Phase Separation Competent TIA1 Couples Glycolytic Shutdown to CD8+ T-Cell Activation and Shapes the Efficacy of Intravesical BCG in Bladder Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Reagents
2.3. Plasmids, siRNA, and Lentiviral Transduction
2.4. RIP-qPCR
2.5. CRISPR/Cas9-Mediated Generation of TIA1-Knock-Off (KO) Cell Lines
2.6. Proliferation, ECAR, and Lactate Assays
2.7. Human CD8+-T-Cell Isolation and Co-Culture
2.8. TIMER and Spatial-Omics Analyses
2.9. Survival Analyses
2.10. Orthotopic Tumor and Intravesical BCG Model
2.11. TIA1 Gain-of-Function Models
2.12. Flow Cytometry of Tumor-Infiltrating Lymphocytes
2.13. Ex Vivo NMIBC Single-Cell Assay
2.14. Immunohistochemistry
2.15. mCherry-BCG Uptake Assay and Immunofluorescence
2.16. Statistics
3. Results
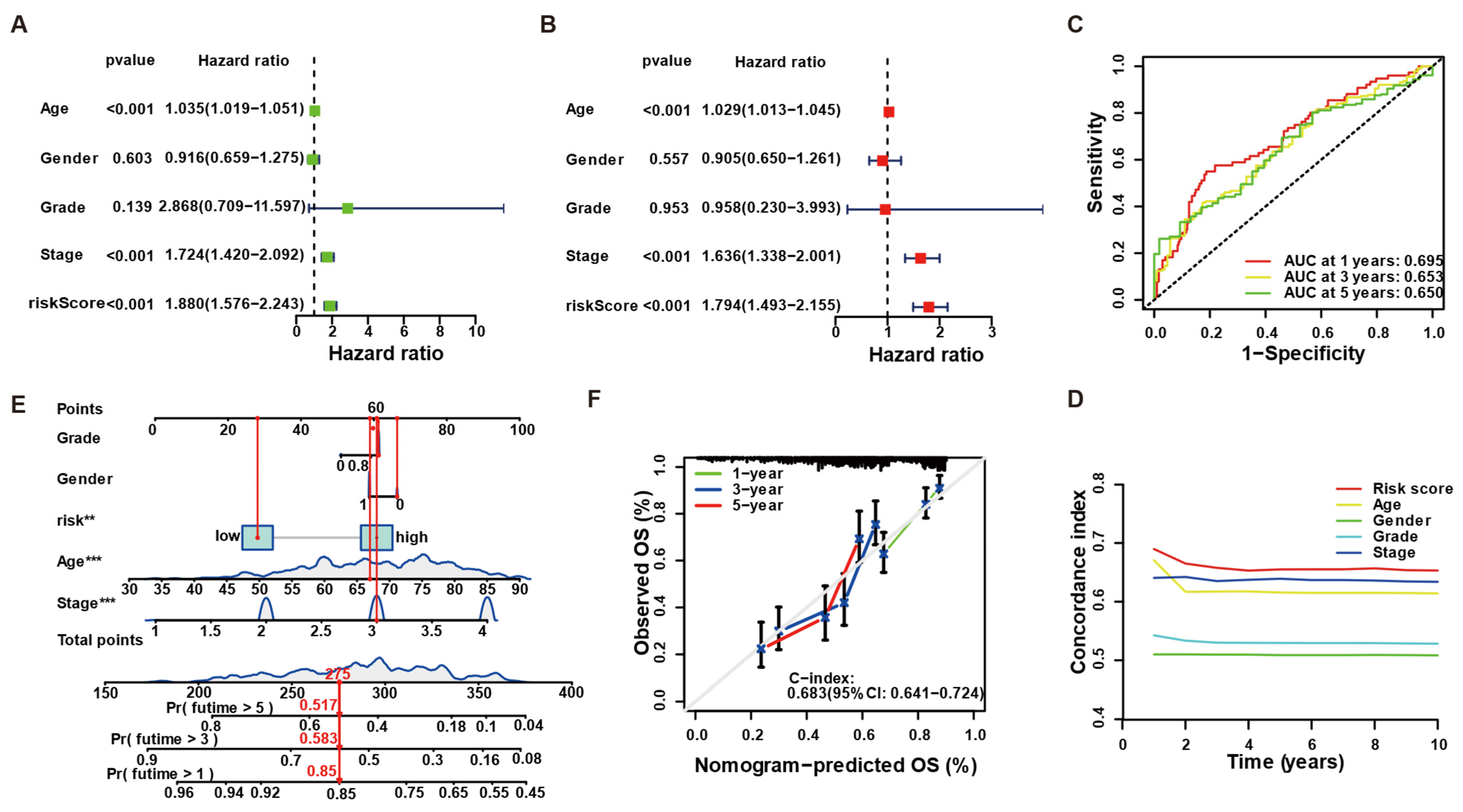
3.1. Construction of an LLPS-Driven Prognostic Model
3.2. The Four-Gene Signature Robustly Stratifies Patient Survival
3.3. LLPS-Related Risk Score Is an Independent Predictor and Improves Clinical Prognostication
3.4. Risk Group-Specific Transcriptional Programs Implicate Cytokine Signaling and Metabolic Homeostasis
3.5. TIA1 Suppresses Lactate Production and Glycolytic Enzyme Expression via Its Low-Complexity Domain
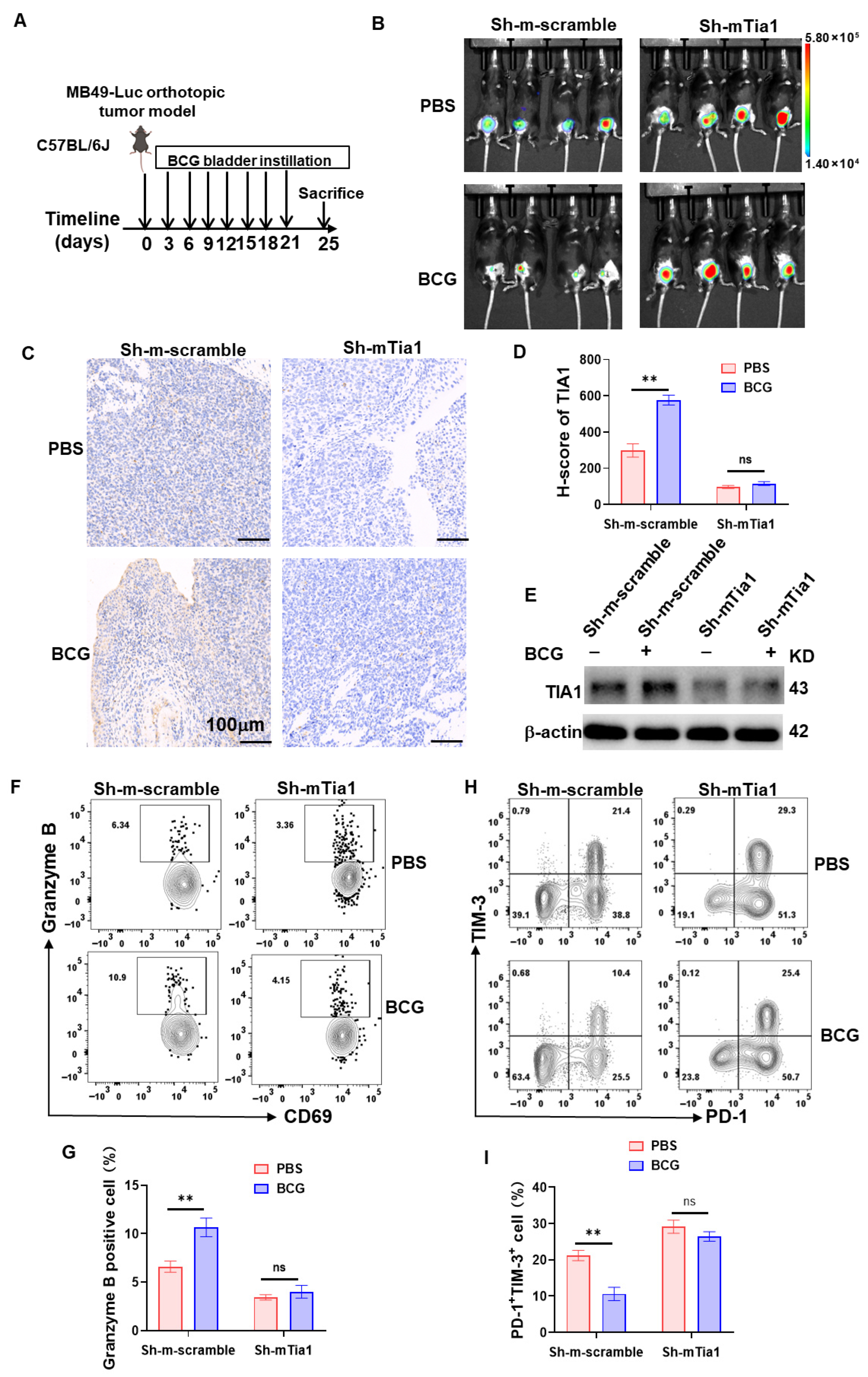
3.6. BCG Suppresses Tumor Glycolysis in a TIA1-Dependent Manner Across Human and Murine Bladder Cancer Models
3.7. TIA1 Is Associated with BCG-Linked Glycolytic Restraint and CD8+-T-Cell Activation in Bladder Cancer
3.8. Correlation and Spatial Convergence of TIA1 with Cytotoxic T-Cell Immunity
4. Discussion
5. Conclusions
6. Patients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guérin |
| NMIBC | Non-muscle-invasive bladder cancer |
| LCD/ΔLCD | Low-complexity domain/deletion of the low-complexity domain |
| LLPS | Liquid–liquid phase separation |
| SGs | Stress granules |
| TIA1 | T-cell-intracellular-antigen-1 |
| TIMER | Tumor Immune Estimation Resource |
| IHC | Immunohistochemistry |
| ECAR | Extracellular Acidification Rate |
References
- Jian, N.; Yu, L.; Ma, L.; Zheng, B.; Huang, W. BCG therapy in bladder cancer and its tumor microenvironment interactions. Clin. Microbiol. Rev. 2025, 38, e0021224. [Google Scholar] [CrossRef]
- Liatsos, G.D.; Mariolis, I.; Hadziyannis, E.; Bamias, A.; Vassilopoulos, D. Review of BCG immunotherapy for bladder cancer. Clin. Microbiol. Rev. 2025, 38, e0019423. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cookson, M.S.; Guercio, B.J.; Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ 2024, 384, e076743. [Google Scholar] [CrossRef]
- Shariat, S.F. Navigating the Challenges of BCG-Unresponsive Non-muscle-invasive Bladder Cancer: Insights and Future Directions. Eur. Urol. 2024, 86, 528–530. [Google Scholar] [CrossRef]
- Rouanne, M.; Adam, J.; Radulescu, C.; Letourneur, D.; Bredel, D.; Mouraud, S.; Goubet, A.G.; Leduc, M.; Chen, N.; Tan, T.Z.; et al. BCG therapy downregulates HLA-I on malignant cells to subvert antitumor immune responses in bladder cancer. J. Clin. Investig. 2022, 132, e145666. [Google Scholar] [CrossRef]
- Huang, S.; Pan, L.; Pang, S.; Guo, H.; Li, M.; Tian, Y.; Shi, W.; Liu, B.; Wang, S.; Fan, Z.; et al. Perforin Generated by CD8(+) T Cells Exacerbates Inflammatory Bowel Disease-Induced Depression by Promoting CXCL9 Production in Intestinal Epithelial Cells. Gastroenterology 2025, 169, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Meghani, K.; Cooley, L.F.; Choy, B.; Kocherginsky, M.; Swaminathan, S.; Munir, S.S.; Svatek, R.S.; Kuzel, T.; Meeks, J.J. First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur. Urol. 2022, 82, 602–610. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, Z.; Shu, G.; Yin, G.; Wang, M. Over-Expression of TNFRSF12A Promotes Immune Suppression and Facilitates Angiogenesis in Triple-Negative Breast Cancer. Biology 2025, 14, 1513. [Google Scholar] [CrossRef]
- Strandgaard, T.; Lindskrog, S.V.; Nordentoft, I.; Christensen, E.; Birkenkamp-Demtröder, K.; Andreasen, T.G.; Lamy, P.; Kjær, A.; Ranti, D.; Wang, Y.A.; et al. Elevated T-cell Exhaustion and Urinary Tumor DNA Levels Are Associated with Bacillus Calmette-Guérin Failure in Patients with Non-muscle-invasive Bladder Cancer. Eur. Urol. 2022, 82, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, S.; Jiao, S.; Fan, Y.; Li, X.; Tan, N.; Fang, J.; Xu, L.; Huang, Y.; Zhao, J.; et al. ATG5-regulated CCL2/MCP-1 production in myeloid cells selectively modulates anti-malarial CD4(+) Th1 responses. Autophagy 2024, 20, 1398–1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Liu, X. Identification of lactylation-associated immune and metabolic regulators in bladder cancer via integrated bulk and single-cell transcriptomics. Front. Immunol. 2025, 16, 1604758. [Google Scholar] [CrossRef]
- Zhi, H.; Yin, W.; Chen, S.; Zhang, X.; Yang, Z.; Man, F.; Li, R.; Cai, Y.; Li, Y.; You, C.; et al. Lactate metabolism regulating nanosystem synergizes cuproptosis and ferroptosis to enhance cancer immunotherapy. Biomaterials 2026, 325, 123538. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, B.; Wang, P.; Ma, Y.; Jiang, Y.; Mo, D.; Wu, J.; Ma, J.; Wang, X.; Miao, Y.; et al. N6-methyladenosine modification of 3’tRF-AlaAGC impairs PD-1 blockade efficacy by promoting lactic acid accumulation in the tumor microenvironment of gastric carcinoma. Drug Resist. Updat. 2025, 79, 101197. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, H.; Yin, J.; Zheng, Y.; Shi, H.; Pan, B.; Li, M.; Zhao, M.; Liang, J.; Bian, Y.; et al. NDRG1-Driven Lactate Accumulation Promotes Lung Adenocarcinoma Progression Through the Induction of an Immunosuppressive Microenvironment. Adv. Sci. 2025, 12, e01238. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.; Yang, W.; Liu, N.; Wang, J.; Li, Z.; Liu, T.; Yan, S.; He, W. Small-molecule-induced liquid-liquid phase separation suppresses the carcinogenesis of β-catenin. Nat. Commun. 2025, 16, 5997. [Google Scholar] [CrossRef]
- Ikenoue, T.; So, M.; Terasaka, N.; Huang, W.E.; Kawata, Y.; Miyanoiri, Y.; Kamagata, K.; Suga, H. De Novo Peptides That Induce the Liquid-Liquid Phase Separation of α-Synuclein. J. Am. Chem. Soc. 2025, 147, 24113–24126. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Tang, R.; Xu, J.; Wang, W.; Zhao, Y.; Yu, X.; Shi, S. Liquid-liquid phase separation in tumor biology. Signal Transduct. Target. Ther. 2022, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, J.; Cristea, I.M. Liquid-liquid phase separation in innate immunity. Trends Immunol. 2024, 45, 454–469. [Google Scholar] [CrossRef]
- Novakovic, M.; Han, Y.; Kathe, N.C.; Ni, Y.; Emmanouilidis, L.; Allain, F.H. LLPS REDIFINE allows the biophysical characterization of multicomponent condensates without tags or labels. Nat. Commun. 2025, 16, 4628. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhang, Z.; Liu, X.; Hu, B.; Tian, F.; Ye, Z.; Shi, L.; Yu, Z. In Situ Liquid-Liquid Phase Separation of Peptides Into Droplets Targeting Membraneless Organelles for Enhanced Cancer Chemotherapy. Adv. Mater. 2025, 37, e2420399. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, Z.; Yu, W.; Ma, Y.; Kim, K.; Rivera, N.; Mohammed, J.; Lantelme, E.; Hsu-Kim, H.; Chilkoti, A.; et al. Biomolecular condensates regulate cellular electrochemical equilibria. Cell 2024, 187, 5951–5966.e5918. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Lu, R.; Yu, A.J.; Yu, A.S.; Cao, Y.; Li, L.; Li, J.; Li, H.; Zhou, Z.; et al. PABPC1 SUMOylation enhances cell survival by promoting mitophagy through stabilizing U-rich mRNAs within stress granules. Nat. Commun. 2025, 16, 7308. [Google Scholar] [CrossRef]
- Hu, S.; Li, X.; Hu, Q.; Wang, C.; Hua, A.; Deng, G.; Huang, W.; Fu, X.; Zhou, H.; Zhang, X.; et al. Cancer Cell-Secreted miR-33a Reduces Stress Granule Formation by Targeting Polyamine Metabolism in Stroma to Promote Tumourigenesis. J. Extracell. Vesicles 2025, 14, e70153. [Google Scholar] [CrossRef] [PubMed]
- Dubinski, A.; Vande Velde, C. Dissolving stress granules. Nat. Chem. Biol. 2025, 21, 1481–1482. [Google Scholar] [CrossRef]
- Wei, Y.; Li, D.; Yang, R.; Liu, Y.; Luo, X.; Zhao, W.; Yang, H.; Chen, Z.; Shen, C.; Wang, Y.; et al. TIA1-mediated stress granules promote neurodegeneration by sequestering HSP70 mRNA in C9orf72 mice. Brain 2025, awaf248. [Google Scholar] [CrossRef]
- Hua, X.; Jin, L.; Fang, Z.; Weng, Y.; Zhang, Y.; Zhang, J.; Xie, D.; Tang, Y.; Guo, S.; Huang, Y.; et al. TIA1-Mediated Stress Granules Promote the Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis through Upregulating IL-31RA Signaling. Adv. Sci. 2025, 12, e2409086. [Google Scholar] [CrossRef]
- Ding, X.; Gu, S.; Xue, S.; Luo, S.Z. Disease-associated mutations affect TIA1 phase separation and aggregation in a proline-dependent manner. Brain Res. 2021, 1768, 147589. [Google Scholar] [CrossRef]
- West, D.L.; Loughlin, F.E.; Rivero-Rodríguez, F.; Vankadari, N.; Velázquez-Cruz, A.; Corrales-Guerrero, L.; Díaz-Moreno, I.; Wilce, J.A. Regulation of TIA-1 Condensates: Zn(2+) and RGG Motifs Promote Nucleic Acid Driven LLPS and Inhibit Irreversible Aggregation. Front. Mol. Biosci. 2022, 9, 960806. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Hsieh, S.M.; Hsieh, T.H.; Jhuang, J.Y.; Kao, Y.C. DUSP22-rearranged primary cutaneous CD30-positive T-cell lymphoproliferative disorders and adult T-cell leukemia/lymphoma frequently share the LEF1+/TIA1- immunophenotype. Hum. Pathol. 2024, 150, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, C.; Shan, Y.; Zhang, Y. RAMP2-AS1 stabilized RAPM2 mRNA through TIA1 to inhibit the progression of non-small cell lung cancer. Cell. Mol. Biol. 2023, 69, 9–14. [Google Scholar] [CrossRef]
- Li, J.; Ren, T.; Liu, R.; Zhang, H.; Wang, N.; Guo, Q.; Xu, L.; Ma, J. Orbital natural killer/T-cell lymphoma: A comprehensive case series and literature review. BMC Cancer 2025, 25, 372. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, L.; Wu, D.; Liu, S.; Pei, S.; Tang, Q.; Wang, Y.; Ou, M.; Zhu, Z.; Ruan, S.; et al. Significance of liquid-liquid phase separation (LLPS)-related genes in breast cancer: A multi-omics analysis. Aging 2023, 15, 5592–5610. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Mao, F. Single cell sequencing analysis and transcriptome analysis constructed the liquid-liquid phase separation(LLPS)-related prognostic model for endometrial cancer. Front. Oncol. 2022, 12, 1005472. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, H.; Ma, S.; Fan, X.; Guo, H.; Sun, M.; Wen, S.; Liu, T.; Yu, G.; Yuan, X.; et al. Functional screening of somatic mutant events in extranodal natural killer/T-cell lymphoma with adrenal involvement. Front. Immunol. 2025, 16, 1566794. [Google Scholar] [CrossRef]
- Bedke, J.; Black, P.C.; Szabados, B.; Guerrero-Ramos, F.; Shariat, S.F.; Xylinas, E.; Brinkmann, J.; Blake-Haskins, J.A.; Cesari, R.; Redorta, J.P. Optimizing outcomes for high-risk, non-muscle-invasive bladder cancer: The evolving role of PD-(L)1 inhibition. Urol. Oncol. 2023, 41, 461–475. [Google Scholar] [CrossRef]
- Jia, Q.; Masleša-Galić, S.; Nava, S.; Horwitz, M.A. Listeria-Vectored Multiantigenic Tuberculosis Vaccine Enhances Protective Immunity against Aerosol Challenge with Virulent Mycobacterium tuberculosis in BCG-Immunized C57BL/6 and BALB/c Mice. mBio 2022, 13, e0068722. [Google Scholar] [CrossRef]
- Burns, J.E.; Hurst, C.D.; Knowles, M.A.; Phillips, R.M.; Allison, S.J. The Warburg effect as a therapeutic target for bladder cancers and intratumoral heterogeneity in associated molecular targets. Cancer Sci. 2021, 112, 3822–3834. [Google Scholar] [CrossRef] [PubMed]
- Gwon, Y.; Maxwell, B.A.; Kolaitis, R.M.; Zhang, P.; Kim, H.J.; Taylor, J.P. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 2021, 372, eabf6548. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.M.; Tauber, D.; Parker, R. G3BP1 promotes intermolecular RNA-RNA interactions during RNA condensation. Mol. Cell 2025, 85, 571–584.e577. [Google Scholar] [CrossRef]
- Sobolewski, C.; Dubuquoy, L.; Legrand, N. MicroRNAs, Tristetraprolin Family Members and HuR: A Complex Interplay Controlling Cancer-Related Processes. Cancers 2022, 14, 3516. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ji, A.H.; Zhang, W.J.; Zhao, N. HuR, TTP, and miR-133b expression in NSCLC and their association with prognosis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2823. [Google Scholar] [PubMed]
- Li, W.J.; He, Y.H.; Yang, J.J.; Hu, G.S.; Lin, Y.A.; Ran, T.; Peng, B.L.; Xie, B.L.; Huang, M.F.; Gao, X.; et al. Profiling PRMT methylome reveals roles of hnRNPA1 arginine methylation in RNA splicing and cell growth. Nat. Commun. 2021, 12, 1946. [Google Scholar] [CrossRef]
- Zhang, Q.; Weng, W.; Gu, X.; Xiang, J.; Yang, Y.; Zhu, M.X.; Gu, W.; He, Z.; Li, Y. hnRNPA1 SUMOylation promotes cold hypersensitivity in chronic inflammatory pain by stabilizing TRPA1 mRNA. Cell Rep. 2023, 42, 113401. [Google Scholar] [CrossRef]
- Bommer, G.T.; Van Schaftingen, E.; Veiga-da-Cunha, M. Metabolite Repair Enzymes Control Metabolic Damage in Glycolysis. Trends Biochem. Sci. 2020, 45, 228–243. [Google Scholar] [CrossRef]
- Krieger, M.R.; Abrahamian, M.; He, K.L.; Atamdede, S.; Hakimjavadi, H.; Momcilovic, M.; Ostrow, D.; Maggo, S.D.; Tsang, Y.P.; Gai, X.; et al. Trafficking of mitochondrial double-stranded RNA from mitochondria to the cytosol. Life Sci. Alliance 2024, 7, e202302396. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhou, K.; Chen, P.; Du, X.; Liu, M. Phase Separation Competent TIA1 Couples Glycolytic Shutdown to CD8+ T-Cell Activation and Shapes the Efficacy of Intravesical BCG in Bladder Cancer. Biology 2025, 14, 1576. https://doi.org/10.3390/biology14111576
Zhang W, Zhou K, Chen P, Du X, Liu M. Phase Separation Competent TIA1 Couples Glycolytic Shutdown to CD8+ T-Cell Activation and Shapes the Efficacy of Intravesical BCG in Bladder Cancer. Biology. 2025; 14(11):1576. https://doi.org/10.3390/biology14111576
Chicago/Turabian StyleZhang, Wenwen, Kailiang Zhou, Pinru Chen, Xuanshuang Du, and Min Liu. 2025. "Phase Separation Competent TIA1 Couples Glycolytic Shutdown to CD8+ T-Cell Activation and Shapes the Efficacy of Intravesical BCG in Bladder Cancer" Biology 14, no. 11: 1576. https://doi.org/10.3390/biology14111576
APA StyleZhang, W., Zhou, K., Chen, P., Du, X., & Liu, M. (2025). Phase Separation Competent TIA1 Couples Glycolytic Shutdown to CD8+ T-Cell Activation and Shapes the Efficacy of Intravesical BCG in Bladder Cancer. Biology, 14(11), 1576. https://doi.org/10.3390/biology14111576




