. Clin. Med. 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Stasiulis, A.; Raugaliene, R. Concentric myocardial hypertrophy after one year of increased training volume in experienced distance runners. Br. J. Sports Med. 2006, 40, 706–709. [Google Scholar] [CrossRef]
- Ellison, G.M.; Waring, C.D.; Vicinanza, C.; Torella, D. Physiological cardiac remodelling in response to endurance exercise training: Cellular and molecular mechanisms. Heart 2012, 98, 5–10. [Google Scholar] [CrossRef]
- Sleeper, M.M.; Durando, M.M.; Holbrook, T.C.; Payton, M.E.; Birks, E.K. Comparison of echocardiographic measurements in elite and nonelite Arabian endurance horses. Am. J. Vet. Res. 2014, 75, 893–898. [Google Scholar] [CrossRef]
- Schweitzer, R.; de Marvao, A.; Shah, M.; Inglese, P.; Kellman, P.; Berry, A.; Statton, B.; O’Regan, D.P. Establishing cardiac MRI reference ranges stratified by sex and age for cardiovascular function during exercise. Radiol. Cardiothorac. Imaging 2025, 7, e240175. [Google Scholar] [CrossRef]
- Vernemmen, I.; Vera, L.; Van Steenkiste, G.; van Loon, G.; Decloedt, A. Reference values for 2-dimensional and M-mode echocardiography in Friesian and Warmblood horses. J. Vet. Intern. Med. 2020, 34, 2701–2709. [Google Scholar] [CrossRef]
- Young, L.E.; Marlin, D.J.; Deaton, C.; Brown-Feltner, H.; Roberts, C.A.; Wood, J.L. Heart size estimated by echocardiography correlates with maximal oxygen uptake. Equine Vet. J. 2010, 34, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Endurance sport and cardiovascular health. BMC Sports Sci. Med. Rehabil. 2015, 7 (Suppl. S1), O13. [Google Scholar] [CrossRef]
- Wang, T.; Meng, J.; Yang, X.; Zeng, Y.; Yao, X.; Ren, W. Differential metabolomics and cardiac function in trained vs. untrained Yili performance horses. Animals 2025, 15, 2444. [Google Scholar] [CrossRef]
- Burtscher, J.; Strasser, B.; Burtscher, M.; Millet, G.P. The impact of training on the loss of cardiorespiratory fitness in aging masters endurance athletes. Int. J. Environ. Res. Public Health 2022, 19, 11050. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, H.; Keithahn, A.; Hertel, G.; Drexel, V.; Stern, H.; Schuster, T.; Lorang, D.; Beer, A.J.; Schmidt-Trucksass, A.; Nickel, T.; et al. Magnetic resonance imaging of myocardial injury and ventricular torsion after marathon running. Clin. Sci. 2011, 120, 143–152. [Google Scholar] [CrossRef]
- Ball, D. Metabolic and endocrine response to exercise: Sympathoadrenal integration with skeletal muscle. J. Endocrinol. 2015, 224, R79–R95. [Google Scholar] [CrossRef] [PubMed]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolontà, V.; Fischetti, F. The Importance of lipidomic approach for mapping and exploring the molecular networks underlying physical exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef]
- Le Moyec, L.; Robert, C.; Triba, M.N.; Billat, V.L.; Mata, X.; Schibler, L.; Barrey, E. Protein catabolism and high lipid metabolism associated with long-distance exercise are revealed by plasma NMR metabolomics in endurance horses. PLoS ONE 2017, 9, e90730. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Suemitsu, H.; Kasai, M.; Kato, K.; Taguchi, M. Medium-chain triglycerides with maltodextrin increase fat oxidation during moderate-intensity exercise and extend the duration of subsequent high-intensity exercise. J. Oleo Sci. 2018, 67, 1455–1462. [Google Scholar] [CrossRef]
- Getzin, A.R.; Milner, C.; LaFace, K.M. Nutrition update for the ultraendurance athlete. Curr. Sports Med. Rep. 2011, 10, 330–339. [Google Scholar] [CrossRef]
- Soria, M.; Ansón, M.; Lou-Bonafonte, J.M.; Andrés-Otero, M.J.; Puente, J.J.; Escanero, J. Fat oxidation rate as a function of plasma lipid and hormone response in endurance athletes. J. Strength Cond. Res. 2020, 34, 104–113. [Google Scholar] [CrossRef]
- Kim, J.; Lim, K. Relationship between FAT/CD36 protein in skeletal muscle and whole-body fat oxidation in endurance-trained mice. J. Exerc. Nutr. Biochem. 2016, 20, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Girona, J.; Soler, O.; Samino, S.; Junza, A.; Martinez-Micaelo, N.; Garcia-Altares, M.; Rafols, P.; Esteban, Y.; Yanes, O.; Correig, X.; et al. Lipidomics reveals myocardial lipid composition in a murine model of insulin resistance induced by a high-fat diet. Int. J. Mol. Sci. 2024, 25, 2702. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef]
- Baranowski, M.; Zabielski, P.; Blachnio, A.; Gorski, J. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol. 2008, 192, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhu, S.; Wu, J.; Zhang, M.; Xu, Y.; Xu, W.; Cui, J.; Yu, B.; Cao, W.; Liu, J.; et al. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasis in the development of diabetic cardiomyopathy. J. Mol. Med. 2020, 98, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Martland, R.; Mondelli, V.; Gaughran, F.; Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 2020, 38, 430–469. [Google Scholar] [CrossRef]
- Fontani, G.; Corradeschi, F.; Felici, A.; Alfatti, F.; Migliorini, S.; Lodi, L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Investig. 2005, 35, 691–699. [Google Scholar] [CrossRef]
- Meienberg, F.; Loher, H.; Bucher, J.; Jenni, S.; Krüsi, M.; Kreis, R.; Boesch, C.; Betz, M.J.; Christ, E. The effect of exercise on intramyocellular acetylcarnitine (AcCtn) concentration in adult growth hormone deficiency (GHD). Sci. Rep. 2019, 9, 19431. [Google Scholar] [CrossRef]
- Zeng, Q.; Isobe, K.; Fu, L.; Ohkoshi, N.; Ohmori, H.; Takekoshi, K.; Kawakami, Y. Effects of exercise on adiponectin and adiponectin receptor levels in rats. Life Sci. 2006, 10, 454–459. [Google Scholar] [CrossRef]
- Kriketos, A.D.; Gan, S.K.; Poynten, A.M.; Furler, S.M.; Chisholm, D.J.; Campbell, L.V. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care 2004, 27, 629–630. [Google Scholar] [CrossRef]
- Kondo, T.; Kobayashi, I.; Murakami, M. Effect of exercise on circulating adipokine levels in obese young women. Endocr. J. 2006, 53, 189–195. [Google Scholar] [CrossRef]
- Rodrigues, A.; De Lucca, L. Acute leptin response after high intensity interval and moderate intensity continuous runs. Eur. J. Hum. Mov. 2020, 45. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. The physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. 2015, 45 (Suppl. S1), 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rustad, P.I.; Sailer, M.; Cumming, K.T.; Jeppesen, P.B.; Kolnes, K.J.; Sollie, O.; Franch, J.; Ivy, J.L.; Daniel, H.; Jensen, J.; et al. Intake of protein plus carbohydrate during the first two hours after exhaustive cycling improves performance the following day. PLoS ONE 2016, 11, e0153229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bian, R.; Xu, X.; Zhang, J.; Zhang, L.; Zheng, Y. Sphingolipid Metabolism and signalling pathways in heart failure: From molecular mechanism to therapeutic potential. J. Inflamm. Res. 2025, 18, 5477–5498. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar]
- Błachnio-Zabielska, A.; Zabielski, P.; Baranowski, M.; Gorski, J. Aerobic training in rats increases skeletal muscle sphingomyelinase and serine palmitoyltransferase activity, while decreasing ceramidase activity. Lipids 2011, 46, 229–238. [Google Scholar] [CrossRef] [PubMed]
| Indicator | Excellent Group | Ordinary Group | Poor Group |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Body weight (kg) | 376.92 ± 5 | 381.19 ± 6.92 | 369.21 ± 23.17 |
| Body surface area (m2) | 5.25 ± 0.05 | 5.29 ± 0.06 | 5.18 ± 0.22 |
| Width height (cm) | 150.33 ± 1.97 | 149.83 ± 4.62 | 148.83 ± 3.19 |
| Body length (cm) | 153.17 ± 1.6 | 151 ± 4.38 | 151.33 ± 4.63 |
| Thoracic circumference (cm) | 168.08 ± 0.67 | 167.83 ± 2.99 | 167 ± 4.2 |
| Circumference of cannon bone (cm) | 18.78 ± 0.68 | 19.58 ± 0.92 | 18.95 ± 0.85 |
| Chest depth (cm) | 63.58 ± 1.66 | 62.25 ± 0.69 | 62.58 ± 1.66 |
| Chest width (cm) | 38.42 ± 1.53 | 36.42 ± 2.46 | 36.33 ± 2.02 |
| Indicator | PCG | OCG | NCG |
|---|---|---|---|
| LVFWd (cm) | 2.15 ± 0.31 | 2.35 ± 0.38 | 2.31 ± 0.29 |
| LVFWs (cm) | 4.32 ± 0.21 Aa | 4.01 ± 0.28 ABb | 3.69 ± 0.25 Bc |
| LVIDd (cm) | 10.56 ± 0.34 Aa | 10.19 ± 0.17 ABb | 10.1 ± 0.09 Bb |
| LVIDs (cm) | 5.66 ± 0.14 | 5.44 ± 0.34 | 5.58 ± 0.25 |
| IVSd (cm) | 2.83 ± 0.39 | 2.72 ± 0.42 | 2.88 ± 0.43 |
| IVSs (cm) | 4.15 ± 0.84 | 4.53 ± 0.34 | 4.53 ± 0.4 |
| RVDd (cm) | 3 ± 0.49 | 2.74 ± 0.38 | 2.84 ± 0.83 |
| RVDs (cm) | 1.79 ± 0.28 | 1.76 ± 0.2 | 1.73 ± 0.77 |
| LVLD (cm) | 15.94 ± 0.42 Aa | 15.25 ± 0.27 Bb | 14.69 ± 0.48 Bc |
| MVD (cm) | 9.75 ± 0.4 | 9.77 ± 0.57 | 9.62 ± 0.62 |
| LADd (cm) | 9.87 ± 0.49 a | 9.11 ± 0.54 b | 8.96 ± 0.66 b |
| LADs (cm) | 11.46 ± 0.42 a | 10.44 ± 0.59 b | 11.04 ± 1.18 |
| AODd (cm) | 6.09 ± 0.33 a | 5.64 ± 0.27 b | 5.71 ± 0.24 b |
| AODs (cm) | 6.89 ± 0.51 | 6.75 ± 0.8 | 6.53 ± 1.2 |
| PADd (cm) | 4.47 ± 0.38 | 4.95 ± 0.46 | 4.82 ± 0.47 |
| PADs (cm) | 5.56 ± 0.49 | 6.08 ± 0.35 | 5.62 ± 0.64 |
| MWTd (cm) | 2.49 ± 0.19 | 2.53 ± 0.25 | 2.6 ± 0.28 |
| RWTd (cm) | 0.47 ± 0.04 | 0.5 ± 0.05 | 0.51 ± 0.06 |
| LVM (g) | 2671.04 ± 285.5 | 2593.37 ± 367.64 | 2646.66 ± 394.5 |
| Heart rate | 35.29 ± 1.42 | 35.09 ± 3.22 | 38.04 ± 5.35 |
| EDV (mL) | 656.37 ± 45.08 Aa | 602.18 ± 34.94 ABb | 581.78 ± 45.63 Bb |
| ESV (mL) | 186.47 ± 11.19 Aa | 207.87 ± 11.49 ABb | 214.76 ± 23.4 Bb |
| EF (%) | 0.72 ± 0.02 Aa | 0.65 ± 0.02 ABb | 0.63 ± 0.06 Bb |
| ET | 0.59 ± 0.02 | 0.58 ± 0.05 | 0.63 ± 0.09 |
| SV (mL) | 469.9 ± 41.34 Aa | 394.31 ± 34.59 ABb | 367.02 ± 59.1 Bb |
| SI | 89.51 ± 7.68 Aa | 74.59 ± 6.96 ABb | 70.96 ± 11.28 Bb |
| CO (mL/min) | 16,571.67 ± 1487.14 | 13,878.4 ± 2170.94 | 14,134.92 ± 3765.31 |
| CI | 3156.5 ± 273.12 | 2626.25 ± 427.28 | 2726.25 ± 692.57 |
| FS% | 48.43 ± 4.23 | 44.5 ± 1.83 | 44.78 ± 2.26 |
| VTI | 3.16 ± 0.27 | 2.63 ± 0.43 | 2.73 ± 0.69 |
| LV MASS-I | 509.09 ± 57.12 | 490.18 ± 67.96 | 512.7 ± 82.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Huang, J.; Ren, W.; Meng, J.; Yao, X.; Chu, H.; Yao, R.; Zhai, M.; Zeng, Y. Cardiac–Metabolic Coupling Revealed by Lipid and Energy Metabolomics Determines 80 km Endurance Performance in Yili Horses. Biology 2025, 14, 1581. https://doi.org/10.3390/biology14111581
Wang T, Huang J, Ren W, Meng J, Yao X, Chu H, Yao R, Zhai M, Zeng Y. Cardiac–Metabolic Coupling Revealed by Lipid and Energy Metabolomics Determines 80 km Endurance Performance in Yili Horses. Biology. 2025; 14(11):1581. https://doi.org/10.3390/biology14111581
Chicago/Turabian StyleWang, Tongliang, Jinlong Huang, Wanlu Ren, Jun Meng, Xinkui Yao, Hongzhong Chu, Runchen Yao, Manjun Zhai, and Yaqi Zeng. 2025. "Cardiac–Metabolic Coupling Revealed by Lipid and Energy Metabolomics Determines 80 km Endurance Performance in Yili Horses" Biology 14, no. 11: 1581. https://doi.org/10.3390/biology14111581
APA StyleWang, T., Huang, J., Ren, W., Meng, J., Yao, X., Chu, H., Yao, R., Zhai, M., & Zeng, Y. (2025). Cardiac–Metabolic Coupling Revealed by Lipid and Energy Metabolomics Determines 80 km Endurance Performance in Yili Horses. Biology, 14(11), 1581. https://doi.org/10.3390/biology14111581






